Abstract
In the title compound, C36H32N2O4S, the piperidine ring adopts a chair conformation, while the five-membered pyrrolidine (with a C atom as the flap atom) and thiazolidine (with the S atom as the flap atom) rings adopt envelope conformations. The naphthalene ring system makes dihedral angles of 18.82 (5) and 40.92 (5)° with the two methoxy-substituted benzene rings. In the crystal, centrosymmetrically-related molecules are linked into dimers via pairs of C—H⋯O and C—H⋯N hydrogen bonds. An intramolecular O—H⋯N hydrogen bond is also observed. The crystal structure is further stabilized by C—H⋯π interactions.
Related literature
For details of cycloaddition, see: Tsuge & Kanemasa (1989 ▶); Nair & Suja (2007 ▶); Aicher et al. (1998 ▶); Lalezari & Schwartz (1988 ▶). For ring conformations, see: Cremer & Pople (1975 ▶). For the stability of the temperature controller used in the data collection, see: Cosier & Glazer (1986 ▶).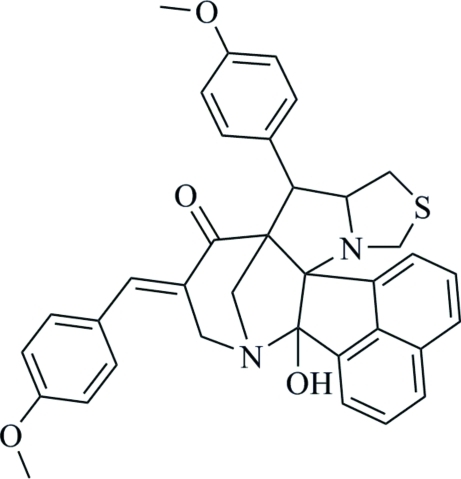
Experimental
Crystal data
C36H32N2O4S
M r = 588.70
Triclinic,

a = 10.6287 (1) Å
b = 11.8672 (2) Å
c = 12.6588 (2) Å
α = 84.439 (1)°
β = 75.105 (1)°
γ = 68.553 (1)°
V = 1436.19 (4) Å3
Z = 2
Mo Kα radiation
μ = 0.16 mm−1
T = 100 K
0.37 × 0.25 × 0.16 mm
Data collection
Bruker SMART APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.943, T max = 0.975
39492 measured reflections
11591 independent reflections
9231 reflections with I > 2σ(I)
R int = 0.036
Refinement
R[F 2 > 2σ(F 2)] = 0.050
wR(F 2) = 0.142
S = 1.02
11591 reflections
394 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.66 e Å−3
Δρmin = −0.38 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S160053681104061X/hb6431sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681104061X/hb6431Isup2.hkl
Supplementary material file. DOI: 10.1107/S160053681104061X/hb6431Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg7 and Cg9 centroids of the C20–C25 and C31–C36 rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1O1⋯N2 | 0.86 (2) | 1.96 (2) | 2.6253 (14) | 133.3 (19) |
| C24—H24A⋯N1i | 0.95 | 2.61 | 3.4447 (15) | 146 |
| C26—H26C⋯O1i | 0.98 | 2.48 | 3.4078 (17) | 157 |
| C19—H19B⋯Cg7ii | 0.98 | 2.82 | 3.4652 (18) | 124 |
| C26—H26B⋯Cg7iii | 0.98 | 2.87 | 3.7720 (14) | 154 |
| C9—H9B⋯Cg9iv | 0.99 | 2.87 | 3.8211 (14) | 161 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Acknowledgments
RSK, HO and ASA thank Universiti Sains Malaysia (USM) for the University Research Grant No. 203/PKIMIA/6711179 and the Ministry of Science, Technology and Innovation Grant No. 09-05-lfn-meb-004. RSK also thanks USM for the award of a post-doctoral fellowship. HKF and MH thank the Malaysian Government and USM for the Research University Grant No. 1001/PFIZIK/811160. MH also thanks USM for a post-doctoral research fellowship.
supplementary crystallographic information
Comment
Three-component reactions involving [3+2]-cycloaddition of azomethine ylides to olefinic dipolarophiles constitutes a facile approach for the construction of five membered heterocyclic rings of biological importance (Tsuge & Kanemasa, 1989; Nair & Suja, 2007). Among these heterocycles, pyrrolo[2,1-b]thiazole is an unusual ring system with antineoplastic (Lalezari & Schwartz, 1988) and hypoglycemic (Aicher et al., 1998) activities. In this paper we wish to report the crystal structure determination of the title compound possessing the biologically-active pyrrolothiazole ring.
The asymmetric unit of the title compound is shown in Fig. 1. The six-membered piperidine (N1/C1–C5) ring adopts a chair conformation [Q = 0.6172 (12) Å; θ = 138.18 (11)° and φ = 119.73 (17)°; Cremer & Pople, 1975] while the five-membered pyrrolidine (N2/C4/C6,C7/C10) and thiazolidine (S1/N2/C7–C9) rings adopt an envelope conformation with the C6 (displacement -0.217 (1) Å) and the S1 (displacement 0.284 (1) Å) atoms as the flap atoms and with puckering parameters, Q = 0.3565 (13) Å; φ = 89.37 (19)°; and Q = 0.5087 (11) Å; and φ = 0.44 (14)° repectively. The naphthalene (C27–C36) ring makes dihedral angles of 18.82 (5)° and 40.92 (5)° with the two methoxy-substituted (C13–C18)/(C20–C25) phenyl rings.
In the crystal structure, (Fig. 2), the centrosymmetrically-related molecules are linked into dimers via pairs of intermolecular C—H···O and C—H···N (Table 1) hydrogen bonds. An intramolecular O—H..N hydrogen bond is also observed. The crystal structure is further stabilized by weak C—H···π interactions involving the centroids Cg7 and Cg9 of the (C20–C25) and (C31–C36) rings, respectively.
Experimental
A mixture of 3,5-bis[(E)-(4-methoxyphenyl) methylidene]-tetrahydro-4(1H)-pyridinone (1 mmol), acenaphthenequinone (1 mmol), and thiazolidine-4-carboxylic acid (1 mmol) were dissolved in methanol (5 mL) and refluxed for 1 hour. After completion of the reaction as evident from TLC, the mixture was poured into water (50 mL). The precipitated solid was filtered and washed with water to obtain the product which was further purified by recrystallisation from pet.ether-ethylacetate mixture to yield colourless blocks of (I).
Refinement
Atom H1O1 was located from a difference Fourier maps and refined freely [O–H = 0.86 (2) Å]. The remaining H atoms were positioned geometrically [C–H = 0.95–1.00 Å] and were refined using a riding model, with Uiso(H) = 1.2 or 1.5 Ueq(C). A rotating group model was applied to the methyl groups.
Figures
Fig. 1.
The asymmetric unit of the title compound, showing 30% probability displacement ellipsoids.
Fig. 2.
The crystal packing of the title compound (I). H atoms not involved in hydrogen bonding are omitted.
Crystal data
| C36H32N2O4S | Z = 2 |
| Mr = 588.70 | F(000) = 620 |
| Triclinic, P1 | Dx = 1.361 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.6287 (1) Å | Cell parameters from 9888 reflections |
| b = 11.8672 (2) Å | θ = 2.3–35.1° |
| c = 12.6588 (2) Å | µ = 0.16 mm−1 |
| α = 84.439 (1)° | T = 100 K |
| β = 75.105 (1)° | Block, colourless |
| γ = 68.553 (1)° | 0.37 × 0.25 × 0.16 mm |
| V = 1436.19 (4) Å3 |
Data collection
| Bruker SMART APEXII CCD diffractometer | 11591 independent reflections |
| Radiation source: fine-focus sealed tube | 9231 reflections with I > 2σ(I) |
| graphite | Rint = 0.036 |
| φ and ω scans | θmax = 34.0°, θmin = 1.7° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −13→16 |
| Tmin = 0.943, Tmax = 0.975 | k = −18→18 |
| 39492 measured reflections | l = −19→19 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.050 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.142 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0751P)2 + 0.5144P] where P = (Fo2 + 2Fc2)/3 |
| 11591 reflections | (Δ/σ)max = 0.001 |
| 394 parameters | Δρmax = 0.66 e Å−3 |
| 0 restraints | Δρmin = −0.38 e Å−3 |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cryosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 100.0 (1) K. |
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.79522 (3) | 0.60158 (3) | 0.48963 (2) | 0.02192 (7) | |
| O1 | 0.36989 (8) | 0.50498 (7) | 0.72474 (7) | 0.01646 (15) | |
| O2 | 0.46670 (9) | 0.92470 (8) | 0.78875 (8) | 0.02136 (17) | |
| O3 | −0.38126 (10) | 1.24757 (10) | 0.79511 (9) | 0.0306 (2) | |
| O4 | 0.89106 (10) | 0.68108 (9) | 1.10873 (8) | 0.02435 (19) | |
| N1 | 0.28341 (9) | 0.66632 (8) | 0.84751 (8) | 0.01489 (16) | |
| N2 | 0.58835 (9) | 0.56730 (9) | 0.64184 (8) | 0.01619 (17) | |
| C1 | 0.18792 (11) | 0.79213 (10) | 0.87130 (9) | 0.01641 (19) | |
| H1A | 0.1511 | 0.8009 | 0.9515 | 0.020* | |
| H1B | 0.1081 | 0.8072 | 0.8385 | 0.020* | |
| C2 | 0.25141 (11) | 0.88926 (10) | 0.83004 (9) | 0.01574 (18) | |
| C3 | 0.40668 (11) | 0.85241 (10) | 0.80088 (9) | 0.01529 (18) | |
| C4 | 0.48625 (10) | 0.71733 (10) | 0.78310 (9) | 0.01380 (17) | |
| C5 | 0.41421 (11) | 0.65015 (10) | 0.87649 (9) | 0.01572 (18) | |
| H5A | 0.4719 | 0.5633 | 0.8785 | 0.019* | |
| H5B | 0.3961 | 0.6867 | 0.9484 | 0.019* | |
| C6 | 0.64462 (11) | 0.67997 (10) | 0.75962 (9) | 0.01479 (18) | |
| H6A | 0.6715 | 0.7393 | 0.7055 | 0.018* | |
| C7 | 0.69662 (11) | 0.55899 (10) | 0.69999 (9) | 0.01564 (18) | |
| H7A | 0.7041 | 0.4916 | 0.7547 | 0.019* | |
| C8 | 0.83679 (11) | 0.53339 (11) | 0.61614 (10) | 0.0188 (2) | |
| H8A | 0.8876 | 0.4451 | 0.6070 | 0.023* | |
| H8B | 0.8953 | 0.5696 | 0.6400 | 0.023* | |
| C9 | 0.64853 (12) | 0.54767 (12) | 0.52515 (10) | 0.0212 (2) | |
| H9A | 0.5784 | 0.5931 | 0.4833 | 0.025* | |
| H9B | 0.6810 | 0.4605 | 0.5078 | 0.025* | |
| C10 | 0.46097 (10) | 0.67354 (10) | 0.68033 (9) | 0.01421 (17) | |
| C11 | 0.32921 (10) | 0.63151 (10) | 0.73099 (9) | 0.01408 (17) | |
| C12 | 0.17995 (11) | 1.00541 (10) | 0.80811 (10) | 0.01742 (19) | |
| H12A | 0.2345 | 1.0553 | 0.7851 | 0.021* | |
| C13 | 0.03109 (11) | 1.06591 (10) | 0.81448 (10) | 0.01782 (19) | |
| C14 | −0.07494 (12) | 1.02983 (10) | 0.88129 (10) | 0.0185 (2) | |
| H14A | −0.0513 | 0.9639 | 0.9303 | 0.022* | |
| C15 | −0.21409 (12) | 1.08765 (11) | 0.87811 (10) | 0.0202 (2) | |
| H15A | −0.2838 | 1.0608 | 0.9239 | 0.024* | |
| C16 | −0.24999 (12) | 1.18508 (11) | 0.80725 (11) | 0.0225 (2) | |
| C17 | −0.14648 (13) | 1.22501 (12) | 0.74238 (12) | 0.0259 (2) | |
| H17A | −0.1708 | 1.2925 | 0.6951 | 0.031* | |
| C18 | −0.00926 (13) | 1.16714 (11) | 0.74650 (11) | 0.0234 (2) | |
| H18A | 0.0595 | 1.1963 | 0.7025 | 0.028* | |
| C19 | −0.48594 (14) | 1.19644 (15) | 0.84307 (14) | 0.0335 (3) | |
| H19A | −0.5714 | 1.2426 | 0.8192 | 0.050* | |
| H19B | −0.5051 | 1.1997 | 0.9229 | 0.050* | |
| H19C | −0.4530 | 1.1120 | 0.8199 | 0.050* | |
| C20 | 0.70703 (11) | 0.67646 (10) | 0.85510 (9) | 0.01501 (18) | |
| C21 | 0.75256 (12) | 0.76997 (11) | 0.86841 (10) | 0.0190 (2) | |
| H21A | 0.7418 | 0.8356 | 0.8180 | 0.023* | |
| C22 | 0.81297 (13) | 0.76854 (11) | 0.95352 (11) | 0.0215 (2) | |
| H22A | 0.8425 | 0.8332 | 0.9612 | 0.026* | |
| C23 | 0.83076 (12) | 0.67281 (11) | 1.02806 (9) | 0.0180 (2) | |
| C24 | 0.78598 (12) | 0.57861 (10) | 1.01762 (9) | 0.01754 (19) | |
| H24A | 0.7968 | 0.5132 | 1.0683 | 0.021* | |
| C25 | 0.72497 (12) | 0.58202 (10) | 0.93148 (9) | 0.01717 (19) | |
| H25A | 0.6945 | 0.5178 | 0.9245 | 0.021* | |
| C26 | 0.90991 (13) | 0.58491 (12) | 1.18668 (10) | 0.0228 (2) | |
| H26A | 0.9547 | 0.6000 | 1.2397 | 0.034* | |
| H26B | 0.9688 | 0.5083 | 1.1490 | 0.034* | |
| H26C | 0.8192 | 0.5803 | 1.2248 | 0.034* | |
| C27 | 0.41283 (11) | 0.76642 (10) | 0.59441 (9) | 0.01534 (18) | |
| C28 | 0.47477 (12) | 0.83835 (11) | 0.52540 (10) | 0.0191 (2) | |
| H28A | 0.5635 | 0.8372 | 0.5288 | 0.023* | |
| C29 | 0.40394 (13) | 0.91443 (11) | 0.44894 (10) | 0.0217 (2) | |
| H29A | 0.4476 | 0.9630 | 0.4006 | 0.026* | |
| C30 | 0.27418 (13) | 0.92003 (11) | 0.44276 (10) | 0.0217 (2) | |
| H30A | 0.2301 | 0.9716 | 0.3906 | 0.026* | |
| C31 | 0.20612 (12) | 0.84865 (10) | 0.51443 (10) | 0.0183 (2) | |
| C32 | 0.07319 (12) | 0.84443 (11) | 0.51840 (11) | 0.0217 (2) | |
| H32A | 0.0199 | 0.8934 | 0.4700 | 0.026* | |
| C33 | 0.02096 (12) | 0.76951 (11) | 0.59229 (11) | 0.0216 (2) | |
| H33A | −0.0680 | 0.7678 | 0.5933 | 0.026* | |
| C34 | 0.09598 (11) | 0.69493 (10) | 0.66679 (10) | 0.0186 (2) | |
| H34A | 0.0577 | 0.6446 | 0.7174 | 0.022* | |
| C35 | 0.22508 (11) | 0.69716 (10) | 0.66413 (9) | 0.01528 (18) | |
| C36 | 0.27902 (11) | 0.77326 (10) | 0.58842 (9) | 0.01567 (18) | |
| H1O1 | 0.459 (2) | 0.4815 (19) | 0.7015 (17) | 0.038 (5)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.01679 (12) | 0.03083 (16) | 0.01653 (13) | −0.00899 (11) | −0.00024 (9) | −0.00062 (11) |
| O1 | 0.0143 (3) | 0.0127 (3) | 0.0219 (4) | −0.0046 (3) | −0.0028 (3) | −0.0023 (3) |
| O2 | 0.0192 (4) | 0.0174 (4) | 0.0302 (5) | −0.0093 (3) | −0.0059 (3) | −0.0015 (3) |
| O3 | 0.0181 (4) | 0.0280 (5) | 0.0396 (6) | −0.0001 (3) | −0.0079 (4) | −0.0013 (4) |
| O4 | 0.0323 (5) | 0.0277 (5) | 0.0232 (4) | −0.0175 (4) | −0.0158 (4) | 0.0060 (4) |
| N1 | 0.0150 (4) | 0.0148 (4) | 0.0147 (4) | −0.0059 (3) | −0.0015 (3) | −0.0021 (3) |
| N2 | 0.0135 (4) | 0.0190 (4) | 0.0157 (4) | −0.0057 (3) | −0.0012 (3) | −0.0052 (3) |
| C1 | 0.0153 (4) | 0.0151 (4) | 0.0181 (5) | −0.0059 (3) | −0.0004 (3) | −0.0037 (4) |
| C2 | 0.0151 (4) | 0.0160 (5) | 0.0164 (5) | −0.0064 (3) | −0.0016 (3) | −0.0035 (4) |
| C3 | 0.0161 (4) | 0.0157 (4) | 0.0149 (4) | −0.0065 (3) | −0.0031 (3) | −0.0026 (4) |
| C4 | 0.0138 (4) | 0.0144 (4) | 0.0140 (4) | −0.0060 (3) | −0.0030 (3) | −0.0010 (3) |
| C5 | 0.0155 (4) | 0.0162 (4) | 0.0158 (4) | −0.0065 (3) | −0.0026 (3) | −0.0007 (4) |
| C6 | 0.0139 (4) | 0.0156 (4) | 0.0153 (4) | −0.0061 (3) | −0.0030 (3) | −0.0004 (3) |
| C7 | 0.0132 (4) | 0.0163 (5) | 0.0166 (5) | −0.0047 (3) | −0.0025 (3) | −0.0017 (4) |
| C8 | 0.0143 (4) | 0.0213 (5) | 0.0193 (5) | −0.0054 (4) | −0.0023 (4) | −0.0022 (4) |
| C9 | 0.0175 (5) | 0.0289 (6) | 0.0174 (5) | −0.0085 (4) | −0.0010 (4) | −0.0085 (4) |
| C10 | 0.0131 (4) | 0.0146 (4) | 0.0151 (4) | −0.0056 (3) | −0.0021 (3) | −0.0017 (3) |
| C11 | 0.0140 (4) | 0.0135 (4) | 0.0154 (4) | −0.0059 (3) | −0.0024 (3) | −0.0017 (3) |
| C12 | 0.0167 (4) | 0.0153 (5) | 0.0203 (5) | −0.0065 (4) | −0.0027 (4) | −0.0020 (4) |
| C13 | 0.0170 (4) | 0.0148 (5) | 0.0209 (5) | −0.0051 (4) | −0.0032 (4) | −0.0025 (4) |
| C14 | 0.0176 (4) | 0.0153 (5) | 0.0213 (5) | −0.0051 (4) | −0.0028 (4) | −0.0026 (4) |
| C15 | 0.0164 (4) | 0.0188 (5) | 0.0238 (5) | −0.0052 (4) | −0.0023 (4) | −0.0041 (4) |
| C16 | 0.0190 (5) | 0.0184 (5) | 0.0269 (6) | −0.0016 (4) | −0.0053 (4) | −0.0050 (4) |
| C17 | 0.0232 (5) | 0.0170 (5) | 0.0318 (7) | −0.0023 (4) | −0.0049 (5) | 0.0022 (5) |
| C18 | 0.0218 (5) | 0.0162 (5) | 0.0293 (6) | −0.0057 (4) | −0.0034 (4) | 0.0019 (4) |
| C19 | 0.0169 (5) | 0.0400 (8) | 0.0387 (8) | −0.0043 (5) | −0.0039 (5) | −0.0079 (6) |
| C20 | 0.0142 (4) | 0.0163 (4) | 0.0155 (4) | −0.0066 (3) | −0.0034 (3) | 0.0003 (4) |
| C21 | 0.0216 (5) | 0.0188 (5) | 0.0210 (5) | −0.0113 (4) | −0.0083 (4) | 0.0043 (4) |
| C22 | 0.0268 (5) | 0.0209 (5) | 0.0245 (6) | −0.0145 (4) | −0.0119 (4) | 0.0047 (4) |
| C23 | 0.0183 (4) | 0.0210 (5) | 0.0177 (5) | −0.0093 (4) | −0.0067 (4) | 0.0015 (4) |
| C24 | 0.0194 (5) | 0.0177 (5) | 0.0169 (5) | −0.0082 (4) | −0.0052 (4) | 0.0022 (4) |
| C25 | 0.0191 (4) | 0.0168 (5) | 0.0178 (5) | −0.0088 (4) | −0.0049 (4) | 0.0010 (4) |
| C26 | 0.0241 (5) | 0.0287 (6) | 0.0194 (5) | −0.0121 (5) | −0.0093 (4) | 0.0050 (4) |
| C27 | 0.0166 (4) | 0.0165 (5) | 0.0145 (4) | −0.0076 (4) | −0.0034 (3) | −0.0013 (4) |
| C28 | 0.0210 (5) | 0.0208 (5) | 0.0183 (5) | −0.0113 (4) | −0.0039 (4) | 0.0005 (4) |
| C29 | 0.0256 (5) | 0.0209 (5) | 0.0207 (5) | −0.0116 (4) | −0.0055 (4) | 0.0030 (4) |
| C30 | 0.0251 (5) | 0.0198 (5) | 0.0212 (5) | −0.0081 (4) | −0.0082 (4) | 0.0036 (4) |
| C31 | 0.0195 (5) | 0.0165 (5) | 0.0198 (5) | −0.0061 (4) | −0.0069 (4) | 0.0003 (4) |
| C32 | 0.0204 (5) | 0.0195 (5) | 0.0263 (6) | −0.0055 (4) | −0.0103 (4) | 0.0010 (4) |
| C33 | 0.0166 (5) | 0.0205 (5) | 0.0291 (6) | −0.0063 (4) | −0.0082 (4) | −0.0007 (4) |
| C34 | 0.0155 (4) | 0.0169 (5) | 0.0238 (5) | −0.0065 (4) | −0.0046 (4) | −0.0005 (4) |
| C35 | 0.0146 (4) | 0.0144 (4) | 0.0171 (5) | −0.0055 (3) | −0.0029 (3) | −0.0021 (4) |
| C36 | 0.0161 (4) | 0.0151 (4) | 0.0167 (5) | −0.0061 (3) | −0.0040 (3) | −0.0017 (4) |
Geometric parameters (Å, °)
| S1—C8 | 1.8072 (12) | C14—C15 | 1.3939 (16) |
| S1—C9 | 1.8318 (12) | C14—H14A | 0.9500 |
| O1—C11 | 1.4066 (13) | C15—C16 | 1.3927 (18) |
| O1—H1O1 | 0.86 (2) | C15—H15A | 0.9500 |
| O2—C3 | 1.2209 (13) | C16—C17 | 1.3966 (19) |
| O3—C16 | 1.3608 (15) | C17—C18 | 1.3789 (18) |
| O3—C19 | 1.4317 (19) | C17—H17A | 0.9500 |
| O4—C23 | 1.3657 (14) | C18—H18A | 0.9500 |
| O4—C26 | 1.4256 (15) | C19—H19A | 0.9800 |
| N1—C5 | 1.4689 (14) | C19—H19B | 0.9800 |
| N1—C1 | 1.4737 (14) | C19—H19C | 0.9800 |
| N1—C11 | 1.4783 (14) | C20—C25 | 1.3985 (15) |
| N2—C9 | 1.4552 (15) | C20—C21 | 1.4018 (15) |
| N2—C10 | 1.4805 (14) | C21—C22 | 1.3847 (17) |
| N2—C7 | 1.4879 (14) | C21—H21A | 0.9500 |
| C1—C2 | 1.5257 (15) | C22—C23 | 1.3941 (16) |
| C1—H1A | 0.9900 | C22—H22A | 0.9500 |
| C1—H1B | 0.9900 | C23—C24 | 1.3943 (16) |
| C2—C12 | 1.3499 (16) | C24—C25 | 1.3953 (16) |
| C2—C3 | 1.4971 (15) | C24—H24A | 0.9500 |
| C3—C4 | 1.5219 (15) | C25—H25A | 0.9500 |
| C4—C6 | 1.5300 (14) | C26—H26A | 0.9800 |
| C4—C5 | 1.5549 (14) | C26—H26B | 0.9800 |
| C4—C10 | 1.5645 (15) | C26—H26C | 0.9800 |
| C5—H5A | 0.9900 | C27—C28 | 1.3768 (15) |
| C5—H5B | 0.9900 | C27—C36 | 1.4159 (15) |
| C6—C20 | 1.5115 (15) | C28—C29 | 1.4237 (17) |
| C6—C7 | 1.5336 (16) | C28—H28A | 0.9500 |
| C6—H6A | 1.0000 | C29—C30 | 1.3785 (17) |
| C7—C8 | 1.5352 (15) | C29—H29A | 0.9500 |
| C7—H7A | 1.0000 | C30—C31 | 1.4233 (16) |
| C8—H8A | 0.9900 | C30—H30A | 0.9500 |
| C8—H8B | 0.9900 | C31—C36 | 1.4077 (16) |
| C9—H9A | 0.9900 | C31—C32 | 1.4199 (16) |
| C9—H9B | 0.9900 | C32—C33 | 1.3806 (17) |
| C10—C27 | 1.5169 (15) | C32—H32A | 0.9500 |
| C10—C11 | 1.6150 (14) | C33—C34 | 1.4204 (17) |
| C11—C35 | 1.5071 (15) | C33—H33A | 0.9500 |
| C12—C13 | 1.4629 (15) | C34—C35 | 1.3738 (15) |
| C12—H12A | 0.9500 | C34—H34A | 0.9500 |
| C13—C14 | 1.4005 (16) | C35—C36 | 1.4111 (15) |
| C13—C18 | 1.4072 (17) | ||
| C8—S1—C9 | 86.85 (5) | C15—C14—C13 | 122.04 (11) |
| C11—O1—H1O1 | 103.5 (14) | C15—C14—H14A | 119.0 |
| C16—O3—C19 | 117.38 (11) | C13—C14—H14A | 119.0 |
| C23—O4—C26 | 116.72 (10) | C16—C15—C14 | 119.42 (11) |
| C5—N1—C1 | 108.80 (8) | C16—C15—H15A | 120.3 |
| C5—N1—C11 | 103.25 (8) | C14—C15—H15A | 120.3 |
| C1—N1—C11 | 115.53 (9) | O3—C16—C15 | 125.09 (12) |
| C9—N2—C10 | 119.67 (10) | O3—C16—C17 | 115.42 (12) |
| C9—N2—C7 | 110.77 (8) | C15—C16—C17 | 119.49 (11) |
| C10—N2—C7 | 110.49 (8) | C18—C17—C16 | 120.48 (12) |
| N1—C1—C2 | 115.45 (9) | C18—C17—H17A | 119.8 |
| N1—C1—H1A | 108.4 | C16—C17—H17A | 119.8 |
| C2—C1—H1A | 108.4 | C17—C18—C13 | 121.44 (11) |
| N1—C1—H1B | 108.4 | C17—C18—H18A | 119.3 |
| C2—C1—H1B | 108.4 | C13—C18—H18A | 119.3 |
| H1A—C1—H1B | 107.5 | O3—C19—H19A | 109.5 |
| C12—C2—C3 | 115.93 (10) | O3—C19—H19B | 109.5 |
| C12—C2—C1 | 125.44 (10) | H19A—C19—H19B | 109.5 |
| C3—C2—C1 | 118.37 (9) | O3—C19—H19C | 109.5 |
| O2—C3—C2 | 123.17 (10) | H19A—C19—H19C | 109.5 |
| O2—C3—C4 | 121.64 (10) | H19B—C19—H19C | 109.5 |
| C2—C3—C4 | 115.15 (9) | C25—C20—C21 | 117.17 (10) |
| C3—C4—C6 | 114.65 (8) | C25—C20—C6 | 123.15 (10) |
| C3—C4—C5 | 107.25 (8) | C21—C20—C6 | 119.67 (10) |
| C6—C4—C5 | 117.84 (9) | C22—C21—C20 | 121.30 (10) |
| C3—C4—C10 | 110.70 (9) | C22—C21—H21A | 119.4 |
| C6—C4—C10 | 104.10 (8) | C20—C21—H21A | 119.4 |
| C5—C4—C10 | 101.35 (8) | C21—C22—C23 | 120.42 (11) |
| N1—C5—C4 | 103.40 (8) | C21—C22—H22A | 119.8 |
| N1—C5—H5A | 111.1 | C23—C22—H22A | 119.8 |
| C4—C5—H5A | 111.1 | O4—C23—C22 | 115.36 (10) |
| N1—C5—H5B | 111.1 | O4—C23—C24 | 124.82 (10) |
| C4—C5—H5B | 111.1 | C22—C23—C24 | 119.81 (10) |
| H5A—C5—H5B | 109.0 | C23—C24—C25 | 118.84 (10) |
| C20—C6—C4 | 117.40 (9) | C23—C24—H24A | 120.6 |
| C20—C6—C7 | 114.59 (9) | C25—C24—H24A | 120.6 |
| C4—C6—C7 | 102.88 (8) | C24—C25—C20 | 122.46 (10) |
| C20—C6—H6A | 107.1 | C24—C25—H25A | 118.8 |
| C4—C6—H6A | 107.1 | C20—C25—H25A | 118.8 |
| C7—C6—H6A | 107.1 | O4—C26—H26A | 109.5 |
| N2—C7—C6 | 105.66 (8) | O4—C26—H26B | 109.5 |
| N2—C7—C8 | 108.84 (9) | H26A—C26—H26B | 109.5 |
| C6—C7—C8 | 113.73 (9) | O4—C26—H26C | 109.5 |
| N2—C7—H7A | 109.5 | H26A—C26—H26C | 109.5 |
| C6—C7—H7A | 109.5 | H26B—C26—H26C | 109.5 |
| C8—C7—H7A | 109.5 | C28—C27—C36 | 118.55 (10) |
| C7—C8—S1 | 105.83 (7) | C28—C27—C10 | 132.36 (10) |
| C7—C8—H8A | 110.6 | C36—C27—C10 | 109.08 (9) |
| S1—C8—H8A | 110.6 | C27—C28—C29 | 119.05 (10) |
| C7—C8—H8B | 110.6 | C27—C28—H28A | 120.5 |
| S1—C8—H8B | 110.6 | C29—C28—H28A | 120.5 |
| H8A—C8—H8B | 108.7 | C30—C29—C28 | 122.19 (11) |
| N2—C9—S1 | 107.06 (8) | C30—C29—H29A | 118.9 |
| N2—C9—H9A | 110.3 | C28—C29—H29A | 118.9 |
| S1—C9—H9A | 110.3 | C29—C30—C31 | 120.14 (11) |
| N2—C9—H9B | 110.3 | C29—C30—H30A | 119.9 |
| S1—C9—H9B | 110.3 | C31—C30—H30A | 119.9 |
| H9A—C9—H9B | 108.6 | C36—C31—C32 | 116.57 (10) |
| N2—C10—C27 | 116.57 (9) | C36—C31—C30 | 116.52 (10) |
| N2—C10—C4 | 103.90 (8) | C32—C31—C30 | 126.90 (11) |
| C27—C10—C4 | 118.45 (9) | C33—C32—C31 | 120.32 (11) |
| N2—C10—C11 | 110.50 (8) | C33—C32—H32A | 119.8 |
| C27—C10—C11 | 103.54 (8) | C31—C32—H32A | 119.8 |
| C4—C10—C11 | 103.06 (8) | C32—C33—C34 | 122.12 (10) |
| O1—C11—N1 | 108.47 (9) | C32—C33—H33A | 118.9 |
| O1—C11—C35 | 112.32 (9) | C34—C33—H33A | 118.9 |
| N1—C11—C35 | 114.67 (9) | C35—C34—C33 | 118.58 (11) |
| O1—C11—C10 | 110.43 (8) | C35—C34—H34A | 120.7 |
| N1—C11—C10 | 105.60 (8) | C33—C34—H34A | 120.7 |
| C35—C11—C10 | 105.07 (8) | C34—C35—C36 | 119.45 (10) |
| C2—C12—C13 | 129.67 (10) | C34—C35—C11 | 131.91 (10) |
| C2—C12—H12A | 115.2 | C36—C35—C11 | 108.64 (9) |
| C13—C12—H12A | 115.2 | C31—C36—C35 | 122.96 (10) |
| C14—C13—C18 | 117.06 (11) | C31—C36—C27 | 123.53 (10) |
| C14—C13—C12 | 125.30 (11) | C35—C36—C27 | 113.51 (10) |
| C18—C13—C12 | 117.63 (10) | ||
| C5—N1—C1—C2 | 47.19 (12) | C3—C2—C12—C13 | 172.17 (11) |
| C11—N1—C1—C2 | −68.33 (12) | C1—C2—C12—C13 | −1.9 (2) |
| N1—C1—C2—C12 | 156.00 (11) | C2—C12—C13—C14 | 25.1 (2) |
| N1—C1—C2—C3 | −17.89 (14) | C2—C12—C13—C18 | −153.53 (13) |
| C12—C2—C3—O2 | 20.91 (17) | C18—C13—C14—C15 | 2.62 (18) |
| C1—C2—C3—O2 | −164.63 (11) | C12—C13—C14—C15 | −176.01 (11) |
| C12—C2—C3—C4 | −156.99 (10) | C13—C14—C15—C16 | −0.56 (18) |
| C1—C2—C3—C4 | 17.47 (14) | C19—O3—C16—C15 | −12.1 (2) |
| O2—C3—C4—C6 | 5.30 (15) | C19—O3—C16—C17 | 168.17 (13) |
| C2—C3—C4—C6 | −176.76 (9) | C14—C15—C16—O3 | 178.80 (12) |
| O2—C3—C4—C5 | 138.19 (11) | C14—C15—C16—C17 | −1.47 (19) |
| C2—C3—C4—C5 | −43.87 (12) | O3—C16—C17—C18 | −178.89 (13) |
| O2—C3—C4—C10 | −112.08 (12) | C15—C16—C17—C18 | 1.3 (2) |
| C2—C3—C4—C10 | 65.86 (11) | C16—C17—C18—C13 | 0.8 (2) |
| C1—N1—C5—C4 | −74.32 (10) | C14—C13—C18—C17 | −2.74 (19) |
| C11—N1—C5—C4 | 48.90 (10) | C12—C13—C18—C17 | 175.99 (12) |
| C3—C4—C5—N1 | 72.44 (10) | C4—C6—C20—C25 | 77.87 (14) |
| C6—C4—C5—N1 | −156.42 (9) | C7—C6—C20—C25 | −43.01 (14) |
| C10—C4—C5—N1 | −43.66 (10) | C4—C6—C20—C21 | −103.47 (12) |
| C3—C4—C6—C20 | 75.97 (12) | C7—C6—C20—C21 | 135.65 (11) |
| C5—C4—C6—C20 | −51.72 (13) | C25—C20—C21—C22 | 0.13 (17) |
| C10—C4—C6—C20 | −162.95 (9) | C6—C20—C21—C22 | −178.61 (11) |
| C3—C4—C6—C7 | −157.21 (9) | C20—C21—C22—C23 | 0.42 (19) |
| C5—C4—C6—C7 | 75.10 (11) | C26—O4—C23—C22 | 179.62 (11) |
| C10—C4—C6—C7 | −36.13 (10) | C26—O4—C23—C24 | 0.61 (18) |
| C9—N2—C7—C6 | 123.03 (10) | C21—C22—C23—O4 | −179.83 (12) |
| C10—N2—C7—C6 | −11.96 (11) | C21—C22—C23—C24 | −0.76 (19) |
| C9—N2—C7—C8 | 0.54 (13) | O4—C23—C24—C25 | 179.52 (11) |
| C10—N2—C7—C8 | −134.45 (9) | C22—C23—C24—C25 | 0.54 (17) |
| C20—C6—C7—N2 | 158.44 (8) | C23—C24—C25—C20 | 0.01 (17) |
| C4—C6—C7—N2 | 29.85 (10) | C21—C20—C25—C24 | −0.34 (17) |
| C20—C6—C7—C8 | −82.24 (11) | C6—C20—C25—C24 | 178.34 (10) |
| C4—C6—C7—C8 | 149.17 (9) | N2—C10—C27—C28 | −60.95 (16) |
| N2—C7—C8—S1 | 29.46 (10) | C4—C10—C27—C28 | 64.25 (16) |
| C6—C7—C8—S1 | −88.03 (10) | C11—C10—C27—C28 | 177.51 (12) |
| C9—S1—C8—C7 | −39.36 (8) | N2—C10—C27—C36 | 117.99 (10) |
| C10—N2—C9—S1 | 100.24 (10) | C4—C10—C27—C36 | −116.81 (10) |
| C7—N2—C9—S1 | −30.08 (11) | C11—C10—C27—C36 | −3.56 (11) |
| C8—S1—C9—N2 | 40.67 (9) | C36—C27—C28—C29 | −1.56 (17) |
| C9—N2—C10—C27 | −8.80 (14) | C10—C27—C28—C29 | 177.29 (12) |
| C7—N2—C10—C27 | 121.64 (10) | C27—C28—C29—C30 | 1.05 (19) |
| C9—N2—C10—C4 | −141.06 (9) | C28—C29—C30—C31 | 0.2 (2) |
| C7—N2—C10—C4 | −10.62 (11) | C29—C30—C31—C36 | −0.86 (18) |
| C9—N2—C10—C11 | 109.00 (10) | C29—C30—C31—C32 | 179.89 (13) |
| C7—N2—C10—C11 | −120.56 (9) | C36—C31—C32—C33 | 0.20 (18) |
| C3—C4—C10—N2 | 152.82 (8) | C30—C31—C32—C33 | 179.46 (12) |
| C6—C4—C10—N2 | 29.13 (10) | C31—C32—C33—C34 | 0.3 (2) |
| C5—C4—C10—N2 | −93.66 (9) | C32—C33—C34—C35 | −0.56 (19) |
| C3—C4—C10—C27 | 21.66 (12) | C33—C34—C35—C36 | 0.29 (17) |
| C6—C4—C10—C27 | −102.03 (10) | C33—C34—C35—C11 | 179.41 (11) |
| C5—C4—C10—C27 | 135.18 (9) | O1—C11—C35—C34 | 57.75 (16) |
| C3—C4—C10—C11 | −91.86 (9) | N1—C11—C35—C34 | −66.69 (16) |
| C6—C4—C10—C11 | 144.45 (8) | C10—C11—C35—C34 | 177.83 (12) |
| C5—C4—C10—C11 | 21.66 (10) | O1—C11—C35—C36 | −123.06 (10) |
| C5—N1—C11—O1 | 84.67 (9) | N1—C11—C35—C36 | 112.51 (10) |
| C1—N1—C11—O1 | −156.69 (8) | C10—C11—C35—C36 | −2.97 (11) |
| C5—N1—C11—C35 | −148.89 (9) | C32—C31—C36—C35 | −0.47 (17) |
| C1—N1—C11—C35 | −30.25 (12) | C30—C31—C36—C35 | −179.81 (11) |
| C5—N1—C11—C10 | −33.72 (10) | C32—C31—C36—C27 | 179.65 (11) |
| C1—N1—C11—C10 | 84.92 (10) | C30—C31—C36—C27 | 0.31 (17) |
| N2—C10—C11—O1 | −0.31 (12) | C34—C35—C36—C31 | 0.23 (17) |
| C27—C10—C11—O1 | 125.23 (9) | C11—C35—C36—C31 | −179.08 (10) |
| C4—C10—C11—O1 | −110.79 (9) | C34—C35—C36—C27 | −179.88 (10) |
| N2—C10—C11—N1 | 116.76 (9) | C11—C35—C36—C27 | 0.81 (13) |
| C27—C10—C11—N1 | −117.70 (9) | C28—C27—C36—C31 | 0.91 (17) |
| C4—C10—C11—N1 | 6.28 (10) | C10—C27—C36—C31 | −178.19 (10) |
| N2—C10—C11—C35 | −121.64 (9) | C28—C27—C36—C35 | −178.98 (10) |
| C27—C10—C11—C35 | 3.90 (11) | C10—C27—C36—C35 | 1.92 (13) |
| C4—C10—C11—C35 | 127.88 (9) |
Hydrogen-bond geometry (Å, °)
| Cg7 and Cg9 centroids of the C20–C25 and C31–C36 rings, respectively. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1O1···N2 | 0.86 (2) | 1.96 (2) | 2.6253 (14) | 133.3 (19) |
| C24—H24A···N1i | 0.95 | 2.61 | 3.4447 (15) | 146 |
| C26—H26C···O1i | 0.98 | 2.48 | 3.4078 (17) | 157 |
| C19—H19B···Cg7ii | 0.98 | 2.82 | 3.4652 (18) | 124 |
| C26—H26B···Cg7iii | 0.98 | 2.87 | 3.7720 (14) | 154 |
| C9—H9B···Cg9iv | 0.99 | 2.87 | 3.8211 (14) | 161 |
Symmetry codes: (i) −x+1, −y+1, −z+2; (ii) −x, −y+2, −z+2; (iii) −x+2, −y+1, −z+2; (iv) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB6431).
References
- Aicher, T. D., Balkan, B., Bell, P. A., Brand, L. J., Cheon, S. H., Deems, R. O., Fell, J. B., Fillers, W. S., Fraser, J. D., Gao, J., Knorr, D. C., Kahle, G. G., Leone, C. L., Nadelson, J., Simpson, R. & Smith, H. C. (1998). J. Med. Chem. 41, 4556–4566. [DOI] [PubMed]
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst. 19, 105–107.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Lalezari, I. & Schwartz, E. L. (1988). J. Med. Chem. 31, 1427–1429. [DOI] [PubMed]
- Nair, V. & Suja, T. D. (2007). Tetrahedron, 63, 12247–12275.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Tsuge, O. & Kanemasa, S. (1989). Advances in Heterocyclic Chemistry, edited by A. R. Katritzky, Vol. 45, p. 231. San Diego: Academic Press.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S160053681104061X/hb6431sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681104061X/hb6431Isup2.hkl
Supplementary material file. DOI: 10.1107/S160053681104061X/hb6431Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




