Abstract
OBJECTIVES
To evaluate the effects of a behavioral intervention, Tai Chi Chih (TCC) on circulating markers of inflammation in older adults.
DESIGN
A prospective, randomized, controlled trial with allocation to two arms, TCC and health education (HE), 16 weeks of intervention administration, and 9 weeks follow-up.
PARTICIPANTS
A total of 83 healthy older adults, aged 59 to 86 years.
MEASUREMENTS
The primary endpoint was circulating levels of interleukin 6 (IL-6). Secondary outcomes were circulating levels of C-reactive protein (CRP), soluble IL-1 receptor antagonist (sIL-1RA), sIL-6R, soluble intercellular adhesion molecule (sICAM) and IL-18. Severity of depressive symptoms, sleep quality, and physical activity was also assessed over the treatment trial.
RESULTS
Among those older adults with high levels IL-6 at entry, a trend for a treatment group by time interaction was found (F(1,70)=3.48, P = .07), in which TCC produced a drop of IL-6 levels comparable to those found in TCC and HE subgroups who had low levels of IL-6 at entry (t’s(72)=0.80, 1.63, P’s>0.10), whereas IL-6 in HE remained higher than the TCC- and HE subgroups with low entry IL-6 (t(72)=2.47, P=0.02; t(72)=1.71, P=0.09). Decreases in depressive symptoms in the two treatment groups correlated with decreases of IL-6 (r=.28, P <.05). None of the other cellular markers of inflammation changed in TCC vs. HE.
CONCLUSION
TCC can be considered a useful behavioral intervention to reduce circulating levels of IL-6 in older adults who show elevated levels of this inflammatory marker and are at risk for inflammation-related morbidity.
Keywords: Tai Chi, Inflammation, Stress, Aging
INTRODUCTION
Inflammation plays an increasingly prominent role in health and wellbeing as people age.1 Circulating levels of inflammatory markers rise with age even in healthy individuals,2–6 and the proportion of person with elevated levels of IL-6 rises markedly among persons older than 70 years.7 In the elderly, many of the diseases that contribute most to disability, morbidity, and mortality stem in part from aberrant inflammation (e.g., cardiovascular disease and heart failure, cancer, Alzheimer’s Disease and other neurodegenerative diseases, metabolic alterations, and diabetes).8–11 Indeed, increases in inflammatory signaling molecules occur and have impacts far beyond the site and time of their initial production. Persistent inflammatory signaling can induce localized remodeling of tissue (chronic inflammation) that produces long-term changes in functional capacity (e.g., sclerosis, muscle wasting, depression).12 Inflammatory signals can also act systemically to alter the function of other tissues and organs. For example, proinflammatory cytokines such as IL-6 can release an integrated package of “sickness behaviors” from the brain that affect global aspects of well-being, including mood, energy, sleep, social behavior, and cognitive function.13 The mechanisms that contribute to age-related increases of IL-6 are not fully known, although biobehavioral factors such as low physical activity and stress response pathways likely play a substantial role independent of other lifestyle- and health factors such as smoking and alcohol use, for example.14, 15 Despite the strong association between aging, inflammation, and morbid outcomes, no definitive treatments have yet been developed that effectively target inflammation especially in those older adults who show elevated levels of IL-6, for example, and are at risk for inflammatory disorders.
Recent controlled trial data provide some promising evidence that exercise training may be an independent means of mitigating inflammation in older adults, although findings are limited.16, 17 Two studies in older adults have found that long-term (i.e., 10 months; 12 months) exercise interventions led to lower circulating levels of interleukin-6 (IL-6)17, 18 with one study suggesting that this effect was only apparent in older adults who show elevated levels of IL-6.17 Other exercise intervention trials that have targeted either healthy adults or various clinical populations have yielded mixed results.16
Given evidence that psychosocial stress can drive increases of inflammation,19, 20 it is possible that treatments that target stress response pathways might also have effects on inflammation. Indeed, Tai Chi, a traditional Chinese martial art that incorporates aerobic activity, relaxation, and meditation, has been found to decrease measures of psychological stress.21 In addition, we have found that Tai Chi Chih, a westernized and manualized form of Tai Chi, can reduce sympathetic stress effector mechanisms22 and can also boost viral specific- and vaccination response in older adults.23, 24 Moreover, as compared to exercise training, TCC is a particularly attractive intervention for use in the elderly, as this “movement meditation” is a type of physical activity that is readily accessed by older adults who often have age-related limitations in their ability to tolerate even moderate intensity exercise.21
This clinical trial was conducted to determine the effects of TCC vs. health education (HE) on circulating levels of IL-6 in older adults, given evidence that levels of IL-6, but not necessarily other measures of inflammation, are modifiable by exercise training. 16, 17 Furthermore, based on prior evidence that exercise training only has detectable effects on reducing IL-6 when levels of this cytokine are elevated (>2.46 pg/ml),17 we hypothesize that TCC, as compared to HE, will reduce IL-6 only in those older adults who show elevated levels of IL-6 at baseline. We explore whether TCC vs. HE might alter other markers of inflammation including C-reactive protein (CRP), soluble IL-1 receptor antagonist (sIL-1RA), sIL-6R, soluble intercellular adhesion molecule (sICAM) and IL-18. Finally, we examine whether change in measures of depressive symptoms, sleep quality, and physical activity are associated with changes in IL-6.
METHODS
Design and population
This randomized, controlled clinical trial allocated older adults to receive either TCC or HE (active control intervention) in a 1:1 ratio at study sites in San Diego and Los Angeles between 2001 and 2005. As previously reported, subjects in both groups received a single dose of live attenuated Oka/Merck varicella vaccine, VARIVAX™ at week 16.24 Institutional review boards at both study sites approved this study.
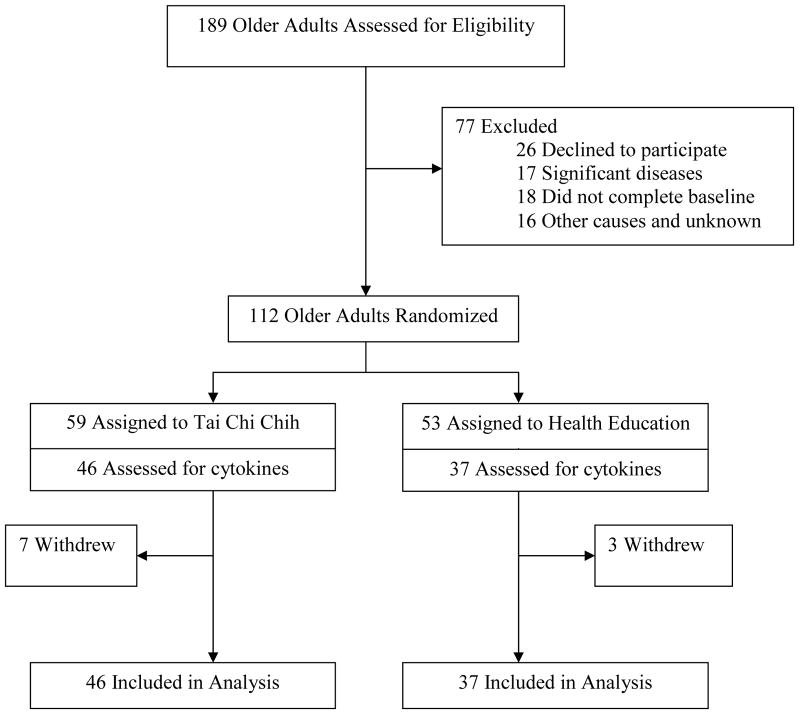
Older adults were recruited through community newspaper advertisements that stated the aim of the study as comparing the effects of TCC vs. HE on “health and well-being in healthy older adults.” Subjects were offered compensation for participation and completion of the study. A total of 112 community dwelling participants aged 59 to 86 years who responded to advertisements were enrolled and randomly assigned to TCC (n= 59) or HE (n = 53) (Figure 1). Randomization was performed using a computer-generated schedule independent of treatment personnel. Major eligibility exclusions were evidence of immunocompromise resulting from disease, corticosteroids, or other immunosuppressive/cytotoxic therapy; chronic liver disease or kidney disease; receipt of immunizations (e.g., hepatitis B vaccine; influenza vaccine) within one month prior to study entry or scheduled over the course of the intervention; any acute intercurrent illness (e.g., thyroid disease, sinusitis, urinary tract infections) that might interfere with interpretation of the study; and presence of a current major psychiatric disorder as determined by the Structured Clinical Interview for DSM-IV diagnoses.25 Additional exclusions were alcohol intake greater than 3 drinks per day, and an unwillingness to adhere to study protocol or ongoing participation in Tai Chi. None of the participants were current smokers.
Figure 1.
Participant flow and distribution of subjects in study
Intervention
Subjects received either 16 weeks of TCC or HE administered to groups of 7 to 10 persons. TCC sessions lasted 40 minutes and were given 3 times per week for a total 120 minutes of weekly instruction. HE was also allocated over a 120-minute period of instruction per week; hence, an identical amount of instructor time was provided to both intervention groups. The rationale communicated to subjects was that TCC is a health management intervention that incorporates meditation and repetitive physical activity to promote well-being in aging, whereas HE aims to promote healthy behaviors and well-being by providing knowledge about health management. For TCC, objectives and learning activities related to the specific set of 20 exercises were delivered according to a therapist manual 26 with serial teaching of 1–2 new movements each week, weekly supervision by master’s level TCC instructors, and verification of skills attainment by week 16. Further practice and mastery of TCC occurred during follow-up to week 25. The HE intervention involved sixteen didactic presentations on a series of health-related themes, which were provided by a physician or licensed clinical psychologists with group discussion, as previously described.27 We assessed treatment credibility and expectation for change after the second treatment session using a 5-point likert scale.28
Assessment and outcome measures
The primary outcome variable was circulating levels of IL-6, with secondary assessment of CRP, sIL-1RA, sIL-6R, sICAM and IL-18 on three occasions: at baseline (before randomization), at 16 weeks (post-intervention), and at 25 weeks (9 weeks follow-up). At baseline, we also assessed a number of factors that have been associated with variations in inflammation including age, sex, socioeconomic status, marital status, physical activity, medical impairment, severity of depressive symptoms, and sleep quality as previously described.14, 15 The Beck Depression Inventory (BDI) was used to assess severity of depressive symptoms and the Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality;29, 30 both measures were administered prior at baseline, 16 weeks, and 25 weeks. Because subjects had a regular sleep-wake schedule as previously described,31 blood sampling was routinely scheduled to occur between 8 a.m. and 10 a.m. to control for circadian variation in IL-6.
Assay of cellular markers of inflammation
Plasma levels of IL-6, as well as CRP, sIL-1RA, sIL-6R, sICAM and IL-18 were quantified by means of enzyme-linked immunosorbent assay methods (R & D Systems, Minneapolis, MN). All samples at baseline, 16 weeks, and 25 weeks were assayed at the same time, in a single run with a single lot number of reagents and consumables employed by a single operator. The intra-assay coefficients of variation for all variables were less than 5%. Testing for cytokines was not implemented for some participants due to scheduling issues. This reduced the sample available for analysis to n=46 for TCC and n=37 for HE (see Figure 1). In addition, there were some additional missing assay values for each of the cytokines due to adequate sample availability and/or technical issues.
Statistical Analysis
Comparison of treatment groups at entry was performed using unpaired t tests for continuous data and χ2 for discrete data. The general effects of the intervention over time on IL-6 were assessed using a treatment group (TCC vs. HE) x time (baseline, week 16, and week 25) repeated measures mixed models analysis of variance. The primary hypothesis is that TCC, as compared to HE, will reduce IL-6 only in those older adults who show elevated levels of IL-6 at baseline; this hypothesis was tested by stratifying the treatment groups by high vs. low IL-6, with the high group being defined by IL-6 values in the highest quartile at study entry (>2.8 pg/ml). Secondary analyses of the effects of intervention over time on other markers of inflammation including CRP, sIL-1RA, sIL-6R, sICAM and IL-18 were analyzed using a treatment group x time repeated measures mixed models analysis of variance. As the covariance structure did not significantly deviate from sphrericity (Mauchly test), compound symmetry was assumed. Due to some missing data, a mixed models approach was used, and all analyses used an intention to treat approach.
RESULTS
Adherence to intervention
Of 112 subjects allocated to the intervention, 102 persons (91%) completed the intervention and were followed to 25 weeks. (Figure 1) Of the 7 withdrawals in TCC, 6 withdrew due to the difficulties with time commitments and/or transportation and 1 did not like the class. Of the 3 withdrawals in HE, 2 withdrew due to difficulties with the time commitment and 1 dropped due to health problems. Attendance at treatment sessions was high; TCC participants attended 83% ± 20% (mean ± SD) and HE subjects attended 80% ± 20% of all sessions (t(110)=.73, P>0.40). As previously reported, the two interventions were perceived as equally credible.24 Over the course of the intervention period, TCC participants showed a significant increase in the number of minutes of at-home TCC practice per week with specific comparisons showing an increase from 111 ± 61 minutes at week 8 to 213 ± 146 minutes at week 16 (t(45)=5.36; P < 0.001), and 149 ± 122 minutes at week 25 (t(45)=2.16, P<0.04). Although there was a significant decrease of TCC practice per week from week 16 to week 25 (t(45)=3.21, P<0.01), participants maintained practice of TCC after completion of the intervention. Despite these increases in TCC practice, overall physical activity, as measured by metabolic equivalents expended per week, did not change over the course of the trial in either group (t(35.6)=0.51, P =0.50), which suggests that participants in the TCC group substituted TCC for other aerobic activity.24
Characteristics of the cytokine assay sub-sample
There were no significant pretreatment differences with respect to age, sex, ethnicity, marital status, educational level, annual income, severity of depressive symptoms, sleep quality, and medical impairments or weekly physical activity between the treatment groups (Table 1). The sub-sample was also largely equivalent with those from the full sample who were not tested, except those in the untested group were younger (mean=66.7, sd=6.0, t(110)=3.06, P<0.005) and with higher incomes (mean = $81.0k, sd=$50.6k, t(90)=2.92, P<0.01) as compared to those tested.
Table 1.
Baseline Characteristics of the Study Participants
| Characteristic | Tai Chi Chih (n = 46) | Health Education a (n = 37) |
|---|---|---|
| Demographics | ||
| Age in years, mean (sd) | 70.7 (5.9) | 71.4 (7.7) |
| Sex, Number (%) | ||
| Male | 14 (30.4) | 18 (48.6) |
| Female | 32(69.6) | 19 (51.4) |
| Ethnicity, Number (%) | ||
| White | 36 (83.8) | 31 (78.3) |
| Non-white | 10 (16.2) | 6 (21.7) |
| Marital Status, Number. (%) | ||
| Married | 24 (52.2) | 22 (59.5) |
| Not married | 22 (47.8) | 15 (40.5) |
| Education in years, mean (sd) | 16.5 (2.4) | 15.9 (2.6) |
| Annual income ($k), mean (sd) | 50.6 (30.7) | 55.2 (43.2) |
| Psychological | ||
| Pittsburgh Sleep Quality Index, mean (sd) | 4.8 (2.4) | 5.2 (3.6) |
| Beck Depression Scores, mean (sd) | 5.3 (4.2) | 5.0 (4.7) |
| Physical Activity and Medical Impairments | ||
| Metabolic equivalents per wk, mean (sd)b | 253.9 (25.6) | 252.5 (23.9) |
| Chronic Disease Score, mean (sd) | 0.9 (1.6) | 1.5 (2.1) |
Group comparisons were performed using unpaired t tests for continuous data and χ2 for discrete data; df=81; comparisons were not different with p’s > 0.15; sex comparison was p=.09.
Metabolic equilavents (METS) calculated according to the methods of Sallis 38
The outcome of interest was change in circulating levels of IL-6. As shown in Table 2, there was statistical trend for a treatment group by time interaction for circulating levels of IL-6, which was characterized by modest decreases of IL-6 in the TCC group and increases IL-6 in the HE group. For CRP, sIL-1RA, sIL-6R, sICAM and IL-18, no treatment group x time interactions were found (Table 2). For IL-6, TCC showed a within-group effect size of 0.25, which was similar to the effect size for aerobic exercise.16 Together these data suggest that about 220 subjects per group yields 80% power to detect a statistical (P<0.05) change in IL-6 following administration of exercise. For the other markers of inflammation, the effect sizes ranged from 0.03 to 0.11, indicating over 500 subjects per group to achieve 80% power.
Table 2.
Inflammatory Markers Across Three Assessments in the Tai Chi Chih and Health Education Groups
| Tai Chi Chih | Health Education | Time | Time × Group | |
|---|---|---|---|---|
| IL-6 (pg/ml) | F(1,72)=1.52, p=.23 | F(1,72)=3.71, p<.06 | ||
| Baseline | 2.25 (1.43) | 1.87 (1.09) | ||
| Week 16 | 2.07 (1.14) | 1.95 (1.43) | ||
| Week 25 | 2.04 (1.36) | 2.64 (2.60) | ||
| CRP (mg/l) | F(1,48)=.50, p=.61 | F(1,48)=.60, p=.55 | ||
| Baseline | 1.66 (1.12) | 1.56 (1.69) | ||
| Week 16 | 1.58 (1.24) | 1.75 (1.88) | ||
| Week 25 | 1.96 (1.54) | 1.67 (1.63) | ||
| IL-18 (pg/ml) | F(1,44)=2.02, p=.14 | F(1,44)=.74, p=.48 | ||
| Baseline | 485 (262) | 445 (245) | ||
| Week 16 | 525 (332) | 551 (366) | ||
| Week 25 | 562 (268) | 548 (372) | ||
| sIL-6r (pg/ml ×1000) | ||||
| Baseline | 33.5 (8.3) | 34.6 (8.6) | F(1,51)=1.98, p=.15 | F(1,51)=.58, p=.56 |
| Week 16 | 33.0 (9.0) | 35.1 (9.7) | ||
| Week 25 | 33.7 (8.6) | 33.8 (10.0) | ||
| IL-1ra (pg/ml) | F(1,29)=.06, p=.94 | F(1,29)=.29, p=.75 | ||
| Baseline | 254 (187) | 234 (143) | ||
| Week 16 | 238 (144) | 240 (137) | ||
| Week 25 | 223 (131) | 254 (131) | ||
| sICAM (pg/ml) | F(1,49)=.24, p=.78 | F(1,49)=2.43, p=.10 | ||
| Baseline | 229 (136) | 208 (86) | ||
| Week 16 | 198 (98) | 203 (102) | ||
| Week 25 | 222 (115) | 205 (108) |
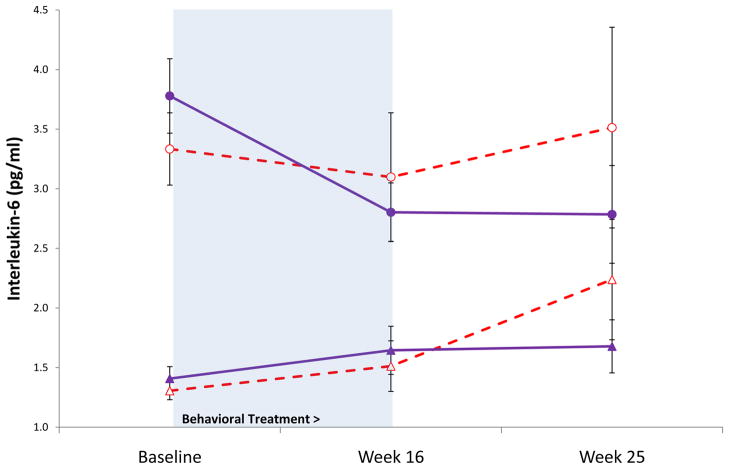
Given prior evidence that exercise training is primarily associated with a reduction of IL-6 in older adults with high median levels of IL-6 (>2.5 pg/ml) with an effect size of about 0.91,16 analyses stratified the sample by entry levels of IL-6 with generation of treatment subgroups (i.e., the top quartile had high levels of IL-6 >2.8 pg/ml). Those participants in the top quartile were significantly older (t(79)=2.67, P<0.01) and more likely to be male (χ2(1)=8.77, P<0.005) as compared to subjects in the bottom three quartiles, but the TCC and HE groups within the top quartiles were similar on all other demographic, psychological, and medical impairment variables. Figure 2 displays the subgroup analysis; a significant quartile group by time interaction (F(1,70)=3.88, P<0.05) and a trend for a treatment group by time interaction (F(1,70)=3.48, P = .07) were found for circulating levels of IL-6. These analyses suggest that while IL-6 increased in the HE groups, it remained steady (low entry IL-6) or decreased (high entry IL-6) in the TCC groups. Post-hoc comparisons showed that the TCC subgroup with high entry IL-6 had levels of IL-6 at week 25 comparable to levels found in the TCC- and HE subgroups with low entry IL-6 (t’s(72)=0.80, 1.63, P’s>0.10). In contrast, the HE subgroup with high entry IL-6 had levels of IL-6 at week 25 that remained higher than the TCC- and HE subgroups with low entry IL-6 (t(72)=2.47, P=0.02; t(72)=1.71, P=0.09, respectively).
Figure 2.
Effects of Tai Chi Chih vs. health education on circulating levels of interleukin-6 over 25 weeks. TCC is stratified by entry levels of IL-6 into high quartile (λ) and lower three quartiles (σ), and health education is stratified by entry levels of IL-6 into high quartile (μ) and lower three quartiles (∇). The shaded area indicates the duration of the intervention from baseline to week 16.
Given that participants were part of a vaccination manipulation, 24 adjustment for receipt of vaccination vs. placebo at week 16 did not alter the results. For example, the HE subgroup with high entry IL-6 were still elevated at week 25 compared to the TCC- and HE subgroups with low entry IL-6 (t(68)=2.43; P=.02; t(68)=1.68; P=0.09).
None of the demographic, psychological (i.e., BDI and PSQI scores), physical activity, or medical impairment variables were correlated with IL-6 at any assessment point (all |r’s|<.20, p’s>.10). In addition, neither the average number of sessions attended (t(13)=0.52, P>0.20) nor weekly minutes of TCC practice at week 25 (t(13)=0.69, P>0.20) was related to change of IL-6 (weekly minutes of TCC practice at baseline was zero). However, we have previously reported that change in BDI-, and PSQI scores occurs during this intervention with similar decreases of BDI scores in the TCC and HE groups, and with changes in PSQI scores found only in those participants who had high PSQI scores >5 at entry.31 Given these prior results, additional analyses examined change from baseline to week 25 in BDI (without sleep items), as well as change in PSQI scores, with change in IL-6 during this interval. Change in BDI was positively correlated with change in IL-6 (r=.28, P <.05), and this relationship was somewhat stronger among those individuals in the upper quartile of IL-6 at baseline (r=.32, P <.05). There was, however, no interaction by treatment condition either in the overall sample (F(1,66)=.18, P >.50) or within the upper quartile (F(1,19)=.02, P >.50), consistent with our prior findings that BDI scores change similarly in the TCC and HE groups. 31 Change in PSQI scores was not associated with change in IL-6 from baseline to week 25.
DISCUSSION
This randomized, controlled trial shows that a “movement meditation”, TCC as compared to HE, appears to result in lower circulating levels of IL-6 in older adults who have elevated levels at baseline. However, the effects of TCC are primarily identified by comparison with the HE group who showed increases of IL-6; a finding consistent with Nicklas et al who found among older adults with elevated levels of IL-6 that circulating concentrations of this cytokine remained unchanged in those who participated in a physical activity intervention, but rose in those participating in an active comparator control condition.17 These data provide novel information that this behavioral treatment, which targets both physical activity and stress response pathways, may be a potentially effective therapy for reducing systemic concentrations of IL-6 in older adults who are most at risk for inflammatory disorder. In other words, given that IL-6 is an important biological predictor of increased risk for disability1 and that the effects of TCC were primarily among those older adults who have high levels of IL-6, TCC might be most beneficial in those older adults who are at the greatest risk for disability, subsequent loss of independence, and morbidity due to inflammatory disorders. Indeed, findings that TCC normalized high levels of IL-6 to levels at or below a concentration of 2.5 pg/ml has potential clinical implications, as healthy older adults above this level are approximately two-thirds more likely to develop disability over the next four years.1
In the present study, decreases in depressive symptoms from baseline to week 25 were associated with decreases in IL-6 over the same interval. Whereas it is not possible to make a causal inference about the direction of this association, the present findings are consistent with high rates of depressive disorders in persons with inflammatory disorders,32 and evidence that experimental activation of inflammatory signaling induce feelings of social withdrawal, depressed mood, and anhedonia.33, 34,35 Finally, we have further found that the complementary addition of TCC to escitalopram treatment of depression in older adults accelerates and augments remission of depressive symptoms, along with inducing decreases of systemic inflammation as indexed by CRP.36
The effects of TCC on inflammation in older adults extend evidence of prior randomized controlled trials, which have used long-term (i.e., 12 months) behavioral interventions to induce decreases of IL-6 in older adults.16 These data suggest that even a shorter- term treatment has the potential to impact this key marker of inflammation, although the benefits of TCC were not found until subjects fully learned all of the various movements by week 16 and were able to practice and consolidate skill acquisition during follow-up by week 25. Moreover, in contrast to prior work that focused solely on exercise training,16 TCC employs components of both physical activity as well as meditation. Interestingly, we have previously found that implementation of this TCC intervention does not impact average weekly levels of physical activity, which suggests that other aspects of TCC are driving effects on inflammation. Finally, consistent with prior studies of exercise training in older adults,16 “anti-inflammatory” effects were found only for IL-6 with no changes in other markers of inflammation, except that Kohut et al. also observed decreases of IL-18.18 However, we caution, given the effect sizes for these other marker of inflammation, that the study did not have sufficient statistical power to evaluate the effects of TCC on these additional indices, nor on change in IL-6 levels for the total sample but only possibly on change of IL-6 within the group with high levels of IL-6 at entry.
In addition to the limitations of sample size, the mechanisms that might contribute to changes in IL-6, but not alterations in other markers of inflammation, are not known. However, activation of IL-6 signaling pathways occurs early in the inflammatory cascade with subsequent downstream effects on CRP and markers of endothelial activation. Hence, changes in other markers of inflammation might have been identified if the treatment and/or follow-up period was of longer duration, allowing time for a decrease in this “early” signal to drive subsequent decreases in other markers of inflammation. Alternatively, it might be that the reduction of IL-6 is due to decreases in the production of IL-6 by non-immune cells, as opposed to immune cells. For example, with the exception of CRP and IL-18, which are reduced by exercise trainining,18 other markers of inflammation are derived primarily from immune cells. In contrast, CRP is an acute phase protein that is produced by the liver in response to IL-6 signaling, and IL-18 is a pleiotropic inflammatory cytokine that is produced in part by adipocytes. Indeed, adipose tissue is also a significant source of circulating IL-6. However, there is no evidence to suggest that the TCC intervention led to significant changes in body composition, because overall physical activity as indexed by metabolic equivalents expended per week did not change. Although we did not assess changes in body weight over the course of the intervention, the absence of changes in physical activity makes it unlikely that alterations in adipocyte production of IL-6 account for these effects, similar to the findings of Kohut et al. who found no relationship between changes in IL-6 and body mass index following exercise training.18 Nevertheless, other studies have found that long-term exercise training can reduce the stimulated cellular production of proinflammatory cytokines,37, 38 suggesting that the reduction of IL-6 may be due to decreased immune cell expression of this cytokine.
Behavioral treatments such as TCC may modulate IL-6 via decreases of sympathetic outflow. Aging is associated with increases in circulating levels of catecholamines39 that are known to increase IL-6. We have found that TCC decreases sympathetic activity, 22 and TCC training over 12 months is reported to improve aerobic fitness as measured by an increase of VO2 MAX and decreases of blood pressure.40 Whereas Tai Chi is unique in bringing together exercise, relaxation, and meditation as one behavioral intervention, it is not known whether these individual components induce similarly changes in IL-6.
TCC is highly accessible to older adults, which contributed to high levels of treatment attendance, adherence, and maintenance, which persisted even after formal administration of TCC had ended. Nevertheless, this study has several limitations. Participants were in good health relative to their age-matched peers, and we do not know whether TCC would be associated with similar decreases in older adults with significant medical morbidity. Second, participants included in this study received a small dose of varicella vaccine vs. placebo vaccine at week 16, which might have an effect on circulating levels of IL- 6 in the days to weeks following vaccine. However, we found no evidence that vaccine administration influenced IL-6 levels at week 25, or 9 weeks after vaccine vs. placebo doses. Third, sampling of older adults with higher social status and income may have influenced the high levels of treatment adherence. In addition, it is not known whether these results would generalize to older adults with lower social status and education. Fourth, our study was only 6 months in duration, and an extended follow-up period was not carried out to determine whether the practice of TCC was maintained or whether its effects on inflammation were durable. Fifth, the non-blinding of subjects may have been a source of bias. Finally, we did not assess whether TCC decreased the incidence of clinical morbidity related to inflammation. The study reported here did demonstrate promising positive effects of TCC on inflammation as indexed by high levels of IL-6; thus, the clinical implications of the present study persist despite its limitations.
Acknowledgments
Funding/Support
This work was supported by grants from the National Institute of Health (R01-AG 18367; R21-AT00255; T32-MH18399, R01 HL 079955, R01- AG 026364, R01-AG 034588, R01-CA 10014152, M01-RR00865, P30-AG028748, General Clinical Research Centers Program, the UCLA Cousins Center at the Semel Institute for Neurosciences, and the UCLA Older Americans Independence Center Inflammatory Biology Core.
Role of the Sponsor
No funding source had any direct role in the study design; in the collection, analysis, or interpretation of the data; in the writing of the report; or in the decision to submit the report for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Clinical Trials Registration ClinicalTrials.gov Identifier: NCT00118885
Contributions: Drs. Irwin was responsible for the study’s concept and design. Dr. Olmstead was responsible for data management and statistical analysis. The report was drafted by Drs Irwin and Olmstead and approved by both authors.
Conflict of interest statement
Dr. Irwin, the corresponding author, has had full access to all the data in the study and has final responsibility for the decision to submit for publication. Drs. Irwin and Olmstead declare that they have no conflicts of interest.
References
- 1.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 2.Thomas DR. The relationship between functional status and inflammatory disease in older adults. J Gerontol A Biol Sci Med Sci. 2003;58:995–998. doi: 10.1093/gerona/58.11.m995. [DOI] [PubMed] [Google Scholar]
- 3.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Butcher SK, Lord JM. Stress responses and innate immunity: aging as a contributory factor. Aging Cell. 2004;3:151–160. doi: 10.1111/j.1474-9728.2004.00103.x. [DOI] [PubMed] [Google Scholar]
- 5.Franceschi C, Bonafe M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18:1717–1720. doi: 10.1016/s0264-410x(99)00513-7. [DOI] [PubMed] [Google Scholar]
- 6.Ershler WB, Keller ET. Age-associated increases in interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000:51. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 7.Giuliani N, Sansoni P, Girasole G, et al. Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp Gerontol. 2001;36:547–557. doi: 10.1016/s0531-5565(00)00220-5. [DOI] [PubMed] [Google Scholar]
- 8.Maggio M, Ceda GP, Lauretani F, et al. Relationship between higher estradiol levels and 9-year mortality in older women: the Invecchiare in Chianti study. J Am Geriatr Soc. 2009;57:1810–1815. doi: 10.1111/j.1532-5415.2009.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maggio M, Guralnik JM, Longo DL, et al. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 11.Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005;66:265–275. doi: 10.1016/j.cardiores.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 13.Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Connor MF, Bower JE, Cho HJ, et al. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor MF, Irwin MR. Links between behavioral factors and inflammation. Clin Pharmacol Ther. 87:479–482. doi: 10.1038/clpt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicklas BJ, Brinkley TE. Exercise training as a treatment for chronic inflammation in the elderly. Exerc Sport Sci Rev. 2009;37:165–170. doi: 10.1097/JES.0b013e3181b7b3d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicklas BJ, Hsu FC, Brinkley TJ, et al. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56:2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohut ML, McCann DA, Russell DW, et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20:201–209. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Pace TW, Mletzko TC, Alagbe O, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 20.Brydon L, Walker C, Wawrzyniak A, et al. Synergistic effects of psychological and immune stressors on inflammatory cytokine and sickness responses in humans. Brain Behav Immun. 2009;23:217–224. doi: 10.1016/j.bbi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Collet JP, Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: a systematic review. Arch Intern Med. 2004;164:493–501. doi: 10.1001/archinte.164.5.493. [DOI] [PubMed] [Google Scholar]
- 22.Motivala S, Thayer DT, Irwin MR. Tai Chi Chih acutely decreases sympathetic outflow in older adults. Psychosomatic Medicine. 2005 doi: 10.1093/gerona/61.11.1177. [DOI] [PubMed] [Google Scholar]
- 23.Irwin MR, Pike JL, Cole JC, et al. Effects of a behavioral intervention, Tai Chi Chih, on varicella-zoster virus specific immunity and health functioning in older adults. Psychosom Med. 2003;65:824–830. doi: 10.1097/01.psy.0000088591.86103.8f. [DOI] [PubMed] [Google Scholar]
- 24.Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of Tai Chi. J Am Geriatr Soc. 2007;55:511–517. doi: 10.1111/j.1532-5415.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition, Version 2.0. New York, New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- 26.Stone JF. Tai Chi Chih, Joy Through Movement. Good Karma Publishing, Incorporated; 1996. [Google Scholar]
- 27.Nicassio P, Greenberg MA. The effectiveness of cognitive-behavioral and psychoeducational interventions in the management of arthritis. In: Weisman MH, Weinblatt M, Louie J, editors. Treatment of Rheumatic Diseases. I. Orlando: William Saunders; 2001. pp. 147–161. [Google Scholar]
- 28.Borkovec T, Nau SD. Credibility of analogue therapy rationales. Journal of Behavior Therapy and Experimental Psychiatry. 1972;3:257–260. [Google Scholar]
- 29.Steer RA, Rissmiller DJ, Beck AT. Use of the Beck Depression Inventory-II with depressed geriatric inpatients. Behaviour Research and Therapy. 2000;38:311–318. doi: 10.1016/s0005-7967(99)00068-6. [DOI] [PubMed] [Google Scholar]
- 30.Cole JC, Motivala SJ, Buysse DJ, et al. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29:112–116. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- 31.Irwin MR, Olmstead R, Motivala SJ. Improving sleep quality in older adults with moderate sleep complaints: A randomized controlled trial of Tai Chi Chih. Sleep. 2008;31:1001–1008. [PMC free article] [PubMed] [Google Scholar]
- 32.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenberger NI, Inagaki TK, Rameson LT, et al. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenberger NI, Inagaki TK, Mashal NM, et al. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisenberger NI, Berkman ET, Inagaki TK, et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavretsky H, Altshtein L, Olmstead R, et al. Complementary use of Tai Chi Chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. American Journal of Geriatric Psychiatry. doi: 10.1097/JGP.0b013e31820ee9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith JK, Dykes R, Douglas JE, et al. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999;281:1722–1727. doi: 10.1001/jama.281.18.1722. [DOI] [PubMed] [Google Scholar]
- 38.Sloan RP, Shapiro PA, Demeersman RE, et al. Aerobic exercise attenuates inducible TNF production in humans. J Appl Physiol. 2007;103:1007–1011. doi: 10.1152/japplphysiol.00147.2007. [DOI] [PubMed] [Google Scholar]
- 39.Irwin M, Brown M, Patterson T, et al. Neuropeptide Y and natural killer cell activity: findings in depression and Alzheimer caregiver stress. FASEB Journal. 1991;5:3100–3107. doi: 10.1096/fasebj.5.15.1743441. [DOI] [PubMed] [Google Scholar]
- 40.Lan C, Lai JS, Chen SY, et al. 12-month Tai Chi training in the elderly: its effect on health fitness. Med Sci Sports Exerc. 1998;30:345–351. doi: 10.1097/00005768-199803000-00003. [DOI] [PubMed] [Google Scholar]