Abstract
Selective electrochemically activated biofunctionalization of In2O3 nanowires (NWs) has been achieved, using monolayer coatings of para-dimethoxybenzene derivatives. Monolayer coatings of 4-(2,5-dimethoxyphenyl)butyl-phosphonic acid (DMP-PA) were deposited on planar indium-tin oxide (ITO) electrodes and In2O3 NWs. The electrochemical behavior of the monolayer coating was first studied using ITO electrodes, as a model system for In2O3 nanowires. When a potential of 950 mV vs. a Ag/AgCl reference electrode is applied to an ITO electrode coated with DMP-PA in PBS buffer, the para-dimethoxyphenyl groups are converted to para-benzoquinone (BQ). The electrochemically formed benzoquinone groups react readily with alkyl thiol groups via a Michael addition. The reaction strategy optimized on ITO was applied to an In2O3 NW mat sample coated with DMP-PA. Applying a potential of 950 mV to metal electrodes deposited on NWs converts the DMP-PA NW coating to BQ-PA, which reacts with a thiol-terminated 20-base oligonucleotide. These NWs showed strong fluorescence response after paring with the dye labeled compliment, demonstrating that the probe was bound to the NW surface and that it remained active toward hybridization with its compliment. The unactivated DMP-PA coated NWs showed no response, demonstrating the selective electrochemical functionalization of NWs and the potential of using them in multiplex sensing. We also compared the para-dimethoxybenzene derivative to the conventional hydroquinone analog. The results show that the former can largely enhance the selectivity during the functionalization of both ITO and In2O3 NWs.
Keywords: selective biofunctionalization, electrochemical activation, Indium oxide nanowire, indium-tin oxide, para-dimethoxybenzene, benzoquinone
Introduction
There is growing interest in detecting biological materials in a multiplexed fashion, which are demonstrated to be essential to the diagnosis and treatment of a range of diseases and the identification of infectious agents.1–4 Among various techniques for multiplexed sensing, nanoscale devices utilizing nanowires (NWs) as active channels are of particular interest due to the high sensitivity and biocompatibility.5 In the past few years, these devices have been employed in the detection of not only individual biomolecules but also a combination of multiple targets.6–8 To construct sensor arrays for multiplexed biosensing, the NWs must be selectively functionalized with different capturing probes against their designated analytes.
Efforts have been made to achieve the selective functionalization of NW-based devices, by using microfluidic chips, microspotting techniques7 and electroactive monolayers.9, 10 Among these approaches, the use of electroactive monolayers possesses a number of unique advantages including low cost and simplicity of instrumentation. In this method, the key step is to design a bifunctional molecule, which bears a NW-anchoring group on one end and an electroactive moiety on the other. A monolayer of this molecule is assembled on NWs to create a controllable interface between devices and capture probes. After being covalently linked to the NWs, the molecule can be activated from the chemically inert “OFF” state to its “ON” state by applying an external voltage to device electrodes. In the “ON” state the electroactive moiety reacts with the desired capture probe to covalently anchor it to the NW surface. This method holds great potential in fabricating high-density sensor arrays for multiplexed biosensing because it is only limited by the ability to electronically address the individual sensors.10 By controlling the potential applied to each sensor, selected devices within an array can be functionalized with a desired biomolecule, without contaminating the other devices within the array.
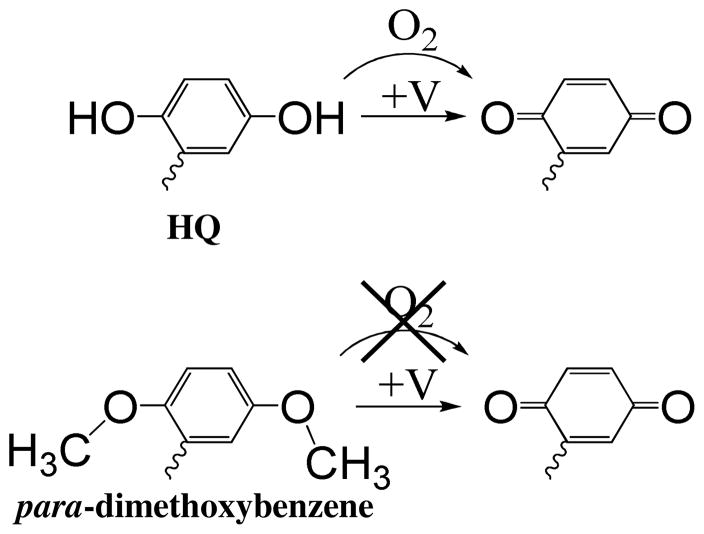
Derivatives of 1,4-hydroquinone (HQ) have been employed in electrochemically activated surface functionalization of gold, by Mrksich and co-workers.11 Since that initial report, this family of molecules has been used in selective functionalization of various surfaces including In2O3 NWs and Si NWs. 9–13 The oxidized form, 1,4-benzoquinone (BQ, the “ON” state) readily reacts with a variety of functional groups such as thiols, primary amines and azides, while HQ (the “OFF” state) is inactive towards these functional groups. Thus, one can choose a particular device in an array for surface coating by electrochemically activating it for probe binding, while leaving the other devices in the array inert. The conversion from HQ derivatives to BQ takes place at relatively low potentials (ca. 200 mV vs. Ag/AgCl) and can be done in physiological solutions such as phosphate buffered saline (PBS). Another advantage of HQ derivatives is their compatibility with biomolecules. However, a major drawback of these molecules is that the HQ moiety can be gradually oxidized to BQ under aerobic conditions (Scheme 1).14 As a result, during the functionalization of a large number of devices, HQ will be unintentionally converted to BQ (the “ON” state) over time without applying any external voltage, eliminating the selectivity of this method. Therefore, in order to fabricate high-density sensor arrays for multiplexed biosensing, it is desirable to design an electrically activated bioconjugate group that possess the advantages of HQ derivatives, and are stable under aerobic conditions.
Scheme 1.
Here we report that para-dimethoxybenzene derivatives can serve as reliable, air-stable, electroactive surface modifiers in selective functionalization of nano-structured surfaces. In2O3 NWs were chosen in this work due to its simple and well studied surface derivatization methods. Also, In2O3 NWs are recognized as good candidates in biosensing,15, 16 because the surface of In2O3 NWs does not possess an insulating, native oxide layer (e.g., SiO2 on Si nanowires) that may decrease the nanowire sensitivity.17 A para-dimethoxybenzene derivative, 4-(2,5-dimethoxyphenyl)butyl-phosphonic acid (DMP-PA), was synthesized and preliminarily studies were carried out on indium-tin oxide (ITO)-coated glass in order to understand the electrochemical behavior of this molecule. Next, a monolayer of this molecule was formed on an In2O3 NW mat and a thiol-terminated DNA oligonucleotide was selectively coupled to the region where the para-dimethoxybenzene moiety was oxidized. We also compared DMP-PA to its HQ analog, the conventional “OFF” state used in previous reports. The results show that the former can largely enhance the selectivity during the functionalization of both ITO and In2O3 NWs.
Experimental section
Materials and instruments
4-(2,5-dimethoxyphenyl)butyl-phosphonic acid (DMP-PA) was synthesized according to a reported procedure.9 Phosphate Buffered Saline (PBS), dodecanethiol and 3-mercaptopropanol was purchased from Sigma-Aldrich. Tris (2-carboxyethyl) phosphine hydrochloride (TCEP) was purchased from Strem Chemicals. Both the probe and the target DNA oligonucleotide were purchased from Integrated DNA Technologies, Inc. The electrochemistry was performed with Potentiostat/Galvanostat Model 263A (Princeton Applied Research). The fluorescence images were captured using a Nikon Eclipse LV-100D-U fluorescence microscope.
In2O3 nanowire growth and electrode deposition
In2O3 nanowires were synthesized via laser ablation on Si/SiO2 substrates.18 Metal electrodes were fabricated on as-grown nanowire samples by photolithography, followed by Ti (5 nm)/Au (40 nm)/Ni (5 nm) deposition. After lift-off, the chip was carefully cleaned before further modification.
Formation of monolayer coatings on ITO-coated glass slides and In2O3 nanowire mats
ITO slides were boiled for 5 minutes each in trichloroethylene, acetone, and finally, ethanol. The slides were then placed in an ozone/UV chamber for 10 minutes. The NW mats were cleaned by the same procedure as ITO, but placed in the ozone/UV chamber for 2 minutes. The pre-cleaned ITO slides or NW mats were soaked into a solution of DMP-PA in ethanol (1mM) for 16 hours, and then washed extensively with ethanol and dried under nitrogen. The ITO slides or NW mats were then annealed at 120°C in nitrogen atmosphere for 12 hours. For NW mats, a further step was taken to passivate the gold electrodes. The sample was soaked in a 1mM solution of dodecanethiol in hexane overnight.
Electrochemistry
Electrochemistry of the DMP-PA monolayer on both ITO and In2O3 NWs were performed in an electrochemical cell filled with PBS buffer (pH 7.4), with Pt wire as the counter electrode and Ag/AgCl as the reference electrode. All electrochemical procedures were carried out in the air. The determination of the BQ-PA monolayer coverage was carried out by chronocoulometry.
Preliminary experiments on ITO slides
For the thiol addition experiment, the DMP-PA monolayer on ITO was first converted to BQ-PA. Then the sample was continuously scanned by CV in a solution of 3-mercaptopropanol (60 μM) in PBS. For the stability test experiment, a DMP-PA-ITO sample was incubated in 3-mercaptopropanol solution for 30 minutes and then washed extensively by PBS buffer. The sample was then oxidized to BQ-PA before CV measurements. Another DMP-PA-ITO sample was first converted to HQ-PA and then treated with 3-mercaptopropanol solution in the same manner.
Selective functionalization of In2O3 NWs
The probe DNA oligonucleotide used for NW mats functionalization was a 20-base DNA with the sequence of [5′-XGCT TTG AGG TGC GTG TTT GT-3′], where X was a 5′ thiol modifier C6. To reduce the thiol modifier, the probe DNA (7 nmol) was first incubated with TCEP (10.5 nmol) in PBS buffer at room temperature for 2 hours. The excess TCEP was then removed by passing the mixture through a desalting column. The electrochemically treated NW mats were immediately immersed into a solution of the probe DNA (~15 μM in PBS) and stored in a humidity chamber for 2 hours. The devices were then carefully rinsed with PBS to remove any unbound DNA. For the DNA hybridization, the NW mats were exposed to a solution of the target DNA (5′-RhoR-XN/ACA AAC ACG CAC CTC AAA GC-3′) for 20 minutes, followed by extensive washing with PBS. The NW mats were then examined under a fluorescence microscope and the images were captured using NIS-elements software.
Results and discussions
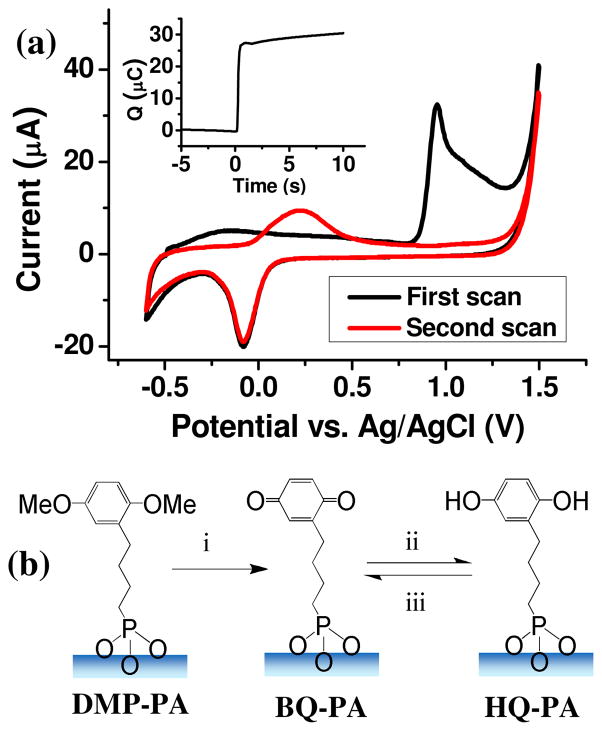
In aqueous solutions the para-dimethoxybenzene moiety can be electrochemically oxidized to produces BQ19 (Scheme 1), which serves as the “ON” state, reacting to form covalent linkages with molecules containing a range of functional groups. In order to incorporate this group onto In2O3 NWs, we synthesized DMP-PA, a para-dimethoxybenzene derivative with phosphonic acid terminus, which covalently binds to metal oxides and metal oxide based NWs.6 The phosphonic acid group is also known to bind to the common transparent conducting oxide indium-tin oxide (ITO), which has a surface composition very similar to that of In2O3 NWs.20 Therefore, preliminary studies were carried out on ITO-coated glass slides in order to understand the electrochemical behavior of the molecule. Freshly-cleaned ITOs were submerged into a 1mM ethanol solution of DMP-PA for 16 hours to form a self-assembled monolayer of DMP-PA. They were then baked at 120°C for 12 hours in a nitrogen atmosphere to promote the binding of the monolayer to the substrate. The resultant monolayer binds to ITO surface predominantly via bidentate/tridentate binding that involves P-O-In bonds.20 In our cyclic voltammetry (CV) studies of the DMP-PA derivatized ITO samples (Fig 1a), we observed that the DMP-PA molecule was irreversibly oxidized to BQ-PA at 950 mV, and the BQ-PA was then reversibly converted to HQ-PA in the following scans, with the oxidation and reduction potentials centered at +220 mV and −80 mV, respectively. The corresponding reaction pathway of DMP-PA is shown in Fig 1b. The mechanism of reaction i in figure 1b is still under investigation. Judging from the CV traces, the peak area at +950 mV is much larger than the one for HQ/BQ conversion, which is known to be a two-electron process. This suggests that the oxidation of para-dimethoxybenzene group doesn’t follow the two-electron mechanism proposed for the oxidation of HQ diesters, a group of molecules with similar structures.21 The surface coverage of BQ-PA/HQ-PA monolayer on ITO was determined by chronocoulometry (Fig 1a, inset). The DMP-PA coating on an ITO with a fixed area (0.64 cm2) was first converted to HQ-PA by applying an oxidizing potential of 950 mV and subsequent reduction at −80 mV. The HQ-PA monolayer was then held at −300 mV for 5 seconds and raised to 500 mV and held for 10 seconds to ensure the complete oxidation to BQ-PA. A charge of 30 μC was consumed for the oxidation process and the calculated molecular coverage was 69Å2/molecule or 2.4×10−10 mol/cm2, which is approximately half of the coverage of a monolayer directly assembled from HQ-PA molecules.9 The relative low surface coverage of BQ-PA/HQ-PA can be attributed to the low yield of anodic oxidation of para-dimethoxybenzene group.19
Figure 1.
(a) CV characterization of a DMP-PA derivatized ITO sample. Inset: A chronocoulometry trace showing the amount of charge necessary to oxidize a pre-defined area of HQ-PA to BQ-PA. (b) The corresponding reaction pathway of the electrochemical process shown in (a).
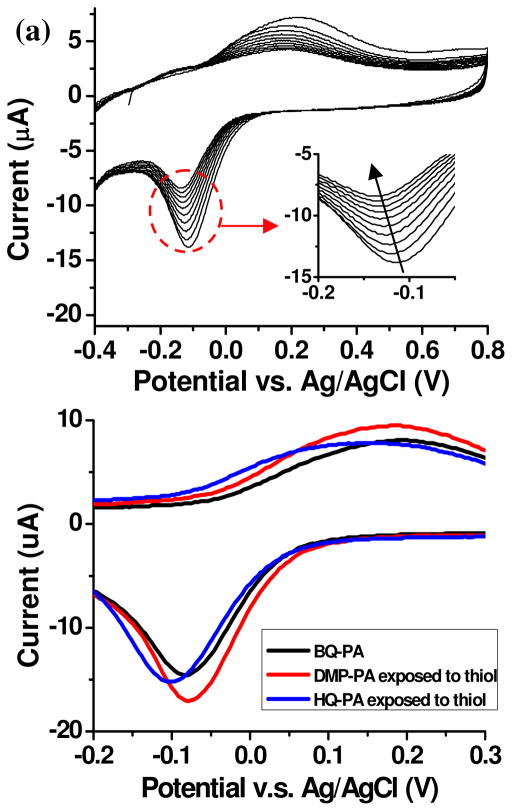
In selective surface functionalization, the “OFF” state should be completely inert toward the functional groups present in biomolecules, which will be used to attach the target biomolecule to the surface. Before we were able to perform selective functionalization using DMP-PA, we first compared it to HQ-PA, the previously used “OFF” state, and studied their stabilities when they are exposed to thiols in air. Thiol is a common group present in antibodies and is readily incorporated into oligonucleotides. Nucleophiles such as thiols and amines react with BQ derivatives by Michael addition, and the addition typically causes a negative shift of the redox potential due to the electron-donating effect.22–24 The effect of thiol addition to BQ-PA monolayer is shown by the CV traces in Figure 2a. A DMP-PA derivatized ITO sample was first electrochemically oxidized, and the resulting BQ-PA monolayer was continuously scanned by CV in presence of 10 μM 3-mercaptopropanol (in PBS, pH 7.4). During 10 scans, the redox potential of the monolayer gradually shifted in the negative direction, which corresponds to the addition of 3-mercaptopropanol to the BQ head group. Based on this result, we can use the negative shift in redox potential as an indicator of thiol addition. In order to study the stability of DMP-PA when exposed to thiols electrochemically, a DMP-PA derivatized ITO sample was submerged to 3-mercaptopropanol solution for 30 minutes and then washed extensively with PBS to remove any unbound thiols. The sample was then subject to an oxidative potential at 950 mV, followed by CV scans (Fig 2b). The CV trace of this sample (red) showed good alignment with the black trace collected from a pure BQ-PA monolayer on ITO. This indicates that DMP-PA is stable under aerobic conditions and is unreactive toward thiols. In parallel, a HQ-PA-ITO sample was also treated with 3-mercaptopropanol in the same manner and examined by CV (blue trace in Fig 2b). It is clear that the redox potential of this monolayer showed a negative shift compared to the pure BQ-PA monolayer. As previously mentioned, the HQ group is prone to aerobic oxidation to BQ under aerobic conditions. We attribute the potential shift to the fact that some HQ-PA molecules were oxidized to BQ-PA during the thiol-incubation and reacted with the thiol. From this experiment, we can conclude that DMP-PA has better stability compared to HQ-PA and can be used as a reliable “OFF” state in selective functionalization methods.
Figure 2.
(a) The first 10 CV scans of a BQ-PA monolayer in presence of 10 μM 3-mercaptopropanol (in PBS, pH 7.4). The peak current decreases during the scans. Inset: The region of the reduction peak. The arrow indicates the shift of the peak current during the 10 scans. (b) CV characterization of ITO samples with BQ-PA monolayer (black), DMP-PA monolayer after exposed to thiols (red) and HQ-PA monolayer after exposed to thiols (blue). The DMP-PA monolayer was first subject to an oxidative potential at 950 mV before CV measurement.
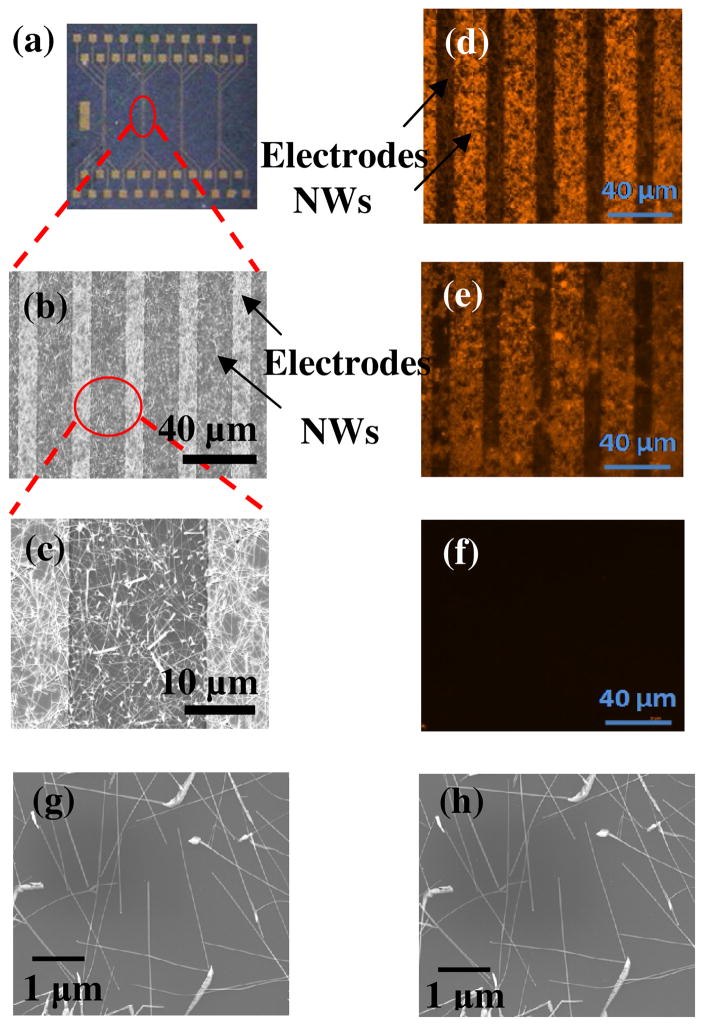
After we demonstrated the good stability of DMP-PA on ITO electrodes, we performed selective functionalization of In2O3 NW mats with thiolated-DNA. Single-crystalline In2O3 NWs (average diameter of 20 nm and length of 5–10 μm) were grown on a Si/SiO2 substrate with a SiO2 layer of 500 nm using a laser ablation technique. The detailed synthesis method and the characterization of these NWs can be found elsewhere.18, 25 Metal electrodes (Ti/Au/Ni, 5 nm/40 nm/5 nm) were patterned on as-grown NW mats by photolithography and metal deposition. Figure 3a shows an optical image of four groups of electrodes deposited on In2O3 NW mats. SEM images of NW mat and electrodes are shown in Figure 3b and 3c. The mat sample was submerged in 1mM solution of DMP-PA overnight and baked at 120°C for 12 hours in a nitrogen atmosphere. The size and length of NWs didn’t show notable changes after DMP-PA coating, according to the SEM images of NWs shown in Figure 3g and 3h. The chip was then soaked in an ethanol solution of dodecane-1-thiol overnight to passivate the metal electrodes. An oxidizing potential of 950 mV was applied to the first group of electrodes, 1, converting the NW surface coating of DMP-PA to BQ-PA. The second group, 2, was subjected to an oxidizing potential of 950 mV, followed by a reducing potential of −80 mV, converting the DMP-PA coating to a HQ-PA coating. The third group, 3, has the original DMP-PA coating. The entire chip was then soaked in a solution of a thiol-terminated probe DNA for 2 hours. The BQ-PA coating on 1 is expected to bind the thiol-DNA efficiently, while 2 and 3 are not expected to bind the DNA. After extensive rinsing with PBS buffer, the chip was treated with a solution of complementary DNA labeled with a fluorescence dye, and again washed with PBS buffer. The three groups of electrodes were examined by fluorescence microscopy and are shown in Figure 3 (d), (e) and (f), showing 1, 2 and 3, respectively. In these figures the gold electrodes appear as dark stripes and any bound fluorescently labeled DNA appears as a bright network. As expected, the NWs in 1 show intense fluorescence, indicating that the BQ-PA surface did bind the thiol-DNA and then the labeled compliment. In contrast, 3, which went through the same DNA treatment, did not show any fluorescence, as seen in Figure 3(f), and thus is inert under these conditions. The NWs in 2, however, show some fluorescence after the DNA treatment, albeit at a brightness lower than that seen for 1, but nonetheless there was binding of the thiol-DNA to the HQ-PA surface. This is in agreement with the results shown in Fig 2(b), indicating that HQ-PA reacts with thiols due to the aerobic oxidation of HQ moiety.
Figure 3.
(a) An optical image of In2O3 NW mats with four groups of electrode deposited on top. (b) A typical SEM image taken in the center region of one group of electrodes. (c) The same image at higher magnification. Figure (d)–(f) are fluorescence images of In2O3 NWs near three groups of electrodes. NWs were first functionalized with DMP-PA. An oxidizing potential of 950 mV was applied to the first group (d), converting the DMP-PA coating to BQ-PA. The second group (e) was subjected to an oxidizing potential of 950 mV, followed by a reducing potential of −80 mV, converting the DMP-PA coating to a HQ-PA. The third group (f) has the original DMP-PA coating. The three groups were treated with thiolated single strand DNA, and then its complementary DNA labeled with a fluorescent dye. After extensive rinsing with PBS buffer, the NWs near the three groups of electrodes were examined under fluorescent microscope. Figure (g) and (h) are SEM images of the same area of a NW mat sample before and after DMP-PA coating, respectively. The size and length of NWs didn’t change after the coating.
Conclusions
In this work we demonstrate that a para-dimethoxybenzene derivative, DMP-PA, can serve as an air-stable, reliable, electroactive surface modifier in selective functionalization of In2O3 NWs. By tailoring the molecular structure, the para-dimethoxybenzene group shows improved stability under aerobic conditions and significantly enhanced selectivity in surface functionalization compared to the conventional HQ moiety. The approach described herein allows for the functionalization of a single In2O3 NW device within an array with a desired capture probe, without contamination of the other devices/sensors in the array. While this work is focused on In2O3 NW based devices, the same approach can be used for selective, electrochemically driven functionalization of devices within an array for nearly any semiconductor NW based device. This can be considered a key step for the future fabrication of high-density biosensor arrays for multiplexed biosensing.
Acknowledgments
We acknowledge financial support from the Whittier Foundation and the National Institute of Health (RO1EB008275).
References
- 1.Ferrari M. Nat Rev Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 2.Kozak KR, Su F, Whitelegge JP, Faull K, Reddy S, Farias-Eisner R. Proteomics. 2005;5:4589–4596. doi: 10.1002/pmic.200500093. [DOI] [PubMed] [Google Scholar]
- 3.Mukundan H, Xie HZ, Price D, Kubicek-Sutherland JZ, Grace WK, Anderson AS, Martinez JS, Hartman N, Swanson BI. Anal Chem. 82:136–144. doi: 10.1021/ac901497g. [DOI] [PubMed] [Google Scholar]
- 4.Wei F, Patel P, Liao W, Chaudhry K, Zhang L, Arellano-Garcia M, Hu S, Elashoff D, Zhou H, Shukla S, Shah F, Ho CM, Wong DT. Clin Cancer Res. 2009;15:4446–4452. doi: 10.1158/1078-0432.CCR-09-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curreli M, Zhang R, Ishikawa FN, Chang HK, Cote RJ, Zhou C, Thompson ME. IEEE Trans Nanotechnol. 2008;7:651–667. [Google Scholar]
- 6.Li C, Curreli M, Lin H, Lei B, Ishikawa FN, Datar R, Cote RJ, Thompson ME, Zhou CW. J Am Chem Soc. 2005;127:12484–12485. doi: 10.1021/ja053761g. [DOI] [PubMed] [Google Scholar]
- 7.Zheng GF, Patolsky F, Cui Y, Wang WU, Lieber CM. Nat Biotechnol. 2005;23:1294–1301. doi: 10.1038/nbt1138. [DOI] [PubMed] [Google Scholar]
- 8.Bunimovich YL, Shin YS, Yeo WS, Amori M, Kwong G, Heath JR. J Am Chem Soc. 2006;128:16323–16331. doi: 10.1021/ja065923u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curreli M, Li C, Sun YH, Lei B, Gundersen MA, Thompson ME, Zhou CW. J Am Chem Soc. 2005;127:6922–6923. doi: 10.1021/ja0503478. [DOI] [PubMed] [Google Scholar]
- 10.Bunimovich YL, Ge GL, Beverly KC, Ries RS, Hood L, Heath JR. Langmuir. 2004;20:10630–10638. doi: 10.1021/la047913h. [DOI] [PubMed] [Google Scholar]
- 11.Yousaf MN, Mrksich M. J Am Chem Soc. 1999;121:4286–4287. [Google Scholar]
- 12.Kim K, Yang H, Jon S, Kim E, Kwak J. J Am Chem Soc. 2004;126:15368–15369. doi: 10.1021/ja0459330. [DOI] [PubMed] [Google Scholar]
- 13.Rohde RD, Agnew HD, Yeo WS, Bailey RC, Heath JR. J Am Chem Soc. 2006;128:9518–9525. doi: 10.1021/ja062012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roginsky V, Barsukova T. J Chem Soc, Perkin Trans. 2000;2:1575–1582. [Google Scholar]
- 15.Ishikawa FN, Curreli M, Chang HK, Chen PC, Zhang R, Cote RJ, Thompson ME, Zhou CW. ACS Nano. 2009;3:3969–3976. doi: 10.1021/nn9011384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa FN, Chang HK, Curreli M, Liao HI, Olson CA, Chen PC, Zhang R, Roberts RW, Sun R, Cote RJ, Thompson ME, Zhou CW. ACS Nano. 2009;3:1219–1224. doi: 10.1021/nn900086c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunimovich YL, Shin YS, Yeo WS, Amori M, Kwong G, Heath JR. J Am Chem Soc. 2006;128:16323–16331. doi: 10.1021/ja065923u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Zhang DH, Han S, Liu XL, Tang T, Zhou CW. Adv Mater. 2003;15:143–146. [Google Scholar]
- 19.Fichter F, Dietrich W. Helv Chim Acta. 1924;7:131–142. [Google Scholar]
- 20.Paramonov PB, Paniagua SA, Hotchkiss PJ, Jones SC, Armstrong NR, Marder SR, Bredas JL. Chem Mater. 2008;20:5131–5133. [Google Scholar]
- 21.Meier EP, Chambers JQ, Chambers CA, Eggins BR, Liao CS. J Electroanal Chem. 1971;33:409. [Google Scholar]
- 22.Villalba MM, Litchfield VJ, Smith RB, Franklin AM, Livingstone C, Davis J. J Biochem and Biophys Methods. 2007;70:797–802. doi: 10.1016/j.jbbm.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Katz EY, Borovkov VV, Evstigneeva RP. J Electroanal Chem. 1992;326:197–212. [Google Scholar]
- 24.Budavari V, Szucs A, Somlai C, Novak M. Electrochim Acta. 2002;47:4351–4356. [Google Scholar]
- 25.Li C, Zhang D, Han S, Liu X, Tang T, Lei B, Liu Z, Zhou C. In: Molecular Electronics Iii. Reimers JR, Picconatto CA, Ellenbogen JC, Shashidhar R, editors. Vol. 1006. New York Acad Sciences; New York: 2003. pp. 104–121. [DOI] [PubMed] [Google Scholar]