Abstract
Background
Research indicates that gender mismatch of organ donor and recipient may adversely affect outcomes in heart transplant (HT) patients. However, there is a paucity of literature on gender-mismatched outcomes in patients receiving a HT, and only a few outcomes have been investigated.
Objectives
Objectives were to: (1) determine if gender-mismatched HT recipients experienced decreased survival, more post-transplant complications, and more days hospitalized during the first post-operative year as compared to gender-matched recipients; and (2) identify risk factors for decreased survival.
Methods
Patients were 347 HT recipients; 21.3% (74) received a heart from the opposite gender. Three groups were compared: Group 1: same gender donor-recipient (273, 78.7%: 36 women, 237 men); Group 2: female donor-male recipient (40, 11.5%); Group 3: male donor-female recipient (34, 9.8%). Ten outcomes were compared with Kaplan-Meier survival analysis, logistic regression, and MANCOVA, using a Bonferroni-adjusted P ≤.005. Risk factors for decreased survival were examined with Cox regression.
Results
Gender-mismatched HT patients with a male donor and a female recipient (Group 3) had more treated acute rejections and were rehospitalized more days after HT discharge during the first post-operative year as compared to gender-matched patients. No significant differences were found in 8 other first-year outcomes: number of deaths, survival time, hospital length of stay for HT surgery, cardiac allograft vasculopathy, severe renal dysfunction, new-onset steroid-induced diabetes, non-skin cancers, or the number of infections treated with an intravenous (IV) antibiotic. Risk factors for decreased year 1 survival were higher year 1 cholesterol, earlier IV-treated infection, severe renal dysfunction, earlier treated rejection, and diabetes (both pre-existing and new-onset steroid-induced diabetes).
Conclusion
Gender-mismatched HT recipients had more complications due to rejection and higher resource utilization due to more rehospitalization during the first post-operative year as compared to gender-matched recipients. Therefore, these problem areas may provide targets for possible interventions.
Keywords: donor-recipient gender mismatch, heart transplant outcomes
Some studies have shown that gender mismatch of organ donor and recipient leads to adverse outcomes in heart transplant (HT) patients,1-14, 19, 20 although other studies found no difference in outcomes.15-18, 21 However, there is a paucity of literature on outcomes in gender-mismatched patients receiving a HT, and only a few outcomes have been investigated. Most of the studies on this topic examined patient survival, and a few studies examined acute rejection, infection, cardiac allograft vasculopathy (CAV), or graft survival.
Differences in various HT outcomes have been found with both types of gender mismatch (Table 1). Many of the studies reported higher mortality when the heart donor was female and the recipient male,1-7, 9-12, 14, 20 possibly due to weight mismatch or size mismatch (body surface area ratio) in which the heart from a smaller female donor could not meet the cardiac demands of the larger male patient.3, 20 A greater number of episodes of severe acute rejection4 and worse graft survival8, 12, 19 due to CAV have also been found with female donor-male recipient mismatch. In addition, higher mortality2, 3, 9, 11 and more rejection episodes10,13 have also been reported with the opposite mismatch: when the donor was male and the recipient female. Registry data from the International Society for Heart and Lung Transplantation (ISHLT) show that gender mismatch is a risk factor for both short-term and long-term mortality in HT recipients.2, 22
Table 1. Summary of previous research findings on significant outcomes in gender-mismatched heart transplant recipients.
Objectives
Because of the limited types of outcomes examined in previous HT studies, more research is needed to investigate the impact of gender mismatch on additional outcomes in HT patients. Therefore, the objectives of this study were to: (1) determine if gender-mismatched HT recipients experienced worse clinical outcomes of decreased survival, more post-transplant complications, and a greater number of days hospitalized (either during or after the surgical admission) during the first post-operative year as compared to gender-matched recipients; and (2) identify risk factors for decreased survival (such as rejection, infection, and co-morbidities). We assessed outcomes during the first year after surgery because HT mortality during this period is higher than the next 4 years combined, according to the ISHLT registry report.2
Methods
Patients
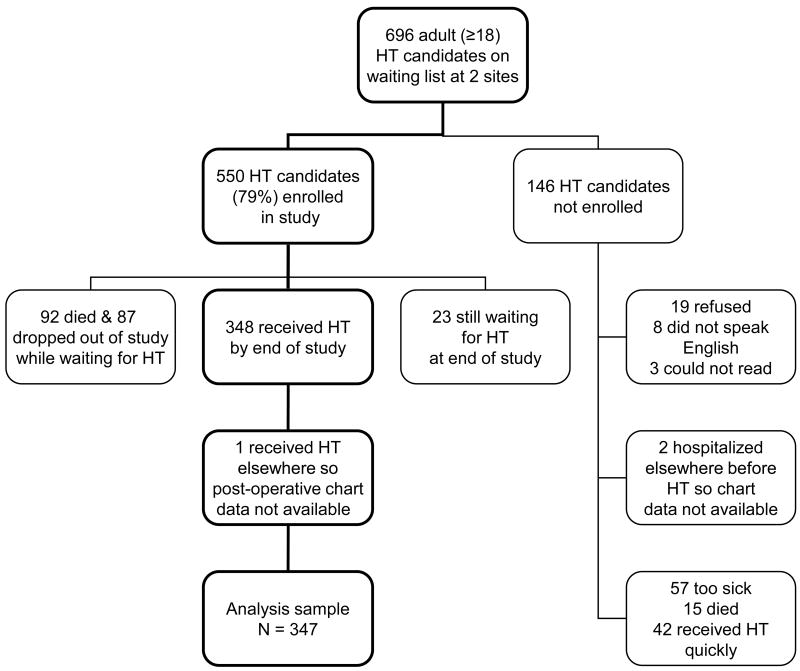
The patients for this report were 347 adult HT recipients (70 women, 20.2%, and 277 men, 79.8%) from 2 medical centers: Loyola University of Chicago and University of Alabama at Birmingham. The percentage of women in this sample is similar to the ISHLT registry data.2 Ages at transplant ranged from 20 to 71 years (mean = 52 ± 10, median = 54). This sample was from a pool of 696 adult (≥18) HT candidates at the 2 sites (Figure 1).
Figure 1.
Flow chart showing how the sample size evolved for analysis of 347 heart transplant (HT) recipients.
Seventy-four of the 347 patients (21.3%) received a heart from the opposite gender, which is similar to the average of 24% gender mismatch in other HT studies reporting on outcomes. Three groups of patients were compared in this study: Group 1: same gender donor and recipient (N = 273, 78.7%: 36 women and 237 men); Group 2: female donor and male recipient mismatch (N = 40, 11.5%); and Group 3: male donor and female recipient mismatch (N = 34, 9.8%).
Procedure
Patients were enrolled in the study while awaiting HT and were followed for up to 5 years after surgery. Both medical data and patient-completed questionnaire data were collected at standardized intervals during the study by nurses experienced in cardiac care, as reported previously by Grady et al23 and Jalowiec et al.24 Pre-operative data were collected every 3 months while patients were on the HT waiting list and first-year post-operative data were collected at 1, 3, 6, 9, and 12 months after transplant. The medical data collection covered the entire time both pre- and post-HT. Patients gave written informed consent to participate in this study. The research was approved by the Institutional Review Board at each site.
Outcomes
For this report, differences in 10 first-year outcomes were examined in the 3 donor-recipient gender groups: (1) mortality/survival: the incidence of death and length of survival; (2) post-transplant complications: the number of treated acute rejections (based on established ISHLT criteria), the number of IV-treated infections (infections treated with an intravenous antibiotic), the incidence of CAV, new-onset steroid-induced diabetes, non-skin cancers, and severe renal dysfunction (which was defined as a serum creatinine >2.5 mg/dl, a diagnosis of renal failure, or being on dialysis, using the definition of the ISHLT registry report2); and (3) hospitalization: hospital length of stay for the HT surgery and the number of days rehospitalized after discharge.
Data
Pre-operative and post-operative clinical data collected included medical and surgical history, intra-operative and post-operative complications, hospitalizations at the transplant site (dates and diagnoses), medications and treatments (immunosuppressant and non-immunosuppressant), laboratory results, and donor data.24 Several methods were used to assess the reliability of the chart review data (of retrieval, recording, coding, computer entry), as described previously by Jalowiec et al.24 The main method was a comparison of the original chart data to a sample of 25% of the study data, systematically chosen so that the data on many of the patients would be verified for some of the pre-operative and post-operative time periods.24
Statistics
Data were analyzed with SPSS. Baseline characteristics of the 3 groups were compared with either: (1) Pearson chi square for 3 × 2 nominal contingency tables with examination of standardized residuals (if >2) to determine which group(s) influenced the statistic; or (2) ANOVA (analysis of variance) for continuous variables with the Tukey post-hoc test to identify which group comparisons were significant (P ≤.05).
Because of the multiple outcomes and multiple risk factors examined, a Bonferroni-adjusted P ≤.005 was used to determine significant group differences in outcomes and mortality risk factors. For objective #1 (outcomes), logistic regression was used to identify significant differences among the 3 groups in the incidence of 5 dichotomous outcomes during the first post-transplant year: deaths, severe renal dysfunction, new-onset steroid-induced diabetes, CAV, and non-skin cancers (using contrasts with Group 1 [no gender mismatch] as the reference group and covariates of patient age at HT and donor age).
For objective #1 (outcomes), MANCOVA (multivariate analysis of covariance) was used to identify significant group differences in 4 continuous outcomes: HT length of stay, number of days rehospitalized after the surgical discharge, number of treated rejections, and number of IV-treated infections (using contrasts with Group 1 as the reference group and covariates of patient HT age and donor age).
For objective #1 (outcomes), Kaplan-Meier time-to-event analysis (with pairwise comparisons and the log rank significance test) was used to calculate and compare survival curves for each donor-recipient gender group during the first post-operative year. For objective #2 (risk factors), Cox regression was used to identify risk factors for decreased survival. Univariate Cox regression was first used to identify potential individual covariates, which were then combined and tested in a multivariate Cox model to determine significant risk factors for decreased survival (using backward stepwise elimination and contrasts with Group 1 as the reference group).
For both Kaplan-Meier and Cox analyses, the dependent time variable for length of survival for censored cases (those who did not die within the first year and those who did not finish the first-year follow-up for reasons other than death) was the number of days of study follow-up during the first post-operative year. For Cox regression, variables that failed the crucial assumption of proportional hazards across time were converted to time-dependent covariates by creating an interaction term with time.
Results
Baseline Characteristics
Significant differences in the baseline characteristics (pre-operative and peri-operative) of the 3 donor-recipient gender groups were: (1) Group 2 (female donor-male recipient) had older donors and more patients with CMV (cytomegalovirus) mismatch (donor positive/patient negative); and (2) Group 3 (male donor-female recipient) was younger, had more minority patients, had fewer patients with pre-HT coronary artery disease (as opposed to non-ischemic heart disease), weighed less pre-HT (based on body mass index), and had lower serum creatinine at HT (Table 2).
Table 2. Baseline characteristics of 347 heart transplant patients by donor-recipient gender group.
| Characteristica | Group 1b (N = 273) |
Group 2b (N = 40) |
Group 3b (N = 34) |
χ2 or Fc |
P |
|---|---|---|---|---|---|
| Age at HT (years) | 52.7 ± 9.6 | 52.8 ± 11.4 | 47.7 ± 12.0 | 3.81 | .023 |
| Minority race | 33 (12.1) | 5 (12.5) | 13 (38.2) | 16.66 | .000 |
| Urgent priority for HT | 132 (48.4) | 20 (50.0) | 15 (44.1) | 0.28 | .869 |
| Coronary artery disease | 150 (54.9) | 25 (62.5) | 9 (26.5) | 11.47 | .003 |
| Diabetes | 50 (18.3) | 9 (22.5) | 6 (17.6) | 0.43 | .806 |
| VAD or TAH | 25 (9.2) | 4 (10.0) | 2 (5.9) | 0.46 | .794 |
| IABP | 22 (8.1) | 3 (7.5) | 1 (2.9) | 1.14 | .565 |
| Ventilator | 13 (4.8) | 0 | 0 | 3.66 | .160 |
| IV inotropes | 154 (56.4) | 22 (55.0) | 21 (61.8) | 0.41 | .814 |
| Serum creatinine (mg/dl) | 1.4 ± .5 | 1.5 ± .4 | 1.1 ± .3 | 7.36 | .001 |
| PRA >10% | 22 (8.1) | 4 (10.0) | 3 (8.8) | 0.18 | .913 |
| Ischemic time (minutes) | 181 ± 58 | 182 ± 58 | 169 ± 60 | 0.67 | .511 |
| Donor age (years) | 25.9 ± 9.2 | 31.9 ± 12.3 | 23.7 ± 8.8 | 8.46 | .000 |
| Repeat HT | 14 (5.1) | 1 (2.5) | 0 | 2.29 | .319 |
| Patient BMI (kg/m2) | 25.5 ± 4.1 | 24.4 ± 3.9 | 23.9 ± 4.3 | 3.38 | .035 |
| Weight mismatch >20%d | 77 (28.2) | 13 (32.5) | 14 (41.2) | 2.56 | .278 |
| CMV mismatche | 31 (11.4) | 11 (27.5) | 2 (5.9) | 9.79 | .007 |
| Smoking history | 184 (67.4) | 26 (65.0) | 21 (61.8) | 0.48 | .786 |
BMI, body mass index; CMV, cytomegalovirus; HT, heart transplant; IABP, intra-aortic balloon pump; IV, intravenous; PRA, panel reactive antibody; TAH, total artificial heart; VAD, ventricular assist device.
Characteristic is pre-HT unless otherwise noted.
Group 1: same gender donor-recipient; Group 2: female donor-male recipient; Group 3: male donor-female recipient.
Chi square (χ2) was used for categorical variables, which are expressed as N (%); ANOVA (F) was used for continuous variables, which are expressed as mean ± standard deviation.
Weight mismatch >20% is based on the percent difference between patient weight and donor weight in kilograms using the formula of Costanzo-Nordin.25
CMV mismatch: donor positive/patient negative.
Mortality/Survival Outcomes
Fifty-two of the 347 patients (15.0%) died during the first post-HT year; 38.5% of the deaths (20/52) occurred within the first month. Both gender-mismatched groups (2 and 3) had a higher percentage of deaths than Group 1 (no mismatch) but this difference was not significant (adjusted for patient age at HT and donor age) (Table 3). Infection and acute rejection were the leading causes of death during year 1 (Table 4).
Table 3. First-year outcomes in 347 heart transplant patients by donor-recipient gender group.
| Outcome | Group 1a (N = 273) |
Group 2a (N = 40) |
Group 3a (N = 34) |
χ2 or Fb |
Pc |
|---|---|---|---|---|---|
| Deaths | 38 (13.9) | 8 (20.0) | 6 (17.6) | 1.39 | .498 |
| Severe renal dysfunctiond | 93 (34.1) | 19 (47.5) | 6 (17.6) | 8.51 | .014 |
| Steroid-induced diabetes | 26 (9.5) | 4 (10.0) | 3 (8.8) | 0.59 | .744 |
| CAV | 19 (7.0) | 3 (7.5) | 4 (11.8) | 0.07 | .966 |
| Non-skin cancers | 10 (3.7) | 0 | 2 (5.9) | 5.48 | .065 |
| HT length of stay (days)e | 20 ± 18 | 23 ± 14 | 18 ± 9 | 0.73 | .571 |
| Number of days rehospitalizede | 23 ± 25 | 19 ± 19 | 38 ± 38 | 3.76 | .005 |
| Number of treated rejections | 2.9 ± 2.7 | 2.3 ± 2.6 | 4.2 ± 3.7 | 4.11 | .003 |
| Number of IV-treated infections | 1.5 ± 2.3 | 1.6 ± 3.1 | 1.5 ± 1.7 | 0.61 | .652 |
CAV, cardiac allograft vasculopathy; HT, heart transplant; IV, intravenously.
Group 1: same gender donor-recipient; Group 2: female donor-male recipient; Group 3: male donor-female recipient.
Logistic regression (χ2), adjusting for patient age at HT and donor age, was used for dichotomous outcomes, which are expressed as N (%); MANCOVA (F), adjusting for patient age and donor age, was used for continuous outcomes, which are expressed as mean ± standard deviation.
Significance determined at P ≤.005.
Defined as a serum creatinine >2.5 mg/dl, a diagnosis of renal failure, or dialysis, using the definition of the International Society for Heart and Lung Transplantation registry report.2
N = 320: excludes 27 patients who died during the HT admission; Group 1 = 254; Group 2 = 34; Group 3 = 32.
Table 4. Cause of death for 52 of 347 patients (15.0%) who died during the first year after heart transplant surgery by donor-recipient gender group.
| Cause of deatha | Group 1b (N = 273) |
Group 2b (N = 40) |
Group 3b (N = 34) |
Total |
|---|---|---|---|---|
| Cytomegalovirus infection | 7 | 7 | ||
| Bacterial infection | 4 | 2 | 6 | |
| Fungal infection | 4 | 1 | 5 | |
| Protozoal infection | 1 | 1 | ||
| Acute rejection | 3 | 2 | 2 | 7 |
| Cardiac allograft vasculopathy | 2 | 1 | 3 | |
| Right ventricular failure | 2 | 1 | 3 | |
| Respiratory failure | 1 | 1 | 2 | |
| Multi-organ failure | 2 | 1 | 3 | |
| Lymphoma | 3 | 3 | ||
| Cerebral hemorrhage | 2 | 2 | ||
| Stroke | 3 | 3 | ||
| Myocardial infarction | 1 | 1 | ||
| Pulmonary embolism | 1 | 1 | ||
| Pancreatitis | 1 | 1 | ||
| Intra-operative pump failure | 2 | 1 | 3 | |
| Intra-operative hemorrhage | 1 | 1 | ||
|
| ||||
| Number (%) of deaths | 38 (13.9) | 8 (20.0) | 6 (17.6) | 52 |
Source: autopsy report (25) or death certificate (12) or physician note on the chart (15).
Group 1: same gender donor-recipient; Group 2: female donor-male recipient; Group 3: male donor-female recipient.
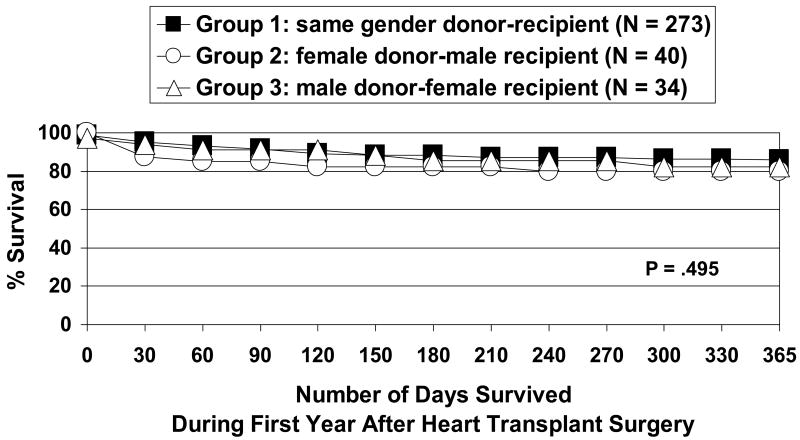
For the overall sample (N = 347), the length of first-year survival ranged from 0 days (4 intra-operative deaths) to 365 days (mean = 307 ± 114, median = 343). Although Group 2 (female donor-male recipient mismatch) showed worse survival than the other 2 groups both at 30 days and at 1 year, survival was not significantly different among the 3 groups (Figure 2).
Figure 2.
Kaplan-Meier survival curves during the first year after heart transplant surgery by donor-recipient gender group (N = 347). Log rank test not significant: 30-day survival: Group 1 = 95.2%, Group 2 = 87.5%, Group 3 = 94.1%; 1-year survival: Group 1 = 85.8%, Group 2 = 79.7%, Group 3 = 82.2%. Mean number of days survived during the first post-operative year: Group 1 = 326, Group 2 = 302, Group 3 = 320.
Mortality Risk Factors
Significant risk factors for decreased year 1 survival were: higher year 1 cholesterol (Group 3: male donor-female recipient), earlier IV-treated infection (Group 2: female donor-male recipient), severe renal dysfunction (Group 2: female donor-male recipient), earlier treated rejection (Group 3: male donor-female recipient), and diabetes (both pre-existing and new-onset steroid-induced diabetes, Group 2: female donor-male recipient) (Table 5).
Table 5. Risk factors for decreased first-year survival in 347 heart transplant patients by donor-recipient gender group.
| Risk factora | Group 1b (N = 273) |
Group 2b (N = 40) |
Group 3b (N = 34) |
RRc (95% CI) |
Wald | Pd |
|---|---|---|---|---|---|---|
| Higher year 1 cholesterol (mg/dl) | 220 ± 46 | 210 ± 54 | 226 ± 56 | 35.72 | .000 | |
| Earlier IV-treated infection (days) | 174 ± 158 | 126 ± 148 | 178 ± 152 | 15.39 | .000 | |
| Severe renal dysfunctione | 93 (34.1) | 19 (47.5) | 6 (17.6) | 1.01 (1.00-1.02) | 10.59 | .001 |
| Earlier treated rejection (days) | 83 ± 119 | 85 ± 121 | 64 ± 115 | 7.86 | .005 | |
| Diabetes (pre-existing & new-onset) | 76 (27.8) | 13 (32.5) | 9 (26.5) | 3.20 (1.41-7.27) | 7.73 | .005 |
CI, confidence interval; IV, intravenously; RR, relative risk.
From multivariate Cox regression. Categorical variables are expressed as N (%); continuous variables are expressed as mean ± standard deviation. Nonsignificant variables: pre-transplant cardiac diagnosis, total artificial heart or ventricular assist device before surgery, ventricular assist device after surgery, repeat heart transplant, patient age, patient gender, patient race, donor age, weight mismatch >20%, cytomegalovirus mismatch (donor positive/patient negative), cardiac allograft vasculopathy, respiratory failure, liver failure, stroke, non-skin cancers.
Group 1: same gender donor-recipient; Group 2: female donor-male recipient; Group 3: male donor-female recipient.
Relative risk applies to dichotomous risk factors.
Significance determined at P ≤.005.
Defined as a serum creatinine >2.5 mg/dl, a diagnosis of renal failure, or dialysis, using the definition of the International Society for Heart and Lung Transplantation registry report.2
Post-Transplant Complications Outcomes
The number of treated acute rejections during year 1 (N = 347) ranged from 0 (19.6% of the patients) to 15 (mean = 3.0 ± 2.8). This was significantly different among the groups (adjusted for patient HT age and donor age): Group 3 (male donor-female recipient mismatch) had more treated rejections than the other 2 groups (Table 3). The number of IV-treated infections during the first year (N = 347) ranged from 0 (44.1% of the patients) to 14 (mean = 1.5 ± 2.4), which was not significantly different among the groups (adjusted for patient HT age and donor age) (Table 3). Also, there were no significant group differences (N = 347) in the first-year incidence of severe renal dysfunction (not significant at the Bonferroni-adjusted P ≤.005 but P = .014; Group 2 mismatch had more cases), new-onset steroid-induced diabetes, CAV, or non-skin cancers (adjusted for patient HT age and donor age) (Table 3).
Hospitalization Outcomes
For those who were discharged after transplant (N = 320, 92.2%; 27 patients died during the surgical admission), the hospital length of stay for HT surgery ranged from 5 days to 169 days (mean = 20 ± 17, median = 17), and 13.1% (42/320) were hospitalized for more than a month after surgery. Length of HT hospital stay was not significantly different among the 3 groups (adjusted for patient HT age and donor age) (Table 3).
After discharge from the HT surgery, 88.4% of the patients (283/320) were readmitted during the first post-operative year, and of those rehospitalized, 39.6% (112/283) were readmitted within the first month. The most common reasons for first-year readmissions were acute rejection, infection, cardiac complications, and gastro-intestinal or hepatic problems.
The number of days rehospitalized after the HT discharge during the first year ranged from 0 days (11.6% of the patients) to 141 days (mean = 24 ± 26, median = 15), which was significantly different among the groups (adjusted for patient HT age and donor age). Group 3 (male donor-female recipient mismatch) spent more days rehospitalized after the surgical discharge during year 1 than the other 2 groups (Table 3).
Discussion
Outcomes
Two outcomes were significantly worse for gender-mismatched HT patients: Group 3 (male donor-female recipient) had more treated acute rejections and was rehospitalized more days during the first post-operative year than the other 2 groups (using a Bonferroni-adjusted P ≤.005). However, there were no significant group differences in 8 other first-year outcomes: number of deaths or length of survival, hospital length of stay for the HT surgery, number of IV-treated infections, or the incidence of severe renal dysfunction, new-onset steroid-induced diabetes, CAV, or non-skin cancers.
Because only 2 of the 10 outcomes were significant in this sample of HT patients, it would seem that gender mismatch of heart donor and recipient need not be a relevant issue when selecting donors, although the significant differences were in important outcomes (treated rejections and time rehospitalized). However, outcomes beyond the first year were not examined in this report so the impact of gender mismatch on later outcomes in this sample is not known.
Contrary to our nonsignificant result on survival in gender-mismatched patients, most of the studies reported that gender mismatch had a detrimental impact on HT survival. However, similar to our findings, 4 other research groups also found no significant difference in HT survival due to gender mismatch.15-17, 21 Several possible reasons could explain our lack of a significant difference in the length of HT survival due to gender mismatch: (1) the important mortality risk of weight mismatch >20% was not a significant risk factor for decreased survival in the Cox regression model; (2) this report included only the first post-transplant year; (3) there were not enough gender-mismatched patients to detect a significant difference in survival; or (4) no difference in survival due to gender mismatch existed in this sample. In addition, our nonsignificant results on some of the other outcomes may have been due to only a small number of cases with that complication within the first year (such as CAV and cancer).
However, our study did demonstrate that gender-mismatched patients in Group 3 (male donor-female recipient) were rehospitalized a significantly greater number of days after the surgical discharge during the remainder of year 1, so these patients may need closer monitoring for complications. None of the studies on gender-mismatched HT outcomes reported the number of days rehospitalized after HT discharge so this finding provides new information.
In this study, Group 3 (male donor-female recipient mismatch) also had significantly more treated acute rejections during the first post-transplant year, which may partially explain why they were rehospitalized for more days. In addition, Group 3 experienced their first treated rejection earlier after the transplant. Keogh et al13 and Prendergast et al10 also found more rejections in gender-mismatched HT patients with a male donor and a female recipient.
Several reasons have been postulated to explain the higher rate of rejection in female recipients with a male donor: different hormonal profiles, gender differences in antigens, pre-sensitization in previously pregnant women, more auto-immune diseases in women, female HT recipients being younger than male recipients (as in this study) and increased immune responsiveness with younger age.3, 13, 26-30 Therefore, female HT patients with a male donor may require higher doses of steroids to offset this propensity for more rejections.13
ISHLT registry data demonstrate that gender mismatch impacts HT survival, especially after the first year. These data showed that, although gender mismatch was a significant risk factor for first-year mortality only in patients older than 60 years (male donor-female recipient mismatch), it was significant for all ages at 5 years (female donor-male recipient mismatch), 10 years (both types of mismatch), and 15 years (both types of mismatch).2, 22 Other studies also reported decreased patient survival,1, 3-7, 9-12, 14, 20 more acute rejection episodes,4, 10, 13 and decreased graft survival8, 12, 19 due to CAV in gender-mismatched HT recipients. Therefore, even though gender matching does not occur in HT organ allocation, tailoring post-operative care based on the documented impact of gender mismatch on various HT outcomes may be reasonable.
Mortality Risk Factors
Because of the wide range in first-year survival times (0 days to 365 days), risk factors for decreased survival were examined. Significant dichotomous risk factors were severe renal dysfunction (most likely due to calcineurin-inhibitor immunosuppressants31-33) and diabetes (pre-existing and steroid-induced); both risk factors were greater in Group 2 (female donor-male recipient mismatch). ISHLT registry data2 and other research16, 34, 35 have identified pre-existing diabetes as a risk factor for both short-term and long-term mortality after HT. In addition, HT recipients are at even greater risk for developing new-onset diabetes and its complications because of post-transplant steroid therapy.36-38
Significant continuous risk factors for decreased survival were: higher year 1 cholesterol (Group 3: male donor-female recipient), earlier IV-treated infection (Group 2: female donor-male recipient), and earlier treated rejection (Group 3: male donor-female recipient). Higher post-transplant cholesterol conveyed the greatest risk for decreased first-year survival in this HT sample (based on the Wald statistic from Cox regression) and also has been identified as a risk factor for death in other HT studies.16, 39 In addition, hyperlipidemia is a well documented risk factor for developing post-transplant CAV, which can eventually lead to graft failure and death.40-44 In this sample, Group 3 (male donor-female recipient mismatch) who had significantly higher cholesterol also had the highest percentage of CAV, although this was not significant, probably due to the small number of cases in year 1 because CAV often takes longer to develop. Therefore, HT recipients with any of these 5 mortality risk factors (renal dysfunction, diabetes, elevated cholesterol, early infection, early rejection) will need aggressive treatment for these conditions to reduce the incidence of early death.
Limitations
Limitations of this research include: (1) only short-term outcomes were examined; (2) gender-mismatched patients accounted for only 21.3% of the sample, which may have decreased the power to detect significant differences in some of the outcomes; and (3) 2 of the 10 post-transplant outcomes (CAV and cancer) have a higher incidence later after HT and thus may not have been fully captured by this first-year analysis. Therefore, this study needs to be replicated with a larger number of gender-mismatched HT recipients, and post-operative data needs to be examined for longer than 1 year.
Conclusion
Gender-mismatched HT patients with a male donor and a female recipient had more complications due to rejection and higher resource utilization due to more rehospitalization during the first post-operative year as compared to gender-matched HT patients. Therefore, these problem areas may provide targets for possible interventions. However overall, our sample of gender-mismatched HT recipients fared as well as gender-matched patients in the vast majority of outcomes examined during the first year after HT surgery.
What's New?
Gender-mismatched HT patients with a male donor and a female recipient had more treated acute rejections and were rehospitalized a greater number of days after the surgical discharge during the first post-operative year, so these patients may need more intensive immunosuppression and closer monitoring for complications.
There were no significant differences in: deaths, survival time, hospital length of stay for HT surgery, IV-treated infections, severe renal dysfunction, new-onset steroid-induced diabetes, cardiac allograft vasculopathy, or non-skin cancers, so gender-mismatched HT recipients fared as well as gender-matched patients in 8 of the 10 outcomes examined during the first postoperative year.
HT recipients with higher cholesterol, severe renal dysfunction, diabetes, earlier treated rejection, or earlier IV-treated infection were at greater risk for decreased first-year survival, so these patients will need to be treated aggressively for these conditions to reduce the incidence of early death.
Acknowledgments
Funding: National Institutes of Health (NINR: #NR01693, #NR01693/S, #5R01-NR01693; NHLBI: #HL49336), Sandoz Pharmaceuticals Corporation, Earl Bane Estate, American Association of Critical-Care Nurses, Sigma Theta Tau, Loyola University Research Committee, Loyola University School of Nursing, Loyola University Medical Center. Study PI: Dr Anne Jalowiec.
References
- 1.Stehlik J, Feldman DS, Brown RN, et al. Interactions among donor characteristics influence post-transplant survival: a multi-institutional analysis. J Heart Lung Transplant. 2010;29:291–298. doi: 10.1016/j.healun.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DO, Stehlik J, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-sixth official adult heart transplant report – 2009. J Heart Lung Transplant. 2009;28:1007–1022. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Weiss ES, Allen JG, Patel ND, et al. The impact of donor-recipient sex matching on survival after orthotopic heart transplantation of 18,000 transplants in the modern era. Circ Heart Fail. 2009;2:401–408. doi: 10.1161/CIRCHEARTFAILURE.108.844183. [DOI] [PubMed] [Google Scholar]
- 4.Welp H, Spieker T, Erren M, et al. Sex mismatch in heart transplantation is associated with increased number of severe rejection episodes and shorter long-term survival. Transplant Proc. 2009;41:2579–2584. doi: 10.1016/j.transproceed.2009.06.098. [DOI] [PubMed] [Google Scholar]
- 5.Izquierdo MT, Almenar L, Martinez-Dolz L, et al. Analysis of the impact of donor gender on early mortality. Transplant Proc. 2007;39:2375–2376. doi: 10.1016/j.transproceed.2007.07.059. [DOI] [PubMed] [Google Scholar]
- 6.Al-Khaldi A, Oyer PE, Robbins RC. Outcome analysis of donor gender in heart transplantation. J Heart Lung Transplant. 2006;25:461–468. doi: 10.1016/j.healun.2005.11.456. [DOI] [PubMed] [Google Scholar]
- 7.Smits JM, Vanhaecke J, Haverich A, et al. Three-year survival rates for all consecutive heart-only and lung-only transplants performed in Eurotransplant, 1997-1999. Clin Transpl. 2003:89–100. [PubMed] [Google Scholar]
- 8.Zeier M, Dohler B, Opetz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002;13:2570–2576. doi: 10.1097/01.asn.0000030078.74889.69. [DOI] [PubMed] [Google Scholar]
- 9.John R, Rajasinghe H, Chen JM, et al. Impact of current management practices on early and late death in more than 500 consecutive cardiac transplant recipients. Ann Surg. 2000;232:302–311. doi: 10.1097/00000658-200009000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prendergast TW, Furukawa S, Beyer AJ, 3rd, et al. The role of gender in heart transplantation. Ann Thorac Surg. 1998;65:88–94. doi: 10.1016/s0003-4975(97)01105-3. [DOI] [PubMed] [Google Scholar]
- 11.Kirsch M, Baufreton C, Naftel DC, Benvenuti C, Loisance DY. Pretransplantation risk factors for death after heart transplantation: the Henri Mondor experience. J Heart Lung Transplant. 1998;17:268–277. [PubMed] [Google Scholar]
- 12.Sharples LD, Caine N, Mullins P, et al. Risk factor analysis for the major hazards following heart transplantation – rejection, infection, and coronary occlusive disease. Transplantation. 1991;52:244–252. doi: 10.1097/00007890-199108000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Keogh AM, Valantine HA, Hunt SA, Schroeder JS, Oyer PE. Increased rejection in gender-mismatched grafts: amelioration by triple therapy. J Heart Lung Transplant. 1991;10:106–110. [PubMed] [Google Scholar]
- 14.Schlechta B, Kocher AA, Ofner P, et al. Impact of gender mismatch on the outcome of heart transplantation. Transplant Proc. 1999;31:3340–3342. doi: 10.1016/s0041-1345(99)00818-0. [DOI] [PubMed] [Google Scholar]
- 15.Tsao CI, Chen RJ, Chou NK, et al. The influence of gender on survival after heart transplantation. Transplant Proc. 2008;40:2634–2635. doi: 10.1016/j.transproceed.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Radovancevic B, Konuralp C, Vrtovec B, et al. Factors predicting 10-year survival after heart transplantation. J Heart Lung Transplant. 2005;24:156–159. doi: 10.1016/j.healun.2003.11.399. [DOI] [PubMed] [Google Scholar]
- 17.De Santo LS, Marra C, De Feo M, et al. The impact of gender on heart transplantation outcomes: a single center experience. Ital Heart J. 2002;3:419–423. [PubMed] [Google Scholar]
- 18.Yamani MH, Erinc K, McNeill A, et al. The impact of donor gender on cardiac peri-transplantation ischemia injury. J Heart Lung Transplant. 2005;24:1741–1744. doi: 10.1016/j.healun.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Erinc K, Yamani MH, Starling RC, et al. The influence of donor gender on allograft vasculopathy: evidence from intravascular ultrasound. Transplant Proc. 2004;36:3129–3131. doi: 10.1016/j.transproceed.2004.10.072. [DOI] [PubMed] [Google Scholar]
- 20.Young JB, Naftel DC, Bourge RC, et al. Matching the heart donor and heart transplant recipient. Clues for successful expansion of the donor pool: a multivariable, multiinstitutional report. The Cardiac Transplant Research Database Group. J Heart Lung Transplant. 1994;13:353–364. [PubMed] [Google Scholar]
- 21.Fabbri A, Bryan AJ, Sharples LD, et al. Influence of recipient and donor gender on outcome after heart transplantation. J Heart Lung Transplant. 1992;11:701–707. [PubMed] [Google Scholar]
- 22.International Society for Heart and Lung Transplantation registry data: 2009. online slides: www.ishlt.org/registries/
- 23.Grady KL, Jalowiec A, White-Williams C. Predictors of quality of life in patients at one year after heart transplantation. J Heart Lung Transplant. 1999;18:202–210. doi: 10.1016/s1053-2498(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 24.Jalowiec A, Grady KL, White-Williams C. Predictors of rehospitalization time during the first year after heart transplant. Heart Lung. 2008;37:344–355. doi: 10.1016/j.hrtlng.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costanzo-Nordin MR, Liao YL, Grusk BB, et al. Oversizing of donor hearts: beneficial or detrimental? J Heart Lung Transplant. 1991;10:717–730. [PubMed] [Google Scholar]
- 26.Csete M. Gender issues in transplantation. Anesth Analg. 2008;107:232–238. doi: 10.1213/ane.0b013e318163feaf. [DOI] [PubMed] [Google Scholar]
- 27.Johnson MR, Naftel DC, Hobbs RE, et al. The incremental risk of female sex in heart transplantation: a multiinstitutional study of peripartum cardiomyopathy and pregnancy. Cardiac Transplant Research Database Group. J Heart Lung Transplant. 1997;16:801–812. [PubMed] [Google Scholar]
- 28.George JF, Pamboukian SV, Tallaj JA, et al. Balancing rejection and infection with respect to age, race, and gender: clues acquired from 17 years of cardiac transplantation data. J Heart Lung Transplant. 2010;29:966–972. doi: 10.1016/j.healun.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Kubo SH, Naftel DC, Mills RM, Jr, et al. Risk factors for late recurrent rejection after heart transplantation: a multiinstitutional, multivariable analysis. Cardiac Transplant Research Database Group. J Heart Lung Transplant. 1995;14:409–418. [PubMed] [Google Scholar]
- 30.Bourge RC, Naftel DC, Costanzo-Nordin MR, et al. Pretransplantation risk factors for death after heart transplantation: a multiinstitutional study. J Heart Lung Transplant. 1993;12:549–562. [PubMed] [Google Scholar]
- 31.Garrido IP, Crespo-Leiro MG, Paniagua MJ, et al. Independent predictors of renal dysfunction after heart transplantation in patients with normal pretransplant renal function. J Heart Lung Transplant. 2005;24:1226–1230. doi: 10.1016/j.healun.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Al Aly Z, Abbas S, Moore E, et al. The natural history of renal function following orthotopic heart transplant. Clin Transplant. 2005;19:683–689. doi: 10.1111/j.1399-0012.2005.00408.x. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez V, Delgado JF, Morales JM, et al. Chronic cyclosporine-induced nephrotoxicity in heart transplant patients: long-term benefits of treatment with mycophenolate mofetil and low-dose cyclosporine. Transplant Proc. 2004;36:2823–2825. doi: 10.1016/j.transproceed.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 34.Weiss ES, Nwakanma LU, Patel ND, Yuh DD. Outcomes in patients older than 60 years of age undergoing orthotopic heart transplantation: an analysis of the UNOS database. J Heart Lung Transplant. 2008;27:184–191. doi: 10.1016/j.healun.2007.11.566. [DOI] [PubMed] [Google Scholar]
- 35.Meyer SR, Modry DL, Norris CM, et al. Pretransplant diabetes, not donor age, predicts long-term outcomes in cardiac transplantation. J Card Surg. 2006;21:117–124. doi: 10.1111/j.1540-8191.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- 36.Ye X, Kuo HT, Sampaio MS, et al. Risk factors for development of new-onset diabetes mellitus in adult heart transplant recipients. Transplantation. 2010;89:1526–1532. doi: 10.1097/TP.0b013e3181dd6bd9. [DOI] [PubMed] [Google Scholar]
- 37.Bodziak KA, Hricik DE. New-onset diabetes mellitus after solid organ transplantation. Transpl Int. 2009;22:519–530. doi: 10.1111/j.1432-2277.2008.00800.x. [DOI] [PubMed] [Google Scholar]
- 38.Mogolian Jimenez MV, Sobrino Marquez JM, Arizon Munoz JM, et al. Incidence and importance of de novo diabetes mellitus after heart transplantation. Transplant Proc. 2008;40:3053–3055. doi: 10.1016/j.transproceed.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 39.Arora S, Aukrust P, Andreassen A, et al. The prognostic importance of modifiable risk factors after heart transplantation. Am Heart J. 2009;158:431–436. doi: 10.1016/j.ahj.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez Lazaro IJ, Almenar Bonet L, Moro Lopez J, et al. Influence of traditional cardiovascular risk factors in the recipient on the development of cardiac allograft vasculopathy after heart transplantation. Transplant Proc. 2008;40:3056–3057. doi: 10.1016/j.transproceed.2008.08.115. [DOI] [PubMed] [Google Scholar]
- 41.Wang SS. Treatment and prophylaxis of cardiac allograft vasculopathy. Transplant Proc. 2008;40:2609–2610. doi: 10.1016/j.transproceed.2008.08.073. [DOI] [PubMed] [Google Scholar]
- 42.Valantine H. Cardiac allograft vasculopathy after heart transplantation: risk factors and management. J Heart Lung Transplant. 2004;23(Suppl 5):S187–193. doi: 10.1016/j.healun.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Parameshwar J, Foote J, Sharples L, Wallwork J, Large S, Schofield P. Lipids, lipoprotein (a) and coronary artery disease in patients following cardiac transplantation. Transpl Int. 1996;9:481–485. doi: 10.1007/BF00336826. [DOI] [PubMed] [Google Scholar]
- 44.Eich D, Thompson JA, Ko DJ, et al. Hypercholesterolemia in long-term survivors of heart transplantation: an early marker of accelerated coronary artery disease. J Heart Lung Transplant. 1991;10:45–49. [PubMed] [Google Scholar]