Abstract
The asymmetric unit of the 5:3 title co-crystal of 2-amino-4-phenyl-5,6-dihydrobenzo[h]quinoline-3-carbonitrile and 3-amino-1-phenyl-9,10-dihydrophenanthrene-2,4-dicarbonitrile, 0.625C20H15N3.0.375C22H15N3, has the atoms of the fused-ring system and those of the amino, cyano and phenyl substitutents overlapped. The fused-ring system is buckled owing to the ethylene linkage in the central ring, the two flanking aromatic rings being twisted by 20.1 (1)°. This ethylene portion is disordered over two positions in a 1:1 ratio. The phenyl ring is twisted by 69.5 (1)° relative to the amino- and cyano-bearing aromatic ring. In the crystal, two molecules are linked by an N—H⋯N hydrogen bond, generating a a helical chain along [010].
Related literature
For the synthesis, see: Aly et al. (1991 ▶); Paul et al. (1998 ▶). For related structures, see: Asiri et al. (2011a
▶,b
▶).
Experimental
Crystal data
0.625C20H15N3·0.375C22H15N3
M r = 306.36
Orthorhombic,
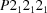
a = 6.9611 (2) Å
b = 12.6093 (2) Å
c = 17.4933 (3) Å
V = 1535.47 (6) Å3
Z = 4
Cu Kα radiation
μ = 0.62 mm−1
T = 100 K
0.30 × 0.20 × 0.02 mm
Data collection
Agilent SuperNova Dual diffractometer with an Atlas detector
Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2010 ▶) T min = 0.835, T max = 0.988
6293 measured reflections
1794 independent reflections
1707 reflections with I > 2σ(I)
R int = 0.018
Refinement
R[F 2 > 2σ(F 2)] = 0.040
wR(F 2) = 0.119
S = 1.05
1794 reflections
240 parameters
24 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.19 e Å−3
Δρmin = −0.23 e Å−3
Data collection: CrysAlis PRO (Agilent, 2010 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811040505/zs2145sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811040505/zs2145Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811040505/zs2145Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
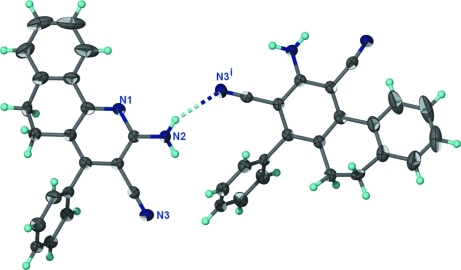
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H1⋯N3i | 0.88 (1) | 2.37 (2) | 3.175 (2) | 152 (3) |
Symmetry code: (i) 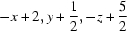 .
.
Acknowledgments
We thank King Abdulaziz University and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
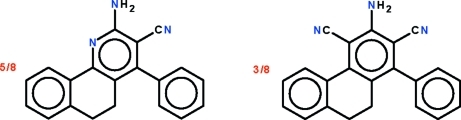
2-Amino-4-phenyl-5,6-dihydrobenzoquinoline-3-carbonitrile is synthesized from the reaction of the α-substituted cinnamonitrile, C6H5CH═C(CN)2, with α-tetralone in a reaction that is catalyzed by ammonium acetate (Aly et al., 1991). The synthesis when conducted under microwave irradiation leads to an improved yield (Paul et al., 1998). In previous studies, we obtained instead di-carbonitrile substituted dihydrophenanthrenes (3-amino-1-(4-methoxyphenyl)-9,10- dihydrophenanthrene-2,4-dicarbonitrile and 3-amino-1-(2H-1,3-benzodioxol-5-yl)- 9,10-dihydrophenanthrene-2,4-dicarbonitrile) with 4-methoxybenzaldehyde and piperonaldehyde in syntheses that differed slightly from the reported ones as we used substituted benzaldehydes, α-tetralone and ethyl cyanoacetate along with a molar excess of ammonium acetate (Asiri et al., 2011a; 2011b).
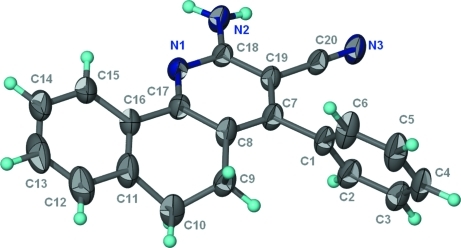
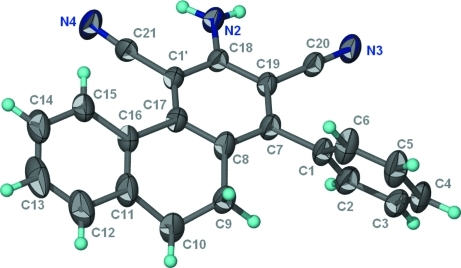
In this study, the reaction of benzaldehyde, α-tetralone and ethyl cyanoacetate yielded the co-crystal of 2-amino-4-phenyl-5,6-dihydrobenzoquinoline-3-carbonitrile (C20H15N3) and 3-amino-1-phenyl-9,10-dihydrophenanthrene-2,4-dicarbonitrile (C22H15N3), with the two components present in a 5: 3 molar ratio (Scheme I). The fused-ring system is buckled owing to the ethylene linkage in the central ring with the two flanking aromatic rings twisted by 20.1 (1)°. Relative to the amino- and cyano-bearing aromatic ring, the phenyl ring is twisted by 69.5 (1) ° (Fig. 1 and Fig. 2). Two molecules are linked by an N—H···N hydrogen bond to generate a helical chain (Table 1 and Fig. 3). The ethylene portion is disordered over two positions in a 1:1 ratio.
Experimental
A mixture of benzaldehyde (1.06 g,10 mmol), α-tetralone (1.46 g, 10 mmol), ethyl cyanoacetate (1.13 g, 10 mmol) and ammonium acetate (6.16 g, 80 mmol) in absolute ethanol (50 ml) was refluxed for 6 h. The mixture was allowed to cool and the precipitate that formed was filtered, washed with water, dried and recrystallized from DMF.
Refinement
Carbon-bound H-atoms were placed in calculated positions [C–H = 0.95–0.99 Å; Uiso(H) 1.2Ueq(C)] and were included in the refinement in the riding model approximation. The amino H-atoms were located in a difference Fourier map and were refined with a distance restraint of N—H = 0.88±0.01 Å and with their isotropic displacement parameters refined.
The crystal is a co-crystal of 2-amino-4-phenyl-5,6-dihydrobenzoquinoline-3-carbonitrile (C20H15N3) and 3-amino-1-phenyl-9,10-dihydrophenanthrene-2,4-dicarbonitrile (C22H15N3). The first component is a dihydrobenzoquinoline and has only one cyano substituent. The second component is a dihydrophenanthrene with two cyano substituents. The two-coordinate N atom of the first molecule occupies the same site as the three-coordinate C atom of the second molecule. As the occupancy refined to an almost 5:3 ratio, the occupancy was then fixed as this ratio. The ethylene –CH2CH2– portion (whose atoms lie on general positions) is disordered over two sites. The occupancy could not be refined, and was fixed as 1:1. The 1,2-connected carbon-carbon distances were restrained to 1.54±0.01 Å and the 1,3-related ones to 2.51±0.01 Å. The displacement parameters of the primed atoms were set to those of the unprimed ones, and the were restrained to be nearly isotropic. Despite the use of low temperature, copper radiation, long exposure times and a large number of redundant reflections, the Flack parameter could not be refined. 1252 Friedel pairs were merged.
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of C20H15N3 at the 70% probability level. Hydrogen atoms are drawn as spheres of arbitrary radius.
Fig. 2.
Thermal ellipsoid plot (Barbour, 2001) of C22H15N3 at the 70% probability level. Hydrogen atoms are drawn as spheres of arbitrary radius.
Fig. 3.
Thermal ellipsoid plot (Barbour, 2001) of C20H15N3 (62.5% component) and C22H15N3 (37.5% component) related by twofold screw axial symmetry. For symmetry code (i), see Table 1.
Crystal data
| 0.625C20H15N3·0.375C22H15N3 | F(000) = 642 |
| Mr = 306.36 | Dx = 1.325 Mg m−3 |
| Orthorhombic, P212121 | Cu Kα radiation, λ = 1.54184 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 3717 reflections |
| a = 6.9611 (2) Å | θ = 3.5–74.3° |
| b = 12.6093 (2) Å | µ = 0.62 mm−1 |
| c = 17.4933 (3) Å | T = 100 K |
| V = 1535.47 (6) Å3 | Plate, brown-orange |
| Z = 4 | 0.30 × 0.20 × 0.02 mm |
Data collection
| Agilent SuperNova Dual diffractometer with an Atlas detector | 1794 independent reflections |
| Radiation source: SuperNova (Cu) X-ray Source | 1707 reflections with I > 2σ(I) |
| Mirror | Rint = 0.018 |
| Detector resolution: 10.4041 pixels mm-1 | θmax = 74.5°, θmin = 4.3° |
| ω scans | h = −8→7 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2010) | k = −13→15 |
| Tmin = 0.835, Tmax = 0.988 | l = −21→20 |
| 6293 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.040 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.119 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0796P)2 + 0.2871P] where P = (Fo2 + 2Fc2)/3 |
| 1794 reflections | (Δ/σ)max = 0.001 |
| 240 parameters | Δρmax = 0.19 e Å−3 |
| 24 restraints | Δρmin = −0.23 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| N1 | 0.9898 (3) | 0.26050 (14) | 1.05516 (10) | 0.0294 (4) | 0.625 |
| C1' | 0.9898 (3) | 0.26050 (14) | 1.05516 (10) | 0.0294 (4) | 0.375 |
| N2 | 1.0082 (3) | 0.28041 (13) | 1.18789 (10) | 0.0339 (4) | |
| H1 | 1.035 (5) | 0.3483 (10) | 1.1827 (17) | 0.052 (8)* | |
| H2 | 1.031 (4) | 0.254 (2) | 1.2335 (9) | 0.045 (8)* | |
| N3 | 0.9475 (4) | 0.02407 (14) | 1.26992 (10) | 0.0396 (5) | |
| C1 | 0.9186 (4) | −0.07603 (16) | 1.08021 (11) | 0.0369 (5) | |
| C2 | 1.0684 (4) | −0.13801 (17) | 1.10772 (12) | 0.0414 (6) | |
| H2A | 1.1883 | −0.1063 | 1.1200 | 0.050* | |
| C3 | 1.0422 (5) | −0.24706 (17) | 1.11721 (12) | 0.0463 (7) | |
| H3 | 1.1450 | −0.2896 | 1.1355 | 0.056* | |
| C4 | 0.8687 (5) | −0.29307 (17) | 1.10017 (12) | 0.0500 (8) | |
| H4 | 0.8513 | −0.3671 | 1.1075 | 0.060* | |
| C5 | 0.7187 (5) | −0.23191 (18) | 1.07229 (14) | 0.0521 (7) | |
| H5 | 0.5990 | −0.2641 | 1.0603 | 0.063* | |
| C6 | 0.7439 (5) | −0.12301 (18) | 1.06183 (13) | 0.0478 (6) | |
| H6 | 0.6418 | −0.0811 | 1.0422 | 0.057* | |
| C7 | 0.9453 (4) | 0.04111 (15) | 1.06991 (11) | 0.0336 (5) | |
| C8 | 0.9536 (4) | 0.08663 (16) | 0.99730 (11) | 0.0391 (6) | |
| C9 | 0.9790 (10) | 0.0170 (5) | 0.9251 (4) | 0.0390 (15) | 0.50 |
| H9A | 1.0343 | −0.0525 | 0.9396 | 0.047* | 0.50 |
| H9B | 0.8524 | 0.0043 | 0.9010 | 0.047* | 0.50 |
| C10 | 1.1119 (9) | 0.0727 (5) | 0.8684 (3) | 0.0440 (15) | 0.50 |
| H10A | 1.1216 | 0.0310 | 0.8206 | 0.053* | 0.50 |
| H10B | 1.2422 | 0.0801 | 0.8905 | 0.053* | 0.50 |
| C9' | 0.9024 (9) | 0.0270 (5) | 0.9245 (4) | 0.0390 (15) | 0.50 |
| H9'C | 0.9220 | −0.0500 | 0.9322 | 0.047* | 0.50 |
| H9'D | 0.7656 | 0.0390 | 0.9117 | 0.047* | 0.50 |
| C10' | 1.0290 (10) | 0.0659 (5) | 0.8592 (3) | 0.0440 (15) | 0.50 |
| H10C | 1.1628 | 0.0426 | 0.8683 | 0.053* | 0.50 |
| H10D | 0.9847 | 0.0335 | 0.8108 | 0.053* | 0.50 |
| C11 | 1.0247 (5) | 0.18419 (19) | 0.85163 (13) | 0.0485 (7) | |
| C12 | 1.0305 (5) | 0.2290 (2) | 0.77912 (15) | 0.0561 (7) | |
| H12 | 1.0608 | 0.1859 | 0.7362 | 0.067* | |
| C13 | 0.9929 (5) | 0.3351 (3) | 0.76887 (17) | 0.0606 (8) | |
| H13 | 0.9963 | 0.3651 | 0.7191 | 0.073* | |
| C14 | 0.9497 (5) | 0.3981 (3) | 0.8318 (2) | 0.0699 (10) | |
| H14 | 0.9246 | 0.4716 | 0.8250 | 0.084* | |
| C15 | 0.9431 (5) | 0.3542 (2) | 0.90409 (18) | 0.0593 (9) | |
| H15 | 0.9134 | 0.3976 | 0.9469 | 0.071* | |
| C16 | 0.9798 (4) | 0.24669 (16) | 0.91469 (12) | 0.0355 (5) | |
| C17 | 0.9738 (3) | 0.19760 (15) | 0.99194 (12) | 0.0331 (5) | |
| C18 | 0.9871 (3) | 0.21628 (14) | 1.12653 (11) | 0.0281 (4) | |
| C19 | 0.9622 (3) | 0.10550 (15) | 1.13457 (11) | 0.0291 (4) | |
| C20 | 0.9531 (4) | 0.05995 (15) | 1.20987 (11) | 0.0308 (5) | |
| N4 | 1.0072 (11) | 0.4629 (4) | 1.0526 (3) | 0.0471 (15) | 0.375 |
| C21 | 0.9965 (10) | 0.3721 (4) | 1.0492 (3) | 0.0337 (12) | 0.375 |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0427 (10) | 0.0207 (8) | 0.0249 (8) | 0.0010 (8) | 0.0045 (8) | 0.0015 (7) |
| C1' | 0.0427 (10) | 0.0207 (8) | 0.0249 (8) | 0.0010 (8) | 0.0045 (8) | 0.0015 (7) |
| N2 | 0.0607 (12) | 0.0210 (7) | 0.0200 (7) | −0.0002 (9) | −0.0011 (8) | −0.0010 (6) |
| N3 | 0.0672 (14) | 0.0253 (8) | 0.0263 (8) | 0.0000 (9) | −0.0015 (9) | 0.0044 (7) |
| C1 | 0.0703 (16) | 0.0214 (9) | 0.0189 (8) | −0.0041 (10) | −0.0016 (10) | 0.0008 (7) |
| C2 | 0.0705 (16) | 0.0260 (10) | 0.0277 (9) | 0.0011 (11) | −0.0012 (11) | −0.0002 (8) |
| C3 | 0.087 (2) | 0.0257 (10) | 0.0259 (10) | 0.0058 (12) | 0.0027 (12) | 0.0009 (8) |
| C4 | 0.106 (2) | 0.0201 (9) | 0.0239 (10) | −0.0060 (13) | 0.0035 (12) | 0.0016 (8) |
| C5 | 0.089 (2) | 0.0300 (10) | 0.0370 (12) | −0.0174 (13) | −0.0096 (13) | 0.0029 (10) |
| C6 | 0.0785 (18) | 0.0283 (10) | 0.0365 (11) | −0.0092 (12) | −0.0159 (13) | 0.0053 (9) |
| C7 | 0.0539 (13) | 0.0211 (9) | 0.0258 (9) | −0.0025 (9) | −0.0007 (10) | 0.0002 (8) |
| C8 | 0.0704 (16) | 0.0228 (9) | 0.0241 (9) | −0.0043 (11) | −0.0002 (11) | 0.0017 (8) |
| C9 | 0.069 (4) | 0.0245 (14) | 0.0237 (10) | 0.001 (3) | −0.002 (3) | 0.0004 (10) |
| C10 | 0.082 (5) | 0.0283 (13) | 0.0211 (15) | −0.013 (3) | −0.001 (2) | −0.0071 (11) |
| C9' | 0.069 (4) | 0.0245 (14) | 0.0237 (10) | 0.001 (3) | −0.002 (3) | 0.0004 (10) |
| C10' | 0.082 (5) | 0.0283 (13) | 0.0211 (15) | −0.013 (3) | −0.001 (2) | −0.0071 (11) |
| C11 | 0.0804 (19) | 0.0357 (11) | 0.0293 (11) | −0.0080 (14) | 0.0008 (12) | 0.0083 (9) |
| C12 | 0.080 (2) | 0.0562 (15) | 0.0321 (12) | −0.0134 (16) | 0.0003 (13) | 0.0126 (11) |
| C13 | 0.0538 (14) | 0.0720 (19) | 0.0559 (16) | 0.0019 (16) | 0.0020 (14) | 0.0404 (15) |
| C14 | 0.0675 (19) | 0.0549 (16) | 0.087 (2) | 0.0299 (16) | 0.0384 (18) | 0.0452 (16) |
| C15 | 0.0674 (18) | 0.0408 (13) | 0.0698 (18) | 0.0201 (14) | 0.0372 (16) | 0.0274 (13) |
| C16 | 0.0429 (11) | 0.0291 (10) | 0.0347 (11) | 0.0004 (10) | 0.0053 (9) | 0.0096 (9) |
| C17 | 0.0456 (11) | 0.0240 (9) | 0.0297 (10) | 0.0003 (10) | 0.0041 (10) | 0.0026 (8) |
| C18 | 0.0405 (11) | 0.0215 (8) | 0.0224 (9) | 0.0005 (9) | 0.0001 (8) | −0.0002 (7) |
| C19 | 0.0432 (11) | 0.0224 (9) | 0.0218 (9) | −0.0005 (9) | 0.0001 (9) | 0.0028 (7) |
| C20 | 0.0480 (12) | 0.0195 (8) | 0.0249 (9) | −0.0013 (9) | −0.0017 (9) | −0.0011 (7) |
| N4 | 0.094 (5) | 0.021 (2) | 0.026 (2) | −0.002 (3) | 0.001 (3) | −0.0014 (18) |
| C21 | 0.055 (3) | 0.027 (3) | 0.018 (2) | 0.003 (3) | 0.005 (2) | −0.0001 (19) |
Geometric parameters (Å, °)
| N1—C17 | 1.366 (3) | C9—H9B | 0.9900 |
| N1—C18 | 1.367 (2) | C10—C11 | 1.560 (7) |
| N2—C18 | 1.352 (2) | C10—H10A | 0.9900 |
| N2—H1 | 0.88 (1) | C10—H10B | 0.9900 |
| N2—H2 | 0.88 (1) | C9'—C10' | 1.523 (7) |
| N3—C20 | 1.144 (3) | C9'—H9'C | 0.9900 |
| C1—C6 | 1.391 (4) | C9'—H9'D | 0.9900 |
| C1—C2 | 1.389 (4) | C10'—C11 | 1.498 (7) |
| C1—C7 | 1.499 (3) | C10'—H10C | 0.9900 |
| C2—C3 | 1.397 (3) | C10'—H10D | 0.9900 |
| C2—H2A | 0.9500 | C11—C12 | 1.389 (3) |
| C3—C4 | 1.373 (4) | C11—C16 | 1.391 (3) |
| C3—H3 | 0.9500 | C12—C13 | 1.375 (4) |
| C4—C5 | 1.387 (4) | C12—H12 | 0.9500 |
| C4—H4 | 0.9500 | C13—C14 | 1.390 (5) |
| C5—C6 | 1.396 (3) | C13—H13 | 0.9500 |
| C5—H5 | 0.9500 | C14—C15 | 1.382 (4) |
| C6—H6 | 0.9500 | C14—H14 | 0.9500 |
| C7—C19 | 1.397 (3) | C15—C16 | 1.392 (3) |
| C7—C8 | 1.395 (3) | C15—H15 | 0.9500 |
| C8—C17 | 1.410 (3) | C16—C17 | 1.487 (3) |
| C8—C9' | 1.521 (7) | C18—C19 | 1.415 (2) |
| C8—C9 | 1.548 (7) | C19—C20 | 1.438 (3) |
| C9—C10 | 1.527 (7) | N4—C21 | 1.149 (7) |
| C9—H9A | 0.9900 | ||
| C17—N1—C18 | 120.11 (16) | C10'—C9'—C8 | 109.5 (4) |
| C18—N2—H1 | 122 (2) | C10'—C9'—H9'C | 109.8 |
| C18—N2—H2 | 120.8 (19) | C8—C9'—H9'C | 109.8 |
| H1—N2—H2 | 115 (3) | C10'—C9'—H9'D | 109.8 |
| C6—C1—C2 | 119.8 (2) | C8—C9'—H9'D | 109.8 |
| C6—C1—C7 | 120.0 (2) | H9'C—C9'—H9'D | 108.2 |
| C2—C1—C7 | 120.2 (2) | C11—C10'—C9' | 112.1 (5) |
| C1—C2—C3 | 119.8 (3) | C11—C10'—H10C | 109.2 |
| C1—C2—H2A | 120.1 | C9'—C10'—H10C | 109.2 |
| C3—C2—H2A | 120.1 | C11—C10'—H10D | 109.2 |
| C4—C3—C2 | 120.3 (3) | C9'—C10'—H10D | 109.2 |
| C4—C3—H3 | 119.8 | H10C—C10'—H10D | 107.9 |
| C2—C3—H3 | 119.8 | C12—C11—C16 | 120.0 (2) |
| C3—C4—C5 | 120.2 (2) | C12—C11—C10' | 119.0 (3) |
| C3—C4—H4 | 119.9 | C16—C11—C10' | 119.9 (3) |
| C5—C4—H4 | 119.9 | C12—C11—C10 | 121.8 (3) |
| C4—C5—C6 | 119.9 (3) | C16—C11—C10 | 116.7 (3) |
| C4—C5—H5 | 120.0 | C13—C12—C11 | 120.6 (3) |
| C6—C5—H5 | 120.0 | C13—C12—H12 | 119.7 |
| C1—C6—C5 | 119.9 (3) | C11—C12—H12 | 119.7 |
| C1—C6—H6 | 120.1 | C12—C13—C14 | 119.6 (2) |
| C5—C6—H6 | 120.1 | C12—C13—H13 | 120.2 |
| C19—C7—C8 | 119.64 (17) | C14—C13—H13 | 120.2 |
| C19—C7—C1 | 119.05 (17) | C15—C14—C13 | 120.1 (3) |
| C8—C7—C1 | 121.31 (17) | C15—C14—H14 | 119.9 |
| C7—C8—C17 | 118.24 (18) | C13—C14—H14 | 119.9 |
| C7—C8—C9' | 123.3 (3) | C14—C15—C16 | 120.4 (3) |
| C17—C8—C9' | 117.3 (3) | C14—C15—H15 | 119.8 |
| C7—C8—C9 | 120.9 (3) | C16—C15—H15 | 119.8 |
| C17—C8—C9 | 119.8 (3) | C15—C16—C11 | 119.2 (2) |
| C10—C9—C8 | 109.7 (5) | C15—C16—C17 | 121.4 (2) |
| C10—C9—H9A | 109.7 | C11—C16—C17 | 119.40 (18) |
| C8—C9—H9A | 109.7 | N1—C17—C8 | 122.06 (18) |
| C10—C9—H9B | 109.7 | N1—C17—C16 | 119.48 (18) |
| C8—C9—H9B | 109.7 | C8—C17—C16 | 118.46 (18) |
| H9A—C9—H9B | 108.2 | N2—C18—N1 | 118.65 (16) |
| C9—C10—C11 | 107.5 (5) | N2—C18—C19 | 121.67 (16) |
| C9—C10—H10A | 110.2 | N1—C18—C19 | 119.68 (17) |
| C11—C10—H10A | 110.2 | C7—C19—C18 | 120.24 (17) |
| C9—C10—H10B | 110.2 | C7—C19—C20 | 120.37 (17) |
| C11—C10—H10B | 110.2 | C18—C19—C20 | 119.39 (17) |
| H10A—C10—H10B | 108.5 | N3—C20—C19 | 179.3 (3) |
| C6—C1—C2—C3 | −0.4 (3) | C10—C11—C12—C13 | −165.4 (4) |
| C7—C1—C2—C3 | 179.9 (2) | C11—C12—C13—C14 | 0.4 (5) |
| C1—C2—C3—C4 | −0.6 (3) | C12—C13—C14—C15 | −0.5 (5) |
| C2—C3—C4—C5 | 1.0 (4) | C13—C14—C15—C16 | 0.1 (5) |
| C3—C4—C5—C6 | −0.3 (4) | C14—C15—C16—C11 | 0.5 (5) |
| C2—C1—C6—C5 | 1.1 (4) | C14—C15—C16—C17 | 179.9 (3) |
| C7—C1—C6—C5 | −179.3 (2) | C12—C11—C16—C15 | −0.7 (5) |
| C4—C5—C6—C1 | −0.7 (4) | C10'—C11—C16—C15 | −168.7 (4) |
| C6—C1—C7—C19 | 110.6 (3) | C10—C11—C16—C15 | 165.6 (3) |
| C2—C1—C7—C19 | −69.8 (3) | C12—C11—C16—C17 | 179.9 (3) |
| C6—C1—C7—C8 | −69.5 (3) | C10'—C11—C16—C17 | 11.8 (5) |
| C2—C1—C7—C8 | 110.1 (3) | C10—C11—C16—C17 | −13.8 (4) |
| C19—C7—C8—C17 | −1.7 (4) | C18—N1—C17—C8 | 0.2 (4) |
| C1—C7—C8—C17 | 178.4 (2) | C18—N1—C17—C16 | −179.2 (2) |
| C19—C7—C8—C9' | −169.0 (4) | C7—C8—C17—N1 | 1.5 (4) |
| C1—C7—C8—C9' | 11.0 (5) | C9'—C8—C17—N1 | 169.6 (3) |
| C19—C7—C8—C9 | 166.8 (4) | C9—C8—C17—N1 | −167.1 (4) |
| C1—C7—C8—C9 | −13.1 (5) | C7—C8—C17—C16 | −179.1 (2) |
| C7—C8—C9—C10 | −141.5 (4) | C9'—C8—C17—C16 | −11.0 (4) |
| C17—C8—C9—C10 | 26.9 (6) | C9—C8—C17—C16 | 12.3 (5) |
| C9'—C8—C9—C10 | 115.4 (13) | C15—C16—C17—N1 | −19.7 (4) |
| C8—C9—C10—C11 | −55.9 (6) | C11—C16—C17—N1 | 159.7 (2) |
| C7—C8—C9'—C10' | −146.4 (4) | C15—C16—C17—C8 | 160.9 (3) |
| C17—C8—C9'—C10' | 46.2 (6) | C11—C16—C17—C8 | −19.7 (4) |
| C9—C8—C9'—C10' | −56.5 (11) | C17—N1—C18—N2 | 178.3 (2) |
| C8—C9'—C10'—C11 | −52.0 (6) | C17—N1—C18—C19 | −1.8 (3) |
| C9'—C10'—C11—C12 | −143.3 (4) | C8—C7—C19—C18 | 0.2 (4) |
| C9'—C10'—C11—C16 | 24.9 (6) | C1—C7—C19—C18 | −179.9 (2) |
| C9'—C10'—C11—C10 | 113.0 (11) | C8—C7—C19—C20 | 179.7 (2) |
| C9—C10—C11—C12 | −142.0 (4) | C1—C7—C19—C20 | −0.4 (3) |
| C9—C10—C11—C16 | 51.9 (5) | N2—C18—C19—C7 | −178.5 (2) |
| C9—C10—C11—C10' | −52.2 (9) | N1—C18—C19—C7 | 1.6 (3) |
| C16—C11—C12—C13 | 0.2 (5) | N2—C18—C19—C20 | 2.0 (4) |
| C10'—C11—C12—C13 | 168.4 (4) | N1—C18—C19—C20 | −177.9 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H1···N3i | 0.88 (1) | 2.37 (2) | 3.175 (2) | 152 (3) |
Symmetry codes: (i) −x+2, y+1/2, −z+5/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: ZS2145).
References
- Agilent (2010). CrysAlis PRO Agilent Technologies, Yarnton, England.
- Aly, A. S., El-Ezabawy, S. R. & Abdel-Fattah, A. M. (1991). Egypt. J. Pharm. Sci. 32, 827–834.
- Asiri, A. M., Al-Youbi, A. O., Faidallah, H. M., Ng, S. W. & Tiekink, E. R. T. (2011a). Acta Cryst. E67, o2438. [DOI] [PMC free article] [PubMed]
- Asiri, A. M., Al-Youbi, A. O., Faidallah, H. M., Ng, S. W. & Tiekink, E. R. T. (2011b). Acta Cryst. E67, o2449. [DOI] [PMC free article] [PubMed]
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
- Paul, S., Gupta, R. & Loupy, A. (1998). J. Chem. Res. (S), pp. 330–331.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811040505/zs2145sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811040505/zs2145Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811040505/zs2145Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report