Abstract
Rationale
Given evidence for age-related differences in the effects of drugs of abuse, surprisingly few preclinical studies have explored effects of opioids in adolescents (versus adults).
Objectives
This study compared the motor stimulating, ataxic, and hypothermic effects of morphine in adolescent, late-adolescent and adult mice. Plasma and brain levels of morphine were assessed to examine possible pharmacokinetic differences among the age groups.
Methods
Locomotion was measured as occlusions of horizontal infrared light beams, ataxia as failing the horizontal wire test, body temperature by rectal probe, and morphine levels by HPLC-UV.
Results
Morphine (3.2 – 56 mg/kg, i.p.) increased locomotion along an inverted U-shaped dose-response curve in adolescent, late adolescent, and adult male C57BL/6J mice. Its potency to stimulate locomotion was similar in all age groups. However, maximal stimulation was higher in adolescents than in late adolescents, and higher in late adolescents than in adults. In contrast, adolescents showed less ataxia than adults when given morphine (5.6 – 100 mg/kg, i.p.). The hypothermic effects of morphine did not differ among the age groups. Morphine levels, which peaked in plasma at 15 min after i.p. injection and in brain at 45 min, did not show age-related differences.
Conclusions
The finding that adolescents are not generally more sensitive to morphine than adults, but differ in their sensitivity to effects involving nigrostriatal/mesolimbic dopamine systems, is consistent with evidence of overactivity of these dopamine systems during adolescence relative to adulthood. The age-related differences observed here are unlikely due to pharmacokinetic factors.
Keywords: Morphine, Locomotion, Ataxia, Hypothermia, Pharmacokinetics, Adolescent, Adult, Mouse
The misuse and abuse of prescription opioids, particularly in adolescents, has become a major public health concern (Compton and Volkow 2006). Epidemiological evidence indicates that in the United States in 2003, opioid analgesics were among the most frequently abused illicit drugs among secondary students (12th graders), second only to marijuana (Johnston et al. 2004). Although the overall use of illicit drugs by young people has dropped, the nonprescription use of opioids has risen, with about 12% of high school seniors reporting illicit use of prescription opioids (Johnston et al. 2009). The increased abuse of opioid analgesics (e.g., oxycodone and the prototypical compound, morphine) is especially worrisome since at the doses that these substances are sometimes abused respiratory depression and death can result. In addition, opioid analgesic abuse is particularly problematic for adolescents because of uncertain implications for future addiction. Like other drug-related conditions, opioid analgesic abuse is mostly concentrated in adolescents and young adults (Substance Abuse and Mental Health Services Administration 2003), yet little is known about opioid effects in adolescence. Most of what we know about opioid abuse and addiction has been learned from heroin addiction in 20 to 40-year-old individuals, and from research in adult animals.
Most drugs of abuse, including prescription opioids, interact directly or indirectly with the dopamine system. This system changes during development, and appears to be overactive during adolescence relative to either childhood or adulthood (Wahlstrom et al. 2010). Conceivably, this overactivity could be involved in the different balance of rewarding and aversive effects of drugs of abuse in adolescents compared with adults (Schramm-Sapyta et al. 2009): adolescents may be more sensitive to the rewarding effects of drugs and less sensitive to withdrawal effects. Evidence for this differential sensitivity has been obtained in animal models primarily with nicotine, ethanol, THC, amphetamine, and cocaine. Unfortunately, only a few studies have examined dopamine-related effects of opioids in adolescent rats (for example, see White and Holtzman 2005; White et al. 2008) or mice (for example, see Hodgson et al. 2009; Zhang et al. 2009).
The present study is part of an effort to examine effects of morphine in adolescent mice in comparison with its effects in adults. Morphine-induced locomotion, motor impairment, and hypothermia were examined because of the involvement of dopaminergic systems in these effects (Zarrindast and Zarghi 1992; Cook and Beardsley 2003; Zarrindast et al. 1994; Baker and Meert 2003). Also, motor activity and hypothermia likely involve dopamine systems in different brain regions (i.e., nigrostriatal/mesolimbic and hypothalamic dopamine systems, respectively). Further, sensitivities to the motor- and hypothermic effects of morphine are not always related (Belknap et al. 1998). To examine a possible role for pharmacokinetic factors in differential effects of morphine in adolescents and adults, plasma and brain levels of morphine were assessed at various times after its administration to the different age groups.
Methods
Subjects
Because adolescence is the gradual period of transition from childhood to adulthood, it is difficult to characterize its precise onset and offset. A conservative age range during which adolescent neurobehavioral characteristics are evident in rodents ranges from approximately postnatal days 28–42 [P28-42, i.e., two weeks (Spear, 2000)]. However, some adolescent transitions may also occur in animals slightly younger or older than this prototypic age range. In an effort to broadly cover the adolescent period, the present study examined mice between P28-42, described as prototypical adolescence by Spear (2000), and mice between P44-56, described as late adolescence (Adriani et al. 2002; Varlinskaya and Spear 2006). Adolescent, late adolescent, and adult male C57BL/6J mice were obtained by breeding C57BL/6J mice bought from the Jackson Laboratory (Bar Harbor, ME). Adolescent mice ranged in age from 29 to 35 days (mean (± SEM) age=30.5±0.1 days; mean body weight=16.2±0.1 g), late adolescent mice ranged in age from 44 to 49 days (mean age=45.1±0.1 days; mean body weight=21.5±0.1 g), and adult mice ranged in age from 64 to 72 days (mean age=65.3±0.1 days; mean body weight=25.2±0.1 g). All animals were housed in a temperature-controlled (24°C) vivarium maintained on a 14/10-hr light/dark cycle (lights on at 07:00, experiments conducted during the light period) in plastic cages containing rodent bedding (Sani-chips, Harlan Teklad, Madison, WI) with free access to food (Rodent sterilizable diet, Harlan Teklad, Madison, WI) and water. On the day after birth, litters were culled to 8 to ensure optimal growth of the selected animals. Litters were housed until weaning with their mothers in standard maternity cages (47×25×30 cm). When they were 21 days old, pups were weaned and pair-housed in plastic cages (29×18×13 cm) with a same-sex sibling; males were used in the experiments described here. To eliminate the possible confounding of litter with treatment effects, no more than one subject from a given litter was assigned to a particular treatment group (Varlinskaya and Spear 2006). Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, 1996).
Procedures
Locomotor activity
Locomotor activity was assessed using eight 30×15×15 cm customized acrylic boxes (Instrumentation Services, University of Texas Health Science Center, San Antonio) that were separately enclosed in commercially available sound-attenuating chambers (model no. ENV-022M; MED Associates, St. Albans, VT). Four infrared light beams were spaced 6 cm apart and located 2 cm above the floor of each box. Occlusions of the infrared light beams were counted using commercially available computer software (Multi-Varimex version 1.00, Columbus Instruments, Columbus, OH). The floor of the boxes consisted of a parallel grid of 2.3-mm stainless steel rods mounted 6.4 mm apart. Between tests, the floor and inside of the boxes were wiped, and the litter paper beneath the floor was changed. On the first day of the experiment, basal activity was measured for 2 h. Twenty-four h later, animals received an i.p. injection of saline or a particular dose of morphine (3.2 – 56 mg/kg, n=6 per dose; each animal received only one injection), and activity was measured again for 2 h.
Ataxia
In the horizontal wire test, mice were lifted by the tail and allowed to grasp a horizontally strung wire with the forepaws. When the mouse grasped the wire with both forepaws its tail was released. The number of animals that did not grasp the wire with at least one hindpaw within 3 s was determined. Mice were tested immediately before and repeatedly at different intervals (ranging from 15 to 120 min) after an i.p. injection of saline or a particular dose of morphine (5.6 – 100 mg/kg, n=8–10 per dose; each animal received only one injection).
Body temperature
Body temperature was measured with a digital thermometer (model BAT7001H) and a thermistor probe (model RET-3), both manufactured by Physitemp Instruments, Inc. (Clifton, NJ). Immediately before drug administration, baseline temperature was measured by inserting the lubricated probe 2 cm into the rectum. Thereafter, body temperature was recorded repeatedly in the same animals at different intervals (ranging from 15 to 240 min) after an i.p. injection of saline or a particular dose of morphine (3.2 – 100 mg/kg, n=8 per dose; each animal received only one injection).
Morphine levels
Animals received an i.p. injection of 17.8 mg/kg morphine, and were sedated with isoflurane either immediately after the injection or after having been placed in individual cages for 5, 10, 15, 20, 30, 45, 60, 75, 90, or 120 min post-injection. Following sedation then decapitation, the brains were quickly removed and frozen at −80°C immediately. Trunk blood was collected and quickly mixed with 60 μL of a solution of 7.5%(w/v) EDTA, then centrifuged to separate plasma from packed red blood cells. Once separated, plasma was rapidly frozen and stored at −80°C.
On the day of assay for plasma morphine, samples were thawed at room temperature, then 200 μL of plasma samples, calibrators, and controls were mixed with 10 μL of 100 μg/ml nalorphine (internal standard). Next, 4 mL of 100 mM K2HPO4 (pH 6.0) were added to each sample, the mixture was vortexed vigorously, and the samples were applied to Varian Bond Elut Certify (130 mg) solid phase extraction columns. The columns/samples were washed sequentially with 2 ml of water, 100 mM acetate buffer (pH 4), and methanol, then the columns were allowed to dry under vacuum. Morphine was eluted from the columns with 2 ml of methanol:ammonia (98:2), which was dried to residue under a stream of nitrogen. Once resuspended in 150 μL of mobile phase, the samples were filtered through a nylon micro-spin filter tube (0.45 micron) and 100 μL of the final samples were injected into the HPLC system. Plasma calibrators contained 0, 100, 500, 1500, and 6000 ng morphine/mL. Final results were expressed in ng morphine/mL plasma.
On the day of the assay for brain morphine levels, samples were thawed at room temperature, then approximately 400 mg of each brain sample were weighed out and homogenized with 10 volumes of saline. Next, 500 μL of the brain homogenate, calibrator, and control samples were assayed using the same procedure that was used for plasma analysis. Brain calibrators contained 0, 52, 103, 207, 413, 825, and 1650 pg/mg of brain wet weight. Final results were expressed as pg morphine/mg brain wet weight.
All reagents were HPLC grade and were purchased from Sigma Chemical Co (St. Louis, MO). The HPLC system consisted of a Waters autosampler, pump, and UV detector (214 nm), and a Grace Alltima C18 analytical column (4.6 × 150 mm, 5 μ). The mobile phase was 6.8 mM K2HPO in water:acetonitrile (97:3). Morphine concentration in each sample was quantified using Waters Empower chromatographic software. Morphine/IS peak area ratios for samples and controls were compared against a linear regression of calibrator ratios to determine morphine levels in plasma and brain.
Data analyses
Locomotion upon initial exposure to the activity chambers was analyzed by means of ANOVA with age (adolescent, late adolescent, adult) as between-subjects factor and time period (four consecutive 30-min periods) as within subjects factor, followed by multiple comparisons with Newman-Keuls tests. Locomotion in animals treated with saline during the second session was analyzed by ANOVA with age as between-subjects factor and session (basal, after saline) and time period as within-subjects factors. There were significant baseline differences among the groups during the first session, and during the second session in animals treated with saline (see results). In these latter animals, activity during the second session was lower than during the first session, but the magnitude of the decrease differed among the age groups. Thus, activity during the second session was not simply predictable from activity during the first session. Therefore, drug effects on locomotion, assessed during the second session, were not expressed as a percentage of within-subjects basal locomotion in the manner described by (White et al. 2008), but were expressed as a percentage of locomotion in saline controls. Effects of morphine on locomotion were examined by ANOVA with age and dose as between factors and time period as within factor, performed on locomotor data expressed as a percentage of locomotion observed in saline controls. Multiple comparisons among age groups were conducted with Newman-Keuls test, and Dunnett’s test was used to compare each dose with saline.
Morphine-induced ataxia was examined by plotting the percentage of animals failing the horizontal wire test as a function of dose, and by testing the statistical significance effects by the method of Fray et al. (1980) as described in detail in Koek and Colpaert (1990). Briefly, the data were arranged in a contingency table with two columns (number of mice failing and number of mice passing the test), one row for each drug dose, and a row for vehicle. From this table, the information statistic 2I, analogous to Chi-square but not constrained by small cell frequencies, was calculated as 2I=2Σij[N.ln(N/E)], where N is each observation in the table, E is the expected value (row total x column total/grand total), and i and j are the numbers of rows and columns, respectively. 2I is distributed as χsquare with (i-1) (j-1) df. If 2I was statistically significant, the minimum significant dose was determined using the following method of planned contrasts. First, a 2 × 2 table was constructed using the data obtained with vehicle and with the lowest drug dose, and 2I was again calculated. If the lowest dose differed significantly from vehicle, the lowest dose was considered the minimum significant dose. If the lowest dose did not differ significantly from vehicle, the next higher drug dose was compared with the combined results obtained with the lowest drug dose and the vehicle. This process was repeated until the minimum significant dose was obtained, or until the highest dose was reached.
Morphine-induced hypothermia was analyzed by ANOVA with age and dose as between-subjects factors and time as within-subjects factor, and with Dunnett’s test to compare each dose with saline control values. In addition, dose-response data obtained at each time point were analyzed separately for each age group by non-linear curve fitting [i.e., four parameter sigmoid function, with a maximum of 38 °C and a minimum of 34 °C (see results)] to estimate ED50 values. These latter values were plotted for each age group as a function of time after morphine administration, and the resulting plots were fitted with quadratic curves (i.e., Y=B0 + B1*X +B2*X^2). GraphPad prism version 5.04 for Windows (GraphPad Software, San Diego CA) was used to conduct non-linear curve fitting and to compare the quadratic curves with F ratio tests.
Plasma and brain levels of morphine were analyzed separately by ANOVA with age and post-injection interval as between-subjects factors. In addition, time - morphine concentration data were fitted by a three exponential function [i.e., Y=A*exp(-Ka*X) + D*exp(-Kd*X) + E*exp(-Ke*X)] by means of GraphPad Prism, which was used to estimate the half-life of morphine in plasma.
Drugs
Morphine sulfate (National Institute on Drug Abuse, Research Technology Branch, Research Triangle Park, NC) was dissolved in physiological saline and injected i.p. in a volume of 10 ml/kg.
Results
Locomotor activity
Adolescents showed significantly less basal locomotion than adults upon their initial exposure to the activity chambers (Fig. 1, upper panel). The effects of age (adolescent, late adolescent, adult), time (4 30-min periods), and their interaction were statistically significant [F(2,123)=120, p<0.0001; F(3,369)=576, p<0.0001; F(6,369)=3.79, P=0.001, respectively]. Adolescents showed less activity than late adolescents, and late adolescents less than adults, at each time period. Activity decreased during the session, but less in adolescents than in the other age groups, which showed a similar decrease [interaction between age and time: adolescent and late adolescent, F(3,246)=7.02, p=0.00015; adolescent and adult, F(3,246)=3.90, p=0.0095; late adolescent and adult, F(3,246)=0.68, p>0.50]. In animals treated with saline during the second session, which took place 24 h after the first, activity decreased not only within sessions, but also between sessions (Fig. 1, lower panel). However, the magnitude of the between session decrease differed among the age groups (compare solid and dotted lines per age group). Overall activity during the second session, which was preceded by an injection of saline, was significantly lower than the activity during the first, basal session in adults and late adolescents, but not in adolescents (p<0.05, Newman-Keuls). Thus, adolescents showed slower within- and between-session habituation of locomotion than older animals. Because of differences in habituation, drug effects on locomotion, assessed during the second session, were not expressed as a percentage of within-subjects basal locomotion, but as a percentage of locomotion in saline controls at each time period.
Fig. 1.
Upper panel: Locomotor activity, measured as mean (± SEM) number of breaks of horizontal infrared beams per 30-min period, in adolescent, late adolescent, and adult male C57/BL6J mice (n=42 per age group), during an initial 2 h exposure to the activity chambers. Error bars that are not shown are contained within the symbol. Asterisks indicate p<0.05 compared with adults, and pound signs indicate p<0.05 compared with late adolescents. Lower panel: locomotor activity during the initial 2h exposure (marked “basal”) and, 24 h later, during a second 2 h exposure (marked “saline”) preceded by an i.p. injection of saline. Data of both sessions were obtained in the same animals (n=6 per age group).
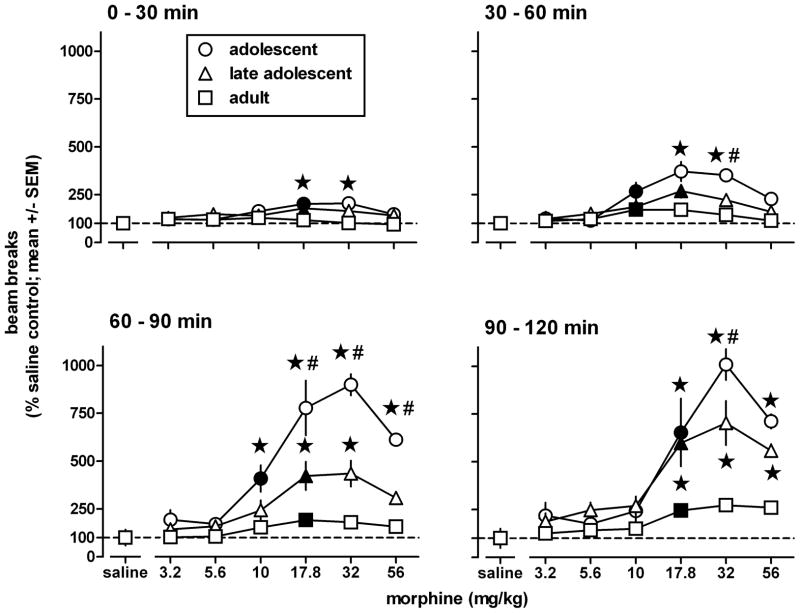
Morphine stimulated locomotion in a dose-, time-, and age-related manner (Fig. 2), evidenced by statistically significant main effects [dose: F(6,105)=30.1, p<0.0001; time: F(3,315)=222.2, P<0.0001; age: F(2,105)=42.5, P<0.0001] and a three factor interaction [dose × time × age: F(36,315)=5.58, p<0.0001]. Between 0 and 30 min after its administration, morphine significantly increased locomotion in adolescents and late adolescents (minimum significant dose: 17.8 mg/kg; Dunnett’s test), but not in adults. Between 30 and 120 min, morphine stimulated locomotion in all age groups (minimum significant dose: 10–17.8 mg/kg). Morphine stimulated locomotion significantly more in adolescents than in adults during the entire 2 h session, and significantly more than in late adolescents from 30–120 min. In the second half of the 2 h session, morphine stimulated locomotion significantly more in late adolescents than in adults. Morphine had maximal effects in all three age groups between 90 and 120 min after its administration. During this interval, morphine increased locomotion to 1000, 720, and 270% of saline control values in adolescent, late adolescents, and adults, respectively. These percentages are based on the number of beam breaks after saline (i.e., 235±119 [mean ± SEM], 345±71, and 889±149, in adolescents, late adolescent, and adults, respectively), which differed significantly among age groups [F(2,15)=8.88, p<0.005], and on the maximum number of beam breaks after morphine (i.e., 2369±191, 2482±401, and 2424±257, in adolescents, late adolescent, and adults, respectively), which did not differ significantly among age groups (F(2,15)=0.01, p>0.9). However, the magnitude of the observed effects of morphine on locomotion, assessed by comparing the results obtained in morphine-treated animals with those obtained in age-matched saline controls, differed among the age groups. Taken together, the results show that morphine increased locomotion with similar potency in all age groups, but increased locomotion more in adolescents than in late adolescents, and more in late adolescents than in adults.
Fig. 2.
Locomotor activity in adolescent, late adolescent, and adult male C57/BL6J mice during a 2h exposure to the activity chambers immediately after an i.p. injection of morphine or its vehicle (n=6 per dose; each animal received only one injection). Results are shown as mean (± SEM) values for each of the four consecutive 30-min periods after the injection. Error bars that are not shown are contained within the symbol. Data obtained in morphine-treated animals were expressed as a percentage of the data obtained in their corresponding age-matched saline controls. Asterisks indicate p<0.05 compared with adults, and pound signs indicate p<0.05 compared with late adolescents. Filled symbols indicate the minimum significant dose (p<0.05) of each dose-response curve.
Ataxia
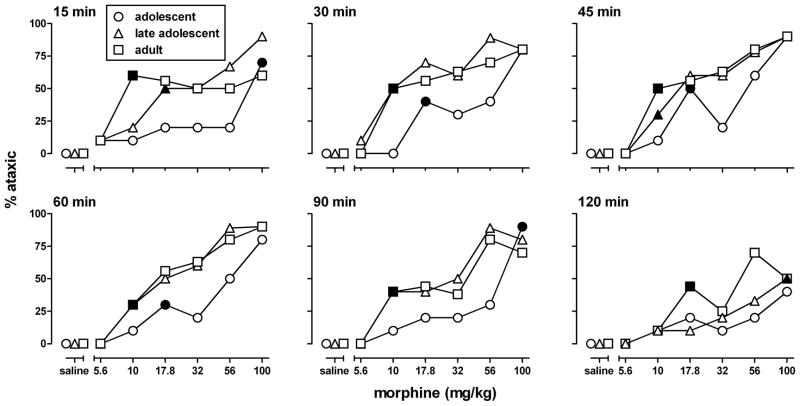
Morphine increased the percentage of animals that failed the wire test in a dose-, time-, and age-related manner (Fig. 3). Morphine significantly increased ataxia in all age groups and at all times (2I≥15.4, df=6, p≤0.017), except in adolescents at 120 min (2I =10.9, df=6, p=0.09). Ataxia reached a maximum of 90% at 45 min after injection in all three age groups; at 120 min, its maximum decreased to 40–70%. Morphine was less potent to produce ataxia in adolescents than in late adolescents and adults: its minimum significant dose was higher in adolescents than in the other age groups at all time points, and did not differ between late adolescents and adults at most of the time points.
Fig. 3.
Morphine-induced ataxia (measured as the percentage of animals failing the horizontal wire test) at different times after its i.p. administration to adolescent, late adolescent, and adult male C57/BL6J mice (n=8–10 per dose; each animal received only one injection). Filled symbols indicate the minimum significant dose (p<0.05) of each dose-response curve.
Body temperature
Morphine decreased body temperature in a dose-, time-, and age-related manner (Fig. 4, upper panels, lower left panel) from control values around 38 °C to a minimum of about 34 °C. The main effects of dose [F(7,164)=122.29, p<0.0001] and time [F(7,1148)=365.73, p<0.0001] were statistically significant; the main effect of age [F(2,164)=1.03, p>0.20] was not significant, but age interacted significantly with time [F(14,1148)=4.27, p<0.0001] and with dose x time [F(98,1148)=2.30, p<0.0001]. The minimum significant dose of morphine was 17.8 mg/kg at 60 min postinjection in adolescents, 10 mg/kg at 60 min in late adolescents, and 10 mg/kg at 15 min in adults, suggesting that its onset of action was slower in adolescents than in adults. However, morphine maximally decreased body temperature earlier in adolescents (at 45 min postinjection:) than in late adolescents and adults (at 90 min postinjection: 33.6± 0.12 and 34.6±0.52 °C, respectively). Differences among the maximum effects of morphine in each age group (lowest observed temperature: 34.0± 0.49 °C in adolescents, 33.6 ± 0.12 °C in late adolescents, and 34.6±0.52 °C in adults) were not statistically significant [F(2,21)=1.63, p>0.20). Morphine’s potency to produce hypothermia increased from 15 to 60 min postinjection, reached an apparent minimum of about 20 mg/kg between 60 and 90 min, and increased thereafter (Fig. 4, lower right panel). The onset of hypothermia, represented by the descending limb of the time-ED50 curve, was similar among the age groups. However, the offset of hypothermia, represented by the ascending limb of the curve, appeared to be somewhat faster in adolescents than in late adolescents and adults. The simplest model that adequately fitted the time-ED50 data consisted of a second order polynomial Y=B0 + B1*X +B2*X^2 with B1 shared among the age groups, but with a value of B2 in adolescents significantly different from its value in late adolescents and adults [F(2,12)=5.01, p=0.026].
Fig. 4.
Morphine-induced hypothermia at different times after its i.p. administration to adolescent, late adolescent, and adult male C57/BL6J mice (n=8 per dose; each animal received only one injection). Results are shown as mean (± SEM) body temperature, in °C, measured repeatedly in the same animals immediately before (marked on the abscissa with a 0) and at various intervals after the injection. Error bars that are not shown are contained within the symbol.
Pharmacokinetics
Plasma and brain levels of morphine varied as a function of time since the i.p. administration of 17.8 mg/kg, and reached an apparent peak at 15 min in plasma and at 45 min in brain (Fig. 5). Plasma levels showed a main effect of time [F(10,99)=10.69, p<0.0001], a main effect of age [F(2,99)=3.11, p=0.049], and no interaction between time and age [F(20,99)=0.78, p>0.20]. Plasma levels averaged across age were significantly higher at 5, 10, and 15 min after the injection than at 0 min. Plasma levels averaged across time were significantly higher in late adolescents than in adults, but were not significantly different in adolescents than in late adolescents or adults. Brain levels differed significantly as a function of time [F(10,97)=2.43, p=0.013] ) but not as a function of age [F(2,97)=0.20, p>0.20], and the interaction between time and age was not significant [F(20,97)=0.39, p>0.20)]. Brain levels averaged across age were significantly higher at 45 min after the injection than at 0 min. Time - morphine concentration data could be fitted by a three exponential function that, when fitted to the plasma values, yielded an estimated half-life of 23 min.
Fig. 5.
Morphine levels in plasma (upper panel) and brain (lower panel) at different times after the i.p. administration of 17.8 mg/kg morphine to adolescent, late adolescent, and adult male C57/BL6J mice (n=3–4 for each dose and each postinjection interval). Data are expressed as mean (± SEM). Error bars not shown are contained within the symbol. The solid lines represent three-exponential functions fitted to the data.
Discussion
The main finding of the present study is that morphine induced more locomotion and less ataxia in adolescent than in adult male mice, and induced similar hypothermia in both age groups. This suggests that adolescents are not generally more sensitive to morphine than adults, but differ from adults in their sensitivity to effects of morphine that are mediated predominantly by nigrostriatal/mesolimbic dopamine systems. Age-related differences in the locomotor stimulating effects of morphine are consistent with evidence that adolescence is associated with overactivity of these systems relative to adulthood. Pharmacokinetic factors are unlikely to be involved in the age-related differences observed here.
Adolescent and late adolescent male mice showed significantly less activity than adults upon their initial exposure to the locomotor activity chambers. This contrasts with the notion that adolescent animals are typically hyperactive in novel situations compared with adults (Spear 2000). However, there are reports that adolescent males are less active when first exposed to a test environment, in studies using rats (Faraday et al. 2003; Collins and Izenwasser 2004; White et al. 2008) and mice (Adriani and Laviola 2000; Hefner and Holmes 2007), and there are other reports showing normal or greater locomotion in adolescent rats (White and Holtzman 2005; Spear and Brake 1983) and mice (Zombeck et al. 2010; Adriani et al. 1998). The novelty of the test situation has been proposed to be a factor that affects differences in locomotion between adolescents and adults (Laviola et al. 2003). Thus, differences in the relative novelty of the test environment may account for the apparent discrepancies among studies.
Morphine is well-know to stimulate motor activity in mice (Shuster et al. 1963; Goldstein and Sheehan 1969). Consistent with this, in the present study morphine dose-dependently increased locomotion, and did so along an inverted dose-response curve. At high doses, the motor-stimulating effects of morphine are likely limited by other effects (e.g., stereotypy, ataxia, sedation) that interfere with locomotion, thereby giving the dose-response curve a biphasic appearance. The locomotor-stimulating effects of morphine increased during the 2 h test period and reached a maximum during the last 30 min of the 2 h session in all age groups. Morphine increased locomotion in all age groups with similar potency. Its maximal effects, expressed as percentage of saline control values, were higher in adolescents than in late adolescents, and were higher in late adolescents than in adults. The maximum number of beam breaks observed with morphine did not differ significantly among age groups, which suggests the possibility that a ceiling effect could have limited the detection of locomotor differences. However, in experiments examining the locomotor effects of morphine after repeated administration, the maximum number of beam breaks between 90 and 120 min after the administration of morphine (i.e., 4804; unpublished observations in our laboratory) was almost two-fold higher than the maximum observed in the present study (i.e., 2482), which suggests that the current results do not reflect a ceiling effect. The present finding that morphine enhanced locomotion more in adolescents that in adults extends previous findings in rats (Spear et al. 1982; White et al. 2008) to mice. Morphine stimulates dopamine systems indirectly by inhibiting GABAergic interneurons that inhibit dopaminergic neurons (Johnson and North 1992). Overactivity of this system during adolescence, relative to adulthood (Wahlstrom et al. 2010), conceivably underlies the age-related effects of morphine on locomotion observed in the present study.
In addition to its ability to stimulate locomotion, morphine can also interfere with motor coordination. Drug effects on motor coordination in mice are often studied in the inverted screen test (Coughenour et al. 1977), which has been shown to be sensitive to morphine (Ginski and Witkin 1994). In preliminary studies, we found the horizontal wire test (Courvoisier 1956; Irwin 1968; Bonetti et al. 1982) to provide a more sensitive measure of morphine-induced ataxia than the inverted screen test (unpublished observations). This was confirmed in the present study, in which morphine produced motor deficits at a minimum significant dose (10 mg/kg) three-fold lower than the minimum effective dose (30 mg/kg) reported in the inverted screen test (Ginski and Witkin 1994). Adolescent mice were less sensitive to morphine-induced ataxia than adults. Because morphine produced less ataxia in adolescents over the dose range in which it produced more locomotion in adolescents, these two effects might be related. However, a similar relationship was not apparent in the late adolescents, which showed enhanced locomotion but generally similar ataxia when compared with adults. Also, preliminary findings in our laboratory showed that repeated administration enhanced the locomotor effects of morphine without altering its ataxic effects (unpublished observations). Together, these findings suggest that sensitivity to morphine-induced ataxia plays only a limited role in the enhanced morphine-induced locomotion observed in adolescents and late adolescents.
Morphine dose-dependently affects body temperature (Adler et al. 1988). Doses lower than 10 mg/kg may produce hyperthermia, whereas at higher doses hypothermia is often observed. In the present study, the effects of morphine on body temperature were examined over a wide dose range. Under the conditions of the present experiments, hyperthermia was not observed, perhaps due to these effects being dependent on various conditions, such as ambient temperature (Adler et al. 1988). However, hypothermic effects were consistently observed, and were dose-dependently produced by morphine with similar potency and maximal effects in adolescents and adults. Thus, adolescents do not appear to be differentially sensitive to all of the effects of morphine. The suggestion that the age-related difference in sensitivity to morphine is not global is consistent with findings in rats that morphine-induced analgesia varied in an assay-dependent manner between adolescents and adults (White et al. 2008), and with findings that mu-opioid receptor function assessed by GTPgammaS binding does not differ between adolescents and adults (Talbot et al., 2005).
Taken together, the findings of the present study suggest the possibility that morphine produces hyperlocomotion in adolescents through a normal opioid system coupled to an overactive dopamine system (assuming the nigrostriatal and mesolimbic dopamine systems to be involved more in the effects of morphine on locomotion than on body temperature). If correct, this hypothesis would predict adolescents to be more sensitive to other effects of morphine mediated indirectly through the dopamine system, such as its rewarding effects. Rewarding properties of drugs of abuse are often examined using conditioned place preference and self-administration procedures. Surprisingly, adolescent rats have been reported not to exhibit morphine-induced place preference (Bolanos et al., 1996), or to show morphine-induced place preference similar in magnitude to that shown by adults (Campbell et al., 2000). In contrast, adolescent rats self-administered less morphine than adults, an effect that may reflect higher drug sensitivity (Doherty et al., 2009). Also, compared with adult mice, adolescent mice were found to self-administer a lower number of infusions of the opioid oxycodone (Zhang et al. 2009). In the latter study, adolescents showed an enhanced response to oxycodone, shown by greater increases in oxycodone-induced striatal dopamine levels. This enhanced response is consistent with the interpretation that lower rates of self-administration could reflect a leftward shift of the inverted U-shaped dose-response function, i.e., a higher sensitivity to the reinforcing effects of oxycodone in adolescents. Understanding the mechanisms that underlie different effects of opioids in adolescents and adults should lead to better strategies for prevention and treatment.
An alternative explanation of the differences between adolescents and adults observed here would be that the pharmacokinetic characteristics of morphine change with age. After the i.p. administration of a dose of morphine (i.e., 17.8 mg/kg) that had near-maximal effects on locomotion, levels of morphine in plasma and in brain were similar across time points in all age groups. The plasma concentration of morphine reached its maximum at 15 min after its administration and its half-life was estimated to be 23 min. These results are consistent with other values obtained with morphine in mice [e.g., 10 min to peak plasma concentration, and an estimated half-life of 28 min (Handal et al. 2002)]. In the present study, brain levels reached a maximum at 45 min, consistent with previous findings (Andersen et al. 2009). Because plasma and brain levels were similar across ages, the age-related effects of morphine reported here are unlikely due to developmental differences in the pharmacokinetic properties of morphine, but likely involve pharmacodynamic differences among the age groups.
In summary, morphine enhanced locomotion in adolescent and late adolescent mice compared with adults, produced less ataxia in adolescents than in the other age groups, and had similar hypothermic effects and pharmacokinetic characteristics in all three age groups. Overactivity of nigrostriatal/mesolimbic dopamine systems during adolescence relative to adulthood may underlie the age-related differences in the effects of morphine observed here.
Acknowledgments
Supported by DA23261 (WK), DA17918 (CPF)
The authors thank Jason Persyn, Chris Limas, Bindumahi Sudaabattula, Sonia Cano, Carlos Herrera, and Tom King for technical assistance. The work was supported by the US Public Health Service Grants DA23261 (W.K.) and DA17918 (C.P.F.)
Contributor Information
Wouter Koek, Department of Psychiatry, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, Mail Code 7792, San Antonio, TX 78229-3900, USA, koek@uthscsa, Tel: (210) 567 5478; Fax: (210) 567 5381, Department of Pharmacology, University of Texas Health Science Center at San Antonio, San Antonio, TX.
Charles P. France, Department of Psychiatry, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, Mail Code 7792, San Antonio, TX 78229-3900, USA. Department of Pharmacology, University of Texas Health Science Center at San Antonio, San Antonio, TX
Martin A. Javors, Department of Psychiatry, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, Mail Code 7792, San Antonio, TX 78229-3900, USA
References
- Adler MW, Geller EB, Rosow CE, Cochin J. The opioid system and temperature regulation. Annu Rev Pharmacol Toxicol. 1988;28:429–449. doi: 10.1146/annurev.pa.28.040188.002241. [DOI] [PubMed] [Google Scholar]
- Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self- administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–346. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Andersen JM, Ripel A, Boix F, Normann PT, Morland J. Increased locomotor activity induced by heroin in mice: pharmacokinetic demonstration of heroin acting as a prodrug for the mediator 6-monoacetylmorphine in vivo. J Pharmacol Exp Ther. 2009;331:153–161. doi: 10.1124/jpet.109.152462. [DOI] [PubMed] [Google Scholar]
- Baker A, Meert T. Morphine and d-amphetamine nullify each others’ hypothermic effects in mice. Pharmacol Toxicol. 2003;92:64–70. doi: 10.1034/j.1600-0773.2003.t01-1-920202.x. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Riggan J, Cross S, Young ER, Gallaher EJ, Crabbe JC. Genetic determinants of morphine activity and thermal responses in 15 inbred mouse strains. Pharmacol Biochem Behav. 1998;59:353–360. doi: 10.1016/s0091-3057(97)00421-8. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Garmsen GM, Clair MA, McDougall SA. Effects of the kappa-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developing rat. Eur J Pharmacol. 1996;317:1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- Bonetti EP, Pieri L, Cumin R, Schaffner R, Pieri M, Gamzu ER, Muller RK, Haefely W. Benzodiazepine antagonist Ro 15–1788: neurological and behavioral effects. Psychopharmacology (Berl) 1982;78:8–18. doi: 10.1007/BF00470579. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cook CD, Beardsley PM. The modulatory actions of dopamine D2/3 agonists and antagonists on the locomotor-activating effects of morphine and caffeine in mice. Pharmacol Biochem Behav. 2003;75:363–371. doi: 10.1016/s0091-3057(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Coughenour LL, Mclean JR, Parker RB. A new device for the rapid measurement of impaired motor function in mice. Pharmacol Biochem Behav. 1977;6:351–353. doi: 10.1016/0091-3057(77)90036-3. [DOI] [PubMed] [Google Scholar]
- Courvoisier S. Pharmacodynamic basis for the use of chlorpromazine in psychiatry. J Clin Exp Psychopathol. 1956;17:25–37. [PubMed] [Google Scholar]
- Doherty J, Ogbomnwan Y, Williams B, Frantz K. Age-dependent morphine intake and cue-induced reinstatement, but not escalation in intake, by adolescent and adult male rats. Pharmacol Biochem Behav. 2009;92:164–172. doi: 10.1016/j.pbb.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Phillips JM, Grunberg NE. Adolescent and adult male rats differ in sensitivity to nicotine’s activity effects. Pharmacol Biochem Behav. 2003;74:917–931. doi: 10.1016/s0091-3057(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Ginski MJ, Witkin JM. Sensitive and rapid behavioral differentiation of N-methyl-D-aspartate receptor antagonists. Psychopharmacology (Berl) 1994;114:573–582. doi: 10.1007/BF02244987. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Sheehan P. Tolerance to opioid narcotics. I. Tolerance to the “running fit” caused by levorphanol in the mouse. J Pharmacol Exp Ther. 1969;169:175–184. [PubMed] [Google Scholar]
- Handal M, Grung M, Skurtveit S, Ripel A, Morland J. Pharmacokinetic differences of morphine and morphine-glucuronides are reflected in locomotor activity. Pharmacol Biochem Behav. 2002;73:883–892. doi: 10.1016/s0091-3057(02)00925-5. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Wellman PJ, Eitan S. Different affective response to opioid withdrawal in adolescent and adult mice. Life Sci. 2009;84:52–60. doi: 10.1016/j.lfs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Overview of Key Findings, 2009. National Institute on Drug Abuse; Bethesda, MD: 2009. Monitoring the Future: National Results on Adolescent Drug Use. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future: National Survey Results on Drug Use 1975–2003, vol. I, Secondary School Students. National Institute on Drug Abuse; Bethesda MD: 2004. [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster L, Hannam RV, Boyle WE., Jr A simple method for producing tolerance to dihydromorphinone in mice. J Pharmacol Exp Ther. 1963;140:149–154. [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP, Horowitz GP, Lipovsky J. Altered behavioral responsivity to morphine during the periadolescent period in rats. Behav Brain Res. 1982;4:279–288. doi: 10.1016/0166-4328(82)90005-5. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Overview of Findings from the 2002 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville MD: 2003. [Google Scholar]
- Talbot JN, Happe HK, Murrin LC. Mu opioid receptor coupling to Gi/o proteins increases during postnatal development in rat brain. J Pharmacol Exp Ther. 2005;314:596–602. doi: 10.1124/jpet.104.082156. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Michaels CC, Holtzman SG. Periadolescent male but not female rats have higher motor activity in response to morphine than do adult rats. Pharmacol Biochem Behav. 2008;89:188–199. doi: 10.1016/j.pbb.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Holtzman SG. Periadolescent morphine exposure alters subsequent behavioral sensitivity to morphine in adult rats. Eur J Pharmacol. 2005;528:119–123. doi: 10.1016/j.ejphar.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Vahedy A, Heidari MR, Ghazi Khansari M. On the mechanism(s) of morphine-induced hypothermia. J Psychopharmacol. 1994;8:222–226. doi: 10.1177/026988119400800405. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Zarghi A. Morphine stimulates locomotor activity by an indirect dopaminergic mechanism: possible D-1 and D-2 receptor involvement. Gen Pharmacol. 1992;23:1221–1225. doi: 10.1016/0306-3623(92)90315-b. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology. 2009;34:912–922. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck JA, Lewicki AD, Patel K, Gupta T, Rhodes JS. Patterns of neural activity associated with differential acute locomotor stimulation to cocaine and methamphetamine in adolescent versus adult male C57BL/6J mice. Neuroscience. 2010;165:1087–1099. doi: 10.1016/j.neuroscience.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]