Abstract
Aims
To assess treatment decision and outcome in patients referred for transcatheter aortic valve implantation (TAVI) in addition to predictive factors of mortality after TAVI.
Methods
Three-centre prospective observational study including 358 patients. Endpoints were defined according to the Valve Academic Research Consortium.
Results
Of the 358 patients referred for TAVI, TAVI was performed in 235 patients (65%), surgical aortic valve replacement (AVR) in 24 (7%) and medical therapy (MT) in 99 (28%). Reasons to decline TAVI in favour of AVR/MT were patient preference (29%), peripheral vascular disease (15%) and non-severe aortic stenosis (11%). The logistic EuroSCORE was significantly higher in patients who underwent TAVI and MT in comparison with those undergoing AVR (19 vs. 10%, p = 0.007). At 30 days, all-cause mortality and the combined safety endpoint were 9 and 24% after TAVI and 8 and 25% after AVR, respectively. All-cause mortality was significantly lower in the TAVI group compared with the MT group at 6 months, 1 year and 2 years (12% vs. 22%, 21% vs. 33% and 31% vs. 55%, respectively, p < 0.001). Multivariable analysis revealed that blood transfusion (HR: 1.19; 95% CI: 1.05–1.33), pre-existing renal failure (HR: 1.18; 95% CI: 1.06–1.33) and STS score (HR: 1.06; 95% CI: 1.02–1.10) were independent predictors of mortality at a median of 10 (IQR: 3–23) months after TAVI.
Conclusions
Approximately two-thirds of the patients referred for TAVI receive this treatment with gratifying short- and long-term survival. Another 7% underwent AVR. Prognosis is poor in patients who do not receive valve replacement therapy.
Keywords: Aortic stenosis, Transcatheter aortic valve implantation, Surgical aortic valve replacement, Treatment decision, Complications, Prognosis
Introduction
Transcatheter aortic valve implantation (TAVI) is a catheter-based treatment for patients with aortic stenosis (AS) who are considered poor candidates for surgical aortic valve replacement (AVR). Although it is increasingly being considered the standard of care in such patients [1], not all patients receive TAVI but instead continue medical therapy (MT). This may bear important consequences in terms of prognosis and quality of life.
Historically, the treatment decision heavily depended upon the assessment of risk of valve replacement by using the Logistic EuroSCORE (LES) or Society of Thoracic Surgeons (STS) score [2, 3]. These scores were developed to assess the operative risk of patients undergoing open-heart surgery but not for the subset of patients who are referred for TAVI. Therefore, careful patient-to-patient case evaluation by Heart Team meetings are strongly encouraged and play a crucial role in the design of randomised clinical trials [1, 4–7]. In this study, we sought to explore the reasons for the treatment decision, the treatment-specific complications and survival in patients referred for TAVI in addition to the predictive factors of mortality in those undergoing TAVI.
Methods
Patients and eligibility
The population consists of all 358 patients who were referred for TAVI at the Department of Cardiology of the Erasmus Medical Centre, Rotterdam, the Netherlands and the Departments of Cardiology and Cardio-Thoracic Surgery of Angiografia de Occidente S.A., Cali, Colombia and Fundacion Clinica Cardio Infantil, Bogota, Colombia between November 2005 and January 2011. In the three institutions, a similar database and structure of data collection and follow-up was set up at the initiation of TAVI as previously described [8].
Treatment decision (TAVI, AVR, MT) was taken by consensus during the Heart Team meeting. Details of eligibility for Medtronic Corevalve System (MCS) implantation, the bioprosthesis and technique of implantation have previously been described [8–10]. All patients underwent transfemoral (n = 228) or trans-subclavian TAVI (n = 7) with the 18 Fr third-generation MCS except for the first five patients treated in 2005 and 2006, in whom a 21 Fr second-generation MCS was implanted.
AVR was performed through mid-sternotomy using standard surgical techniques. In all patients a biological prosthesis was used.
Patients not undergoing valve intervention continued MT. Balloon aortic valvuloplasty (BAV) was performed in patients with AS and worsening symptoms as a bridge to TAVI or as a palliative approach in patients who could not undergo TAVI/AVR.
Data collection
All data were prospectively collected and entered in a dedicated database. Source verification of the baseline data and clinical events was performed by the first author at each participating centre (within a Master of Science Programme of the Netherlands Institute for Health Sciences, Erasmus University Rotterdam, supported by the Erasmus-Columbus Latin-European Exchange Grant - www.erasmus-columbus.eu).
All endpoints were selected and defined according to the Valve Academic Research Consortium (VARC) [11].
Cerebrovascular events were evaluated and adjudicated by a vascular neurologist. A full blood and chemistry sample was taken before and up to 3 days after the procedure to assess the occurrence and severity of periprocedural vascular, bleeding and kidney complications. Data on red blood cell (RBC) transfusions were recorded by the institution’s blood bank. The occurrence and timing of new atrial fibrillation and postprocedural 3rd degree atrioventricular block was assessed by continuous telemetry recording.
The VARC combined safety endpoint at 30 days consisted of all-cause death, major stroke, major vascular complication, life-threatening bleeding, acute kidney injury (AKI) stage III and any in-hospital re-intervention due to prosthesis dysfunction (interventional/surgical).
Follow-up
Follow-up information of patients treated at the Erasmus Medical Centre (TAVI, AVR, MT) was collected by first checking the vital status via the civil registries every 6 months. In case of survival, a questionnaire was sent to the patient for the assessment of symptoms, (cardiac) events and readmission(s). Also surviving patients were contacted by telephone to confirm hospital readmission and reason after which events were verified with the treating hospital. All medical records were revised and general practitioners were contacted when necessary. Follow-up was complete for all patients.
Follow-up information of patients treated in Colombia was obtained by the regular office visit and/or telephone contact (dedicated local research nurse or doctor) with the treating physician and/or general practitioner and/or patient or family followed by verification of the event with the treating hospital. Follow-up was complete for all except for 3 patients.
Statistical analysis
Categorical variables are presented as frequencies and percentages and were compared with the Chi-square test or Fisher’s exact test. Normal and skewed continuous variables are presented as means ± SD and medians (IQR), respectively. To compare the three treatment groups, analysis of variance was used for continuous variables and the Chi-square test for categorical variables. Kaplan-Meier survival methods were used to calculate the cumulative survival at different time intervals and the log-rank test was used to assess differences in survival. A stepwise Cox regression analysis including all variables with p < 0.10 in the univariable analysis was used to determine independent predictors of late mortality in patients undergoing TAVI. A two-sided p < 0.05 was considered to indicate significance and all analyses were performed with SPSS software (version 17.0).
Results
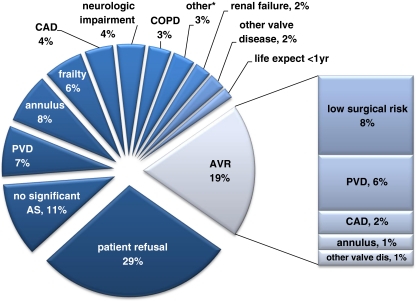
Of the 421 patients, 60 (14%) died on the waiting list at a median (IQR) of 48 (14–110) days after first medical contact and 3 (1%) were lost to follow-up. Therefore, the total study population consists of 358 patients of whom 235 (65%) underwent TAVI at a median (IQR) interval of 71 (30–119) days and 24 (7%) AVR at an interval of 63 (33–122) days. The remaining 99 patients (28%) continued MT. The reasons why AVR or MT was chosen instead of TAVI are depicted in Fig. 1. The main reason to reject TAVI in favour of AVR or MT were patient preference (29%), peripheral vascular disease (PVD, 13%) and non-severe AS (11%). The baseline characteristics of the three treatment groups are summarised in Table 1. Not unexpectedly, patients who underwent TAVI or continued MT had a significantly higher LES.
Fig. 1.
Reasons to decline TAVI in favour of AVR and MT. *Four patients had another reason: severe left ventricular dysfunction (LVEF <20%); bleeding diathesis; abusive alcohol use; unknown. AS = aortic stenosis; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; PVD = peripheral vascular disease
Table 1.
Baseline characteristics of patients undergoing TAVI, AVR and medical therapy
| TAVI | AVR | Medical | p-value | |
|---|---|---|---|---|
| n = 235 | N = 24 | n = 99 | ||
| Age (years), mean ± SD | 80 ± 7 | 78 ± 9 | 80 ± 8 | 0.26 |
| Male, n (%) | 116 (49) | 13 (54) | 42 (42) | 0.32 |
| Height (cm), mean ± SD | 166 ± 11 | 169 ± 8 | 166 ± 8 | 0.50 |
| Weight (kg), mean ± SD | 71 ± 13 | 75 ± 11 | 69 ± 16 | 0.14 |
| BMI, mean ± SD | 26.7 ± 4 | 26.4 ± 4 | 24.9 5 | 0.34 |
| BSA, mean ± SD | 1.81 ± 0.19 | 1.87 ± 0.15 | 1.77 ± 0.24 | 0.14 |
| NYHA class ≥III, n (%) | 177 (75) | 12 (50) | 53 (53) | 0.091 |
| Previous MI, n (%) | 45 (19) | 5 (21) | 16 (16) | 0.78 |
| Previous CABG, n (%) | 54 (23) | 0 | 21 (21) | 0.032 |
| Previous PCI, n (%) | 60 (26) | 5 (21) | 26 (26) | 0.92 |
| PVD, n (%) | 30 (13) | 6 (25) | 28 (28) | 0.002 |
| Diabetes mellitus, n (%) | 57 (24) | 5 (21) | 28 (28) | 0.64 |
| Hypertension, n (%) | 132 (56) | 12 (50) | 34 (34) | 0.029 |
| Creatinine, mean ± SD | 123 ± 131 | 104 ± 56 | 129 ± 68 | 0.75 |
| Chronic haemodialysis, n (%) | 11 (5) | 1 (5) | 0 | 0.20 |
| COPD, n (%) | 78 (33) | 5 (21) | 31 (31) | 0.59 |
| Permanent pacemaker, n (%) | 26 (11) | 3 (13) | 10 (10) | 0.96 |
| Atrial fibrillation, n (%) | 49 (21) | 8 (33) | 25 (25) | 0.23 |
| Aortic valve area (cm2), mean ± SD | 0.67 ± 0.21 | 0.81 ± 0.39 | 0.77 ± 0.27 | 0.001 |
| LV, n (%) | ||||
| - Poor (EF <30%) | 34 (14) | 2 (8) | 10 (10) | 0.89 |
| - Moderate (EF 30–59%) | 82 (35) | 6 (25) | 24 (24) | 0.66 |
| Mitral regurgitation grade ≥III, n (%) | 28 (12) | 2 (8) | 5 (5) | 0.29 |
| Aortic regurgitation grade ≥III, n (%) | 45 (19) | 0 | 3 (3) | 0.001 |
| Logistic Euroscore, mean ± SD | 19.1 ± 13.7 | 10.1 ± 4.3 | 18.9 ± 12.1 | 0.007 |
| STS score, mean ± SD | 6.1 ± 5.5 | 4.1 ± 2.4 | 5.8 ± 3.8 | 0.17 |
BMI body mass index, BSA body surface area, CABG coronary artery bypass graft, COPD chronic obstructive pulmonary disease, EF ejection fraction, NYHA New York Heart Association, LV left ventricular, MI myocardial infarction, PCI percutaneous coronary intervention, PVD peripheral vascular disease, STS Society of Thoracic Surgeons
Thirty-day clinical outcome
Thirty-day all-cause and cardiovascular mortality was 9 and 6% in the TAVI group and 8 and 8% in the AVR group, respectively (Table 2). In the TAVI group, six patients died during the procedure. The cause of death was hypotension during induction of anaesthesia (n = 1), electromechanical dissociation (n = 2), coronary obstruction (n = 1), left ventricular outflow tract rupture (n = 1) and retroperitoneal haemorrhage (n = 1). Another 14 patients died at a median of 7 (IQR: 3–13) days after TAVI due to stroke (n = 3), heart failure (n = 2), sudden death (n = 2: unrecognised alternating left and right bundle branch block leading to asystole at day 8 and sudden death 1 day after discharge on day 29), sepsis (n = 2), pneumonia (n = 2), retroperitoneal haemorrhage (n = 2) and cardiac tamponade (n = 1). In the surgical group, the cause of death was ventricular fibrillation (n = 1) and severe paravalvular aortic regurgitation (n = 1).
Table 2.
Thirty-day clinical outcome in patients undergoing TAVI and AVR
| TAVI | AVR | p-value | |
|---|---|---|---|
| n = 235 | n = 24 | ||
| Mortality, n (%) | |||
| - All-cause | 20 (9) | 2 (8) | 1.0 |
| - Cardiovascular cause | 13 (6) | 2 (8) | 0.64 |
| Myocardial infarction, n (%) | |||
| - All | 3 (1) | 1 (4) | 0.32 |
| - Periprocedural (<72 h) | 2 (1) | 0 | 1.0 |
| Cerebrovascular complication, n (%) | |||
| - All1 | 20 (9) | 2 (8) | 1.0 |
| - Major stroke | 11 (5) | 1 (4) | 1.0 |
| Vascular complication, n (%) | |||
| - All | 42 (18) | 0 | 0.036 |
| - Major | 24 (10) | 0 | 0.14 |
| Bleeding complication, n (%) | |||
| - All | 67 (29) | 2 (8) | 0.049 |
| - Life-threatening or disabling | 21 (9) | 2 (8) | 1.0 |
| Acute kidney injury, n (%) | |||
| - All | 40 (17) | 8 (33) | 0.058 |
| - Stage III | 5 (2) | 2 (8) | 0.13 |
| Cardiac re-intervention, n (%) | |||
| - AVR | 1 (1) | 0 | 1.0 |
| - BAV | 1 (1) | 0 | 1.0 |
| - Other2 | 1 (1) | 2 (8) | 0.023 |
| New pacemaker implantation | |||
| - All | 48 (21) | 1 (4) | 0.056 |
| - For 3rd degree AV block | 40 (17) | 0 | 0.032 |
| New atrial fibrillation | 9 (5) | 2 (11) | 0.60 |
| Repeat hospitalisation, n (%)3 | 3 (1) | 0 | 1.0 |
| Combined 30-day safety endpoint, n (%)4 | 55 (24) | 6 (25) | 1.0 |
AV atrioventricular, AVR aortic valve replacement, BAV balloon aortic valvuloplasty
Mutually non-exclusive analysis (≥1 event/patient possible)
1Including TIA
2Closure of severe paravalvar aortic regurgitation with Amplatzer closure device (n = 1) in TAVI group and resternotomy for severe aortic regurgitation (n = 1) and bleeding (n = 1) in AVR group
3For symptoms of valve-related dysfunction or cardiac decompensation
4Composite all-cause mortality, major stroke, major vascular complication, life-threatening bleeding, acute kidney injury - stage 3, peri-procedural myocardial infarction, repeat procedure for valve-related dysfunction (surgical or interventional)
Cardiac re-intervention after the index procedure was required in 3 patients in the TAVI group: immediate conversion to AVR (n = 1), closure of a paravalvar leak 14 days after TAVI (n = 1) and post-implantation dilatation of the MCS 21 days after TAVI (n = 1). In the AVR group, two patients underwent re-thoracotomy for severe aortic regurgitation (n = 1) and cardiac tamponade (n = 1) 1 day after AVR.
Although the total rate of vascular and bleeding complications was higher in the TAVI group in comparison with the AVR group, the combined 30-day safety endpoint did not differ between the two groups (24 vs. 25%, p = 1.0).
Follow-up
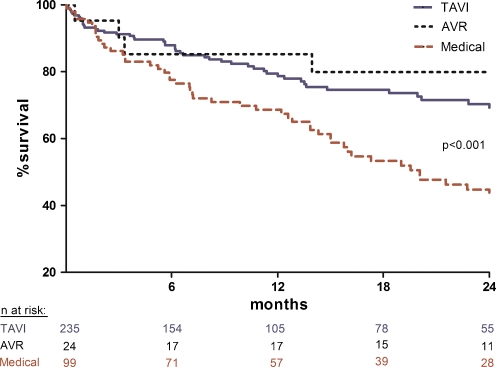
The median follow-up was 298 (IQR: 107–688) days in the TAVI group, 836 (IQR: 327–1269) days in the surgical group and 456 (IQR: 187–869) days in the medical group. Kaplan-Meier estimates of survival are shown in Fig. 2. Estimated survival at 2 years was 80% in the AVR group, 69% in the TAVI group and 45% in patients who continued MT (p < 0.001). The median time between treatment (AVR or TAVI) or first medical contact (MT) and death was 96 (IQR: 11–679) days in the surgical group, 171 (IQR: 24–365) days in the TAVI group and 300 (IQR: 98–578) days in the MT group.
Fig. 2.
Kaplan-Meier survival curve for patients undergoing TAVI, AVR and MT
Details of adverse events beyond 30 days are summarised in Table 3. By univariable analysis, PVD, baseline creatinine, STS score, RBC transfusion and AKI were identified as potential determinants of mortality after TAVI. Multivariable analysis retained RBC transfusion (HR: 1.19; 95% CI: 1.05–1.33), pre-existing renal failure (HR: 1.18; 95% CI: 1.06–1.33) and STS score (HR: 1.06; 95% CI: 1.02–1.10) as independent predictors of mortality after TAVI
Table 3.
Adverse events beyond 30 days after TAVI, AVR and medical treatment
| TAVIa | AVRa | Medicala | |||
|---|---|---|---|---|---|
| All (n = 106) | Fatal (n = 40) | All (n = 10) | Fatal (n = 5) | Fatal (n = 59) | |
| Cardiac | 46 (43) | 19 (48) | 5 (50) | 1 (20) | 31 (53) |
| Heart failure | 13 (12) | 4 (10) | 4 (40) | 1 (20) | 19 (32) |
| Sudden death | 8 (8) | 8 (20) | 0 | 0 | 9 (15) |
| Myocardial infarction | 2 (2) | 2 (5) | 1 (20) | 0 | 1 (2) |
| Cardiac re-intervention | 3 (3)1 | 0 | 0 | 0 | 0 |
| Stroke or TIA | 11 (10)2 | 4 (10) | 0 | 0 | 2 (3) |
| Pacemaker implantation | 9 (9) | 1 (3)3 | 0 | 0 | 0 |
| Non-cardiac | 56 (53) | 21 (52) | 5 (50) | 4 (80) | 9 (15) |
| Infection | 17 (16) | 8 (20) | 1 (10) | 1 (20) | 5 (8) |
| Renal failure | 7 (7) | 4 (10) | 2 (20) | 2 (40) | 0 |
| Vascular | 3 (3) | 0 | 0 | 0 | 0 |
| Bleeding (non-cranial) | 3 (3) | 1 (3) | 1 (10) | 0 | 0 |
| Neoplasm | 9 (8) | 4 (10) | 0 | 0 | 1 (2) |
| Metabolic disease | 2 (2) | 2 (5) | 0 | 0 | 0 |
| Other | 15 (14) | 2 (5)4 | 1 (10) | 1 (20)5 | 3 (5)6 |
| Unknown | 4 (4) | 0 | 0 | 0 | 19 (32) |
aMedian follow-up was 298 (IQR: 107–688) days in the TAVI group, 836 (IQR: 327–1269) days in the AVR group and 456 (IQR: 187–869) days in the medical group
1Re-interventions before discharge included AVR (n = 1) and post-implantation balloon aortic valvuloplasty (n = 2)
2Including TIA (n = 4); of the 11 events, 9 were ischaemic of which 2 fatal and 2 were haemorrhagic, both of which fatal
3Pneumothorax following pacemaker implantation (n = 1)
4Blood transfusion reaction (n = 1), euthanasia (n = 1)
5Delirium (n = 1)
6Obstructive pulmonary disease (n = 2), lung emboli (n = 1)
Discussion
We found that the majority of patients referred for TAVI (72%) undergo valve implantation/replacement (TAVI 65%, AVR 7%) but that nearly 30% continue MT mainly because of comorbidity and patient preference not to receive TAVI/AVR. Patients who underwent TAVI had a higher LES than those who underwent AVR, were more symptomatic with a higher prevalence of antecedent CABG and impaired renal function but less PVD. The most frequent complications after TAVI consisted of bleeding and vascular complications.
With respect to treatment allocation, the present findings most likely reflect the current ‘real world’ practice. The two-thirds acceptance and one-third rejection rate contrasts with randomised studies such as the Placement of AoRTic TraNscathetER valve (PARTNER) Cohort-B trial in which only 12% of the referred patients were accepted for randomised treatment allocation. Of note, we observed a significant increase in acceptance for TAVI from 2006 until 2010; it was 20% in 2006, 33% in 2007, 50% in 2008, 57% in 2009 and 81% in 2010. This is not explained by accepting less sick patients since the LES did not change over time but is most likely explained by increased experience and familiarity with the procedure in combination with an increased public awareness resulting in less patients who refuse therapy. This may also explain that – over time - less patients were redirected to surgery (29% of the surgical patients were treated in 2006; 29% in 2007; 25% in 2008; 13% in 2009 and 4% in 2010).
Initially the LES was a critical factor in patient acceptance but a present consensus on treatment allocation by the Heart Team has become the dominant factor. This is not surprising since this score was neither designed nor validated for TAVI and does not capture the spectrum of clinical details allowing a balanced treatment decision [12, 13]. Also the LES is out of synchrony with the STS score [12, 14]. The shortcomings of the risk score models and the value of multidisciplinary patient discussion are illustrated by the web-based conference call system used in the United States to review and approve patients for TAVI, which is subsequently used in the PARTNER trial [1]. Moreover, randomisation to TAVI or AVR in the SURgery and Transcatheter Aortic Valve Implantation (SURTAVI) trial will be based upon clinical judgement by the Heart Team but not a risk score. The latter will only be used as criterion for entry into the Heart Team discussion [6].
The outcome in the TAVI group in the present study is consistent with the findings of the multi-centre observational registries [14–18]. The worst complications are death and stroke. Conceptually safety will increase when less sick patients receive TAVI. It remains to be elucidated whether stroke can be reduced by the use of embolic protection devices during the procedure. As mentioned, the most frequent complications are bleeding and vascular complications. Patient-related factors may play a role but are not the only ones. At present there is no fully proven percutaneous closure technique. Since bleeding and vascular complications are inherently associated with a series of ensuing events (e.g. transfusion) and complications (e.g. anaemia, renal dysfunction) which in turn affect short- and long-term outcome, surgical access and closure of the arterial entry site should be considered [19–23]. The need for pacemaker insertion is predominantly a device-related phenomenon. A consistently higher frequency of new pacemaker implantation after MCS implantation (up to 49%) than after Edwards implantation (up to 27%) is reported [24, 25]. This is not without clinical importance since abnormal conduction may impair left ventricular ejection fraction recovery after TAVI [26].
Follow-up was characterised by a high incidence of cardiac and non-cardiovascular events. As for immediate outcome, it is conceivable that long-term outcome will be better in less sick patients. This is the subject of investigation in an ongoing Danish study in which age ≥70 is the main selection criterion for random allocation to TAVI or AVR. (ClinicalTrials.gov, Identifier: NCT01057173).
In the present study, outcome was poor in patients who continued MT. MT was continued mainly because of Heart Team rejection for TAVI because of comorbidity. Yet, a substantial number of patients refused valve implantation/replacement. Patient preference intrinsically is bi-directional and will play an increasing role in treatment decisions due to increased public awareness. This puts the medical community under pressure since the position statement paper on TAVI advocates not to include patient preference in the treatment decision [4]. It also raises an ethical issue since one may question whether one has the right to refuse a treatment modality if a patient who is adequately informed about treatment options, outcomes and the presence or absence of future treatment possibilities in case of failure of the index treatment persists in his/her treatment preference.
Conclusion
Up to 65 and 7% of the patients with aortic stenosis who are referred for TAVI undergo TAVI and AVR with promising results. Patients who are rejected or refuse valve replacement have a dismal prognosis.
Acknowledgements
This study was supported by the Erasmus-Columbus (ERACOL) Latin-European Exchange Grant (www.erasmus-columbus.eu). Lidsa Cruz, RN (Angiografia de Occidente), is acknowledged for her contribution on database management.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
The questions can be answered after the article has been published in print. You have to log in to: www.cvoi.nl.
References
- 1.Leon M, Smith C, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/S1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2–isolated valve surgery. Ann Thorac Surg. 2009;88:S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 4.Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2008;29:1463–1470. doi: 10.1093/eurheartj/ehn183. [DOI] [PubMed] [Google Scholar]
- 5.Rosengart TK, Feldman T, Borger MA, et al. Percutaneous and minimally invasive valve procedures: a scientific statement from the American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology, Functional Genomics and Translational Biology Interdisciplinary Working Group, and Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2008;117:1750–1767. doi: 10.1161/CIRCULATIONAHA.107.188525. [DOI] [PubMed] [Google Scholar]
- 6.Serruys PW. Design of a novel randomized clinical trial comparing SAVR with TAVI in patients with severe aortic stenosis at intermediate peri-operative risk. Presented at TCT Congress September 21. Washington: 2010.
- 7.Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 8.Nuis RJ, van Mieghem NM, van der Boon RM, et al. Effect of experience on results of transcatheter aortic valve implantation using a medtronic corevalve system. Am J Cardiol. 2011;107:1824–1829. doi: 10.1016/j.amjcard.2011.02.315. [DOI] [PubMed] [Google Scholar]
- 9.Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the European society of cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 10.Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol. 2007;50:69–76. doi: 10.1016/j.jacc.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 11.Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J. 2011;32:205–217. doi: 10.1093/eurheartj/ehq406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Head SJ, Kappetein AP. Transcatheter aortic valve implantation after PARTNER: what is up next? EuroIntervention. 2010;6:560–561. doi: 10.4244/EIJV6I5A94. [DOI] [PubMed] [Google Scholar]
- 13.Rosenhek R, Iung B, Tornos P, et al. ESC Working group on valvular heart disease position paper: assessing the risk of interventions in patients with valvular heart disease. Eur Heart J. 2011 epub ahead of print. [DOI] [PMC free article] [PubMed]
- 14.Piazza N, Wenaweser P, van Gameren M, et al. Relationship between the logistic EuroSCORE and the Society of Thoracic Surgeons Predicted Risk of Mortality score in patients implanted with the CoreValve ReValving system–a Bern-Rotterdam Study. Am Heart J. 2010;159:323–329. doi: 10.1016/j.ahj.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Zahn R, Gerckens U, Grube E, et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J. 2011;32:198–204. doi: 10.1093/eurheartj/ehq339. [DOI] [PubMed] [Google Scholar]
- 16.Rodes-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–1090. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Eltchaninoff H, Prat A, Gilard M, et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32:191–197. doi: 10.1093/eurheartj/ehq261. [DOI] [PubMed] [Google Scholar]
- 18.Lefevre T, Kappetein AP, Wolner E, et al. One year FU of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32:148–157. doi: 10.1093/eurheartj/ehq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 20.Nuis RJ, Van Mieghem NM, Tzikas A, et al. Frequency, determinants, and prognostic effects of acute kidney injury and red blood cell transfusion in patients undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2011;77:881–889. doi: 10.1002/ccd.22874. [DOI] [PubMed] [Google Scholar]
- 21.Van Mieghem NM, Nuis RJ, Tzikas A, et al. Prevalence and prognostic implications of baseline anaemia in patients undergoing transcatheter aortic valve implantation. EuroIntervention. 2011;7:184–191. doi: 10.4244/EIJV7I2A32. [DOI] [PubMed] [Google Scholar]
- 22.Ducrocq G, Francis F, Serfaty JM, et al. Vascular complications of transfemoral aortic valve implantation with the Edwards SAPIEN prosthesis: incidence and impact on outcome. EuroIntervention. 2010;5:666–672. doi: 10.4244/EIJV5I6A110. [DOI] [PubMed] [Google Scholar]
- 23.Van Mieghem NM, Nuis RJ, Piazza N, et al. Vascular complications with transcatheter aortic valve implantation using the 18 Fr Medtronic CoreValve System(R): the Rotterdam experience. EuroIntervention. 2010;5:673–679. doi: 10.4244/EIJV5I6A111. [DOI] [PubMed] [Google Scholar]
- 24.Thielmann M, Wendt D, Eggebrecht H, et al. Transcatheter aortic valve implantation in patients with very high risk for conventional aortic valve replacement. Ann Thorac Surg. 2009;88:1468–1474. doi: 10.1016/j.athoracsur.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 25.Roten L, Wenaweser P, Delacretaz E, et al. Incidence and predictors of atrioventricular conduction impairment after transcatheter aortic valve implantation. Am J Cardiol. 2010;106:1473–1480. doi: 10.1016/j.amjcard.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Tzikas A, van Dalen BM, Van Mieghem NM, et al. Frequency of conduction abnormalities after transcatheter aortic valve implantation with the Medtronic-CoreValve and the effect on left ventricular ejection fraction. Am J Cardiol. 2011;107:285–289. doi: 10.1016/j.amjcard.2010.09.015. [DOI] [PubMed] [Google Scholar]