Summary
Adjuvants are requisite components of many vaccines designed to elicit T-cell immunity although the exact components of commonly used adjuvants are not always fully defined. In 2006, owing to concerns of prion contamination, the formulation of Montanide ISA 51 Incomplete Freund’s Adjuvant (IFA) was changed from using oleic acid isolated from beef tallow to that isolated from olives. In sequential clinical trials in the Surgery Branch, NCI patients at high risk for recurrence of melanoma were immunized with the gp100 melanoma/melanocyte antigenic peptide, gp100: 209-217 (210M), emulsified in the beef-derived IFA or the olive-derived IFA. The in vivo generation of gp100 reactive T cells was significantly less in patients receiving the olive compared with the beef IFA as assessed by both ELISPOT (P2=0.0001) and in vitro sensitization assays (P2=0.0001). Local skin reactions to the peptide emulsion were also far less severe using the olive IFA (P2=0.0003). Thus it seems likely that contaminants in the beef-derived IFA played an important role in the increased adjuvanticity of this preparation compared with the olive-derived IFA. These findings raise serious concerns related to the use of the available olive-derived IFA for immunization in clinical trials. A survey of ongoing clinical trials listed in ClinicalTrials.gov revealed 36 trials currently accruing patients that are using the olive-derived Montanide ISA 51 IFA.
Keywords: Incomplete Freund’s Adjuvant, vaccine, melanoma, immunotherapy
In earlier trials carried out and published in 2005 from the Surgery Branch, NCI we showed that patients at high risk for recurrence from metastatic melanoma could be successfully immunized against the gp100 melanoma/melanocyte antigen using the heteroclitic gpl00:210-217 (210M) peptide emulsified in Incomplete Freund’s Adjuvant (IFA).1,2 Evidence of immunization was obtained by comparing preimmunization and postimmunization peripheral blood samples using tetramer, ELISPOT, and in vitro sensitization assays. The adjuvant used in these earlier studies was Montanide ISA 51 IFA from SEPPIC, Inc. (Fairfield, NJ) a water-in-oil emulsion composed of an aqueous phase of droplets containing the peptide disbursed in a continuous oil phase. The stabilization of water-in-oil emulsions is dependent on the presence of a surfactant, a compound containing a hydrophilic polar group and a nonpolar group, often composed of a fatty chain. Montanide ISA 51 was a mixture of mineral oil and a surfactant composed of a mannide monoleate that comes from the esterification of an oleic acid and a sugar, mannitol. The surfactant is rapidly metabolized and eliminated after injection.
In our earlier studies, Montanide ISA 51 (NSC #675756) contained oleic acid of beef tallow origin. Because of the possible danger of prions in beef tallow, the use of beef oleic acid was discontinued in 2006 and oleic acid of olive origin was substituted.3 Thus, all patients in our clinical trials beginning September 1, 2006, received the same gp100:209- 217 (210M)4 peptide emulsified in the new vegetable Montanide ISA 51 VG (NSC #737063). The exact same amount and schedule of the peptide in IFA was used in the prior trials published in 2005 and in our current immunization trials.
We have prepared this brief report because there has been a significant decrease in our ability to immunize patients with this peptide in our current trials and our studies strongly suggest that the new vegetable formulation has less adjuvanticity than the prior beef formulation of IFA.
MATERIALS AND METHODS
Patients with a confirmed diagnosis of melanoma were enrolled in these protocols. All patients were clinically free of disease and eligible for the protocol if they had primary melanomas that were ≥1.5-mm thick or were ulcerated, had 1 or more resected positive lymph nodes or had completely resected metastaic melanoma. All patients were entered into the protocol within 6 months of surgical resection of their melanoma. No patient had earlier been immunized with gp100.
In the prior protocols published in 2005, patients received 1mg of gp100:209-217 (210)M and tyrosinase: 368-376 (370D) peptides emulsified separately in IFA (Montanide ISA 51 (NSC#675756) with oleic acid of beef tallow origin) and injected s.c. in different extremities.5 In the current protocol patients received 1mg of the gp100: 209-217 (210M) peptide emulsified in IFA (Montanide ISA 51 VG (NSC#737063) with oleic acid of olive origin) injected s.c. either alone or with the application of imiquimod cream (5%) massaged over the skin of the injection site once daily for 5 days. Patients in both protocols received the peptide injections every 3 weeks for up to 12 injections. Patients underwent leukopheresis before immunization and 3 weeks after every 4 immunizations. Peripheral blood mononuclear cell (PBMC) were cryopreserved at −180°C after Ficoll-Hypaque separation.
Media and Tissue Culture
T2 cells (peptide transporter-associated protein-deficient T-B hybrid) or C1R-A2 cells were pulsed with peptide and used as targets. Human lymphocytes were cultured in complete medium (CM) consisting of RPMI 1640, 2mM L-glutamine, 50U/mL penicillin, 50 μg/mL streptomycin (invitrogen Life Technologies), and 10% heat-inactivated human AB serum (Gemini Bio-Product; Valley Biomedical).
Immunologic Assays
For all assays, pretreatment and posttreatment cryopreserved PBMC samples from a patient were evaluated simultaneously.5
ELISPOT Assays
In brief, PBMC were thawed from cryopreservation and cultured overnight in complete medium at a density of 107 cells/well in a 6-well plate. PBMC were then incubated for 24 hours at 105 cells/well with peptide-pulsed C1R-A2 cells in 96-well plates coated with anti-IFN-γ Ab (BD pharmingen), developed with avidin-alkaline phosphatase, and stained. The number of spots per experiment were counted using a Immunospot Analyzer (CTL Analyzers) and were corrected by subtracting background spots resulting from PBMC incubated with unpulsed C1R-A2 cells.
In Vitro Sensitization Assay
In vitro sensitization assays were carried out as earlier described.2 In brief, PBMC were cultured in Iscove medium with 10% heat-inactivated human AB serum with 1-μM specific or control native peptides and 300 IU/mL IL-2. After 11 to 13 days, T cells were harvested and coincubated with peptide-pulsed T2 cells overnight and IFN-γ release in the supernatant measured by ELISA. A positive was defined as IFN-γ release after incubation with the native peptide by postvaccination PBMC of >100 pg/mL and more than twice all controls including the prevaccination PBMC.
Peptide Preparations
The gp100:209-217 (210M) peptide, IMDQVPFSV, was produced to GMP grade by solid phase synthesis techniques by Multiple Peptide Systems (San Diego, CA). The peptide was vialed in 1.5mL containing 1.5mg of peptide. To prepare the 1-mg peptide dose, 1.5mL of Montanide ISA-51 were combined with 1.5mL of the peptide solution and vortexed vigorously for 12 minutes as earlier described. As indicated in the text some patients in the current protocol received the peptide emulsified using a 2-syringe method as recommended by SEPPIC, Inc. The peptide and Montanide ISA-51 VG were drawn into separate syringes connected by a 3-way stopcock.
The emulsion was prepared by carrying out 20 “preemulsion cycles” (a cycle defined as on pass back and forth between the two syringes), followed by 100 additional cycles. The preemulsion cycles were carried out over approximately 1 minute. The 100 additional cycles were carried out over approximately 1 minute.
RESULTS
In the prior and current trials, patients received immunization with 1mg of the gp100:209-217 (210M) peptide injected subcutaneously every 3 weeks by the same personnel. Patients were pheresed prior to immunization and 3 weeks after every course (4 injections) of peptide immunization. Table 1 provides the results of the ELISPOT assay after 12 cycles of immunization utilizing beef IFA in 25 patients in the prior trial and 40 patients who received 12 cycles of immunization of peptide in vegetable IFA in the current trial. In the current trial 23 patients received vortexed emulsion prepared as in our prior trials. Thirteen of those received peptide immunization alone and 10 patients received the same peptide immunization with imiquimod cream applied directly over the immunization site. Only two of 23 patients were successfully immunized in the current trial. Because of concerns that the vegetable IFA emulsion might be unstable, we then treated 17 patients (8 with peptide and 9 with peptide plus imiquimod cream) using the 2 syringe method as specified by the manufacturer. This 2 syringe method resulted in an emulsion that remained usually stable for over 2 months. Using the 2 syringe method, only 2 of 17 patients were successfully immunized. Thus, using vegetable IFA, only 4 of 40 patients (10%) could be successfully immunized compared with our prior results that showed that 15 of 25 patients (60%) could be successfully immunized after 12 immunizations using the beef-derived IFA (P2=0.0001) (Table 1). When comparing just the vortex method of emulsification in the current trial that was used in the prior trial, the difference in immunization between the 2 trials was still significant (P2=0.015).
TABLE 1.
Immunization With gp100:209-217 (210M) Peptide in Incomplete Freund’s Adjuvant Derived From Beef or Vegetable Sources: Elispot Assay*
| Vegetable IFA (Current) | Beef IFA (2005 Study) | |||
|---|---|---|---|---|
| Method of emulsification | Total | #Positive† | Total | #Positive |
| (Number of patients) | ||||
| Vortex: peptide | 13 | 2 | 25 | 15 (P2=0.015) |
| Peptide+IM | 10 | 0 | ||
| 2-syringe peptide | 8 | 1 | ||
| Peptide+IM | 9 | 1 | ||
| Total | 40 | 4 (10%) | 25 | 15 (60%) (P2=0.0001) |
Assay performed 3 weeks after third course (12 immunizations).
Positive: ≥10 spots over control.
IM indicates imiquimod cream.
Because of the concern that the difference in immunization results comparing prior with current protocols might be due to differences in the conduct of the ELISPOT assay, we thawed cryopreserved samples from our prior study in 2005 and we reassayed those samples along with current samples from patients receiving vegetable IFA in the same assay. As shown in the 2 experiments in Table 2, the results of the ELISPOT assay currently done on cryopreserved samples were very similar to the results achieved in 2005. In this same assay, we again failed to find a significant level of immunization using the vegetable IFA. It thus seemed that differences in the conduct of the ELISPOT assay could not explain the differences in immunization in the prior and current trials.
TABLE 2.
ELISPOT Assay: Comparison of Results on Same Samples Tested in 2005 (Prior Study) and 2009 (Current Study)
| Beef IFA | Vegetable IFA* | |||
|---|---|---|---|---|
| Assay (2005)† | Assay (2009)† | Current Study | ||
| Patient | (Number of spots)‡ | |||
| Expt. 1: | 1 | 436 | 381 | 1 |
| 2 | 8 | 0 | 0 | |
| 3 | 99 | 69 | 0 | |
| 4 | 567 | 442 | 0 | |
| 5 | 0 | 0 | 0 | |
| Expt. 2: | 6 | 436 | 207 | 2 |
| 0 | ||||
| 3 | ||||
| 17 | ||||
| 3 | ||||
| 0 | ||||
| 2 | ||||
Samples run in same assay on current patients who received the gp100:209-217 (210M) peptide in vegetable IFA.
Results of assay on aliquots from the same samples tested in 2005 and in 2009.
gp100:209-217 spots minus control.
In our prior study, we also used an in vitro sensitization assay to measure the ability to successfully immunize with peptide in IFA. This same assay was used to test samples from 9 patients in the current trial who received 8 peptide immunizations (Table 3). No evidence of immunization was seen in these 9 patients compared with our prior result in which 14 out of 22 patients (64%) were successfully immunized after 8 immunizations using this in vitro sensitization assay (P2=0.0001). Retesting of cryopreserved samples from the prior trial again showed similar results to those obtained in 2005 (data not shown). Thus, by 2 separate assays in multiple patients, our ability to immunize patients using the identical peptide in vegetable IFA seemed to be inferior to our ability to immunize with this peptide in beef IFA.
TABLE 3.
Immunization With gp100:209-217 (210M) Peptide in Incomplete Freund’s Adjuvant Derived From Beef or Vegetable Sources: In Vitro Sensitization Assay*
| Vegetable IFA | Beef IFA | |||
|---|---|---|---|---|
| (Number of patients) | ||||
| Total | #Positive† | Total | #Positive | |
| Peptide | 5 | 0 | 22 | 14 (P2=0.015) |
| Peptide+IM | 4 | 0 | ||
| Total | 9 | 0 (0%) | 22 | 14 (64%) (P2=0.0001) |
PBMC collected 2 weeks after second course (8 immunizations).
Positive: ≥100 pg/mL IFN-g and at last 2 times greater than all controls.
Local Toxicity of the Peptide in IFA Injection Site
A total of 31 patients in the prior trial (beef IFA) and 67 patients in the current trial (vegetable IFA) received at least 1 full course of immunization (4 immunizations) although not all patients completed all courses of immunization because of tumor progression, local toxicity, or patient withdrawal. After 1 immunization course, 8 of 31 patients in the prior trial achieved grade 2 injection site toxicity (defined in CTCAE Version 3.0 as pain or swelling with inflammation or phlebitis) compared with only 1 of 67 patients in the current trial (P2=0.0003). Some patients in the prior trial developed ulceration at the injection site (as illustrated in Fig. 1) that was not seen in the current trial. Thus local skin inflammation was far more intense in patients receiving peptide in beef-derived IFA than in vegetable-derived IFA. No skin reactions were seen at sites of injection of the tyrosinase peptide.
FIGURE 1.
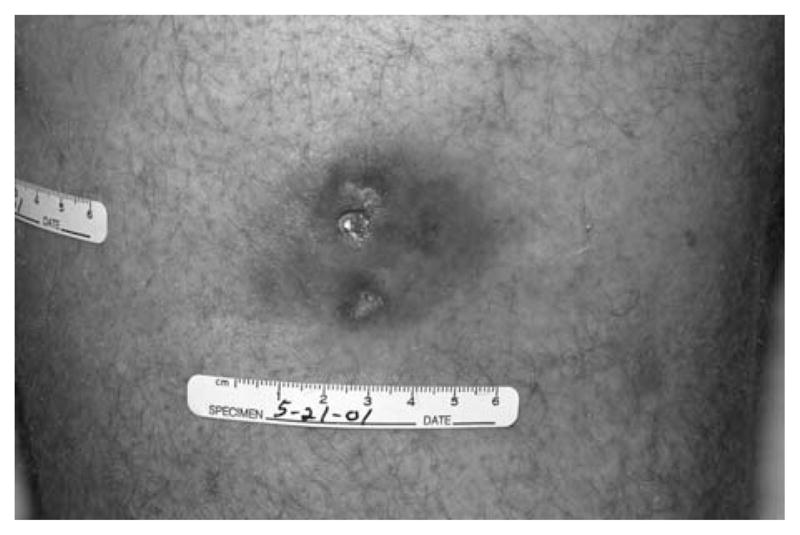
Example of skin reaction and ulceration after immunization with the gp100:209-217 (210M) peptide in beef-derived IFA.
DISCUSSION
In sequential clinical trials, we found that immunization using an immunogenic heteroclitic peptide from the gp100 molecule4 was far more effective in generating T cells when emulsified in IFA that contained oleic acid of beef tallow origin compared with IFA containing oleic acid of olive origin. This change in the production of IFA occurred in 2006 and was mandated by regulatory agencies concerned about the potential of prion-like molecules contaminating materials extracted from bovine sources. This change in the formulation of IFA closely correlated with a substantial decrease in our ability to immunize melanoma patients with the gp100:209-217 (210M) immunogenic peptide emulsified in IFA. By ELISPOT and in vitro sensitization assays the switch from beef to vegetable-derived IFA resulted in a decrease from 60 to 10% of patients generating peptide-specific T cells (P2=0.0001). Although it is difficult to compare the results of assays carried out at different times it does not seems that the conduct of the immunologic assays could account for the differences seen because current reassay of prior cryopreserved samples from the earlier trial gave results similar to those originally obtained. The same vortex method of preparing the emulsion was used in both trials. To test whether a change may have occurred in preparing the emulsion, in the current trial, we also tested an additional cohort of patients using a stable emulsion prepared by a “two-syringe” method as recommended by SEPPIC, Inc. supplier of the IFA, yet very little immunization was seen using this method as well. It should be emphasized that the peptide used were all synthesized by the same company (Multiple Peptide Systems, San Diego, CA) and as peptide lots were used, new lots were synthesized. Because the old lots are not available we could not control for this difference in sequential protocols.
As further evidence of the decreased immunogenicity of the vegetable IFA, severe local skin reactions were seen when patients received the beef-derived IFA and very few skin reactions were seen in patients receiving the vegetable-derived IFA (P2=0.0003).
Immunologic adjuvants have long been known to be essential to enhance the immune response to vaccine antigens. Alum, a chemical family of aluminum salts, is widely used to stimulate antibody responses to vaccines but is relatively ineffective in stimulating vigorous T-cell responses. Adjuvants that contain components of bacterial extracts are potent T-cell stimuli. Although several mechanisms may play a role in the ability of adjuvants to enhance immune responses an important finding to explain their activity was published in 1997 by Medzhitov et al,6 who characterized a human homologue of the Drosophila toll protein that induce innate immune responses in adult Drosophila. These toll-like receptors (TLR), also known as pattern recognition receptors are expressed on antigen presenting cells and bind to components from microorganisms that lead to activation of antigen presenting cells. Ten different functional TLR have been identified that recognize motifs in molecules present in viruses or bacteria.7 Activation of TLR by bacterial components either deliberately administered or inadvertently present in immunizing mixtures have been referred to as the immunologist’s “dirty little secret.”8 These factors may play an important part in understanding the findings described in this paper.
It is possible that contaminants in the beef-derived IFA played an important role in the increase adjuvanticity of this preparation compared with the olive-derived IFA. This presumed difference in the adjuvanticity of the different IFA preparations is particularly important in the light of the recent description of improved response rates to interleukin-2 in patients with metastatic melanoma who were immunized with the gp100:209-217 (210M) peptide in IFA.9 Most of these patients received the beef-derived IFA. A survey of ongoing clinical trials listed in ClinicalTrials.gov revealed 135 trials that used Montanide IFA, 36 of which are currently accruing patients using the olive-derived IFA. If indeed the new olive-derived IFA is less effective in immunizing patients this would have important consequences for the use of this material in ongoing and future clinical trials.
Footnotes
All investigator’s have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Sherry RM, Morton KE, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 3.Bonhoure F, Gaucheron J. Montanide ISA 51 VG as adjuvant for human vaccines. J Immunother. 2009;29:647–648. Ref Type: Abstract. [Google Scholar]
- 4.Parkhurst MR, Salgaller ML, Southwood S, et al. Improved induction of melanoma reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A* 0210 binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 5.Banchereau J, Palucka AK, Dhodapkar M, et al. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 6.Medzhitov R, Preston-Hurlburt P, Janeway C., Jr A human homologue of the drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 7.Barton GM, Kagan JC. A cell biological view of toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symposia on Quantitative Biology. 1989 doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Schwartzentruber DJ, Lawson D, Richards J, et al. A phase III multi-institutional randomized study of immunization with the gp100:209-217(210M) peptide followed by high-dose IL-2 compared with high dose IL-2 alone in patients with metastatic melanoma. J Clin Oncol. 2009;27:18s. Ref Type: Abstract. [Google Scholar]


