Abstract
BACKGROUND
Daily inhaled glucocorticoids are recommended for young children at risk for asthma exacerbations, as indicated by a positive value on the modified asthma predictive index (API) and an exacerbation in the preceding year, but concern remains about daily adherence and effects on growth. We compared daily therapy with intermittent therapy.
METHODS
We studied 278 children between the ages of 12 and 53 months who had positive values on the modified API, recurrent wheezing episodes, and at least one exacerbation in the previous year but a low degree of impairment. Children were randomly assigned to receive a budesonide inhalation suspension for 1 year as either an intermittent high-dose regimen (1 mg twice daily for 7 days, starting early during a predefined respiratory tract illness) or a daily low-dose regimen (0.5 mg nightly) with corresponding placebos. The primary outcome was the frequency of exacerbations requiring oral glucocorticoid therapy.
RESULTS
The daily regimen of budesonide did not differ significantly from the intermittent regimen with respect to the frequency of exacerbations, with a rate per patient-year for the daily regimen of 0.97 (95% confidence interval [CI], 0.76 to 1.22) versus a rate of 0.95 (95% CI, 0.75 to 1.20) for the intermittent regimen (relative rate in the intermittent-regimen group, 0.99; 95% CI, 0.71 to 1.35; P=0.60). There were also no significant between-group differences in several other measures of asthma severity, including the time to the first exacerbation, or adverse events. The mean exposure to budesonide was 104 mg less with the intermittent regimen than with the daily regimen.
CONCLUSIONS
A daily low-dose regimen of budesonide was not superior to an intermittent high-dose regimen in reducing asthma exacerbations. Daily administration led to greater exposure to the drug at 1 year.
Recurrent wheezing episodes in pre-school-age children are usually triggered by respiratory tract infections,1,2 which often progress to severe exacerbations requiring systemic glucocorticoids3 and frequent use of health care services.4,5 In children under the age of 5 years who had at least four wheezing episodes during the previous year and positive values on the modified asthma predictive index (API) (indicating an increased likelihood of persistent asthma in the future),6,7 the National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3) recommends the initiation of long-term daily inhaled glucocorticoid therapy8 on the basis of the results of the Childhood Asthma Research and Education (CARE) Network Prevention of Early Asthma in Kids (PEAK) trial (ClinicalTrials.gov number, NCT00272441).9 In a post hoc analysis, investigators in the PEAK trial found that daily therapy with inhaled glucocorticoids most benefited children who had had at least one exacerbation requiring emergency or hospital care during the previous year.10
Daily use of inhaled glucocorticoids in the PEAK trial was associated with a small but significant reduction in height growth, as compared with placebo, a reduction that was only partially reversed during a 1-year observation period after the discontinuation of study treatments.9 Concern about growth retardation and parental resistance to a daily regimen of inhaled glucocorticoids for young children, who usually have only episodic but often severe symptoms, stimulated a search for alternative strategies — specifically, intermittent therapy with inhaled glucocorticoids. Intermittent 7-day courses of high-dose nebulized budesonide, as compared with placebo, initiated during respiratory tract illnesses led to significant reductions in the severity of respiratory symptoms, without affecting linear growth, in the CARE Network Acute Intervention Management Strategies (AIMS) study (NCT00000622); benefits were most apparent in children with positive values on the modified API.11 In confirmation of these findings, preemptive use of intermittent high-dose fluticasone during upper respiratory illness reduced exacerbations requiring oral glucocorticoids in young children with moderate-to-severe, virus-induced wheezing.12
We conducted the Maintenance and Intermittent Inhaled Corticosteroids in Wheezing Toddlers (MIST) trial to determine whether a daily low-dose regimen of budesonide would be superior to an intermittent high-dose regimen in young children who had positive values on the modified API, along with recurrent wheezing, high-risk asthma (≥1 exacerbation in the previous year), and low impairment (infrequent use of albuterol and infrequent night awakenings between episodes).
METHODS
STUDY PATIENTS
We enrolled children between the ages of 12 and 53 months who met all the following criteria: during the previous year, they had at least four episodes of wheezing (or three episodes of wheezing and controller use for ≥3 months), positive values on the modified API,7 and at least one exacerbation requiring the use of systemic glucocorticoids, urgent or emergency care, or hospitalization, and during a 2-week run-in period, they had fewer than 3 days per week of albuterol use and fewer than 2 nights with awakening. Children were excluded from the study if they had received more than six courses of oral glucocorticoids or had been hospitalized more than two times for wheezing during the previous year. (For additional details on inclusion and exclusion criteria, see the Supplementary Appendix, available with the full text of this article at NEJM.org.)
STUDY DESIGN
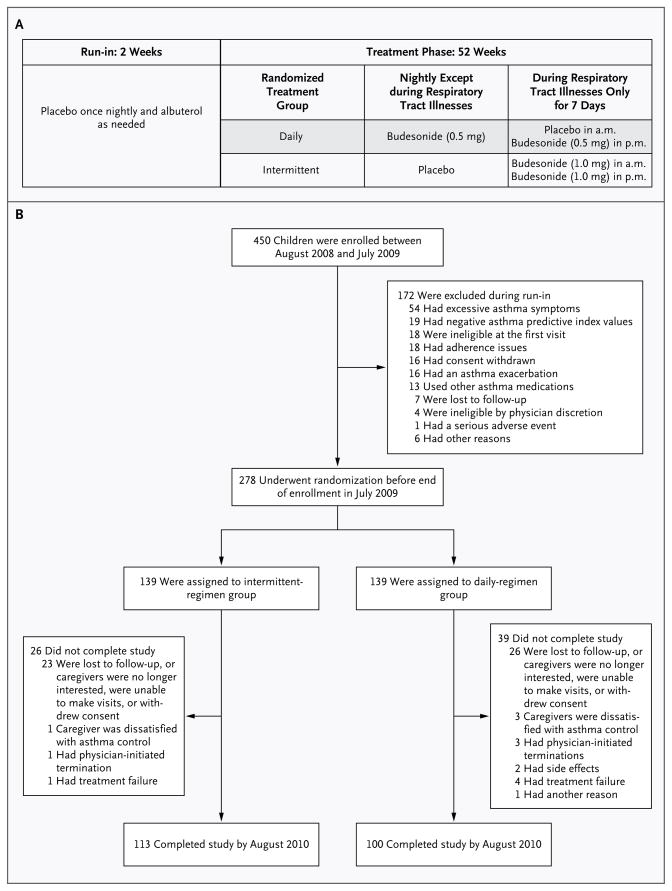
The study, which was conducted at seven sites, was a randomized, double-blind, parallel-group trial. During a 2-week run-in period, all patients received nightly placebo doses of budesonide inhalation suspension (Pulmicort Respules, Astra-Zeneca) and as-needed albuterol, followed by a 52-week treatment period. The full protocol and statistical analysis plan are available at NEJM.org. We compared the use of budesonide inhalation suspension given in a daily low-dose regimen with an intermittent high-dose regimen,13–18 with matching placebos (Fig. 1A). Specific procedures that were performed at all visits are detailed in Figure S1 in the Supplementary Appendix. The institutional review board at each center approved the study. Parents or guardians (hereafter referred to as parents for simplicity, unless otherwise noted) provided written informed consent.
Figure 1. Study Design and Enrollment.
Panel A shows the study design and treatments. Intermittent high-dose nebulized budesonide inhalation suspension was administered at a dose of 1 mg twice daily in the form of Pulmicort Respules for 7 days at the onset of a predefined respiratory tract illness. A matched placebo was administered once nightly on all other days. Daily low-dose nebulized budesonide inhalation suspension was administered at a dose of 0.5 mg once nightly, also in the form of Pulmicort Respules. During respiratory tract illnesses, an appropriately matched morning placebo was used for 7 days. To maintain blinding during respiratory tract illnesses, daily treatments were discontinued for 7 days and respiratory illness kits that were based on the study-group assignment were administered for 7 days. After 7 days, regular daily treatments were restarted. Open-label rescue albuterol was administered per protocol during a respiratory tract illness and as needed. Study medications were administered with the use of a Pari Ultra II compressor with a Pari LC Sprint reusable nebulizer and a mask (Bubbles the Fish II or Pari Baby mask), if needed, or a mouthpiece, depending on the age of the child. Rescue albuterol was administered at a dose of 180 μg per treatment by metered-dose inhalation (Ventolin HFA, GlaxoSmithKline) through AeroChamber Z-STAT Plus with FlowSIGnal Whistle with ComfortSeal Mask (Monaghan Medical) or a solution of 2.5 mg of albuterol per treatment by nebulization according to protocol during a respiratory tract illness (four times daily, while the child was awake, for the first 48 hours) and as needed. Panel B shows the numbers of patients who were enrolled in the study, underwent randomization, and completed the study.
Intermittent high-dose treatments were started for an identified respiratory tract illness on the basis of a published education program (see the Supplementary Appendix).11,19 Parents began a 7-day course of intermittent study medication at the onset of symptoms or signs of a respiratory tract illness that they identified as their child’s usual starting point before the development of wheezing (Fig. S2 in the Supplementary Appendix). These individualized symptoms or signs were reassessed at all study visits. Parents contacted study staff within 72 hours after starting treatment kits for respiratory tract illness, in order to summarize the events that defined the illness. In daily diaries, parents reported symptoms (e.g., nocturnal and daytime coughing, wheezing, difficulty breathing, and symptoms interfering with activities, with the severity of each scored from 0 to 5 and with higher scores indicating greater severity),20 medications, health care visits, and absences from day care or preschool or parental work.
OUTCOME MEASURES
The primary outcome measure was the frequency of exacerbations, which was defined as the number of courses of an oral glucocorticoid (prednisolone) started for acute wheezing after consultation with a physician (by telephone or in person) on the basis of a specific published protocol9,11 during the 12-month treatment period (see the Supplementary Appendix). About 30% of courses of an oral glucocorticoid were not initiated by the study team. Secondary prespecified risk outcomes included the time to exacerbations, rate of treatment failure, rate of wheezing-related health care utilization, and growth effects.7 Secondary prespecified impairment outcomes included the number of episode-free days,11 symptom severity during a respiratory tract illness,11,20 absences related to respiratory symptoms, albuterol use, quality of life according to the Infant Toddler Quality of Life questionnaire,21 and levels of fractional exhaled nitric oxide22 (Fig. S1 in the Supplementary Appendix). Adherence was determined by means of diary recordings of budesonide use. Analyses also examined the relationship between specific nasal respiratory viruses and respiratory tract illnesses.1
STUDY OVERSIGHT
The study was funded by the National Heart, Lung, and Blood Institute and was approved by its protocol review committee and data and safety monitoring board. AstraZeneca donated budesonide and matching placebo and reviewed the protocol with minor comments and the manuscript without commenting; the company had no other role in the study. The authors are fully responsible for the study design and data (collection, analysis, completeness, accuracy, and interpretation), as well as for the fidelity of the report to the study protocol.
STATISTICAL ANALYSIS
The study was designed as a superiority trial of a daily low-dose regimen of budesonide, as compared with an intermittent high-dose regimen, since previous CARE trials had shown the efficacy of both regimens versus placebo in similar high-risk, low-impairment cohorts.9,11 Baseline characteristics were summarized with the use of descriptive statistics. We used the exact Wilcoxon–Mann–Whitney test, stratified according to study center and age group, to determine statistical significance.
Although the determination of statistical significance for the treatment comparison was based on a nonparametric test, the primary research question was framed in terms of the annual rate of exacerbations. For the primary parametric analysis, we used a negative binomial regression model incorporating actual follow-up time so that we could appropriately estimate the rate of exacerbation per patient-year. Secondary analyses examined the effect of treatment on other outcomes. For counted outcomes, such as unscheduled health care visits, a similar model was applied. We used analysis of covariance for outcomes that were measured on a continuous scale, such as linear growth, and appropriate transformations were applied for any outcome that had a skewed distribution. We used a proportional-hazards regression model for time-to-event outcomes, such as the time to the first exacerbation. The total exposure to budesonide in an intention-to-treat model is described in the Supplementary Appendix.
For sample-size determination, we used the results of the PEAK7 and AIMS11 trials to estimate exacerbation rates. The sample size that was required for a power of 90% was determined for a range of exacerbation rates, from 0.6 to 1.2 per year. On the basis of a 10% dropout rate, we calculated that a sample of 250 children would provide a power of 90% (225 total patient-years) if the relative exacerbation rate for one treatment group was at least 40% lower than the rate in the other group. During the course of the trial, it became evident that the actual noncompletion rate might be as high as 20%. With approval from the data and safety monitoring board and the institutional review boards, 28 additional patients underwent randomization, which preserved a power of 90%, since data from 235 patient-years would be available for the intention-to-treat analysis.
All analyses were performed with the use of SAS statistical software, version 9.1, and were adjusted for the randomization strata. A two-sided P value of less than 0.05 for between-group comparisons was considered to indicate statistical significance, with no adjustment for multiple testing of secondary outcomes.
RESULTS
STUDY PATIENTS
Of the 450 children who were originally enrolled in the study, 172 were excluded during the run-in period. A total of 278 children underwent randomization, and 213 completed the study (Fig. 1B). The two study groups had similar demographic and clinical characteristics (Table 1). The noncompletion rate was 23.3%, with no significant differences between the two groups (Tables S1 and S2 in the Supplementary Appendix). Reported rates of adherence to treatment and diary entries were high and similar in the two groups (Table 2). Of the reports of treatments for respiratory tract illness, 95.6% were made by mothers, 3.9% by fathers, 0.4% by grandparents, and 0.1% by legal guardians.
Table 1.
Demographic and Asthma Characteristics of the Patients.*
| Characteristic | Total (N = 278) | Intermittent Regimen (N = 139) | Daily Regimen (N = 139) |
|---|---|---|---|
| Age of 12–32 mo — no. (%) | 127 (45.7) | 64 (46.0) | 63 (45.3) |
| Male sex — no. (%) | 192 (69.1) | 102 (73.4) | 90 (64.7) |
| White race — no. (%)† | 173 (62.2) | 91 (65.5) | 82 (59.0) |
| Height — cm | 94.2±9.1 | 94.0±9.1 | 94.5±9.0 |
| Weight — kg | 15.2±3.1 | 15.0±3.0 | 15.5±3.1 |
| Head circumference — cm | 50.0±1.9 | 50.1±2.0 | 49.9±1.9 |
| Physician diagnosis of asthma — no. (%) | 198 (71.2) | 99 (71.2) | 99 (71.2) |
| No. of wheezing episodes in past year | 6.7±5.4 | 7.0±5.9 | 6.4±4.7 |
| No. of urgent or emergency visits in past year | 4.8±4.2 | 4.6±4.2 | 5.0±4.1 |
| Hospitalizations in past year — no. (%) | 53 (19.1) | 26 (18.7) | 27 (19.4) |
| Tobacco-smoke exposure from birth — no. (%) | 114 (41.0) | 55 (39.6) | 59 (42.4) |
| Medication use in past year — no. (%) | |||
| Asthma controller | 194 (69.8) | 100 (71.9) | 94 (67.6) |
| Inhaled glucocorticoid | 189 (68.0) | 96 (69.1) | 93 (66.9) |
| Oral glucocorticoid | 210 (75.5) | 110 (79.1) | 100 (71.9) |
| Allergy — no./total no. (%) | |||
| Food sensitivity | 95/273 (34.8) | 50/135 (37.0) | 45/138 (32.6) |
| Any aeroallergen sensitivity | 161/276 (58.3) | 82/137 (59.9) | 79/139 (56.8) |
| IgE — IU/ml | |||
| Median | 58 | 50 | 61 |
| Interquartile range | 21–186 | 20–195 | 25–179 |
| Eosinophils ≥4% — no./total no. (%) | 123/260 (47.3) | 61/132 (46.2) | 62/128 (48.4) |
| Eczema — no. (%) | 146 (52.5) | 76 (54.7) | 70 (50.4) |
| Allergic rhinitis — no. (%) | 105 (37.8) | 50 (36.0) | 55 (39.6) |
| Parental asthma — no./total no. (%) | 171/266 (64.3) | 85/131 (64.9) | 86/135 (63.7) |
| Exhaled nitric oxide ≥10 ppb — no./total no. (%) | 78/178 (43.8) | 36/82 (43.9) | 42/96 (43.8) |
| Episode-free days during run-in period — % | 67±30 | 66±30 | 68±29 |
| Diary scores during run-in period‡ | |||
| Coughing | 0.4±0.4 | 0.4±0.5 | 0.4±0.4 |
| Wheezing | 0.1±0.3 | 0.2±0.3 | 0.1±0.3 |
| Trouble breathing | 0.1±0.3 | 0.1±0.3 | 0.1±0.2 |
| Interference with activities | 0.1±0.2 | 0.1±0.2 | 0.1±0.3 |
| General health perceptions from ITQOL§ | 59.2±14.1 | 59.0±14.8 | 59.4±13.5 |
| Any nasal virus identified — no. (%) | 148 (53.2) | 72 (51.8) | 76 (54.7) |
Plus–minus values are means ±SD. There were no significant differences between the two groups.
Race was determined by parents or guardians.
Scores range from 0 to 5, with higher scores indicating more severe symptoms. Scores were calculated from diaries as means ±SD for the 2-week run-in period.
The scores on the Infant Toddler Quality of Life (ITQOL) questionnaire range from 0 to 100, with higher scores indicating better health.
Table 2.
Outcomes at 1 Year.*
| Outcome | Intermittent Regimen (N = 139) | Daily Regimen (N = 139) | Treatment Effect† |
|---|---|---|---|
| Event Rate/Person-Yr (95% CI) | Relative Rate (95% CI) | ||
|
| |||
| No. of prednisolone courses for asthma‡ | 0.95 (0.75 to 1.20) | 0.97 (0.76 to 1.22) | 0.99 (0.71 to 1.35) |
|
| |||
| No. of treatments for respiratory tract illness‡ | 3.61 (3.13 to 4.16) | 3.27 (2.82 to 3.79) | 1.10 (0.91 to 1.35) |
|
| |||
| No. of urgent care visits for asthma‡ | 2.37 (1.89 to 2.97) | 2.40 (1.91 to 3.02) | 0.99 (0.72 to 1.35) |
|
| |||
| No. of days absent from work, school, or day care‡ | 2.72 (2.00 to 3.70) | 3.02 (2.22 to 4.12) | 0.90 (0.59 to 1.37) |
| Proportion (95% CI) | Relative Proportion (95% CI) | ||
| Respiratory tract illnesses in which prednisolone was administered‡ | 0.24 (0.19 to 0.29) | 0.26 (0.21 to 0.32) | 0.90 (0.68 to 1.19) |
|
| |||
| Respiratory tract illnesses with virus detected in nasal sample | 0.83 (0.74 to 0.92) | 0.83 (0.74 to 0.93) | 1.00 (0.85 to 1.17) |
|
| |||
| Respiratory tract illnesses with exacerbations and nasal virus detected | 0.79 (0.61 to 1.02) | 0.78 (0.61 to 1.01) | 1.00 (0.70 to 1.44) |
| Mean Value (95% CI) | Mean Difference (95% CI) | ||
| Episode-free days — % | 78 (75 to 80) | 78 (76 to 81) | −0.7 (−4.0 to 2.0) |
|
| |||
| Episode-free days, excluding days during treatment for respiratory tract illness — % | 85 (82 to 87) | 84 (82 to 86) | 0.5 (−3.0 to 4.0) |
|
| |||
| Days with albuterol use — % | 6 (5 to 7) | 5 (4 to 6) | 0.4 (−1.0 to 2.0) |
|
| |||
| Annualized days of treatment with budesonide — no. | 24 (21 to 27) | 337 (330 to 344) | −314 (−322 to −306) |
|
| |||
| Cumulative dose of budesonide — mg | 46 (39 to 53) | 150 (140 to 160) | −104 (−116 to −92) |
|
| |||
| Change in height from baseline | |||
|
| |||
| Value — cm | 8.01 (7.71 to 8.30) | 7.76 (7.45 to 8.07) | 0.26 (−0.17 to 0.68) |
|
| |||
| Percentile | 4.04 (2.07 to 6.00) | 2.39 (0.14 to 4.64) | 1.65 (−1.34 to 4.63) |
|
| |||
| z score | 0.12 (0.04 to 0.20) | 0.10 (0.02 to 0.18) | 0.02 (−0.10 to 0.13) |
|
| |||
| Change in weight from baseline | |||
|
| |||
| Value — kg | 2.54 (2.37 to 2.71) | 2.38 (2.20 to 2.55) | 0.16 (−0.08 to 0.41) |
|
| |||
| Percentile | 4.11 (2.07 to 6.16) | 0.73 (−1.39 to 2.85) | 3.38 (0.43 to 6.33) |
|
| |||
| z score | 0.15 (0.07 to 0.22) | 0.05 (−0.03 to 0.13) | 0.10 (−0.01 to 0.21) |
|
| |||
| Change in head circumference from baseline | |||
|
| |||
| Value — cm | 0.92 (0.79 to 1.05) | 0.87 (0.74 to 1.00) | 0.05 (−0.14 to 0.23) |
|
| |||
| Percentile | −1.20 (−3.45 to 1.04) | −3.59 (−6.67 to −0.51) | 2.39 (−1.43 to 6.20) |
|
| |||
| z score | −0.07 (−0.17 to 0.03) | −0.13 (−0.24 to −0.02) | 0.06 (−0.09 to 0.21) |
|
| |||
| Days with diary-card adherence — % | 83 (78 to 88) | 88 (84 to 92) | −5 (−11 to 2) |
|
| |||
| Days with adherence to budesonide or placebo — %§ | |||
|
| |||
| Daily | 94 (93 to 96) | 95 (94 to 96) | −1 (−2 to 1) |
|
| |||
| During respiratory tract illness | 81 (75 to 87) | 82 (75 to 89) | −1 (−10 to 8) |
All outcomes have been adjusted for age and clinical center. Growth was measured in 113 children in the intermittent-regimen group and 110 in the daily-regimen group. All other outcomes included data from the entire cohort.
The treatment effect was calculated as the value in the intermittent-regimen group divided by the value in the daily-regimen group.
Models were also adjusted for sex, race, and atopy, since results were similar with adjustment for age and clinical center only.
Treatment adherence was evaluated on days for which diary data were available.
PRIMARY OUTCOME
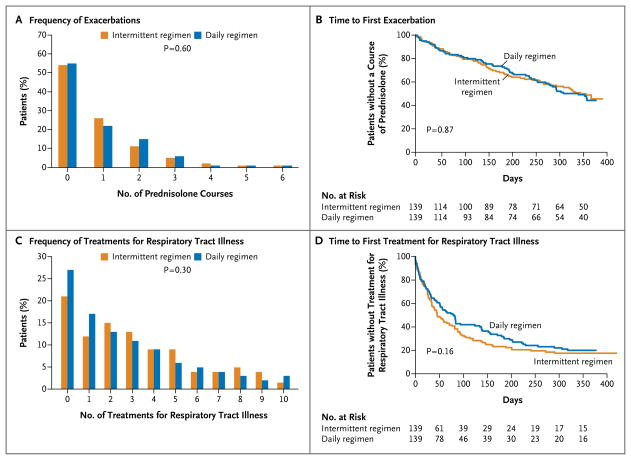
There was no significant difference between a daily regimen of budesonide and an intermittent regimen with respect to the frequency of exacerbations requiring the use of rescue oral glucocorticoids, with a rate per patient-year of 0.97 (95% confidence interval [CI], 0.76 to 1.22) in the daily-regimen group versus a rate of 0.95 (95% CI, 0.75 to 1.20) in the intermittent-regimen group (relative rate in the intermittent-regimen group, 0.99; 95% CI, 0.71 to 1.35; P = 0.60) (Fig. 2A and Table 2). There was also was no significant between-group difference with respect to the time to the first exacerbation (hazard ratio, 0.97; 95% CI, 0.76 to 1.22; P = 0.87) (Fig. 2B), the time to the second exacerbation (hazard ratio, 0.79; 95% CI, 0.49 to 1.32; P=0.38), or the frequency of treatment failure (P=0.12).
Figure 2. Exacerbations of Wheezing and Respiratory Tract Illness.
P values are based on exact Wilcoxon–Mann–Whitney tests for Panels A and C and on Wald tests from a proportional-hazards regression model for Panels B and D. All comparisons have been adjusted for clinical center and age.
SECONDARY PRESPECIFIED OUTCOMES
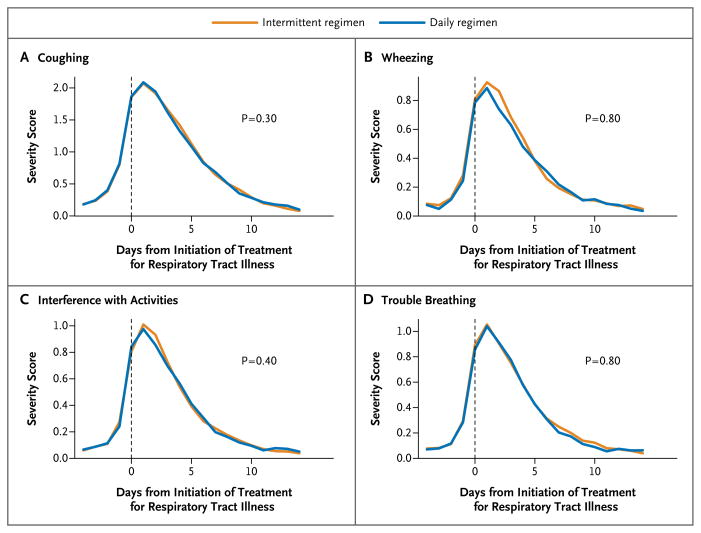
The distribution of the type of symptoms (e.g., coughing or wheezing) that led caregivers to recognize a respiratory tract illness and initiate treatment was similar in the two study groups (P=0.60) (Table S3 in the Supplementary Appendix). There were also no significant differences in the rates of respiratory tract illness per patient-year (3.27 with the daily regimen and 3.61 with the intermittent regimen, P = 0.30), respiratory tract illnesses in which prednisolone was administered (0.26 with the daily regimen and 0.24 with the intermittent regimen, P = 0.50) (Table 2), the frequency of treatments for respiratory tract illnesses (P = 0.30) (Fig. 2C), and the time to the first treatment for respiratory tract illness (P = 0.16) (Fig. 2D). Most exacerbations occurred during treated respiratory tract illnesses, with 102 of 111 exacerbations during treated illnesses (91.9%) in the daily-regimen group and 105 of 115 exacerbations during treated illnesses (91.3%) in the intermittent-regimen group. Similarly, there were no significant between-group differences in symptom scores during respiratory tract illnesses (Fig. 3, and Table S4 in the Supplementary Appendix) or during exacerbations (Fig. S3 in the Supplementary Appendix).
Figure 3. Profiles of Symptom Severity during Respiratory Tract Illness.
Day zero corresponds to the start of treatment for a respiratory tract illness. P values are for comparisons of symptom levels during the first 14 days after the initiation of treatment for respiratory tract illness, with adjustment for baseline symptom levels (on days 13 to 7 before the initiation of treatment). Plotted values are means for the indicated day. Scores range from 0 to 5, with higher scores indicating more severe symptoms.
The proportion of episode-free days across the study was 78%, and the rate of wheezing-related unscheduled physician visits was approximately 2.40 visits per patient-year in the two study groups (Table 2). The rate of absences for children and parents (from day care and work, respectively), the proportion of days with albuterol use (Table 2), and changes in most quality-of-life measures (Table S4 in the Supplementary Appendix) were similar in the two study groups. Given that measurements of fractional exhaled nitric oxide were unsuccessful in 36% of children at baseline, particularly in the youngest children, changes in values for fractional exhaled nitric oxide were not reported. The frequency and distribution of respiratory viruses in nasal secretions during two regularly scheduled clinic visits and during respiratory tract illnesses were similar in the two groups (Table 2, and Table S5 in the Supplementary Appendix).
SAFETY
The total exposure to budesonide over the course of the study was less in the intermittent-regimen group (45.7 mg; 95% CI, 38.9 to 52.8) than in the daily-regimen group (149.9 mg; 95% CI, 140.1 to 159.6), with an average reduction of 104 mg (95% CI, −116 to −92) in the intermittent-regimen group. Between-group differences in changes in height, weight, and head circumference were not significant (Table 2, and Fig. S4 in the Supplementary Appendix).
There were no deaths. The proportions of patients with serious adverse events (including all hospitalizations) and nonserious adverse events did not differ significantly in the two study groups (Table S6 in the Supplementary Appendix). Four children in the daily-regimen group and five in the intermittent-regimen group were hospitalized for asthma exacerbations. Other reasons for hospitalization were pneumonia, gastroenteritis, and diarrhea (one patient each) in the daily-regimen group and concussion, gastroenteritis, influenza, tonsillectomy, and motor-vehicle accident (one patient each) in the intermittent-regimen group.
DISCUSSION
We report that a daily low-dose regimen of budesonide inhalation suspension was not superior to an intermittent high-dose regimen, administered for 7 days during a predefined respiratory tract illness, with respect to the frequency of exacerbations (the primary outcome) in preschool-age children at risk for asthma and future exacerbations. In addition, there was no significant between-group difference in respiratory symptoms (symptom severity during respiratory tract illnesses, episode-free days, and bronchodilator use) or quality of life. These findings occurred in the context of similar rates of reported adherence and identification of nasal viruses in the two study groups.
The treatment of preschool-age children with recurrent wheezing is complex, since most of these children do not have persistent asthma, as defined by regular symptoms, and the diagnosis is difficult to confirm. U.S. guidelines8 recognize this complexity in regard to diagnosis and management and recommend daily therapy with inhaled glucocorticoids as the preferred option for young children with recurrent wheezing and risk factors for persistent asthma (i.e., positive values on the modified API),8 although this treatment does not alter the course of the disease after the inhaled glucocorticoids are discontinued.9 Guidelines of the Global Initiative for Asthma (GINA)23 also recommend daily controller therapy, including the use of inhaled glucocorticoids, for young children with intermittent wheezing, a history suggestive of asthma, and at least three wheezing episodes in the previous year. The guidelines caution against the use of daily high-dose therapy with inhaled glucocorticoids for prolonged periods, given the reported effect of such use on growth, and advise using the lowest dose necessary for asthma control.8 In this context, the inability to show the superiority of a daily low-dose regimen of budesonide over an intermittent high-dose regimen may be important in the preparation of future guidelines.
The efficacy of daily low-dose inhaled glucocorticoids, as compared with placebo, has been documented in studies ranging from 1.5 to 12 months and involving preschool-age children with positive values on the modified API,9 risk factors for asthma,24–26 frequent recurrent wheezing or asthma symptoms,27,28 or physician-diagnosed asthma.13,29 A recent meta-analysis30 and both the EPR-3 national guidelines8 and GINA guidelines23 for infants and preschool-age children support these findings. In the PEAK trial, the daily low-dose inhaled glucocorticoid that was shown to be efficacious was fluticasone administered in a metered-dose inhaler, but there has been no evidence that efficacy differs when other inhaled glucocorticoids are used in clinically similar doses.8,10 In preschool-age children who entered a trial with frequent symptoms, the percentage of symptom-free days was higher among those receiving daily inhaled glucocorticoids than among those receiving placebo, but the comparison between daily use and as-needed use was not significant.31 On the other hand, the use of intermittent high-dose inhaled glucocorticoids for specified periods was more effective than placebo in preschool-age children with intermittent, recurrent wheezing12,32,33 and in those at high risk for asthma but with low levels of respiratory impairment.11 Thus, we determined that a placebo group was not indicated for our study, which involved children who had low impairment but were at high risk for emerging, persistent asthma and recurrence of an asthma exacerbation, a decision that was approved by the independent oversight boards.
Budesonide inhalation suspension was selected for the daily low-dose inhaled glucocorticoid since it was the only such drug approved by the Food and Drug Administration for daily use in children between the ages of 1 and 4 years at recommended starting daily doses of 0.5 to 1.0 mg on the basis of pivotal trials of budesonide versus placebo in children between the ages of 1 and 8 years.13–15 In post hoc analyses, daily doses of 0.5 and 1.0 mg of budesonide were similarly effective in children under the age of 4 years and in those 4 years of age or older.34 Budesonide in a daily dose of 0.5 mg was also associated with fewer exacerbations and less impairment than was cromolyn17 or montelukast16 in young children. The efficacy of once-daily budesonide by nebulization (including a dose of 0.5 mg) has been summarized previously.35 In addition, guidelines recommend a daily dose of 0.5 mg of budesonide as a low-dose inhaled glucocorticoid.8,23 As compared with placebo, budesonide was efficacious without retarding growth when used in an intermittent high-dose regimen of 1 mg twice daily for 7 days with each respiratory tract illness in preschool-age children.11
Our findings may not be applicable to young children whose asthma was different from or more severe than that of the children in our study. Daily36 or intermittent31,37 use of inhaled glucocorticoids or even short courses of oral glucocorticoids started at the onset of wheezing episodes38,39 may not be efficacious in preschool-age children with a first episode, with transient or infrequent wheezing, or without an asthma diagnosis or a high risk of asthma.
Although the observed exacerbation rates in the two study groups were numerically similar, our results do not show with certainty that the two treatments were equally effective. The uncertainty is reflected in the confidence interval for the relative rate of exacerbations, which extends from approximately 0.7 to 1.35, so our data do not rule out the possibility that either treatment could be up to 35% more effective than the other. The advantage of the intermittent regimen over the daily regimen cannot be based on differential effects on growth, but rather on a reduced exposure to inhaled glucocorticoids (approximately 100 mg less during the course of a year, or a reduction in exposure by a factor of 3.3). The non-completion rate was higher than anticipated but similar in the two groups, as were the characteristics of the children who did not complete the study.
A major advantage of an intermittent regimen of inhaled glucocorticoids is that its initiation occurs early during a predefined respiratory tract illness on the basis of individualized symptoms that historically have occurred before the onset of wheezing.11 This strategy avoids the use of inhaled glucocorticoids for each upper respiratory tract illness and thus allows for the benefits of the regimen at considerably lower cumulative levels of exposure. In our study, intermittent budesonide was initiated on average once every 3.5 months, in contrast to a monthly rate when such therapy was started preemptively with each upper respiratory tract infection.12 This finding may explain the adverse effects on growth in the latter study,12 as compared with our approach.11 Nevertheless, parents require careful, individualized instruction on when to start budesonide in order to ensure that this intermittent approach is used appropriately, as detailed previously11,19 (Fig. S2 in the Supplementary Appendix).
In summary, our trial compared the efficacy of an early intermittent high-dose regimen of budesonide for respiratory tract illness with that of a daily low-dose regimen, with the latter recommended by current guidelines for preschool-age children with recurrent wheezing episodes and positive values on the modified API. We conclude that for such children who have had one or more exacerbations requiring the use of systemic glucocorticoids, urgent or emergency medical visits, or hospitalizations during the previous year but a documented history of low impairment from asthma, the daily low-dose regimen of budesonide was not superior to an intermittent high-dose regimen initiated during a predefined respiratory tract illness in reducing exacerbations. Moreover, the daily low-dose regimen was associated with more frequent administration of and greater exposure to budesonide.
Supplementary Material
Acknowledgments
Funded by the National Heart, Lung, and Blood Institute and others; MIST ClinicalTrials.gov number, NCT00675584.
Supported by grants (5U10HL064287, 5U10HL064288, 5U10HL064295, 5U10HL064307, 5U10HL064305, and 5U10HL064313) from the National Heart, Lung, and Blood Institute; a grant (5UL1RR02499204) from the Washington University School of Medicine Clinical and Translational Science Awards (CTSA) Infrastructure for Pediatric Research; a grant (1UL1RR025011) from the Madison CTSA; a grant (UL1RR025780) to the Colorado CTSA from the National Center for Research Resources, National Institutes of Health; and grants to the General Clinical Research Centers at Washington University School of Medicine (M01 RR00036), National Jewish Health (M01 RR00051), and the University of New Mexico (5M01 RR00997).
APPENDIX
The authors’ affiliations are as follows: the Department of Allergy, Kaiser Permanente Southern California (R.S.Z., N.J.F., M.H.M., M.S.), and the Department of Pediatrics, University of California, San Diego (R.S.Z., M.S.) — both in San Diego; the Department of Public Health Sciences, Pennsylvania State University, Hershey (D.M., S.B., V.M.C.); the Department of Pediatrics, Washington University and St. Louis Children’s Hospital, St. Louis (L.B.B., R.C.S., A.B.); the Departments of Medicine and Pediatrics, School of Medicine and Public Health and School of Pharmacy, University of Wisconsin–Madison, Madison (T.W.G., R.F.L., D.J.J., C.A.S., J.E.G.); Arizona Respiratory Center, University of Arizona, Tucson (F.D.M., W.J.M.); the Department of Pediatrics, Divisions of Pediatric Clinical Pharmacology and Allergy and Immunology, National Jewish Health and University of Colorado School of Medicine, Denver (R.C., S.J.S., J.M.-R., L.M.T.); the Department of Pediatrics, University of New Mexico School of Medicine, Albuquerque (H.W.K., H.H.R.); and the Department of Family Medicine, School of Medicine and Public Health, University of Wisconsin–Milwaukee, Milwaukee (E.B.).
Footnotes
The authors’ affiliations are listed in the Appendix.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–7. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Horner CC, Bacharier LB. Management approaches to intermittent wheezing in young children. Curr Opin Allergy Clin Immunol. 2007;7:180–4. doi: 10.1097/ACI.0b013e32807fafd2. [DOI] [PubMed] [Google Scholar]
- 3.Bacharier LB, Phillips BR, Bloomberg GR, et al. Severe intermittent wheezing in preschool children: a distinct phenotype. J Allergy Clin Immunol. 2007;119:604–10. doi: 10.1016/j.jaci.2006.12.607. [DOI] [PubMed] [Google Scholar]
- 4.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma — United States, 1980–1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- 5.Getahun D, Demissie K, Rhoads GG. Recent trends in asthma hospitalization and mortality in the United States. J Asthma. 2005;42:373–8. doi: 10.1081/JAS-62995. [DOI] [PubMed] [Google Scholar]
- 6.Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–6. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 7.Guilbert TW, Morgan WJ, Krawiec M, et al. The prevention of early asthma in kids study: design, rationale, and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 2004;25:286–310. doi: 10.1016/j.cct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma: full report. 2007 ( http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf)
- 9.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–97. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 10.Bacharier LB, Guilbert TW, Zeiger RS, et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. J Allergy Clin Immunol. 2009;123:1077–82. doi: 10.1016/j.jaci.2008.12.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacharier LB, Phillips BR, Zeiger RS, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol. 2008;122:1127–35. doi: 10.1016/j.jaci.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ducharme FM, Lemire C, Noya FJ, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360:339–53. doi: 10.1056/NEJMoa0808907. [DOI] [PubMed] [Google Scholar]
- 13.Kemp JP, Skoner DP, Szefler SJ, Walton-Bowen K, Cruz-Rivera M, Smith JA. Once-daily budesonide inhalation suspension for the treatment of persistent asthma in infants and young children. Ann Allergy Asthma Immunol. 1999;83:231–9. doi: 10.1016/S1081-1206(10)62646-4. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro G, Mendelson L, Kraemer MJ, Cruz-Rivera M, Walton-Bowen K, Smith JA. Efficacy and safety of budesonide inhalation suspension (Pulmicort Respules) in young children with inhaled steroid-dependent, persistent asthma. J Allergy Clin Immunol. 1998;102:789–96. doi: 10.1016/s0091-6749(98)70019-3. [DOI] [PubMed] [Google Scholar]
- 15.Baker JW, Mellon M, Wald J, Welch M, Cruz-Rivera M, Walton-Bowen K. A multiple-dosing, placebo-controlled study of budesonide inhalation suspension given once or twice daily for treatment of persistent asthma in young children and infants. Pediatrics. 1999;103:414–21. doi: 10.1542/peds.103.2.414. [DOI] [PubMed] [Google Scholar]
- 16.Szefler SJ, Baker JW, Uryniak T, Goldman M, Silkoff PE. Comparative study of budesonide inhalation suspension and montelukast in young children with mild persistent asthma. J Allergy Clin Immunol. 2007;120:1043–50. doi: 10.1016/j.jaci.2007.08.063. [DOI] [PubMed] [Google Scholar]
- 17.Leflein JG, Szefler SJ, Murphy KR, et al. Nebulized budesonide inhalation suspension compared with cromolyn sodium nebulizer solution for asthma in young children: results of a randomized outcomes trial. Pediatrics. 2002;109:866–72. doi: 10.1542/peds.109.5.866. [DOI] [PubMed] [Google Scholar]
- 18.Szefler SJ, Eigen H. Budesonide inhalation suspension: a nebulized corticosteroid for persistent asthma. J Allergy Clin Immunol. 2002;109:730–42. doi: 10.1067/mai.2002.122712. [DOI] [PubMed] [Google Scholar]
- 19.Rivera-Spoljaric K, Chinchilli VM, Camera LJ, et al. Signs and symptoms that precede wheezing in children with a pattern of moderate-to-severe intermittent wheezing. J Pediatr. 2009;154:877–81. doi: 10.1016/j.jpeds.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santanello NC, Demuro-Mercon C, Davies G, et al. Validation of a pediatric asthma caregiver diary. J Allergy Clin Immunol. 2000;106:861–6. doi: 10.1067/mai.2000.110478. [DOI] [PubMed] [Google Scholar]
- 21.Raat H, Landgraf JM, Oostenbrink R, Moll HA, Essink-Bot ML. Reliability and validity of the Infant and Toddler Quality of Life Questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res. 2007;16:445–60. doi: 10.1007/s11136-006-9134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beigelman A, Mauger DT, Phillips BR, et al. Effect of elevated exhaled nitric oxide levels on the risk of respiratory tract illness in preschool-aged children with moderate-to-severe intermittent wheezing. Ann Allergy Asthma Immunol. 2009;103:108–13. doi: 10.1016/S1081-1206(10)60162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Global Initiative for Asthma. Global strategy for the diagnosis and management of asthma in children 5 years and younger. doi: 10.1002/ppul.21321. ( http://www.ginasthma.org/uploads/users/files/GINA_Under5_2009_CorxAug11.pdf) [DOI] [PubMed]
- 24.Teper AM, Colom AJ, Kofman CD, Maffey AF, Vidaurreta SM, Bergadá I. Effects of inhaled fluticasone propionate in children less than 2 years old with recurrent wheezing. Pediatr Pulmonol. 2004;37:111–5. doi: 10.1002/ppul.10400. [DOI] [PubMed] [Google Scholar]
- 25.Teper AM, Kofman CD, Szulman GA, Vidaurreta SM, Maffey AF. Fluticasone improves pulmonary function in children under 2 years old with risk factors for asthma. Am J Respir Crit Care Med. 2005;171:587–90. doi: 10.1164/rccm.200408-1088OC. [DOI] [PubMed] [Google Scholar]
- 26.Roorda RJ, Mezei G, Bisgaard H, Maden C. Response of preschool children with asthma symptoms to fluticasone propionate. J Allergy Clin Immunol. 2001;108:540–6. doi: 10.1067/mai.2001.118789. [DOI] [PubMed] [Google Scholar]
- 27.Bisgaard H, Gillies J, Groenewald M, Maden C. The effect of inhaled fluticasone propionate in the treatment of young asthmatic children: a dose comparison study. Am J Respir Crit Care Med. 1999;160:126–31. doi: 10.1164/ajrccm.160.1.9811024. [DOI] [PubMed] [Google Scholar]
- 28.Pao CS, McKenzie SA. Randomized controlled trial of fluticasone in preschool children with intermittent wheeze. Am J Respir Crit Care Med. 2002;166:945–9. doi: 10.1164/rccm.200203-265OC. [DOI] [PubMed] [Google Scholar]
- 29.Wasserman RL, Baker JW, Kim KT, et al. Efficacy and safety of inhaled fluticasone propionate chlorofluorocarbon in 2- to 4-year-old patients with asthma: results of a double-blind, placebo-controlled study. Ann Allergy Asthma Immunol. 2006;96:808–18. doi: 10.1016/S1081-1206(10)61343-9. [DOI] [PubMed] [Google Scholar]
- 30.Castro-Rodriguez JA, Rodrigo GJ. Efficacy of inhaled corticosteroids in infants and preschoolers with recurrent wheezing and asthma: a systematic review with meta-analysis. Pediatrics. 2009;123(3):e519–e525. doi: 10.1542/peds.2008-2867. [DOI] [PubMed] [Google Scholar]
- 31.Papi A, Nicolini G, Baraldi E, et al. Regular vs prn nebulized treatment in wheeze preschool children. Allergy. 2009;64:1463–71. doi: 10.1111/j.1398-9995.2009.02134.x. [DOI] [PubMed] [Google Scholar]
- 32.Connett G, Lenney W. Prevention of viral induced asthma attacks using inhaled budesonide. Arch Dis Child. 1993;68:85–7. doi: 10.1136/adc.68.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svedmyr J, Nyberg E, Thunqvist P, Asbrink-Nilsson E, Hedlin G. Prophylactic intermittent treatment with inhaled corticosteroids of asthma exacerbations due to airway infections in toddlers. Acta Paediatr. 1999;88:42–7. doi: 10.1080/08035259950170583. [DOI] [PubMed] [Google Scholar]
- 34.Scott MB, Ellis MH, Cruz-Rivera M, Fitzpatrick S, Smith JA. Once-daily budesonide inhalation suspension in infants and children < 4 and > or = 4 years of age with persistent asthma. Ann Allergy Asthma Immunol. 2001;87:488–95. doi: 10.1016/s1081-1206(10)62262-4. [DOI] [PubMed] [Google Scholar]
- 35.Shapiro G. Once-daily inhaled corticosteroids in children with asthma: nebulisation. Drugs. 1999;58(Suppl 4):43–9. doi: 10.2165/00003495-199958004-00006. [DOI] [PubMed] [Google Scholar]
- 36.Murray CS, Woodcock A, Langley SJ, Morris J, Custovic A. Secondary prevention of asthma by the use of Inhaled Fluticasone propionate in Wheezy INfants (IFWIN): double-blind, randomised, controlled study. Lancet. 2006;368:754–62. doi: 10.1016/S0140-6736(06)69285-4. [DOI] [PubMed] [Google Scholar]
- 37.Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354:1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 38.Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1–5 years: randomised controlled trial. Lancet. 2003;362:1433–8. doi: 10.1016/S0140-6736(03)14685-5. [DOI] [PubMed] [Google Scholar]
- 39.Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360:329–38. doi: 10.1056/NEJMoa0804897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.