Abstract
The goal of the present study is to understand the probable molecular mechanism of toxicities and the associated pathways related to observed pathophysiology in high PCB-exposed populations. We have performed a microarray-based differential gene expression analysis of children (mean age 46.1 months) of Central European descent from Slovak Republic in a well-defined study cohort. The subset of children having high blood PCB concentrations (>75 percentile) were compared against their low PCB counterparts (<25 percentile), with mean lipid-adjusted PCB values of 3.02±1.3 and 0.06±0.03 ng/mg of serum lipid, for the two groups, respectively (18.1±4.4 and 0.3±0.1 ng/ml of serum). The microarray was conducted with the total RNA from the peripheral blood mononuclear cells of the children using an Affymetrix platform (GeneChip Human genome U133 Plus 2.0 Array) and was analyzed by Gene Spring (GX 10.0). A highly significant set of 162 differentially expressed genes between high and low PCB groups (p value <0.00001) were identified and subsequently analyzed using the Ingenuity Pathway Analysis tool. The results indicate that Cell-To-Cell Signaling and Interaction, Cellular Movement, Cell Signaling, Molecular Transport, and Vitamin and Mineral Metabolism were the major molecular and cellular functions associated with the differentially altered gene set in high PCB-exposed children. The differential gene expressions appeared to play a pivotal role in the development of probable diseases and disorders, including cardiovascular disease and cancer, in the PCB-exposed population. The analyses also pointed out possible organ-specific effects, e.g., cardiotoxicity, hepatotoxicity and nephrotoxicity, in high PCB-exposed subjects. A few notable genes, such as BCL2, PON1, and ITGB1, were significantly altered in our study, and the related pathway analysis explained their plausible involvement in the respective disease processes, as mentioned. Our results provided insight into understanding the associated molecular mechanisms of complex gene-environment interactions in a PCB-exposed population. Future endeavors of supervised genotyping of pathway-specific molecular epidemiological studies and population biomarker validations are already underway to reveal individual risk factors in these PCB-exposed populations.
Keywords: PCB, Microarray, Gene expression, Environmental exposure, Functional analysis, PCB-exposed population
1. Introduction
Exposure to Polychlorinated Biphenyls (PCBs) and its association with different disease risks in humans raises important questions in exposure biology, even after a reasonable period of legal ban on its production and use. Epidemiological studies indicate that different disease risks are associated with PCB exposure. (Engel et al., 2007; Bruner-Tran and Osteen, 2010; Carpenter 2006). PCB exposure may affect and interfere with major physiological functions of the body, such as reproductive functions (Loch-Caruso, 2002; Brouwer et al., 1999), neurological functions (Sanchez-Alonso et al., 2003), endocrinal functions (Tabb et al., 2004; Brouwer et al., 1998), cardiovascular functions (Kopf and Walker, 2009; Sergeev and Carpenter, 2010), and immunological function. (Hertz-Picciotto et al., 2008). The effects of PCB exposure on fetal and infant development (Winneke et al., 2002) have also been frequently reported. However, the carcinogenic effects of PCBs remain highly controversial (Faroon et al., 2001; Laden et al., 2002; Golden et al., 2003).
The average body burden of PCBs in different populations depends on the intake of lipids containing food contaminated with PCBs. The composition and the body burden of PCBs have been widely investigated in different population (Smeds and Saukko, 2001; Duarte-Davidson et al., 1994; Covaci et al., 2002; Kocan et al., 2001; Yu Z et al., 2007). Both coplanar and non-coplanar PCB congeners were identified in humans; however, the non-planar PCBs are the most prevalent and persistent PCB congeners (Humphrey et al., 2000). PCB-153 and PCB-138 have been reported to be the predominant congeners in humans (Hovander et al., 2006; Fangstrom et al., 2005).)
PCBs, because of their structural differences (co-planar and non-co-planar), can modulate gene expression through different pathways. The majority of PCB-related toxicity studies at the molecular and cellular level have focused on co-planar congeners the dioxin-like structure and anti-estrogenic function of co-planar congeners. The most important regulatory action observed in PCB-related toxicities is mediated through the aryl hydrocarbon receptor AhR (Bruner-Tran and Osteen, 2010). Planar PCBs and related congeners, e.g., 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD), induce gene expression by a ligand-dependent transactivating factor, the arylhydrocarbon receptor, and by alternative pathways for gene expression, e.g., c-Src and crosstalk with the MAP kinase pathway (Aoki, 2001). Because of their structure, planar PCB congeners are expected to interact with hormone function and to result in disease processes in steroid responsive tissues, including cancer in the breast and prostate (Ritchie et al., 2005; Rattenborg et al., 2002; Tsuchiya et al., 2007; Howsam et al., 2004; Li et al., 2005). Tabb et al. (2004) reported that certain highly chlorinated PCB congeners were potent activators of rodent pregnane X receptors (PXR). On the other hand, these congeners antagonize their human ortholog, the steroid and xenobiotic receptor (SXR), inhibiting target gene induction. Also, nonplanar PCB-153 antagonized the effect of TCDD on CYP1A gene transcription and on protein levels in primary rat hepatocytes (Chen & Bounce, 2004). Two PCB congeners (153 and 77) were studied to understand their probable role in the activation of an oxidative stress-responsive transcription factor, namely, nuclear factor-kappaB (NF-kappaB) and activator protein-1 (AP-1), as well as their resultant effects on hepatocyte cell proliferation and apoptosis (Tharappel et al., 2002). Their study showed that both PCBs alone or in combination can increase the DNA binding activities of NF-kappaB and AP-1, whereas the DNA binding activities of STAT3 and STAT5 are decreased. The induction of altered hepatic foci appears to be related to compensatory cell proliferation in PCB-77-treated rats, whereas the inhibition of apoptosis appears to be important in PCB-153-treated rats. The two non-ortho (dioxin-like) coplanar PCBs (PCB-126 and PCB-77) showed anti-androgenic properties on the human prostatic carcinoma cell line LNCaP (Endo et al., 2003). Both PCBs reduced androgen-depending prostatic specific antigen (PSA) secretion and cell proliferation. In contrast, the non-coplanar PCB-153 induced cell proliferation and PSA secretion at low concentrations (Endo et al., 2003). However, a study on Chinese Hamster Ovary cells (Bonefeld-Jorgensen et al., 2001) showed that the di-ortho, multiple-chloro substituted biphenyls, PCB-138, PCB-153 and PCB-180 (three non-coplanar PCBs), can compete with the binding of the natural ligand to two nuclear receptors (estrogen receptor and androgen receptor), and thus, these three non-coplanar PCBs possess the ability to interfere with sexual hormone-regulated processes. The coplanar PCB-126-induced changes in membrane properties affect membrane beta-adrenoceptor (beta-AR) affinity and capacity in chicken embryo hepatocytes (Katynski et al., 2004).
The non-coplanar PCB-95 enhanced N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl4-isoxasolepropiate (AMPA)-mediated Ca2+ signals, by changing a functional association of the FKBP12/RyR complex, result in amplification of glutamate signaling in cultured cerebellar granule neurons (Gafni et al., 2004). The non-coplanar PCB-104 may contribute to tumor metastasis by inducing vascular endothelial growth factor (VEGF) overexpression that stimulates endothelial hyperpermeability, as well as transendothelial migration of cancer cells (Eum et al., 2004). Congener- and concentration-dependent antagonism of 17beta-estradiol (E2)-induced gene expression, rather than the induction of ER-dependent gene expression, was observed for the MeSO (2)-PCBs (2, 2′, 4, 5, 5′-pentachlorobiphenyl) (PCB-101) and 2, 2′, 4, 5′-tetrachlorobiphenyl (CB49) on lucifierase activity in stably transfected human breast adenocarcinoma T47D cells (ER-CALUX) (Letcher et al., 2002). A considerable literature exists on neurobehavioral toxicity resulting from pre- and post-natal exposures to PCBs performed in animal studies (Kodavanti et al., 1998; Lilienthal & Winneke, 1991; Goldey et al., 1995; Kuriyama & Chahoud, 2004; Allen et al., 1976; Levin et al., 1988), all indicating neurobehavioral deficits. Lilienthal & Winneke (1991) used a cross-fostering study design to demonstrate that prenatal rather than postnatal exposure to PCBs was responsible for neurobehavioral deficits in rodents. The doses of congeners #138, 153, and 180 to the fetus were lower from prenatal exposure than from postnatal (lactational) exposure, suggesting a greater sensitivity of the brain during gestation as compared with following gestation. Cognitive development in monkeys, measured by performance on a task involving spatial learning and memory, was impaired by PCBs at low exposure levels (Levin et al., 1988). The mothers of the 4–6 year-old rhesus monkeys had been exposed to Aroclor 1248 for an eighteen-month period ending one year prior to pregnancy (Levin et al., 1988).
Several human studies have addressed developmental effects from PCBs and from dibenzo-dioxins and furans. Following high exposures from accidental poisonings in Japan (Kuratsune et al., 1996) and Taiwan (Chen et al., 1994), children born to mothers who consumed cooking oil contaminated with PCBs had dermatologic and dental abnormalities at birth, deficits in development, and behavioral abnormalities, as well as an increased prevalence of speech problems and psychomotor delays. Scores on IQ tests were low. Children born many years after their mothers had been poisoned showed the same abnormalities, and there was no improvement with age (Chen et al., 1994).
The role of the hydroxylated metabolites of PCB 2, 3, 3′, 4′, 5-pentachloro-4-biphenylol (4-OH-CB107) has also been reported for its adverse endocrine-related toxicity (Meerts et al., 2004). Exposure to endocrine-disrupting chemicals such as polychlorinated biphenyls (PCBs) and dioxins and subsequent children’s health problems have been addressed in epidemiological studies, but in-depth studies are needed to understand the genetic susceptibilities of the individual (Kishi et al., 2006). Several non-dioxin-like PCB congeners and hydroxylated PCB compounds suppress thyroid hormone receptor (TR) action by dissociating TR from thyroid hormone response element (TRE) through interaction with TR-DBD (DNA-binding domain) (Miyazaki et al., 2008). The effects of PCBs and their metabolites on the thyroid axis were also exclusively studied because of their structural similarities with thyroid hormone (Khan et al., 2002; Osius and Karmaus 1998). PCBs may interfere with neurochemical parameters (Kodavanti, 2005), and the study of PCBs helps to elucidate the etiology of some neurological disease processes associated with PCB exposure (Corrigan et al., 1998).
To date, global gene expression studies conducted by our laboratory or by other groups have used specific PCB congeners, congeners in combinations, or the Arochlor mix, and have mainly focused on cell culture and animal models such as rat or aquatic species (Vezina et al., 2004; Chen et al., 2006; Ghosh et al., 2007; Dutta et al., 2008; De et al., 2010; Royland 2008a, b; Yum et al., 2010; Nakayama et al, 2008, Ghosh et al., 2011). Royland et al., (2008a, b) extensively used a rat model to study the effects of commercial PCB mixtures using the global gene expression platform to explore the pathophysiology of developmental neurobiology. However, the use of this commercial mixture does not represent the profile of an actual exposure from the bioaccumulated PCB congeners, and the species difference possibly alters the pharmacokinetics of toxicity.
Work so far reported on actual human data was conducted on pooled serum samples from non-smoking men exposed to PCBs and PCDFs and their matched referents. Only 908 genes were considered, which did not represent a picture of the global effects of PCBs on the human genome. The results indicated that the down-regulation of the tumor suppressor gene, von Hippel-Lindau (VHL) (Tsai et al., 2008). So far, no data are available on PCB-induced global gene expression in humans. To obtain the impact of PCBs on different cellular pathways and to explain the possible mode of action, we employed microarray-based global gene expression accompanied by Ingenuity Pathway Analysis (IPA) as a useful method, which could help us to understand better the overall effects of high PCB exposure on functional genomics.
2. Methods
2.1 Study population and selection of subjects
Different parts of Eastern Slovakia are highly contaminated by hazardous chemicals, particularly polychlorinated biphenyl-like compounds, because of the improper waste management of the chemical industries. PCB-related adverse health effects on humans have already been reported in this population. The subjects of the present study were selected from a well-defined cohort of mother-and-children pairs, originally enrolled in the ‘Slovak PCB Effects on Early Child Development Study’ between 2001 and 2004 (Sonneborn 2008a, b). The PCB levels of the children were studied longitudinally from birth, and PCB levels of the respective mothers were also measured at the time of the child’s birth to understand and evaluate the relative contribution of pre- and postnatal sources of exposure to these children (Supplemental Figure 1). Details of the recruitment and characterization of this cohort have been described elsewhere (Park et al., 2010). The children of the Michalovce area in Eastern Slovakia were exposed to high PCB contamination from a chemical manufacturing plant, while their counterparts in the Svidnik area, located 70 km to the northwest, had lower levels of environmental PCB exposure. Concentrations of 15 PCB congeners were measured in serum using high-resolution gas chromatography with electron capture detection. The study cohort included data and PCB concentrations measured at birth and when the children reached 6, 16 and 45 months of age. For this gene expression analysis, the children with an average of 45 months of age with high blood PCB concentration (>75 percentile) were compared to their low PCB counterparts (<25 percentile). Mean lipid-adjusted PCB values were 3.02±1.3 and 0.06±0.03 ng/mg of serum lipid, for the two groups respectively (18.1±4.4 and 0.3±0.1 ng/ml of serum). Detailed demography of this cohort can be found in previously published work (Park et al., 2010).
2.2 Sample collection and RNA isolation
Whole blood was collected in PAXgene® Blood RNA Tubes (BD, Cat. # 762165) in Slovakia and dispatched to the Molecular Genetics Laboratory at Howard University, Washington DC, USA, according to the specific protocol of the manufacturer (Qiagen MD). The study was approved by the Howard University Institutional Review Board (IRB-07-GSAS-30), and the approved format written informed consent was obtained from each study subject prior to the withdrawal of blood. RNA was extracted from the PAXgene tubes using the PAXgene Blood RNA Kit IVD (Quagen, Cat. # 762164), according to the manufacturer’s instructions. Contaminating DNA was removed with the Ambion DNA-free kit. RNA content was determined spectrophotometrically on a nanodrop at 230, 260 and 280 λ. RNA quality was also verified by Agilent bioanalyzer analysis using an RNA 6000 nanochip before microarray chip hybridization, with RNA stored at −80 °C.
2.3 Microarray experiments
The oligonucleotide Microarray experiments were conducted at the Center for Genetic Medicine, Children’s National Medical Center, Washington DC, USA, using the Affymetrix U133 Plus 2.0 Array platform, which has a comprehensive coverage of the whole transcribed human genome on a single array. Chips were run in triplicate form on each individual subject under this study. Expression profiling data were obtained for each sample individually with standard operating procedures and following the protocols of Zhao and Hoffman (2004) for quality control.
2.4 Gene Expression Data analysis
The microarray data (*.cel file) were filtered and normalized by PLIER (http://www.affymetrix.com/support/technical/technotes/plier_technote.pdf) (Seo and Hoffman 2006), and a subsequent statistical analysis was performed using the GeneSpring GX10.0 analysis tool. Differential expression was compared using unpaired t-test statistics. A hierarchical clustering algorithm using the Pearson correlation was then used to temporally group those probe sets based on their expression pattern. The link for the expression data sets is http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=hhcrrgemgsokcdw&acc=GSE22868. We utilized the information in the Ingenuity Knowledge Base (Genes Only) as a reference set and considered both direct and indirect relationships; we included the molecules and/or the relationships only, when species were equal to human. We also used the data sources from ingenuity expert findings or from microRNA-mRNA interactions or protein-protein interactions. We decided to use the “Core Analysis” function to interpret the data in the context of biological processes, pathways and networks. Differentially expressed gene identifiers were defined as value parameters for analysis and identified the relationship between gene expression alterations and related changes in biofunctions under the subcategories of Molecular and Cellular Functions, Physiological System Development and Function, and Disease and Disorders.
Possible toxicological effects of these altered gene functions were also evaluated. The network analysis showed interrelatedness between these gene sets. The focus molecules were the genes that met specified criteria, such as a cutoff value and a directionality that fits in the network. The association of the gene sets with the canonical pathways indicated that there are possible effects on the well-defined biological pathway of the IPA platform, based on the most updated knowledge base. Both up- and down-regulated identifiers were defined as value parameters for the analysis, and these identifiers identified the relationship between gene expression alterations and related changes in biofunctions under the subcategories of Molecular and Cellular Functions, Physiological System Development and Function, and Disease and Disorders.
2.5 Quantitative Real Time Polymerase Chain Reaction (RT-PCR)
The differential expression of these genes was confirmed by quantitative RT-PCR, using the sets of forward and reverse primers (See Supplemental Table 4). Briefly, reverse transcriptions were conducted according to the manufacturer’s protocols (Applied Biosystem, CA, USA). Triplicate samples and no-template controls were included for each set of primers. A validation experiment was performed using serial dilutions of untreated cDNA from one entity to confirm equivalent relative efficiencies for target and housekeeping genes. Replicate raw data (threshold cycle number, or Ct) for each sample were averaged and then adjusted by dividing these values by the corresponding averaged Ct for GAPDH to correct for any differences in the starting quantity of the material. Relative quantification using comparative Ct analyses were performed by using the SDS automation controller software (V 2.3; Applied Biosystem, CA). RT-PCR data analysis was performed using the comparative Ct method (ΔΔCt) (Livak and Schmittgen, 2001). Statistical significance was determined by using the t-test (p<0.05) (see Ghosh et al, 2011 for details).
3. Results
3.1 Differential expression of genes between a High PCB group and a Low PCB group
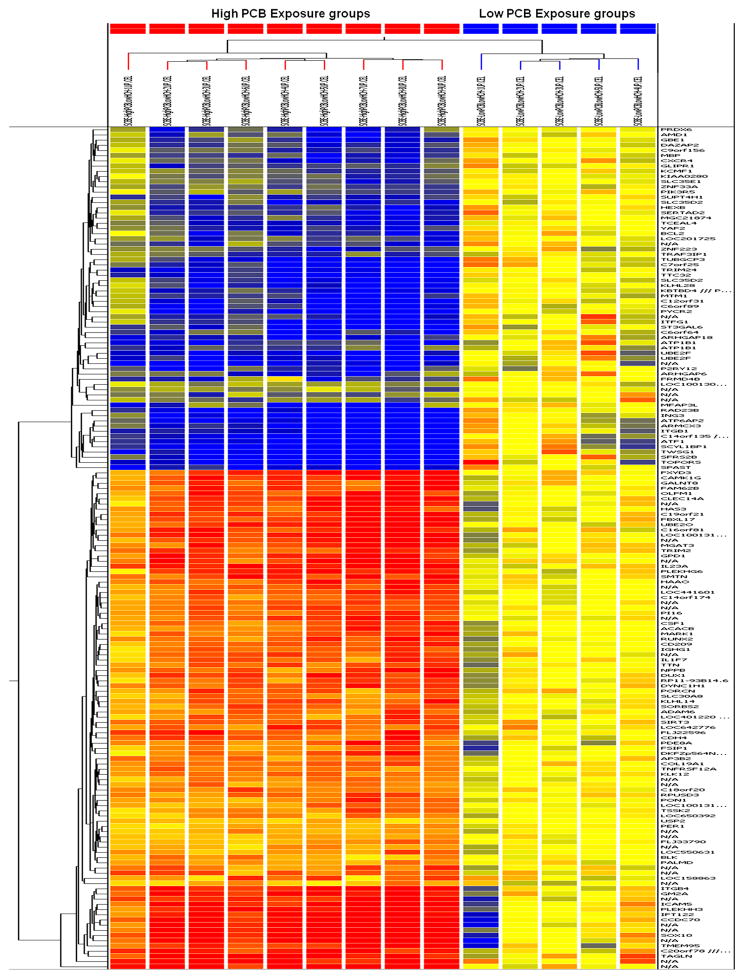
The differentially expressed genes between the High PCB group and the Low PCB group were identified using GeneSpring GX 10.0 on the basis of a stringent p-value (p<0.00001); a total of 162 genes were differentially expressed under this condition, of which 96 were up-regulated and 66 were down-regulated. Hierarchical clustering graphically displayed the different gene expression patterns between these two groups (Figure 1). The up- and down-regulated gene sets, with their functional locations and respective fold changes, were included (See Supplemental Table 1).
Figure 1.
Heat-Map showing the hierarchical clustering of differentially expressed genes (162) that up regulated (red) and down regulated (green) between High PCB and Low PCB groups (p<0.00001). Dendrogram displayed the clustering results systematically.
3.2 The effect of differentially regulated gene sets on biofunctions
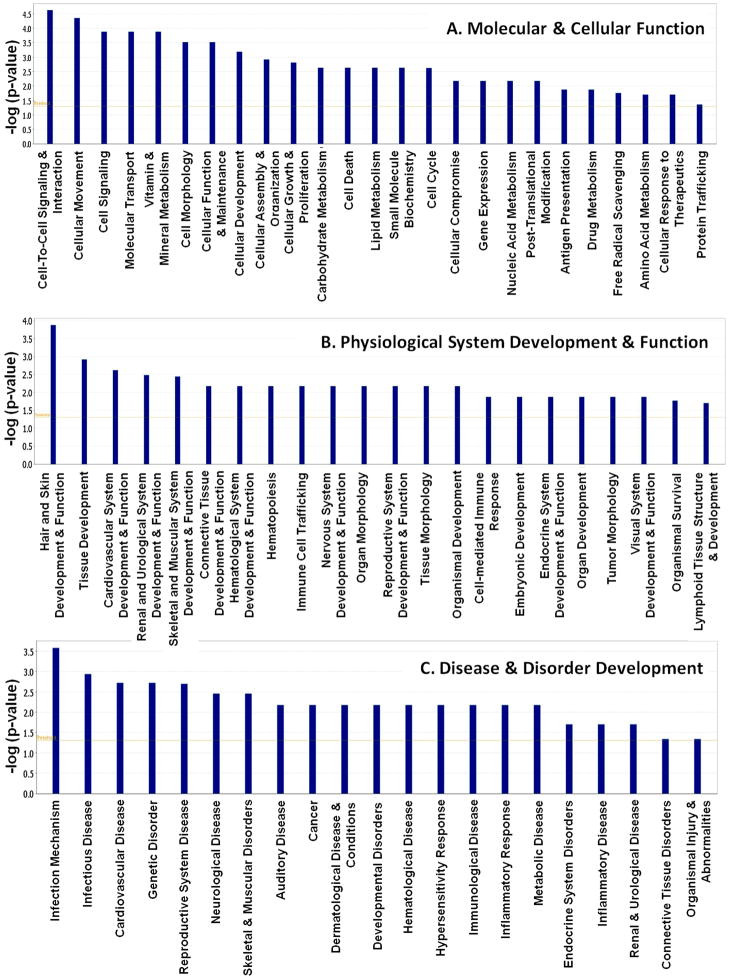
The IPA analysis on these gene sets (162) indicated that 25 different molecular and cellular functions were significantly affected because of high PCB exposure. The major altered events were Cell-To-Cell Signaling and Interaction, Cellular Movement, Cell Signaling, Molecular Transport, and Vitamin and Mineral Metabolism (Figure 2A). Twenty-two different types of Physiological System developments were significantly affected (Figure 2B). The most affected functions were Hair and Skin Development and Function, Tissue Development, Cardiovascular System Development and Function, Renal and Urological System Development and Function and Skeletal and Muscular System Development and Function. IPA analysis also revealed that 21 different disease and disorder developments were significantly associated with our gene set. Out of those, Infection Mechanism, Infectious Disease, Cardiovascular Disease, Genetic Disorder, and Reproductive System Disease are at the top of the list (Figure 2C). The lists of all biofunctions significantly affected by PCB exposure using the IPA program are available in an additional information section (See Supplemental Table 2).
Figure 2.
Relationship between gene expression alterations and relative changes in the top biofunctions obtained through Functional analysis for the dataset of differentially expressed genes (p<0.00001) in High PCB exposure in the subcategory of Molecular and Cellular Functions(A), Physiological System Development and Function (B), and Disease and Disorders development (C).
3.3 Gene Networks affected by PCBs exposure
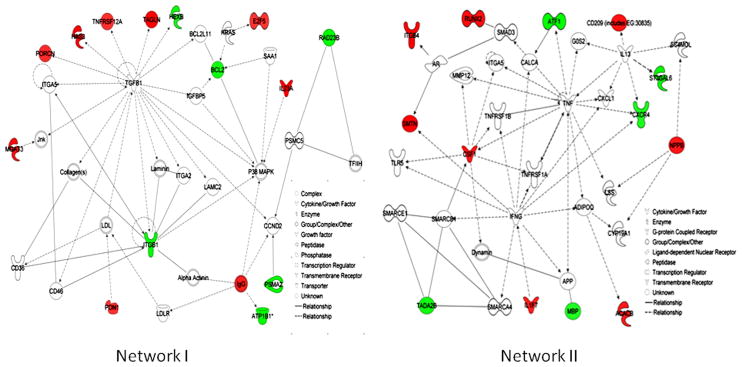
The network indicated the regulatory relationships within the genes in the data set. Eight distinct gene networks were observed. Of these networks, two major networks represented 14 and 13 focus molecules (also called Network Eligible molecules, which interact with other molecules in Ingenuity’s Knowledge Base. These molecules serve as the “seeds”, or focal points, for generating networks). The top functions and diseases related to Network I are Cell Death, Cellular Growth and Proliferation and Cell-To-Cell Signaling and Interaction, and those related to Network II are Cellular Growth and Proliferation, Cell Death, and Cellular Movement (Figure 3, Networks I and II). The respective genes involved in the networks are listed in a table (See Supplemental Table 3).
Figure 3.
The top two of the major networks by differentially expressed gene set in high PCB exposure showing the connectivity of differentially expressed genes. Solid interconnecting lines shows the genes that are directly connected and the dotted lines signify indirect connection between the genes with the gene regulation and functions attributed by their shape and denoted. Net work (I) consist with six down regulated and eight up-regulated genes and Net work (II) consist with five down regulated and eight up-regulated genes.
3.4 Association with the canonical pathways and the gene set
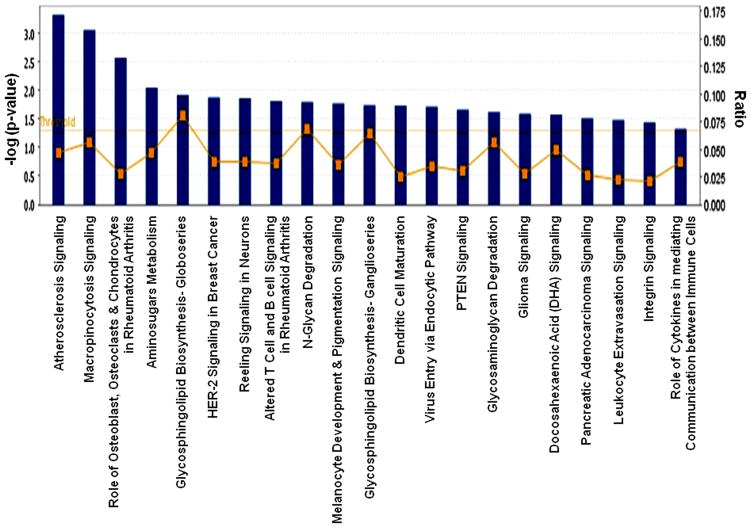
Canonical pathways in the IPA library include both Metabolic Pathways and Signaling Pathways. Significant associations of 21 canonical pathways were observed in our analysis (Figure 4). The ratio value represents the number of molecules in a given pathway that meet the cut criteria, divided by the total number of molecules that make up that pathway. The metabolic pathways were Amino sugars Metabolism, Glycosphingolipid Biosynthesis–Globoseries, N-Glycan Degradation, Glycosphingolipid Biosynthesis–Ganglioseries, and Glycosaminoglycan Degradation. The others were signaling and disease-responsive pathways. Some pathways were affiliated with more than one pathway, such as PTEN Signaling, which was categorized under apoptosis as well as in pathways related to cancer. Different canonical pathways and respective genes are described in Table 1.
Figure 4.
The significant canonical pathways involved in the well-characterized cell signaling and metabolic pathways associated with the genes that differentially expressed in High PCB exposure. The threshold line in the bar chart represents a p-value of 0.05. The ratio (r) is calculated by the number of genes from the our data set of differentially expressed gene set that participate in a Canonical Pathway, and dividing it by the total number of genes in that Canonical Pathway in IPA analysis.
Table 1.
Significant Canonical Pathways from IPA library associated with differentially expressed gene set in High PCB exposure.
| Ingenuity Canonical Pathways | −log(p-value) | Ratio | Molecules |
|---|---|---|---|
| Atherosclerosis Signaling | 3.32E00 | 4.72E-02 | CSF1, CXCR4, IL1F7, PRDX6, TNFRSF12A |
| Macropinocytosis Signaling | 3.06E00 | 5.56E-02 | ITGB1, CSF1, PIK3R5, ITGB4 |
| Role of Osteoblasts, Osteoclasts and Chondrocytes in Rheumatoid Arthritis | 2.57E00 | 2.78E-02 | ITGB1, CSF1, RUNX2, PIK3R5, IL1F7, BCL2 |
| Aminosugars Metabolism | 2.05E00 | 4.69E-02 | PDE8A, GM2A, HEXB |
| Glycosphingolipid Biosynthesis - Globoseries | 1.92E00 | 8E-02 | GM2A, HEXB |
| N-Glycan Degradation | 1.8E00 | 6.9E-02 | GM2A, HEXB |
| HER-2 Signaling in Breast Cancer | 1.88E00 | 3.85E-02 | ITGB1, PIK3R5, ITGB4 |
| Reelin Signaling in Neurons | 1.86E00 | 3.85E-02 | ITGB1, BLK, PIK3R5 |
| Altered T Cell and B Cell Signaling in Rheumatoid Arthritis | 1.82E00 | 3.66E-02 | CSF1, IL1F7, IL23A |
| Melanocyte Development and Pigmentation Signaling | 1.77E00 | 3.57E-02 | SOX10, PIK3R5, BCL2 |
| Glycosphingolipid Biosynthesis - Ganglioseries | 1.74E00 | 6.45E-02 | GM2A, HEXB |
| Dendritic Cell Maturation | 1.73E00 | 2.52E-02 | PIK3R5, IGHG1, IL1F7, IL23A |
| Virus Entry via Endocytic Pathways | 1.72E00 | 3.41E-02 | ITGB1, PIK3R5, ITGB4 |
| PTEN Signaling | 1.66E00 | 3E-02 | ITGB1, PIK3R5, BCL2 |
| Glycosaminoglycan Degradation | 1.62E00 | 5.56E-02 | GM2A, HEXB |
| Glioma Signaling | 1.59E00 | 2.8E-02 | E2F5, CAMK1G, PIK3R5 |
| Docosahexaenoic Acid (DHA) Signaling | 1.58E00 | 5E-02 | PIK3R5, BCL2 |
| Pancreatic Adenocarcinoma Signaling | 1.51E00 | 2.7E-02 | E2F5, PIK3R5, BCL2 |
| Leukocyte Extravasation Signaling | 1.48E00 | 2.17E-02 | ITGB1, ARHGAP6, CXCR4, PIK3R5 |
| Integrin Signaling | 1.44E00 | 2.04E-02 | ITGB1, PIK3R5, ITGB4, TTN |
| Role of Cytokines in Mediating Communication between Immune Cells | 1.33E00 | 3.85E-02 | IL1F7, IL23A |
| Role of BRCA1 in DNA Damage Response | 1.27E00 | 3.45E-02 | ATF1, E2F5 |
3.5 Tox function analysis on differentially regulated genes in the high PCB exposure group
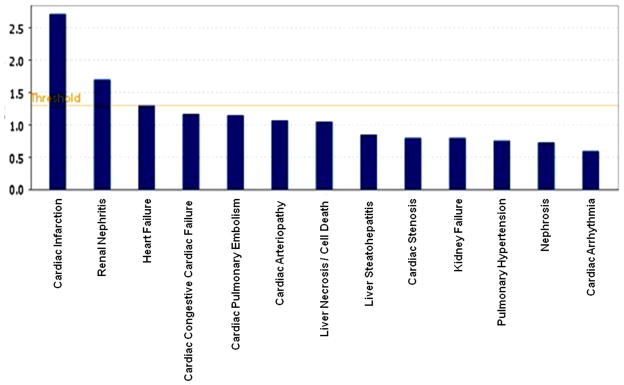
The IPA analysis was also conducted to determine probable toxicological effects due to high PCB exposure (Table 2). The associated toxic effects were categorized in Cardio toxicity, Hepatotoxicity and Nephrotoxicity, and subsequently in various subcategories, such as Cardiac Infarction, Heart Failure, Cardiac Congestive Cardiac Failure, Cardiac Pulmonary Embolism, Cardiac Arteriopathy, Necrosis and Steatohepatitis, Kidney failure, and Nephritis and Renal nephritis. The probability of cardiac infraction and renal nephritis were only above the threshold level of significance, as observed in Figure 5. The findings on the differential gene expression due to high PCB exposure were very interesting, and the possible molecular mechanism of the disease process and the related toxicities in a PCB-exposed population are discussed below.
Table 2.
Tox functions from IPA analysis on differentially expressed gene sets.
| Toxicological Effect | Genes involved |
|---|---|
| Cardiac Infarction | PON1, P2RY12, NPPB |
| Renal Nephritis | PDE8A, BCL2 |
| Heart Failure | PDE8A, NPPB, TTN |
| Cardiac Congestive Cardiac Failure | PDE8A, NPPB |
| Cardiac Pulmonary Embolism | NPPB |
| Cardiac Arteriopathy | COL19A1, CDH4 (includes EG:1002), ATP1B1, GM2A, FRMD4B, UBE2O, BCL2, PDE8A, PON1, ICAM5, CD209 (includes EG:30835), GBE1, P2RY12 |
| Liver Necrosis/Cell Death | BCL2 |
| Liver Steatohepatitis | PDE8A |
| Cardiac Stenosis | RUNX2 |
| Kidney Failure | PDE8A |
| Pulmonary Hypertension | NPPB |
| Nephrosis | PDE8A |
| Cardiac Arrythmia | NPPB |
Figure 5.
The important toxicological functions with the differentially expressed gene set in high PCB exposure as obtained through IPA analysis for toxicological functions. The threshold line in the bar chart represents a p-value of 0.05
4. Discussion
We studied the microarray results of the total RNA obtained from the PBMC cells of a group of Caucasian children (average age 46.1±1.4 month) from the Slovak Republic with differential PCB exposure. A stringent criterion of a p-value <0.00001 was chosen to obtain a highly significant set of differentially expressed genes for functional analysis. This stringent criterion leaves many other genes, which were differentially expressed in PCB exposure, out of the analysis. The analysis of microarray results indicated a low and moderate fold change in gene expression due to the high PCB exposure. However, we observed greater fold changes for in vitro studies, both in transformed cell lines (Dutta et al., 2008) and in PBMC treatment (Ghosh et al., 2011). Some other genes, which were differentially expressed in PCB exposure, but did not qualify under this level of stringency, were not included in the IPA analysis. Expression levels of some of the biologically significant genes, such as ARNT, BCL2, CYP2D6, CCK, and MYC, were differentially expressed in microarray experiments and were validated using the quantitative RT-PCR method. Some of the genes were already reported in PCB-related toxicities. CYP2D6-specific activity was elevated in rat liver microsomes after Aroclor 1254 induction (Easterbrook and Li, 2001). Our recent work on microarray analysis of PCB-treated HepG2 cell lines indicated the involvement of the AhR/ARNT pathway (De et al., 2010). We validated the microarray findings through the quantitative RT-PCR method, and the results were in full agreement with our microarray findings (See Supplemental Figure 2)
In our study subjects, the detectable PCB congeners were PCB-28, 52, 101,105, 114, 118, 123+149, 138+163, 153, 156+171, 157, 167, 170, 180 and 189, of which only congeners 118 and 156 were DL-PCBs (Park et al., 2010). NDL-PCB congeners 138, 153, 170, and 180 were the major contributors to the total PCB load of an individual (Trnovec et al., 2010). Non-ortho PCBs, also known as the coplanar PCBs, bind the aryl hydrocarbon receptor (AhR) and are capable of producing dioxin-like effects within biological systems (Mortensen and Arukwe, 2008).
A chronic exposure pattern is different from acute exposure. Chronic exposure is also different from in vitro and in vivo animal studies, as observed by various research groups (Ulbrich and Stahlmann, 2004; Suvorov and Takser, 2008). In chronic environmental exposure, multiple toxicants can act synergistically to induce a more lethal effect (Loeffler and Peterson, 1999). However, it is also possible for one toxicant to antagonize the effects of another (Bruner-Tran and Osteen, 2010). Our previous in cellulo gene expression studies have shown entirely different effects while comparing dioxin-like and non-dioxin-like PCB exposure (Ghosh et al., 2007; Dutta et al., 2008; De et al., 2010). Even the effect of the two most persistent non-dioxin-like PCB congeners, PCB-138 and 153, appeared to be quite different in their gene expression profile (Ghosh et al., 2011).
To understand the association of altered pathophysiological changes in PCB-related toxicities in epidemiological studies on high PCB exposure, our gene expression results indicated that some potential biochemical changes occur along related functional pathways. Cell-to-cell signaling and interaction were the most significantly affected molecular and cellular biofunctions in this study, as observed in the IPA analysis (Figure 2A). Considering the existing information on the involvement of PCBs in cell signaling (Eum et al., 2009; Dietrich and Kiana, 2010), it was expected that the high PCB exposure could alter signaling events in humans. Down-regulated molecules, such as BCL2 and ITGB1, were the central molecules regulating the different key cellular functions (Supplemental Table 2). This result indicated that the elevated PCB levels can alter the normal physiological functions of the body. The cytokines IL1F7 and IL23A were up-regulated in high PCB exposure involved in T cell and B cell signaling. Il1F7 is a member of the interleukin-1 (IL-1) cytokine family, which elicits a wide variety of biological activities that initiate and promote inflammatory responses and is also associated with arthritis types of disease (Rahman et al., 2006). Altered IL-23 signaling may lead to the dysregulation of T-cell-driven immune responses (Gudjonsson and Johnston, 2009). The integrin molecules ITGB1 and ITGB4 were also key molecules (−1.823 and 1.786) that were linked with different functional pathways (IPA Canonical Pathways). Caveolar-mediated Endocytosis Signaling, Clathrin-mediated Endocytosis Signaling, HER-2 Signaling in Breast Cancer, ILK Signaling, Integrin Signaling, Macropinocytosis Signaling, and Virus Entry via Endocytic Pathways were common in both ITGB1 and ITGB4. The other major pathways that were related to ITGB1 in the IPA analysis were Actin Cytoskeleton Signaling, Cdc42 Signaling, CDK5 Signaling, ERK/MAPK Signaling, FAK Signaling, Germ Cell-Sertoli Cell Junction Signaling, NF-κB Activation by Viruses, Phospholipase C Signaling, PI3K/AKT Signaling, PTEN Signaling, and Rac Signaling. These signaling pathways govern important cellular functions. Our analysis indicated that the differential expression of these genes due to high PCB exposure may affect the integration of the signaling pathways and may be associated with altered physiological function.
The effects of PCBs on different cell signaling events have been studied extensively for in vitro studies. Work on MCF-7 cells indicated that PCB-153 induces an ERK1/2-mediated mitogenic effect. However, the mixture of PCBs induces an antiproliferative effect. When studied in combination, PCB-101, PCB-118, and PCB-138 were ascribable to an apoptotic function (Radice et al., 2008). The non-coplanar PCB congeners with two or more chlorines in the ortho-positions were reported to interfere with intracellular signaling pathways dependent on Ca(2+) homeostasis. The other mode of action reported in these congeners involves changes in protein kinase C translocation, changes in cellular dopamine (DA) uptake, formation of reactive oxygen species, and thyroid effects (Filipov et al., 2005). In our study, the calcium/calmodulin-dependent protein kinase IG (CAMKIG) was up-regulated (1.562-fold) in high PCB exposure. However, the effect on calcium signaling was not observed in IPA analysis. The genes SRY (sex determining region Y-box 10) showed an up-regulation of 1.977-fold and were related to Melanocyte Development and Pigmentation Signaling.
The functional analysis in our study indicated that infectious mechanisms and infectious diseases were the top two functions in disease disorder categories. These effects have not previously been reported in the literature in relation to PCB exposure; however, the impairment of immunological effects was extensively studied (Jusko et al., 2010, Leijs et al., 2009). Choi et al. (2009) found that membrane domains called caveolae may regulate PCB-induced inflammatory parameters. Cardiotoxicity, which is the most significant toxic effect observed in our study, may be due to altered inflammatory response (Li et al., 2005) (Figure 5).
In our effort to investigate and understand disease and disorder development due to high PCB exposure, we utilized IPA analysis software, which demonstrated some key molecular mechanisms involved in PCB-related toxicities. This investigation explained some of the cellular insult and aberration in pathology that is commonly associated with PCB exposure. The effect of PCB exposure and its association with carbohydrate metabolism has been reported in the Slovak population (Klimeš et al., 2003; Radicova et al., 2004; Langer et al., 2008; Langer et al., 2009). A recent report showed that increasing levels of PCBs were also associated with increased prevalence of diabetes (Ukropec et al., 2010). Our microarray result indicated the elevation of PON1 in high PCB exposure; PON1 is an important enzyme, protecting against vascular disease by metabolizing the oxidized lipids, and improving the antioxidant status of the body. Abbot et al. (1995) reported that low serum paraoxonase activity in type 1 and type 2 diabetes were caused by low paraoxonase-specific activity. There were other reports that showed that the PON1 Q192R polymorphism, while not independently associated with myocardial infarction (MI), further increased the risk of MI among the subjects with diabetes, obesity, or both, under conditions associated with high oxidative stress (Li et al., 2005). Thus, the elevation of PON1 may be a protective measure against disease risk that is due to increased PCB levels.
The effect on reproductive system diseases was also observed and was among the top disease disorder categories in this IPA analysis. The effect was reported in epidemiological studies (Schell and Gallo, 2010) on PCB exposure. However, several studies have also been reported on the inverse associations between PCBs and circulating testosterone levels in men (Meeker & Hauser, 2010). It is a common credence that dioxin-like coplanar PCBs are mostly responsible for endocrine-related disease. The mechanism may involve something other than the endocrine disrupter property of PCBs (Bonde et al., 2008). The results from our study showed that the genes responsible for proper reproductive function were altered under environmental exposure to PCBs, in which both the DL and non-DL congeners were present, indicating that the several mechanisms mentioned may be involved.
The risk of auditory disease was observed in our analysis. The auditory problem from high PCB exposure reported in Slovak populations resulted from the deterioration of outer hair cells (OHCs) of the cochlea (Trnovec et al., 2010). PCB-related toxicity was also reported in animals for its effects on brain stem auditory evoked potentials (Meerts et al., 2004). The involvement of Sox10 in the development of cochlear function has been reported previously (Breuskin et al., 2009). We found that the Sox10 was over-expressed in our work. Further mechanistic study is needed to understand the regulatory effect of this gene on cochlear development under PCB exposure. Similarly, the effect on neurological disease development was previously suspected in PCB exposure, and analyses showed that a significant number of differentially expressed genes were associated with neurological disease (Supplemental Table 2).
Strong correlations between mono- and di-ortho PCBs, and p, p′-DDE exposures make it difficult to identify the most important contributor to the suggested immunomodulation, as well as to separate effects due to pre- and postnatal exposure. Children from Taiwan, who were accidentally exposed to high levels of both non-dioxin-like and dioxin-like PCBs and polychlorinated dibenzofurans (PCDFs) prenatally, had higher rates of bronchitis, upper respiratory infections and middle ear infections than normally found in reference populations with low exposure (Guo et al., 2004; Yu et al., 1998). Glynn et al. (2008) suggested that PCB and p, p′-DDE modulation of infection risks may have consequences to healthy development during childhood, because respiratory infections early in life can be risk factors for asthma and middle ear infections. In acute PCB exposure, dermatological effects due to inflammatory responses on sebecious glands (chloracne) were observed by Masuda et al. (2009). In our present study, we also observed the possibility of developing dermatological diseases and dermatological conditions as a downstream effect of the infection mechanism due to altered IL-23 signaling, which could lead to deregulations of T-cell-driven immune responses.
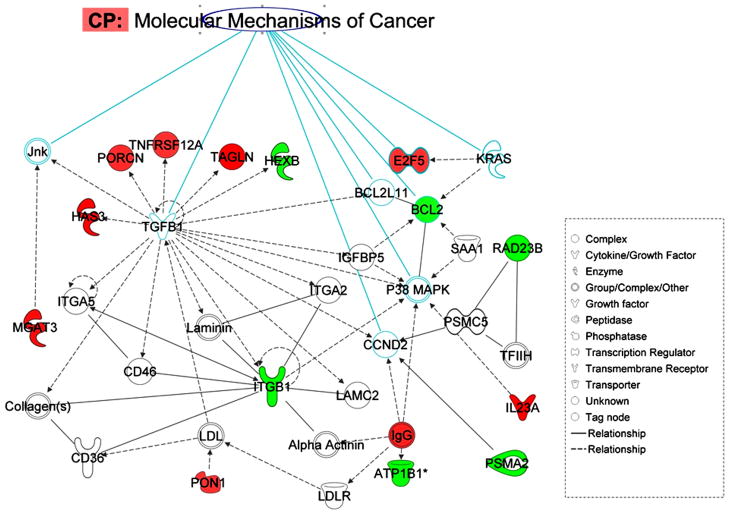
The debate on the risk of cancer due to PCB exposure in humans has yet to be resolved. The carcinogenic effects of PCBs (Faroon et al., 2001; Laden et al., 2002) are, however, highly controversial (Golden et al.,. 2003). Because of their structural resemblance with estrogen and their endocrine-disrupter properties, PCBs were investigated in relation to steroid hormone responsive cancers such as breast and prostate cancer in epidemiological studies. In our gene expression analysis, cancer was identified as one of the disease risks, through IPA analysis. The overlay of canonical pathways related to the molecular mechanisms of cancer in IPA analysis detected eight key molecules in network I (Figure 6). Two genes, E2F5 and BCL2, correspond to key molecules that have changed paths. The transcription regulator E2F5 is an important gene in relation to certain cancers. E2F-5-positive breast cancers were more common in TNBC [ER/progesterone receptor (PgR)/HER2-negative breast cancers, triple negative breast cancer], and in breast cancers with a basal phenotype. In addition, these breast cancer subtypes were also associated with worse clinical outcomes. On the other hand, BCL2 is one of the central molecules of the major network formed by down-regulated genes. The BCL2 gene has been implicated in a number of cancers, including melanoma, breast, prostate, and lung carcinomas, as well as in schizophrenia and autoimmunity. In our analysis, BCL2 appeared to be one of the key molecules, in all of the major disease process. The down-regulation of the BCL2 gene (−1.62) in our study suggested that high PCB exposure can favor the apoptotic pathway, which is in agreement with our previous result with an in vitro setup (Ghosh et al., 2010). The analysis also revealed that certain cancer-related genes were present in the top pathways. One of those genes is colony-stimulating factor 1 (CSF1). The expression of this gene was significantly elevated in high PCB exposure (1.473-fold). The IPA analysis revealed that this gene is related to atherosclerosis signaling, which was the strongest associated canonical pathway in the IPA library for our gene set. This cytokine of extracellular space has been reported both in cancer risk and in the determination of drug efficacy for different cancer types (Tamimi et al., 2008). Its biomarker potential has also been reported in mouse saliva in relation to Spontaneous Sjogren’s syndrome (Delaleu et al., 2008). The chemokine (C-X-C motif) receptor (CXCR4), a plasma membrane protein, has been down-regulated under high PCB exposure (−1.561). The role of this protein was described in tumor genesis under dioxin exposure in AhR signaling pathways (Wang et al., 2010). The protein was also reported as a possible therapeutic target of CXCR4 antagonist molecules in prostate cancer (Mason, 2010). The above finding showed that high PCB exposure could modulate the signaling event in a protective manner against a cancer risk.
Figure 6.
The overlay of significant canonical pathway and the connectivity in the molecular mechanism of cancer, with the eight genes in IPA canonical pathway and with the genes in Network (I) that differentially altered in PCB exposure. Solid interconnecting lines shows the genes that are directly connected and the dotted lines signify indirect connection between the genes with the gene regulation and functions attributed by their shape and denoted.
High PCB exposures have been shown to affect various stages of cell signaling processes via IPA analysis. In the present work, a matched control group was selected for a comparison analysis. The effect of PCBs, as observed on the children, requires validation in a randomized population study, as well as validation through a longitudinal study, to see the effect of PCB exposure on gene expression over time. These studies are currently underway in our laboratory. Some of the differentially expressed genes are destined to produce proteins in extracellular fluid, and they may be very useful and may carry a significant potential for the diagnostic evaluation of PCB exposure of an individual in early life. The present study could only indicate the probable genomic signatures for the disease risk of an individual due to an environmental exposure (PCB here) and may lead to the development of molecular diagnostics and therapeutics in the future, to respond to similar environmental threats. However, the extent of risk and severity of the disease process may depend on the genetic makeup of an individual and thus needs rigorous future work.
5. Conclusions
Our results are in accordance with various reported epidemiological studies on PCB exposure. Our results show how this complex, chronic and sublethal environmental exposure may affect normal cellular processes. Because of the lack of a clear phenotype, it was very difficult to follow the classical case control model for gene expression analysis in chronic PCB exposure. At the same time, it is very difficult to isolate and to identify a particular gene or genes in relation to PCB exposure. The analysis results underline the possible routes of pathogenesis in PCB exposures (Network I and II, Figure 3). The present study is the first in this area, as it provides the molecular basis of some of the pathophysiology observed in an epidemiological situation, and helps us to further understand the possible development of disease risk and disorder development. The stringent criterion of gene selection may have left out many other genes having important biological functions. Still, the analysis identified a reasonable number of genes for functional analysis. The results indicated the involvement of biochemical and disease pathways, which are already reported in pathophysiological conditions related to PCB exposure in epidemiological studies. Analysis reveals that important regulatory molecules, such as BCL2, PON1 and ITGB1, play a significant role in different pathways because of their altered gene expression induced by PCB exposure, and they can act as signature biomarkers of PCB exposures.
Supplementary Material
Children with Low serum PCB: Relationship between Mother Blood, cord Blood and children at different age of their life. b: Children with High serum PCB: Relationship between mother, cord blood, and children at different age of their life.
The Comparison of microarray and RT-PCR data in exposed subjects for the select genes in high exposures. Data are expressed as fold change. Error bars represent standard errors of means (±SEM; n=3).
Research Highlights.
Identified differentially altered gene sets in PCB-exposed children.
Identified pathways underlying PCB-induced pathophysiological events.
Found a probable molecular mechanism of PCB-induced disease processes.
Found putative biomarkers for early diagnosis of PCB-induced exposure.
Acknowledgments
These studies are supported by the 1UO1ES016127-01 grant from the National Institute of Environmental Health Sciences (NIEHS/NIH). Thanks are also due to General Clinical Research Center (GCRC) of Howard University in supporting us towards blood collection from healthy donors as per approved HU IRB # IRB-07- GSAS-30. Its contents are solely the responsibility of the authors. We sincerely acknowledge the efforts of Prof. Emeritus Prabir K. Chakrabaory of USUHS, Bethesda, for his critical reading and valuable suggestions.
Footnotes
Authors’ contributions
PM, SG and SZ have equally contributed to this the work. SG performed the handling of the human subject sample selections and the isolation of RNA in the microarray studies. SZ did the statistical analysis; PM did the IPA analysis and drafted the manuscript. SGM provided the microarray run for the specified subjects. TT, LP and ES were responsible for human subject blood and data collection. IHP and DS provided the required human subject background data needed for this work. EH and SKD provided support and direction, and revised the manuscript. SKD holds the NIEHS/UO1 grant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott CA, Mackness MI, Kumar S, Boulton AJ, Durrington PN. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arteriosclerosis Thrombosis and Vascular Biol. 1995;15:1812–18. doi: 10.1161/01.atv.15.11.1812. [DOI] [PubMed] [Google Scholar]
- Allen JR, Carstens LA, Abrahamson LJ. Responses of rats exposed to polychlorinated biphenyls for fifty-two weeks I. Comparison of tissue levels of PCB and biological changes. Arch Environ Contam Toxicol. 1976;4:404–19. doi: 10.1007/BF02221038. [DOI] [PubMed] [Google Scholar]
- Aoki N, Aoyama K, Nagata M, Matsuda T. A growing family of dual specificity phosphatases with low molecular masses. J Biochem. 2001;130:133–40. doi: 10.1093/oxfordjournals.jbchem.a002952. [DOI] [PubMed] [Google Scholar]
- Bonde JP, Toft G, Rylander L, Rignell-Hydbom A, Giwercman A, Spano M, et al. Fertility and markers of male reproductive function in inuit and european populations spanning large contrasts in blood levels of persistent organochlorines. Environ Health Perspect. 2008;116:269–77. doi: 10.1289/ehp.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM. Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology. 2001;158:141–53. doi: 10.1016/s0300-483x(00)00368-1. [DOI] [PubMed] [Google Scholar]
- Breuskin I, Bodson M, Thelen N, Thiry M, Borgs L, Nguyen L, Lefebvre PP, Malgrange B. Sox10 promotes the survival of cochlear progenitors during the establishment of the organ of Corti. Dev Biol. 2009;335:327–39. doi: 10.1016/j.ydbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Ahlborg UG, van Leeuwen FX, Feeley MM. Report of the WHO working group on the assessment of health risks for human infants from exposure to PCDDs, PCDFs and PCBs. Chemosphere. 1998;37:1627–43. doi: 10.1016/s0045-6535(98)00230-6. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Longnecker MP, Birnbaum LS, Cogliano J, Kostyniak P, Moore J, et al. Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBs. Environ Health Perspect. 1999;107(Suppl 4):639–49. doi: 10.1289/ehp.99107s4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner-Tran KL, Osteen KG. Dioxin-like PCBs and endometriosis. Systems Biology in Reproductive Medicine. 2010;56:132–46. doi: 10.3109/19396360903381023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DO. Polychlorinated biphenyls (PCBs): Routes of exposure and effects on human health. Rev on Environ Health. 2006;21:1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- Chen G, Bunce NJ. Interaction between halogenated aromatic compounds in the Ah receptor signal transduction pathway. Environ Toxicol. 2004;19:480–9. doi: 10.1002/tox.20053. [DOI] [PubMed] [Google Scholar]
- Chen YC, Yu ML, Rogan WJ, Gladen BC, Hsu CC. A 6-year follow-up of behavior and activity disorders in the Taiwan Yu-cheng children. Am J Public Health. 1994;84:415–21. doi: 10.2105/ajph.84.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YQ, De S, Ghosh S, Dutta SK. Congener-specific polychlorinated biphenyl-induced cell death in human kidney cells in vitro: Potential role of caspase. Int J Toxicol. 2006;25:341–47. doi: 10.1080/10915810600840859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Arzuaga X, Kluemper CT, Caraballo A, Toborek M, Hennig B. Quercetin blocks caveolae-dependent pro-inflammatory responses induced by co-planar PCBs. Environ Int. 2010;36:931–34. doi: 10.1016/j.envint.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan FM, Murray L, Wyatt CL, Shore RF. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in parkinson’s disease. Exp Neurology. 1998;150:339–42. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- Covaci A, Jorens P, Jacquemyn Y, Schepens P. Distribution of PCBs and organochlorine pesticides in umbilical cord and maternal serum. The Science of the Total Environment. 2002;298:45–53. doi: 10.1016/s0048-9697(02)00167-5. [DOI] [PubMed] [Google Scholar]
- De S, Ghosh S, Chatterjee R, Chen YQ, Moses L, Kesari A, Hoffman EP, Dutta SK. Identification of PCB Induced Congener Specific Oxidative Stress Response Pathways by Microarray Analysis using Human Liver Cell Line (Accepted, in press Special Issue of Environ Int. 2010;36:907–17. doi: 10.1016/j.envint.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaleu N, Immervoll H, Cornelius J, Jonsson R. Biomarker profiles in serum and saliva of experimental Sjögren’s syndrome: associations with specific autoimmune manifestations. Arthritis Res Ther. 2008;10:R22. doi: 10.1186/ar2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C, Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell-cell contact and tumor growth. Carcinogenesis. 2010;31:1319–28. doi: 10.1093/carcin/bgq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Davidson R, Wilson SC, Jones KC. PCBs and other organochlorines in human tissue samples from the welsh population: II--milk. Environ Pollution. 1994;84:79–87. doi: 10.1016/0269-7491(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Dutta SK, Ghosh S, De S, Hoffman EP. CYP1A1 and MT1K are congener specific biomarker genes for liver diseases induced by PCBs. Environ Toxicol Pharmacol. 2008;25:218–21. doi: 10.1016/j.etap.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Easterbrook J, Fackett D, Li AP. A comparison of aroclor 1254-induced and uninduced rat liver microsomes to human liver microsomes in phenytoin O-deethylation, coumarin 7-hydroxylation, tolbutamide 4-hydroxylation, S-mephenytoin 4′-hydroxylation, chloroxazone 6-hydroxylation and testosterone 6beta-hydroxylation. Chem Biol Interact. 2001;134:243–9. doi: 10.1016/s0009-2797(01)00159-4. [DOI] [PubMed] [Google Scholar]
- Endo F, Monsees TK, Akaza H, Schill WB, Pflieger-Bruss S. Effects of single non-ortho, mono-ortho, and di-ortho chlorinated biphenyls on cell functions and proliferation of the human prostatic carcinoma cell line, LNCaP. Reprod Toxicol. 2003;17:229–36. doi: 10.1016/s0890-6238(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Engel LS, Laden F, Andersen A, Strickland PT, Blair A, Needham LL, et al. Polychlorinated biphenyl levels in peripheral blood and non-hodgkin’s lymphoma: A report from three cohorts. Cancer Res. 2007;67:5545–52. doi: 10.1158/0008-5472.CAN-06-3906. [DOI] [PubMed] [Google Scholar]
- Eum SY, Andras I, Hennig B, Toborek M. NADPH oxidase and lipid raft-associated redox signaling are required for PCB153-induced upregulation of cell adhesion molecules in human brain endothelial cells. Toxicol Appl Pharmacol. 2009;240:299–305. doi: 10.1016/j.taap.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum SY, Lee YW, Hennig B, Toborek M. VEGF regulates PCB 104-mediated stimulation of permeability and transmigration of breast cancer cells in human microvascular endothelial cells. Experi Cell Res. 2004;296:231–44. doi: 10.1016/j.yexcr.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Fangstrom B, Hovander L, Bignert A, Athanassiadis I, Linderholm L, Grandjean P, et al. Concentrations of polybrominated diphenyl ethers, polychlonnated biphenyls, and polychlorobiphenylols in serum from pregnant faroese women and their children 7 years later. Environ Sci Technol. 2005;39:9457–63. doi: 10.1021/es0513032. [DOI] [PubMed] [Google Scholar]
- Faroon OM, Keith S, Jones D, De Rosa C. Carcinogenic effects of polychlorinated biphenyls. Toxicol Industrial Health. 2001;17:41–62. doi: 10.1191/0748233701th098oa. [DOI] [PubMed] [Google Scholar]
- Filipov NM, Lawrence DA, Seegal RF. Influence of polychlorinated biphenyls and turning preference on striatal dopamine metabolism. Jf Toxicol Environ Health Part A. 2005;68:167–83. doi: 10.1080/15287390590890563. [DOI] [PubMed] [Google Scholar]
- Gafni J, Wong PW, Pessah IN. Non-coplanar 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) amplifies ionotropic glutamate receptor signaling in embryonic cerebellar granule neurons by a mechanism involving ryanodine receptors. Toxicoll Sci. 2004;77:72–82. doi: 10.1093/toxsci/kfh004. [DOI] [PubMed] [Google Scholar]
- Ghosh S, De S, Chen YQ, Sutton DC, Ayorinde FO, Dutta SK. Polychlorinated biphenyls (PCB-153) and (PCB- 77) absorptions in human liver (HepG2) and kidney (Hk2) cells in vitro: PCB levels and cell death. Environ Int. 2010;36:893–900. doi: 10.1016/j.envint.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, De S, Dutta SK. Altered protein expressions in chronic PCB-153-induced human liver (HepG2) cells. Int J Toxicol. 2007;26:203–212. doi: 10.1080/10915810701352648. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Zang S, Mitra PS, Ghimbovschi S, Hoffman EP, Dutta SK. Global gene expression and Ingenuity biological functions analysis on PCB 153 and 138 induced human PBMC in vitro reveals differential mode(s) of action in developing toxicities. Environmental International. 2011;37(2011):838–857. doi: 10.1016/j.envint.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A, Thuvander A, Aune M, Johannisson A, Darnerud PO, Ronquist G, Cnattingius S. Immune cell counts and risks of respiratory infections among infants exposed pre- and postnatally to organochlorine compounds: a prospective study. Environ Health. 2008;7:62. doi: 10.1186/1476-069X-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden R, Doull J, Waddell W, Mandel J. Potential human cancer risks from exposure to PCBs: A tale of two evaluations. Crit Rev Toxicol. 2003;33:543–80. [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Johnston A. Current understanding of the genetic basis of psoriasis. Expert Rev Clin Immunol. 2009;5:433–43. doi: 10.1586/eci.09.13. [DOI] [PubMed] [Google Scholar]
- Guo YL, Lambert GH, Hsu CC, Hsu MM. Yucheng: health effects of prenatal exposure to polychlorinated biphenyls and dibenzofurans. Int Arch Occup Environ Health. 2004;77:153–158. doi: 10.1007/s00420-003-0487-9. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Park HY, Dostal M, Kocan A, Trnovec T, Sram R. Prenatal exposures to persistent and non-persistent organic compounds and effects on immune system development. Basic Clinl Pharmacol Toxicol. 2008;102:146–54. doi: 10.1111/j.1742-7843.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- Hovander L, Linderholm L, Athanasiadou M, Athanassiadis I, Bignert A, Fangstrom B, et al. Levels of PCBs and their metabolites in the serum of residents of a highly contaminated area in eastern slovakia. Environ Sci Technol. 2006;40:3696–703. doi: 10.1021/es0525657. [DOI] [PubMed] [Google Scholar]
- Howsam M, Grimalt JO, Guino E, Navarro M, Marti-Rague J, Peinado MA, et al. Organochlorine exposure and colorectal cancer risk. Environ Health Perspec. 2004;112:1460–66. doi: 10.1289/ehp.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey HE, Gardiner JC, Pandya JR, Sweeney AM, Gasior DM, McCaffrey RJ, et al. PCB congener profile in the serum of humans consuming great lakes fish. Environ Health Perspec. 2000;108:167–172. doi: 10.1289/ehp.00108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, De Roos AJ, Schwartz SM, Lawrence BP, Palkovicova L, Nemessanyi T, et al. A cohort study of developmental polychlorinated biphenyl (PCB) exposure in relation to post-vaccination antibody response at 6-months of age. Environ Res. 2010;110:388–95. doi: 10.1016/j.envres.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katynski AL, Vijayan MM, Kennedy SW, Moon TW. 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) impacts hepatic lipid peroxidation, membrane fluidity and beta-adrenoceptor kinetics in chick embryos. Comp Biochem Physiol Toxicol Pharmacol. 2004;137:81–93. doi: 10.1016/j.cca.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Khan MA, Lichtensteiger CA, Faroon O, Mumtaz M, Schaeffer DJ, Hansen LG. The hypothalamo-pituitary-thyroid (HPT) axis: A target of nonpersistent ortho-substituted PCB congeners. Toxicol Sci. 2002;65:52–61. doi: 10.1093/toxsci/65.1.52. [DOI] [PubMed] [Google Scholar]
- Kishi R, Sata F, Saijo Y, Kurahashi N, Kato S, Nakajima S, Sasaki S. Exposure to endocrine disrupting chemicals and children’s health: problems in epidemiological studies. [Article in Japanese] Nippon Eiseigaku Zasshi. 2006;61:19–31. doi: 10.1265/jjh.61.19. [DOI] [PubMed] [Google Scholar]
- Klimeš I, Koška J, Kšinantová L, Bučková K, Èervenáková Ž, Imrich R, Petrík J, et al. Increased frequency of glucose intolerance in the population of specific areas of eastern slovakia biphenyls (PCB) Diabetes. 2003;52(Suppl 1):P-955. [Google Scholar]
- Kodavanti PR, Ward TR. Differential effects of commercial polybrominated diphenyl ether and polychlorinated biphenyl mixtures on intracellular signaling in rat brain in vitro. Toxicol Sci. 2005;85:952–62. doi: 10.1093/toxsci/kfi147. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Ward TR. Interactive effects of environmentally relevant polychlorinated biphenyls and dioxins on [3H]phorbol ester binding in rat cerebellar granule cells. Environ Health Perspect. 1998;106:479–86. doi: 10.1289/ehp.98106479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokan A, Petrik J, Jursa S, Chovancova J, Drobna B. Environmental contamination with polychlorinated biphenyls in the area of their former manufacture in Slovakia. Chemosphere. 2001;43:595–600. doi: 10.1016/s0045-6535(00)00411-2. [DOI] [PubMed] [Google Scholar]
- Kopf PG, Walker MK. Overview of developmental heart defects by dioxins, PCBs, and pesticides. Journal of Environmental Science and Health Part C, Environ Carcinogen Ecotoxicol Rev. 2009;27:276–85. doi: 10.1080/10590500903310195. [DOI] [PubMed] [Google Scholar]
- Kuratsune M, Yoshimura T, Matsuzaka J, Yamaguchi A. Epidemiologic study on Yusho, a Poisoning Caused by Ingestion of Rice Oil Contaminated with a Commercial Brand of Polychlorinated Biphenyls. Environ Health Perspect. 1972;1:119–28. doi: 10.1289/ehp.7201119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama SN, Chahoud I. In utero exposure to low-dose 2,3′,4,4′,5-pentachlorobiphenyl (PCB 118) impairs male fertility and alters neurobehavior in rat offspring. Toxicology. 2004;202:185–97. doi: 10.1016/j.tox.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Kuriyama SN, Chahoud I. In utero exposure to low-dose 2,3′,4,4′,5-pentachlorobiphenyl (PCB 118) impairs male fertility and alters neurobehavior in rat offspring. Toxicology. 2004;202:185–97. doi: 10.1016/j.tox.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Laden F, Ishibe N, Hankinson SE, Wolff MS, Gertig DM, Hunter DJ, et al. Polychlorinated biphenyls, cytochrome P450 1A1, and breast cancer risk in the nurses’ health study. Cancer Epidemiol Biomarkers Prevention. 2002;11:1560–1565. [PubMed] [Google Scholar]
- Langer P, Kocan A, Tajtakova M, Koska J, Radikova Z, Ksinantova L, et al. Increased thyroid volume, prevalence of thyroid antibodies and impaired fasting glucose in young adults from organochlorine cocktail polluted area: Outcome of transgenerational transmission? Chemosphere. 2008;73:1145–50. doi: 10.1016/j.chemosphere.2008.06.067. [DOI] [PubMed] [Google Scholar]
- Langer P, Kocan A, Tajtakova M, Susienkova K, Radikova Z, Koska J, et al. Multiple adverse thyroid and metabolic health signs in the population from the area heavily polluted by organochlorine cocktail (PCB, DDE, HCB, dioxin) Thyroid Res. 2009;2:3. doi: 10.1186/1756-6614-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijs MM, Koppe JG, Olie K, van Aalderen WM, de Voogt P, ten Tusscher GW. Effects of dioxins, PCBs, and PBDEs on immunology and hematology in adolescents. Environ Sci Technol. 2009;43:7946–51. doi: 10.1021/es901480f. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Lemmen JG, van der Burg B, Brouwer A, Bergman A, Giesy JP, et al. In vitro antiestrogenic effects of aryl methyl sulfone metabolites of polychlorinated biphenyls and 2,2-bis(4-chlorophenyl)-1,1-dichloroethene on 17beta-estradiol-induced gene expression in several bioassay systems. Toxicol Sci. 2002;69:362–372. doi: 10.1093/toxsci/69.2.362. [DOI] [PubMed] [Google Scholar]
- Levin ED, Schantz SL, Bowman RE. Delayed spatial alternation deficits resulting from perinatal PCB exposure in monkeys. Arch Toxicol. 1988;62:267–73. doi: 10.1007/BF00332486. [DOI] [PubMed] [Google Scholar]
- Li J, Wang X, Huo Y, Niu T, Chen C, Zhu G, et al. PON1 polymorphism, diabetes mellitus, obesity, and risk of myocardial infarction: Modifying effect of diabetes mellitus and obesity on the association between PON1 polymorphism and myocardial infarction. Genetics in Med. 2005;7:58–63. doi: 10.1097/01.gim.0000151152.78092.ca. [DOI] [PubMed] [Google Scholar]
- Lilienthal H, Winneke G. Sensitive periods for behavioral toxicity of polychlorinated biphenyls: determination by cross-fostering in rats. Fundam Appl Toxicol. 1991;17:368–75. doi: 10.1016/0272-0590(91)90226-t. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–08. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loch-Caruso R. Uterine muscle as a potential target of polychlorinated biphenyls during pregnancy. Int J Hygiene Environ Health. 2002;205:121–30. doi: 10.1078/1438-4639-00137. [DOI] [PubMed] [Google Scholar]
- Loeffler IK, Peterson RE. Interactive effects of TCDD and p, p′-DDE on male reproductive tract development in in utero and lactationally exposed rats. Toxicol Appl Pharmacol. 1999;154:28–39. doi: 10.1006/taap.1998.8572. [DOI] [PubMed] [Google Scholar]
- Mason VL. American association for cancer research - 101st annual meeting - investigating new therapeutic candidates: part 1. Drugs. 2010;13:357–59. [PubMed] [Google Scholar]
- Masuda Y. Toxic effects of PCB/PCDF to human observed in yusho and other poisonings. Fukuoka Igaku Zasshi. 2009;100:141–55. [Article in Japanees] [PubMed] [Google Scholar]
- Meeker JD, Hauser R. Exposure to polychlorinated biphenyls (PCBs) and male reproduction. Syst Biol Reprod Med. 2010;56:122–31. doi: 10.3109/19396360903443658. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Lilienthal H, Hoving S, van den Berg JH, Weijers BM, Bergman A, et al. Developmental exposure to 4-hydroxy-2,3,3′,4′,5-pentachlorobiphenyl (4-OH-CB107): Long-term effects on brain development, behavior, and brain stem auditory evoked potentials in rats. Toxicol Sci. 2004;82:207–18. doi: 10.1093/toxsci/kfh252. [DOI] [PubMed] [Google Scholar]
- Miyazaki W, Iwasaki T, Takeshita A, Tohyama C, Koibuchi N. Identification of the functional domain of thyroid hormone receptor responsible for polychlorinated biphenyl-mediated suppression of its action in vitro. Environ Health Perspect. 2008;116:1231–36. doi: 10.1289/ehp.11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen AS, Arukwe A. Estrogenic effect of dioxin-like aryl hydrocarbon receptor (AhR) agonist (PCB congener 126) in salmon hepatocytes. Marine Environ Res. 2008;66:119–20. doi: 10.1016/j.marenvres.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Sei N, Oshima Y, Tashiro K, Shimasaki Y, Honjo T. Alteration of gene expression profiles in the brain of japanese medaka (oryzias latipes) exposed to KC-400 or PCB126. Marine Poll Bull. 2008;57:460–66. doi: 10.1016/j.marpolbul.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Osius N, Karmaus W. Thyroid hormone level in children in the area of a toxic waste incinerator in south Essen. Gesundheitswesen. 1998;60:107–12. [Article in German] [PubMed] [Google Scholar]
- Park HY, Hertz-Picciotto I, Sovcikova E, Kocan A, Drobna B, Trnovec T. Neurodevelopmental toxicity of prenatal polychlorinated biphenyls (PCBs) by chemical structure and activity: a birth cohort study. Environ Health. 2010;9:51. doi: 10.1186/1476-069X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice S, Chiesara E, Fucile S, Marabini L. Different effects of PCB101, PCB118, PCB138 and PCB153 alone or mixed in MCF-7 breast cancer cells. Food and Chem Toxicol. 2008;46:2561–67. doi: 10.1016/j.fct.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Rádiková Ž, Koška J, Kšinantová L, Imrich R, Kočan A, Petrík J, Hučková M, Wsolová L, Langer P, Trnovec T, Šeböková E, Klimeš I. Increased frequency of diabetes and other forms of dysglycemia in the population of specific areas of eastern slovakia chronically exposed to contamination with polychlorinated biphenyls (PCB) Organohalogen Compounds. 2004;6:3498–502. [Google Scholar]
- Rahman P, Sun S, Peddle L, Snelgrove T, Melay W, Greenwood C, et al. Association between the interleukin-1 family gene cluster and psoriatic arthritis. Arthritis and Rheumatism. 2006;54:2321–25. doi: 10.1002/art.21928. [DOI] [PubMed] [Google Scholar]
- Rattenborg T, Gjermandsen I, Bonefeld-Jorgensen EC. Inhibition of E2-induced expression of BRCA1 by persistent organochlorines. Breast Cancer Res. 2002;4:R12. doi: 10.1186/bcr461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JM, Vial SL, Fuortes LJ, Robertson LW, Guo H, Reedy VE, et al. Comparison of proposed frameworks for grouping polychlorinated biphenyl congener data applied to a case-control pilot study of prostate cancer. Environ Res. 2005;98:104–13. doi: 10.1016/j.envres.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Royland JE, Kodavanti PR. Gene expression profiles following exposure to a developmental neurotoxicant, aroclor 1254: Pathway analysis for possible mode(s) of action. Toxicol Appl Pharmacol. 2008a;231:179–196. doi: 10.1016/j.taap.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Royland JE, Wu J, Zawia NH, Kodavanti PR. Gene expression profiles in the cerebellum and hippocampus following exposure to a neurotoxicant, Aroclor 1254: developmental effects. Toxicol Appl Pharmacol. 2008b Sep 1;231(2):165–78. doi: 10.1016/j.taap.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alonso JA, Lopez-Aparicio P, Recio MN, Perez-Albarsanz MA. Apoptosis-mediated neurotoxic potential of a planar (PCB 77) and a nonplanar (PCB-153) polychlorinated biphenyl congeners in neuronal cell cultures. Toxicol Lett. 2003;144:337–49. doi: 10.1016/s0378-4274(03)00238-8. [DOI] [PubMed] [Google Scholar]
- Schell LM, Gallo MV. Relationships of putative endocrine disruptors to human sexual maturation and thyroid activity in youth. Physiol & Behavior. 2010;99:246–53. doi: 10.1016/j.physbeh.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Hoffman EP. Probe set algorithms: Is there a rational best bet? BMC Bioinformatics. 2006;7:395. doi: 10.1186/1471-2105-7-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeds A, Saukko P. Identification and quantification of polychlorinated biphenyls and some endocrine disrupting pesticides in human adipose tissue from Finland. Chemosphere. 2001;44:1463–71. doi: 10.1016/s0045-6535(00)00313-1. [DOI] [PubMed] [Google Scholar]
- Sonneborn D, Park HY, Petrik J, Kocan A, Palkovicova L, Trnovec T, et al. Prenatal polychlorinated biphenyl exposures in eastern slovakia modify effects of social factors on birth weight. Paedia Perinatal Epidemiol. 2008b;22:202–13. doi: 10.1111/j.1365-3016.2008.00929.x. [DOI] [PubMed] [Google Scholar]
- Sonneborn D, Park HY, Babinska K, Palkovicova L, Trnovec T, Kocan A, et al. Serum PCB concentrations in relation to locally produced food items in eastern Slovakia. J Exposure Sci Environ Epidemiol. 2008a;18:581–587. doi: 10.1038/jes.2008.1. [DOI] [PubMed] [Google Scholar]
- Sergeev AV, Carpenter DO. Increased hospitalizations for ischemic stroke with comorbid diabetes and residential proximity to sources of organic pollutants: a 12-year population-based study. Neuroepidemiology. 2010;35(3):196–201. doi: 10.1159/000316874. Epub 2010 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorov A, Takser L. Facing the challenge of data transfer from animal models to humans: the case of persistent organohalogens. Environ Health. 2008;7:58. doi: 10.1186/1476-069X-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb MM, Kholodovych V, Grun F, Zhou C, Welsh WJ, Blumberg B. Highly chlorinated PCBs inhibit the human xenobiotic response mediated by the steroid and xenobiotic receptor (SXR) Environ Health Perspect. 2004;112:163–169. doi: 10.1289/ehp.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamimi RM, Brugge JS, Freedman ML, Miron A, Iglehart JD, Colditz GA, et al. Circulating colony stimulating factor-1 and breast cancer risk. Cancer Res. 2008;68:18–21. doi: 10.1158/0008-5472.CAN-07-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharappel JC, Lee EY, Robertson LW, Spear BT, Glauert HP. Regulation of cell proliferation, apoptosis, and transcription factor activities during the promotion of liver carcinogenesis by polychlorinated biphenyls. Toxicol Appl Pharmacol. 2002;179:172–84. doi: 10.1006/taap.2001.9360. [DOI] [PubMed] [Google Scholar]
- Trnovec T, Sovcikova E, Pavlovcinova G, Jakubikova J, Jusko TA, Hustak M, et al. Serum PCB concentrations and cochlear function in 12-year-old children. Environ Sci Technol. 2010;44:2884–89. doi: 10.1021/es901918h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PC, Wang YJ, Tsai JH, Guo YL, Ueng TH, Liu HS, et al. Reduced expression of von hippel-lindau gene in subjects exposed to polychlorinated biphenyls and dibenzofurans. Environmental Research. 2008;108:247–51. doi: 10.1016/j.envres.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M, Tsukino H, Iwasaki M, Sasaki H, Tanaka T, Katoh T, et al. Interaction between cytochrome P450 gene polymorphisms and serum organochlorine TEQ levels in the risk of endometriosis. Mol Human Reprod. 2007;13:399–404. doi: 10.1093/molehr/gam018. [DOI] [PubMed] [Google Scholar]
- Ukropec J, Radikova Z, Huckova M, Koska J, Kocan A, Sebokova E, et al. High prevalence of prediabetes and diabetes in a population exposed to high levels of an organochlorine cocktail. Diabetologia. 2010;53:899–906. doi: 10.1007/s00125-010-1683-2. [DOI] [PubMed] [Google Scholar]
- Ulbrich B, Stahlmann R. Developmental toxicity of polychlorinated biphenyls (PCBs): a systematic review of experimental data. Arch Toxicol. 2004;78:252–68. doi: 10.1007/s00204-003-0519-y. [DOI] [PubMed] [Google Scholar]
- Vezina CM, Walker NJ, Olson JR. Subchronic exposure to TCDD, PeCDF, PCB126, and PCB153: effect on hepatic gene expression. Environ Health Perspect. 2004;112:1636–44. doi: 10.1289/txg.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Gavin HM, Arlt VM, Lawrence BP, Fenton SE, Medina D, Vorderstrasse BA. Aryl hydrocarbon receptor (AhR) activation during pregnancy, and in adult nulliparous mice, delays the subsequent development of DMBA-induced mammary tumors. Int J Cancer. 2011;128:1509–23. doi: 10.1002/ijc.25493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winneke G, Walkowiak J, Lilienthal H. PCB-induced neurodevelopmental toxicity in human infants and its potential mediation by endocrine dysfunction. Toxicology. 2002;181–182:161–165. doi: 10.1016/s0300-483x(02)00274-3. [DOI] [PubMed] [Google Scholar]
- Yu ML, Hsin JW, Hsu CC, Chan WC, Guo YL. The immunologic evaluation of the Yucheng children. Chemosphere. 1998;37:1855–1865. doi: 10.1016/s0045-6535(98)00251-3. [DOI] [PubMed] [Google Scholar]
- Yu Z, Palkovicova L, Drobna B, Petrik J, Kocan A, Trnovec T, et al. Comparison of organochlorine compound concentrations in colostrum and mature milk. Chemosphere. 2007;66:1012–18. doi: 10.1016/j.chemosphere.2006.07.043. [DOI] [PubMed] [Google Scholar]
- Yum S, Woo S, Kagami Y, Park HS, Ryu JC. Changes in gene expression profile of medaka with acute toxicity of arochlor 1260, a polychlorinated biphenyl mixture. Comp Biochem Physiol Toxicol Pharmacol. 2010;151:51–56. doi: 10.1016/j.cbpc.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Zhao P, Hoffman EP. Embryonic myogenesis pathways in muscle regeneration. Develop Dynamics. 2004;229:380–392. doi: 10.1002/dvdy.10457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Children with Low serum PCB: Relationship between Mother Blood, cord Blood and children at different age of their life. b: Children with High serum PCB: Relationship between mother, cord blood, and children at different age of their life.
The Comparison of microarray and RT-PCR data in exposed subjects for the select genes in high exposures. Data are expressed as fold change. Error bars represent standard errors of means (±SEM; n=3).