Abstract
Lymphodepletion prior to adoptive cell transfer (ACT)-based immunotherapies can enhance anti-tumor responses by augmenting innate immunity, by increasing access to homeostatic cytokines, and by depressing the numbers of regulatory T cells and myeloid-derived suppressor cells. Although it is clear that high-dose total body irradiation (HD-TBI) given together with hematopoietic stem cell (HSC) transplantation effectively enhances ACT, the relationship between the intensity of lymphodepletion and tumor treatment efficacy has not been systematically studied. Using the pmel-1 mouse model of self/tumor-reactive CD8+ T cells, we observed a strong correlation between the intensity of the conditioning regimen and the efficacy of ACT-based treatments using linear regression analysis. This was the case for preparative TBI administered either as a single dose (R2 = 0.97, p < 0.001) or in fractionated doses (R2 = 0.94, p < 0.001). Increased amounts of preparative TBI were directly correlated with progressively more favorable ratios of transferred tumor-reactive CD8+ T cells towards endogenous cells with the potential for inhibitory activity including: CD4+ cells (potentially T regulatory cells); Gr1+ cells (which are capable of functioning as myeloid-derived suppressor cells): and endogenous CD8+ and NK1.1+ cells (that can act as “sinks” for homeostatic cytokines in the post-ablative setting). With increasing ablation, we also observed elevated LPS levels in the sera and heightened levels of systemic inflammatory cytokines. Thus, increased intensity lymphodepletion triggers enhanced tumor treatment efficacy and the benefits of HD-TBI must be titrated against its risks.
Introduction
Lymphodepleting conditioning regimens using high-dose total body irradiation (HD-TBI) and hematopoietic stem cell (HSC) transplantation prior to adoptive cell transfer (ACT) of tumor-specific T cells can be highly effective in mice and in humans.1–3 HD-TBI works by augmenting innate immunity,4, 5 by increasing access to homeostatic cytokines,6 and by depressing the numbers of regulatory T cells7–9 and myeloid-derived suppressor cells10–12. However, the administration of HD-TBI can carry considerable short- and long-term toxicities including prolonged neutropenia with its associated risk for infection, renal insufficiency, interstitial pneumonitis, veno-occlusive disease of the liver, infertility, secondary solid tumor and hematologic malignancies and other complications.13–16 These toxicities have motivated major efforts in the allogeneic transplant field to develop reduced-intensity conditioning regimens (RIC).17, 18 RIC regimens have successfully achieved durable engraftment of donor stem cells and tolerable toxicities.17 Thus, we wanted to evaluate if we can achieve effective tumor treatment efficacy in the setting of autologous ACT using RIC rather than HD-TBI.
The effects of increased intensity lymphodepleting regimens on the tumor treatment efficacy of adoptively transferred T cells in the setting of solid tumors have not been systematically studied. Most preclinical models have utilized preparative TBI given at a single non-myeloablating dose (~5Gy),4, 6, 19 and some studies have used higher doses of preparative TBI (~9Gy) given together with syngeneic BMT.1, 15, 20 However, these previous experiments have not systematically addressed the impact of preparative TBI given at various doses and fractionation schemes. We sought to evaluate the relationship between the intensity of the lymphodepleting regimen and the effectiveness of ACT-based immunotherapy. We employed the pmel-1 T cell receptor (TCR) transgenic mouse model, which recognizes the mouse homolog of human gp100,21, 22 to study the impact of increasing doses of single-dose or multiply-fractionated TBI followed by syngeneic adoptively transferred tumor-reactive CD8+ T cells. The goal of this study was to elucidate the optimal lymphodepleting conditioning regimen to reach the maximal tumor treatment capacity of adoptively transferred T cells – specifically to measure if the preparative dose reached a plateau after which point increasing doses of irradiation were not optimal or even were detrimental to the tumor treatment efficacy.
Materials and Methods
Mice and tumor lines
All mice used in these experiments were bred and housed at NIH facilities. Female pmel-1 TCR-Tg mice were generated in our laboratory22 and crossed with C57BL/6-Thy1.1+–Tg or C57BL/6-Ly5.1+–Tg mice (The Jackson Laboratory) to derive pmel-1–Thy1.1+ or pmel-1–Ly5.1+ double-Tg mice (we have made these C57BL/6-pmel-1–Thy1.1+ available at http://www.jax.org ). Experiments were conducted with the approval of the National Cancer Institute Animal Use and Care Committee. B16-F10 (H-2b), a spontaneous, transplantable murine melanoma is gp100+. 23
In vitro activation of pmel-1 CD8+ T cells
Pmel-1 splenocytes were isolated as described previously24 and cultured in the presence of the Kb-restricted epitope of 1 μM hgp10025–33 21 and CM containing 30 IU/ml of rhIL-2 (Chiron).25 Cells were used for adoptive transfer 6–7 days after the start of the culture.
Adoptive cell transfer and administration of TBI
Mice 6–12 weeks of age (n = 5–6 for all groups) were injected subcutaneously with 2–5 × 105 B16-F10 melanoma cells and treated 10–14 days later with in vitro-activated pmel-1 CD8+ T cells.
Lymphopenia was induced using TBI delivered using a cesium-137 source. The photon energy of decay is 662 keV which was delivered at a rate of 72.38 rads (cGy) min−1 . Mice received 5, 9, 10.5 or 12 Gy single-dose TBI delivered one day prior to pmel-1 CD8+ T cell transfer. Fractionated irradiation was given at total doses of 12, 15, 18, 21 or 24 Gy fractionated TBI that was equally divided into six doses given twice daily using a schedule that was similar to that described in our recent clinical trial using 12 Gy TBI.2 The last dose was given one day prior to pmel-1 CD8+ T cell transfer. All animals also received a transplantation of 105 lin−/c-kit+ syngeneic HSCs. The maximal tolerated dose (MTD) for single-dose irradiation was 12Gy; the amount could be increased up to 24Gy for the fractionated dose. Exceeding these doses resulted in the need for euthanasia of animals within six days (Supplemental Figure1). Animals were euthanized when they exhibited evidence of distress including poor grooming or weight loss.
Recombinant human (rh) IL-2 (36 μg/dose; Chiron) or 600,000 IU/dose was administered by intraperitoneal injection twice daily for a total of six doses. Tumors were measured using calipers, and the products of the perpendicular diameters were recorded.26 All experiments were performed in a blinded, randomized fashion and performed independently at least twice, with similar results.
Hematopoietic stem cell transplantation
HSCs were extracted from the bone marrow by lineage depletion with streptavidin-coated magnetic beads (Dynabeads M-280 Streptavidin; Dynal Biotech) against biotin-labeled antibodies (γδ T cell receptor, αβ T cell receptor, CD4, CD8α, NK1.1, Gr-1, B220, Ter-119, CD2, CD11b) (BD Biosciences) followed by a c-kit enrichment with CD117 MicroBeads (Miltenyi Biotec) and cultured for 18 hours in 10% DMEM with 50 ng recombinant mouse IL-3 (rmIL-3), 500 ng rmIL-6, and 500 ng rmSCF (PeproTech).
Statistics
Wilcoxon Rank Sum Test was used for comparisons between groups of experimental mice. P values less than 0.05 were considered significant. R2 values for linear regression analyses were determined using the least squares method.
Flow cytometry
All antibodies were purchased from BD Bioscience (San Jose, CA). The H2-Db-mgp10025–33 tetramer was purchased from Beckman Coulter (Fullerton, CA). FACSCalibur flow cytometer and CellQuest software were used to analyze the samples.
Enumeration of cells in vivo
At indicated time points, spleens were resected, and adoptively transferred CD8+Thy1.1+ or CD8+Ly5.1+ pmel-1 CD8+ T cells were enumerated as previously described. Samples were analyzed by flow cytometry for B220, Gr1, CD8α, CD4, and NK1.1 expression (BD Biosciences). Cell numbers were calculated by multiplying the total number of live cells by the percentage of B220+, Gr1+, CD8α+, Foxp3+, CD4+, or NK1.1+ cells.
Detection of serum LPS
A limulus amebocyte lysate (LAL) assay (QCL-1000; Cambrex) was used to analyze serum LPS six days after ACT. Conversion into pg/ml was performed using a standard curve.
Results
Increased intensity of single-dose or fractionated TBI augments adoptive T cell therapy
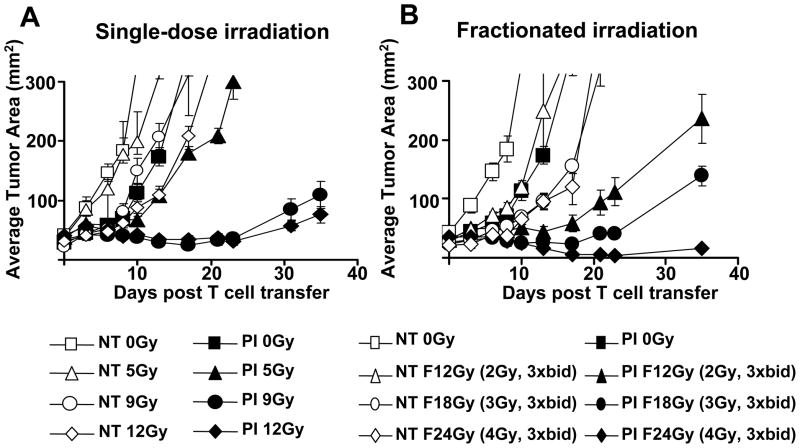
In order to evaluate the optimal dose of irradiation prior to ACT, we administered irradiation as either single-dose or fractionated doses. The single-dose irradiation was given immediately prior to T cell transfer while fractionated doses were given twice daily over 3 days using a regimen similar to that described in our recent clinical trial using 12 Gy TBI.2 All animals also received a transplantation of 105 lin−/c-kit+ syngeneic HSCs. We observed a progressive improvement of tumor treatment dependent on the intensity of TBI up to the highest tolerated dose in the fractionated setting (Figure 1A). High dose irradiation had some impact on the tumor-growth rate even in the absence of ACT. It is likely that some of the tumor treatment effect seen in maximally irradiated animals is occurring through direct impact of irradiation on tumor growth. Using a single-dose regimen of TBI, we reached a plateau at the myeloablating dose of 9Gy with no statistically significant increase when 12Gy was given (12Gy vs. 18Gy (P>0.05), whereas we did not see a plateau using fractionated TBI: 0Gy vs. 12Gy (P=0.006); 12Gy vs. 24Gy (P=0.01); and 18Gy vs. 24Gy (P=0.02). Thus, increase preparative doses of fractionated TBI increased the tumor treatment efficacy.
Figure 1. Tumor treatment curves of adoptively transferred T cells following increased intensity TBI.
One million effector pmel-1 CD8+ T cells (P) were transferred with rhIL-2 (I) in tumor-bearing C57BL/6 mice which were either irradiated with a single dose of irradiation on the day of transfer or a fractionated dose of irradiation divided into six equal doses given twice daily at the amount indicated in the legend. All animals received an HSC transplant. Results for tumor area are the mean of measurements from 7 mice per group (+/−SEM). Statistical analysis is as follows. (A) For single-dose radiation for those treatment groups receiving PI: 0Gy vs. 5Gy (NS), 5Gy vs. 9Gy (P=0.009), 9Gy vs. 12Gy (NS), 5Gy vs. 12Gy (P=0.002). (B) For fractionated irradiation in groups receiving PI: 0Gy vs. 12Gy (P=0.006), 12Gy vs. 18Gy (NS), 18Gy vs. 24Gy (P=0.02), 12Gy vs. 24Gy (P=0.01). Data are representative of two independent experiments.
Linear regression analysis of tumor treatment efficacy vs. the amount of preparative TBI
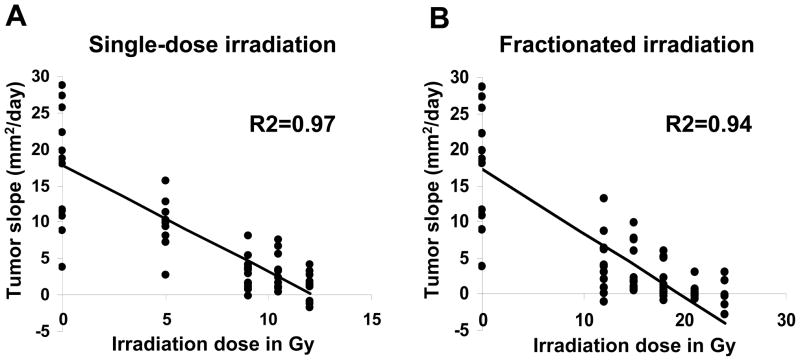
Another way of directly correlating the tumor treatment efficacy vs. the amount of preparative TBI given was using linear regression analysis. To accomplish this, we calculated the tumor growth rate for each mouse using linear regression of the tumor measurements using the least squares method. Tumor growth slopes (mm2 d−1) of treated individual mice were plotted against the dose of irradiation used prior to ACT. We then performed a linear regression on that data. We found a strong correlation for single-dose (left panel, R2=0.97, p < 0.001) as well as fractionated ablation (right panel, R2=0.94, p < 0.001) (Figure 2). The results indicated that administration of HD-TBI was needed in order to reach optimal tumor treatment with syngeneic tumor-reactive CD8+ T cells.
Figure 2. Linear regression analysis of tumor treatment efficacy vs. the amount of preparative TBI.
Experiments were analyzed using linear regression analyses to study the relationship of radiation dose with tumor response. To perform the regression analysis, the tumor growth rates (mm2 d−1) were calculated then plotted against the amount of single-dose TBI administered or as the summed dose of fractionated TBI. Tumor response was elicited by using one million effector pmel-1 CD8+ T cells (P) transferred together with rhIL-2 (I) in tumor-bearing C57BL/6 mice which were either irradiated with (A) single-dose irradiation directly at the day of transfer or (B) fractionated doses divided equally over six doses given twice daily up to the amount indicated in the legend as described in Fig. 1. All animals received a syngeneic HSC transplant. Data are derived from two independently performed experiments. R2 values for linear regression of data sets in single-dose and fractionated radiation are shown.
Impact of ablation on endogenous host cells
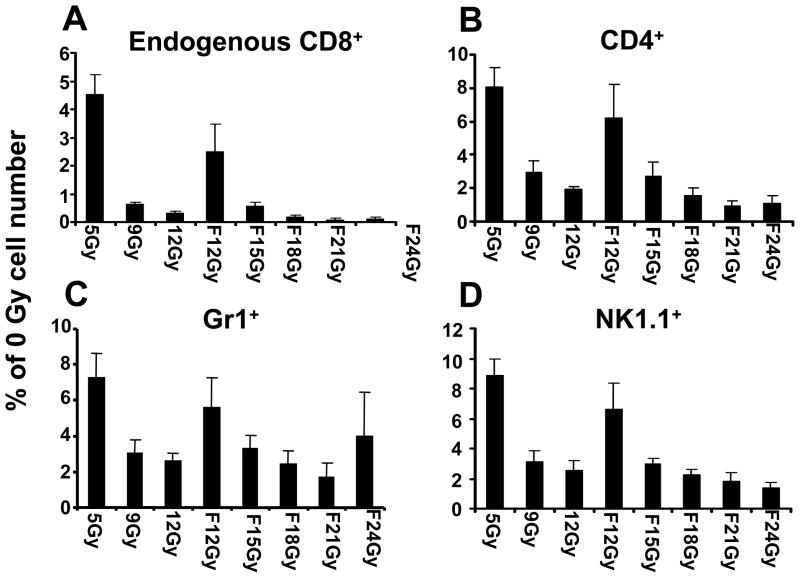
We next studied the impact of the dose of preparative TBI on key populations of endogenous host cells including NK cells, CD4+ T cells and myeloid suppressor cells, which have been found to consume homeostatic cytokines and hamper the tumor treatment efficacy of adoptively transferred CD8+ T cells.6–10, 12, 27–29 We ablated animals with either single-dose or fractionated irradiation followed by a HSC and T cell transfer and administration of IL-2. We found that relatively low doses of irradiation (5Gy, single-dose and 12Gy, fractionated) depleted the majorities of endogenous cells (Figure 3). Fractions of host immune cell subsets were determined cytoflourometrically and multiplied by the absolute number of splenocytes. These values were then plotted as a percent of the number of cells with each marker compared to mice receiving no TBI regimen (0Gy). Decreased endogenous host cells were observed for endogenous CD8+ cells (distinguished from transferred T cells by the absence of the Thy1.1 congenic marker), which potentially can act as “cytokine sinks,” are shown in Panel A. We also observed depressed levels of endogenous CD4+ cells, subsets of which can function as T regulatory cells (Panel B); Gr1+ cells (which can be a marker for myeloid-derived suppressor cells, Panel C); and NK cells and NK-T cells as determined by staining with anti-mouse NK1.1 (Panel D).
Figure 3. Fraction of endogenous host immune cell subsets following TBI regimens.
Thy1.2+ host mice received a preparative regimen with either single-dose irradiation at the day of T cell transfer or a fractionated (F) dose of irradiation over six times twice daily up to the amount indicated in the legend followed by the adoptive transfer of 1 x 106 Thy1.1+ effector pmel-1 CD8+ T cells (P) with rhIL-2 (I). Absolute numbers of Thy1.1+ pmel-1 vs.: (A) endogenous Thy1.2+ CD8+ T cells; (B) endogenous CD4+ T cells; (C) NK 1.1+ cells; and (D) Gr1+ cells were determined six days after the pmel-1 T cell transfer in the spleen. Error bars represent SEM. Experimental data are derived from three mice collected and individually evaluated. Experiment was repeated independently with similar results.
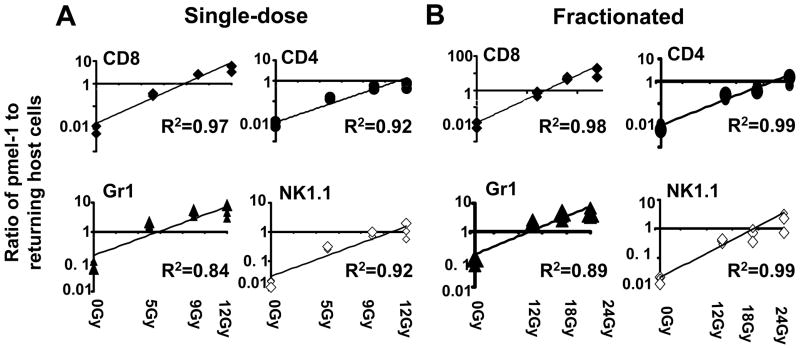
Increased amounts of preparative TBI were directly correlated with progressively more favorable ratios of transferred tumor-reactive CD8+ T cells towards endogenous cells with the potential for inhibitory activity including: CD4+ cells (potentially T regulatory cells); Gr1+ cells (which are capable of functioning as myeloid-derived suppressor cells): and endogenous CD8+ and NK1.1+ cells (that can act as “sinks” for homeostatic cytokines in the post-ablative setting).
We sought to quantify the correlation between increasing doses of TBI to the ratios of adoptively transferred Thy1.1+ effector pmel-1 CD8+ T cells to returning host cells six days after ACT (Figure 4). The ratio of transferred tumor-reactive T cells towards endogenous host cells, especially CD8+ T cells, CD4+ T cells, myeloid suppressor cells and natural killer cells is of critical importance for the tumor treatment efficacy of transferred T cells.1 We found that the ratio of transferred pmel-1+Thy1.1+ T cells towards endogenous host cells strongly correlated with the degree of ablation (Figure 4). This result was also observed at day 14 post transfer (data not shown).
Figure 4. The ratio of adoptively transferred effector pmel-1 CD8+ T cells relative to returning host cells as a function of TBI dose.
Flow cytometry data were analyzed using a linear least squares regression analysis. Three individual mice per group were used to calculate the ratios of adoptively transferred Thy1.1+ pmel-1 CD8+ T cells towards returning host cells six days after ACT. Thy1.2+ host mice received a preparative regimen with either (A) single-dose irradiation at the day of T cell transfer or (B) fractionated dose of irradiation divided into six equal doses given twice daily up to the amount indicated in the legend followed by the adoptive transfer of 1 x 106 Thy1.1+ effector pmel-1 CD8+ T cells with rhIL-2. Four quadrants are shown per panel. These include the ratios of the adoptively transferred Thy1.1+ cells to endogenous (Thy1.2+) CD8+ T cells, endogenous CD4+ T cells; as well as (endogenous) Gr1+ and NK 1.1+ cells as labeled. These ratios were then plotted against the dose of preparative TBI given and analyzed using linear regression with the least squares method. Experiment was repeated independently and similar results were obtained.
Serum LPS and inflammatory cytokines increase with increasing TBI
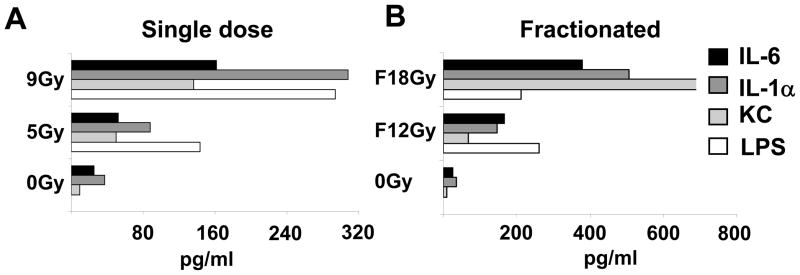
We sought to determine if higher doses of TBI resulted in higher levels of systemic inflammatory cytokines or in increased LPS levels in the sera of irradiated mice. We have previously found that the translocation of commensal gut microflora resulting is an important mechanism by which TBI augments ACT.4, 5 One million effector pmel-1 T cells were adoptively transferred along with rhIL-2 in animals that were either irradiated with a single dose of TBI as indicated directly at the day of transfer (Figure 5A) or irradiated in six equal fractionated (F) doses of TBI given twice daily up to the amount shown. Six days after irradiation sera of three mice were collected and individually tested for the presence of microbial LPS or sera were pooled and analyzed for inflammatory cytokines and chemokines and LPS. Preparative TBI led to increased levels of IL-6 and IL-1α as well as KC (also known as Cxcl 1 and Gro-α). In addition, we observed elevated LPS levels in the sera in dose dependent manner both for non-fractionated (Figure 5A) and fractionated (Figure 5B) TBI. Taken together, these data show a strong correlation of intensity of the lymphodepleting regimen and the release of pro-inflammatory cytokines, the ratio of tumor-reactive T cells towards endogenous inhibitory cells and most importantly the tumor treatment efficacy. The best tumor treatment was observed following the use of the highest intensity lymphodepleting regimens.
Figure 5. Measurements of systemic inflammatory cytokines and LPS levels in the sera of irradiated mice.
One million effector pmel-1 T cells were adoptively transferred along with rhIL-2 in animals that received either a single dose of irradiation as indicated directly at the day of transfer (Panel A) or irradiated in six equal fractionated (F) doses of irradiation given twice daily up to the amount shown (Panel B). Six days after irradiation, sera of three mice were collected, pooled, and analyzed for inflammatory cytokines and chemokines. Shown are IL-6, IL-1α, and chemokine KC (Cxcl1). Microbial LPS was assessed using the limulus amebocyte lysate (LAL) assay.
Discussion
Immunotherapies using the adoptive transfer of tumor-specific T cells have shown considerable efficacy in the treatment of mouse and human tumors, especially when given after TBI based preparative regimens.1, 2 However, the relationship of the intensity of TBI and tumor treatment efficacy has not been systematically studied. We have previously reported that non-myeloablative lymphodepletion with 5Gy TBI prior to transfer of tumor-reactive T cells significantly improves treatment outcome in mice; however, vaccination with an altered-self ligand-based vaccine was still absolutely required in order to mediate robust tumor regression.6 Surprisingly, vaccination was not required in the setting of a myeloabaltive conditioning regimen prior to ACT to achieve CD8+ T cell mediated tumor eradication.1 The central finding of this previous work was that HSC – through a still unknown mechanism – activated adoptively transferred anti-tumor CD8+ T cells to a level sufficient to mediate tumor treatment.
Vaccine-independence is a critical feature in the translation of ACT based on tumor infiltrating lymphocytes (TIL) in humans, as vaccines for adoptively transferred polyclonal T cells are not usually available; especially since approximately 50% of tumor-reactive TILs have specificity against epitopes from unknown cancer antigens. In melanoma patients with advanced metastatic disease, lymphodepletion with a non-myeloablative regimen prior to the transfer of TILs and IL-2 resulted in an objective response rate of 50% without the use of a vaccine.30, 31 Increasing the non-myeloablative conditioning regimen to a myeloablative regimen might increase tumor treatment capacity of TILs in patients and lead to more durable responses. Objective response rates in 18/25 patients with metastatic melanoma were recently observed.2 However, it should be pointed out that this response rate was achieved using a selected group of patients. These patients were required to have a resectable lesion, good performance status, and evidence that T cells from their tumors could be grown to large numbers while retaining tumor-specificity. Even in the case of these optimal tumor-treatment conditions, the reasons why the best treatments can still fail in many patients are unknown. Treatment failures could be due to tumor heterogeneity in antigen expression, in the ability of tumors to process and present these target antigens, or on a failure of the type or specificity of the T cells used in immunotherapy.32–35 Current efforts in our laboratory are focused improving the T cell culture conditions to optimize the state of differentiation or polarization of naturally-occurring or genetically modified T cells.36–46.
The present study was confined to an analysis of the impact of increasing TBI on pmel-1 CD8+ T cells. Although we have previously found that 5Gy TBI augments the function of TRP-1-specific CD4+ T cells26 the impact of higher doses of TBI in that system have not yet been elucidated. Here, we studied the effects of TBI alone as a preparative regimen, rather than in combination with chemotherapy. The reason for this is that the mouse B16 melanoma is relatively resistant to the impact of irradiation; however unlike its human melanoma counterparts, B16 is exquisitely sensitive to cyclophosphamide and fludarabine, the two drugs most commonly used in current protocols for lymphodepletion in man.2
High doses of single-dose as well as fractionated irradiation did show an impact on the tumor-growth even in the absence of ACT (Fig. 1). A more pronounced effect of irradiation on the non-treatment groups was seen in those receiving fractionated irradiation (Fig. 1B). This might be because the fractionated regimen started 3 days earlier than for single-dose irradiated animals. Thus, tumors were slightly smaller at the time fractionated TBI was started thus potentially more susceptible to growth reduction through irradiation. It is likely that some of the tumor treatment effect seen in maximally irradiated animals is a direct impact of irradiation on the tumor growth. Apoptosis of tumor cells triggered by HD-TBI might also lead to inflammation and stimulation from the endogenous reconstituting immune response.47
Great efforts have been made in the hematopoietic stem cell transplantation field to establish reduced-intensity lymphodepleting conditions, which show diminished toxicity and still result in effective transplant engraftment. In the allogeneic setting, establishing the optimal dose for the lymphodepleting regimen is determined by the minimal intensity of lymphodepletion that will eventually result in 100% chimerism. In some cases however, this allogeneic immune mechanism may not be sufficient and significant cytoreduction is required.48 In the setting of advanced solid-tumors, such as melanomas, conditioning is aimed at transiently eliminating host regulatory elements and increasing signals that activate ex vivo generated tumor-specific lymphocytes. The maturation and activation of CD8+ T cells must occur during this relatively short period of lymphopenia.49
Here, we show that optimal tumor treatment is reached with the HD-TBI and that reducing the intensity leads to considerable loss in tumor treatment capacity of transferred tumor-reactive T cells. Although the spectrum and the intensity of toxicities in myeloablated patients who received syngeneic cell transplant seem to be less severe than in patients who received an allogeneic stem-cell transplant,2, 16 these findings require a careful clinical analysis of the risks of high-dose TBI with the benefits of improved tumor treatment outcome. For the future, the implementation of less toxic lymphodepleting methods such as selective antibody depletion used in combination with immunomodulatory substances such as Toll-like receptor agonists might be fruitful for the enhancement of ACT.50, 51
Supplementary Material
Acknowledgments
This study was supported by the intramural program of the National Cancer Institute, National Institutes of Health (Bethesda, MD).
Abbreviations
- HD-TBI
High dose Total Body Irradiation
- HSC
Hematopoietic Stem Cells
- ACT
Adoptive Cell Transfer
- RIC
Reduced Intensity Conditioning
- BMT
Bone Marrow Transplant
- MTD
Maximal Tolerated Dose
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Wrzesinski C, Paulos CM, Gattinoni L, et al. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117(2):492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L, Powell DJ, Jr, Rosenberg SA, et al. Nat Rev Immunol. 2006;6(5):383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulos CM, Kaiser A, Wrzesinski C, et al. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13(18 Pt 1):5280–9. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulos CM, Wrzesinski C, Kaiser A, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117(8):2197–204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antony PA, Paulos CM, Ahmadzadeh M, et al. Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. J Immunol. 2006;176(9):5255–66. doi: 10.4049/jimmunol.176.9.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174(5):2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28(2):120–8. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronte V, Wang M, Overwijk WW, et al. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161(10):5313–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Bronte V, Apolloni E, Cabrelle A, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96(12):3838–46. [PMC free article] [PubMed] [Google Scholar]
- 12.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 13.Chen CI, Abraham R, Tsang R, et al. Radiation-associated pneumonitis following autologous stem cell transplantation: predictive factors, disease characteristics and treatment outcomes. Bone Marrow Transplant. 2001;27(2):177–82. doi: 10.1038/sj.bmt.1702771. [DOI] [PubMed] [Google Scholar]
- 14.Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99(3):731–5. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 15.Anasetti C, Mule JJ. To ablate or not to ablate? HSCs in the T cell driver's seat. J Clin Invest. 2007;117(2):306–10. doi: 10.1172/JCI30973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muranski P, Boni A, Wrzesinski C, et al. Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go? Nat Clin Pract Oncol. 2006;3(12):668–81. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adkins DR, DiPersio JF. Total body irradiation before an allogeneic stem cell transplantation: is there a magic dose? Curr Opin Hematol. 2008;15(6):555–60. doi: 10.1097/MOH.0b013e32831188f5. [DOI] [PubMed] [Google Scholar]
- 18.Sandmaier BM, Mackinnon S, Childs RW. Reduced intensity conditioning for allogeneic hematopoietic cell transplantation: current perspectives. Biol Blood Marrow Transplant. 2007;13(1 Suppl 1):87–97. doi: 10.1016/j.bbmt.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klebanoff CA, Khong HT, Antony PA, et al. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26(2):111–7. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrzesinski C, Restifo NP. Less is more: lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr Opin Immunol. 2005;17(2):195–201. doi: 10.1016/j.coi.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overwijk WW, Tsung A, Irvine KR, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of "self"-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188(2):277–86. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai Y, Yang JC, Spiess P, et al. Cloning and characterization of the genes encoding the murine homologues of the human melanoma antigens MART1 and gp100. J Immunother. 1997;20(1):15–25. doi: 10.1097/00002371-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115(6):1616–26. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111(11):5326–33. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheever MA, Greenberg PD, Fefer A. Specificity of adoptive chemoimmunotherapy of established syngeneic tumors. J Immunol. 1980;125(2):711–4. [PubMed] [Google Scholar]
- 28.Surh CD, Boyman O, Purton JF, et al. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–63. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 29.Blazar BR, Taylor PA. Regulatory T cells. Biol Blood Marrow Transplant. 2005;11(2 Suppl 2):46–9. doi: 10.1016/j.bbmt.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Restifo NP, Esquivel F, Kawakami Y, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177(2):265–72. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Restifo NP, Kawakami Y, Marincola F, et al. Molecular mechanisms used by tumors to escape immune recognition: immunogenetherapy and the cell biology of major histocompatibility complex class I. J Immunother Emphasis Tumor Immunol. 1993;14(3):182–90. doi: 10.1097/00002371-199310000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Restifo NP, Marincola FM, Kawakami Y, et al. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst. 1996;88(2):100–8. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of "tumor escape" phenotypes. Nat Immunol. 2002;3(11):999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8(+) memory stem cells. Nat Med. 2009 doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinrichs CS, Kaiser A, Paulos CM, et al. Type 17 CD8+ T cells display enhanced anti-tumor immunity. Blood. 2009 doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009 doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101(7):1969–74. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102(27):9571–6. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Z, Restifo NP. Cancer vaccines: progress reveals new complexities. J Clin Invest. 2002;110(3):289–94. doi: 10.1172/JCI16216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr Opin Immunol. 2009;21(2):200–8. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer DC, Balasubramaniam S, Hanada K, et al. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J Immunol. 2004;173(12):7209–16. doi: 10.4049/jimmunol.173.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Parkhurst MR, Zheng Z, et al. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling. Cancer Res. 2007;67(6):2425–9. doi: 10.1158/0008-5472.CAN-06-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y, Zheng Z, Cohen CJ, et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13(1):151–9. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schumacher TN, Restifo NP. Adoptive T cell therapy of cancer. Curr Opin Immunol. 2009;21(2):187–9. doi: 10.1016/j.coi.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Restifo NP. Building better vaccines: how apoptotic cell death can induce inflammation and activate innate and adaptive immunity. Curr Opin Immunol. 2000;12(5):597–603. doi: 10.1016/s0952-7915(00)00148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sloand E, Childs RW, Solomon S, et al. The graft-versus-leukemia effect of nonmyeloablative stem cell allografts may not be sufficient to cure chronic myelogenous leukemia. Bone Marrow Transplant. 2003;32(9):897–901. doi: 10.1038/sj.bmt.1704231. [DOI] [PubMed] [Google Scholar]
- 49.Palmer DC, Chan CC, Gattinoni L, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci U S A. 2008;105(23):8061–6. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popat U, Carrum G, May R, et al. CD52 and CD45 monoclonal antibodies for reduced intensity hemopoietic stem cell transplantation from HLA matched and one antigen mismatched unrelated donors. Bone Marrow Transplant. 2005;35(12):1127–32. doi: 10.1038/sj.bmt.1704975. [DOI] [PubMed] [Google Scholar]
- 51.Brenner MK, Wulf GG, Rill DR, et al. Complement-fixing CD45 monoclonal antibodies to facilitate stem cell transplantation in mouse and man. Ann N Y Acad Sci. 2003;996:80–8. doi: 10.1111/j.1749-6632.2003.tb03236.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.