Abstract
When mice are subjected to a Pseudomonas aeruginosa challenge 5 days after cecal ligation and puncture (CLP), clearance of the Pseudomonas is diminished when compared to sham mice. The object of this study was to determine which component(s) of CLP contributed to the impairment of the innate immune response. Mice subjected to either trauma alone or cecal ischemia/necrosis alone did not have impaired ability to clear a subsequent Pseudomonas challenge (determined by colony-forming units (cfu’s) after culture of spleen tissue). However, mice subjected to abdominal contamination with heat-killed cecal contents had reduced ability to clear the subsequent Pseudomonas challenge. In contrast to normobiotic mice, neither CLP performed in germ-free mice nor abdominal contamination of mice with cecal contents from germ-free mice adversely affected clearance of a subsequent Pseudomonas challenge. These data suggest that suppressed immune function after CLP is due to exposure to microbial ligands within the cecal lumen rather than tissue trauma, ischemia, or necrosis. However, suppression of immune function did not appear to be due to exposure to LPS as TLR4-deficient mice subject to abdominal contamination with cecal contents had diminished clearance of a Pseudomonas challenge similar to that seen in wild-type mice.
Keywords: Animal model, sepsis, Mice, Immunosuppression, Pseudomonas
1. Introduction
Cecal ligation and puncture (CLP) was introduced in 1980 as a more clinically relevant model of sepsis than injection of lipopolysaccharide or a single strain of gram-negative bacteria [1], and the CLP model continues to be widely used for that purpose. It is a complex model that entails trauma, tissue ischemia and necrosis, and polymicrobial contamination and infection. The model can be adjusted by either varying the length of cecum that is ligated or by varying the size (gauge) of the needle used for the puncture [1–4]. Thus, the model may vary in severity, ranging from highly lethal within the first 1–2 days to one in which minimal mortality is observed for 2 weeks or longer.
In patients, sepsis occurs most often not as a primary lesion but rather as a sequelae to a major injury. It is commonly thought that patients become more susceptible to the development of sepsis because of injury-induced impairments of immune function, which seems substantiated in part because many of the causative organisms isolated from septic patients do not typically cause disease in normal, immunocompetent individuals [5,6]. In our hospital, burned patients that become septic typically have a mild to moderate infection initially but have subsequent infections that become more severe, which suggests progressive decline in immune function after injury and infection. Based upon that scenario, we have used CLP not as a primary model for which to study sepsis but rather as a ‘first-hit’ injury and infection which causes diminished innate immune function in response to subsequent bacterial challenges. Mice that have been subjected to a minimally-lethal model of CLP and allowed to recover for 5 days have impaired ability to clear a live bacterial challenge when compared to sham control mice [7]. However, it is not clear whether this impaired ability to clear the subsequent bacterial challenge is induced by the surgical trauma, the tissue ischemia/necrosis, or the abdominal contamination induced by CLP. This study examines CLP in mice to discern which component, or components, of the model are responsible for the subsequent decline in innate immune function.
2. Materials & Methods
2.1. Animal models (cecal ligation and puncture, cecal tissue implantation, cecal content implantation)
All experiments were conducted in accordance with the National Institutes of Health’s Guidelines for the Use of Laboratory Animals (National Institutes of Health Publication 85-23, revised 1996) and with the approval of the Institutional Animal Care and Use Committee at the University of Texas Medical Branch. C57BL/6J male mice and C57BL/10ScNJ (TLR4-deficient) were purchased from Jackson Laboratories (Bar Harbour, ME). Male Swiss Webster germ-free mice were purchased from Taconic Farms (Hudson, NY) and tested free of aerobic, anaerobic, and mycotic contamination. The animals were 6–8 weeks of age and were allowed to acclimatize for at least 7 days after delivery. The mice were maintained on 12 hr light-dark cycles with ad libitum food and water at all times.
CLP
Cecal ligation and puncture was performed as previously described (Wichtermann KA, 1980) with some modifications. Isoflurane anesthesia (2.5% in 100% O2) was initiated in an induction chamber and maintained by delivery through a face mask. After shaving and cleaning the ventral abdominal wall with alcohol, a midline incision was made and the cecum was exteriorized. Cecal contents were massaged out of the tip and towards the base of the cecum and the distal 0.5 cm of the apex was ligated with 3-0 silk suture. A 25-gauge needle was used to perforate the ligated portion of the cecum once in a through-and-through manner. Sham CLP mice, in which the cecum was exteriorized but neither ligated nor punctured, were performed as controls. The cecum was returned to the abdominal cavity, the abdominal wall was closed with 4-0 Vicryl suture, and the skin was reapposed with cyanoacrylate tissue adhesive. Mice were allowed to recover for 5 days before bacterial challenge.
Cecal tissue implantation
Donor mice were prepared and anesthetized as described above. After midline incision, the distal 0.5 cm apex of the cecum was amputated and the mice were sacrificed. The amputated cecal tissue was rinsed copiously in sterile saline, then incubated in RPMI with Imipenem antibiotic solution (5mg/ml for 30 mins), before a final rinse in saline. Treatment of the cecal tissue in this fashion resulted in a >99% reduction in viable bacteria. Recipient mice were prepared and anesthetized as described. Following a midline incision, the amputated cecal tissue was placed into the abdominal cavity. The incisions were closed as described and the mice allowed to recover for 5 days before bacterial challenge.
Cecal content implantation
Donor mice were prepared and anesthetized as described. After midline incision, the cecum was exteriorized and ligated and amputated as close to the base as possible, and the mice were sacrificed. The luminal contents of the amputated cecums were pooled together in a sterile tube and incubated in a 56°C water bath for 2 hours. This resulted in a >95% reduction in viable bacteria. The cecal contents were normalized based upon LPS concentration by the addition of sterile saline to yield cecal content solution with an LPS concentration of 500ng/ml. Recipient mice were prepared and anesthetized as described. Following a midline skin incision, 1ml of cecal content solution was injected through the abdominal wall into the abdominal cavity. The incisions were closed as described and the mice allowed to recover for 5 days before bacterial challenge.
2.2. Bacterial challenge and clearance
Pseudomonas aeruginosa (strain 19660, American Type Culture Collection, Rockville, MD) was inoculated into tryptic soy broth and allowed to replicate overnight in a shaking incubator at 37°C. The resulting bacterial culture was washed with 10ml of sterile 0.9% saline. Viable numbers of colony-forming units (cfu) were determined by plating serial dilutions overnight on tryptic soy agar. Bacteria were suspended in sterile 0.9% saline at a final concentration of 1×109 cfu/ml. Mice were challenged with 0.1 ml of this suspension (1×108 cfu; i.v.) 5 days after the CLP (or sham), cecal tissue implantation, or cecal content implantation procedures.
The mice were sacrificed under isoflurane 6 hours after intravenous injection of Pseudomonas aeruginosa. Spleens were aseptically excised, weighed and homogenized in sterile saline using sterile tissue grinders. Serial dilutions of tissue homogenates were plated on tryptic soy agar and incubated overnight at 37°C. Bacterial colony-forming units were counted to assess bacterial burden.
2.3. Measurement of plasma cytokines
Plasma concentrations of IFNγ and IL-10 were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, San Diego, CA) following the manufacturers recommended procedures.
2.4. Data analysis
Statistical analyses were performed using GraphPad Prism 4 software (La Jolla, CA). All data are presented as mean ± SEM. Multiple group data were analyzed by ANOVA and post-hoc Tukeys test while comparisons between 2 groups were performed by an unpaired t-test. A p value less than 0.05 was considered statistically significant.
3. Results
3.1. CLP caused impaired clearance of a subsequent Pseudomonas challenge
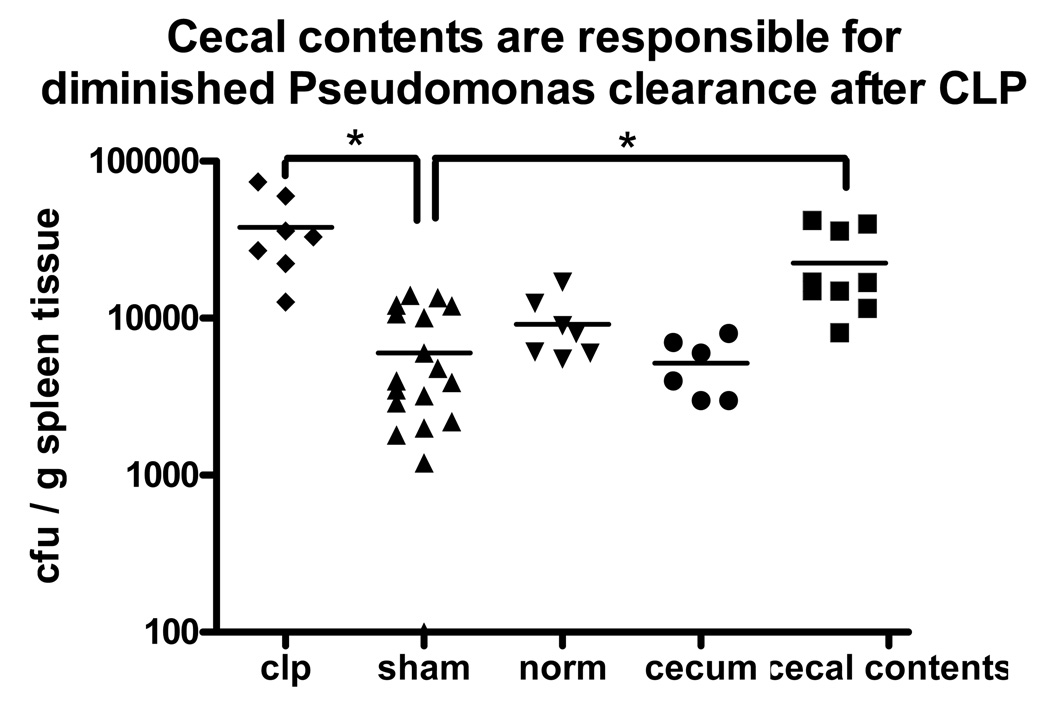
Mice were subjected to CLP or sham CLP and allowed to recover for 5 days. Mice subjected to CLP had overt signs of injury, including piloerection and lethargy for the first 1–2 days, but continued to eat, drink, and maintain near normal levels of activity afterward. There were no deaths in any of the groups. On the 5th day after these procedures, the mice were anesthetized briefly and subjected to an intravenous challenge of live Pseudomonas aeruginosa. The mice were sacrificed 6 hours later for collection of tissues. Mice that had been subjected to CLP had increased number of Pseudomonas bacterial colony-forming units in spleen tissue samples when compared to sham control mice. (Figure 1)
Figure 1. CLP caused impaired clearance of a subsequent Pseudomonas challenge, which could be replicated by abdominal contamination with cecal contents but not surgical trauma or cecal ischemia.
After Pseudomomas challenge, mice previously subjected to CLP had more Pseudomonas growth in their spleen homogenates when compared to sham mice. Neither surgical trauma alone (sham) nor cecal ischemia/necrosis impaired clearance of the Pseudomonas challenge when compared to normal, unmanipulated mice. However, mice subjected to abdominal contamination with cecal contents had more impaired clearance of the Pseudomonas challenge when compared to those from sham control mice. (* = p<0.05, N = 6–9/group).
3.2. Neither trauma (sham CLP) nor cecal ischemia caused impaired bacterial clearance. However, exposure to cecal luminal contents caused impaired clearance of a subsequent Pseudomonas challenge
To determine how the CLP model contributes to post-CLP impairment of innate immune function, each component of the CLP model was investigated individually. To test the effect of anesthesia and surgical trauma on subsequent immune function in the CLP model, the sham group clearance of the Pseudomonas challenge was compared to that of normal mice (no procedures prior to the Pseudomonas challenge) and was not found to differ significantly (Figure 1). To test the effect of cecal ischemia/necrosis on subsequent immune function, mice were subjected to abdominal incision as in the CLP/sham groups and had cecal tissue amputated from other mice placed within the abdomen prior to closure. Clearance of a Pseudomonas challenge 5 days later did not differ from that seen in the sham group of mice (Figure 1). Finally, to test the effect of systemic exposure to a polymicrobial population, mice were subjected to abdominal contamination with heat-killed cecal contents harvested from donor mice. A control group of mice were subjected to intraperitoneal was injected with saline alone. Five days later, the mice were challenged with an intravenous injection of live Pseudomonas aeruginosa. Clearance of the Pseudomonas challenge in the saline-injected control mice did not differ from that of sham or normal mice (data not shown). The mice that had been subjected to abdominal contamination with cecal contents had increased number of Pseudomonas bacterial colony-forming units in spleen tissues when compared to the control groups of mice. (Figure 1)
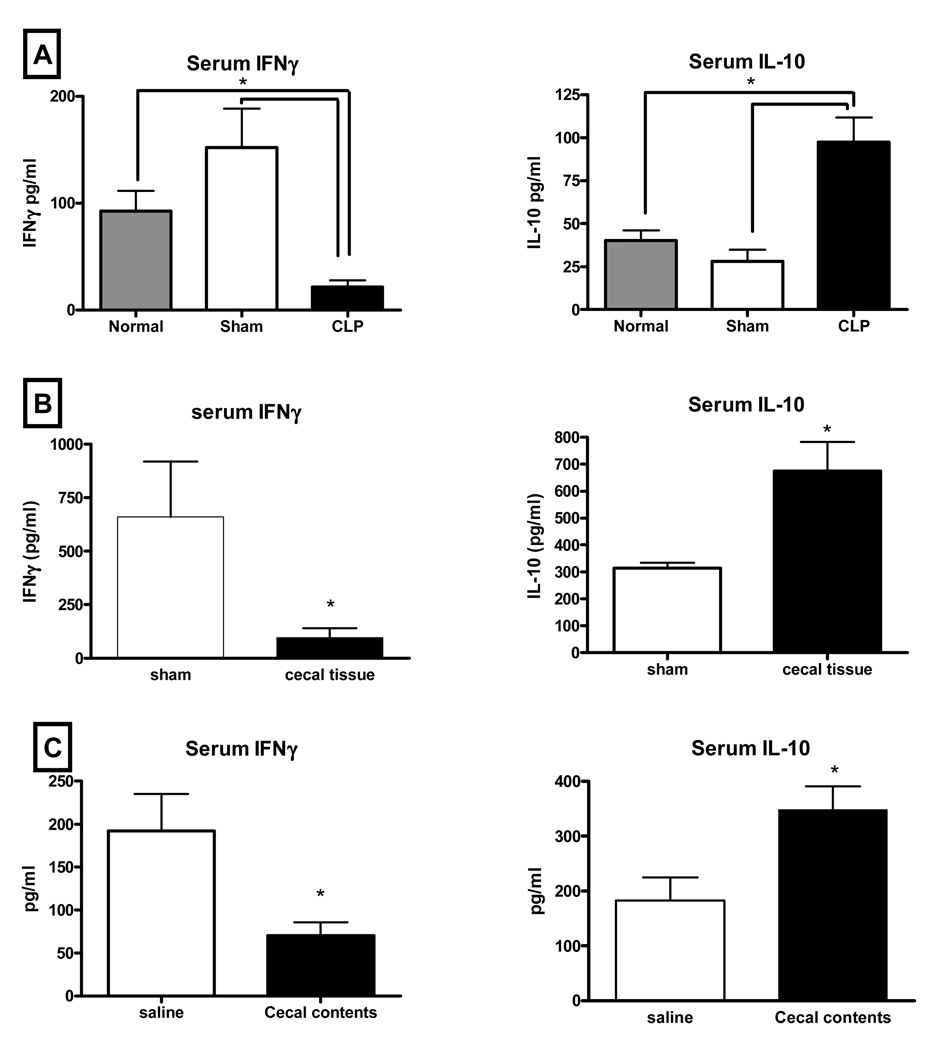
3.3. CLP, cecal ischemia/necrosis, and cecal contents caused increased serum IL-10 and decreased IFNγ responses to subsequent bacterial challenges while prior anesthesia and surgical trauma had no effect on the IFNγ/IL-10 response
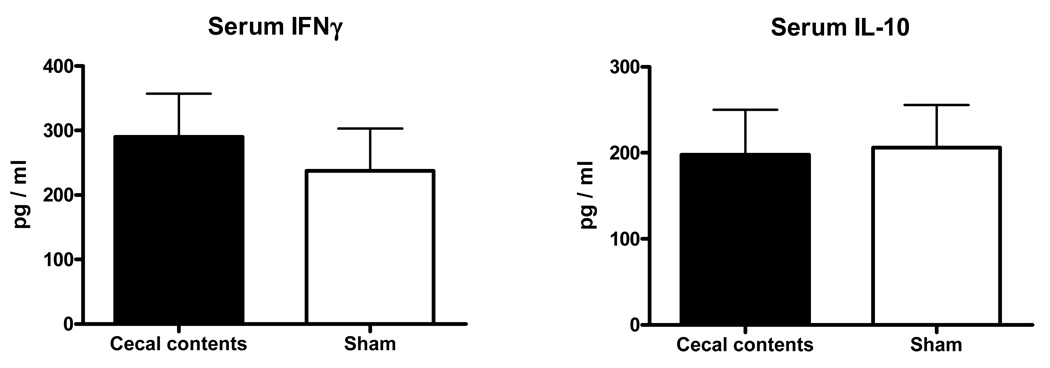
Induction of the TH1 cytokine IFNγ and the anti-inflammatory cytokine IL-10 are often used as indicators of the relative state of immune function. We measured these cytokines in each of the model variables to determine any association between the relative IFNγ/IL-10 balance and competency of bacterial clearance. Prior anesthesia and surgical trauma had no effect of the IFNγ/IL-10 response to Pseudomonas challenge compared to the response of normal mice with no prior manipulation. In these mice, circulating concentrations of IFNγ were measured to be higher than concentrations of IL-10 (Fig 2A). CLP was associated with a reversal of that balance, with IL-10 in higher concentrations in response to the bacterial challenge (Fig 2A). Although cecal ischemia/necrosis had no effect on bacterial clearance, the cytokine balance in those mice was shifted towards IL-10 (Fig 2B). Likewise, IL-10 predominated in the response to the bacterial challenge in mice previously subjected to abdominal contamination with cecal contents (Fig 2C).
Figure 2. Both cecal ischemia/necrosis and abdominal contamination with cecal contents were associated with a reversal of the IFNγ/IL-10 response to the Pseudomonas challenge while trauma alone had no effect.
The cytokine response to a Pseudomonas challenge in normal mice with no prior manipulation was characterized by high IFNγ and low IL-10 plasma concentrations and this was not affected in mice subjected to anesthesia and surgical trauma (shams) 5 days prior to the challenge (2A). However, the IFNγ/IL-10 serum cytokine balance was reversed in mice subjected to implantation of ischemic/necrotic cecal tissue (2B) or mice subjected to abdominal contamination with cecal contents (2C). (* = p<0.05, N = 10–14/group).
3.4. CLP/Sham of germ-free mice is not associated with impaired bacterial clearance
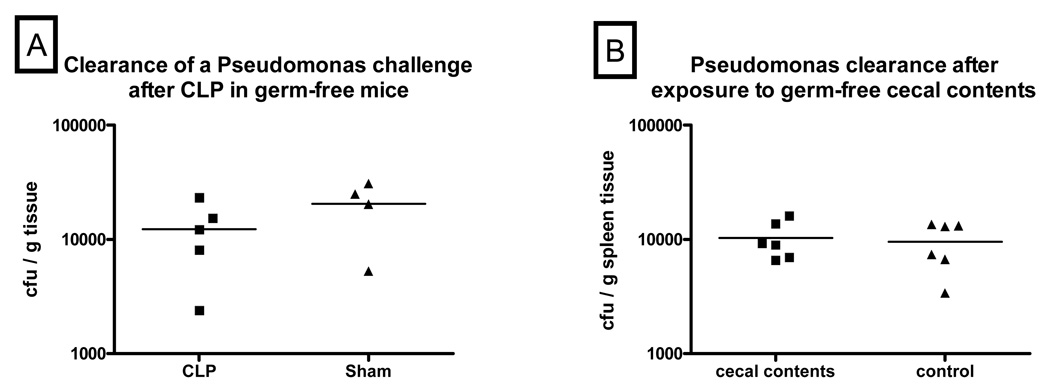
The previous experiments in mice with normal bacterial flora showed that exposure to the cecal contents, rather than surgical trauma or cecal ischemia/necrosis, was the component of CLP responsible for diminishing subsequent innate immune function. To determine whether this was induced specifically by microbial-related ligands in the cecal contents, we tested the effect of CLP on innate immune function in germ-free mice. CLP in germ-free mice was not associated with an impaired ability to clear a subsequent bacterial challenge when compared to sham animals. (Figure 3A)
Figure 3. Germ-free cecal contents did not cause impaired clearance of the Pseudomonas challenge.
Fig 3A – CLP did not have an adverse effect on clearance of a Pseudomonas challenge in germ-free mice as the amount of Pseudomonas growth did not differ between post-CLP and sham germ-free mice. (N = 4–5/group). Fig 3B - Intraperitoneal injection of a solution containing cecal contents harvested from germ-free donor mice into normobiotic, wild-type mice did not affect clearance of a subsequent Pseudomonas challenge as these mice had no difference in the amount of Pseudomonas growth in their spleen homogenates when compared to those from sham control mice. (N = 6/group)
3.5. Transfer of cecal contents from germ-free mice does not cause impaired bacterial clearance
To further test that the immunosuppressing factor in cecal contents was microbial-related, cecal contents from germ-free mice were used for abdominal contamination of C57BL6/J mice. As opposed to cecal contents harvested from normobiotic mice (Fig 1), abdominal contamination with germ-free cecal contents had no adverse effect on clearance of a subsequent bacterial challenge. (Figure 3B)
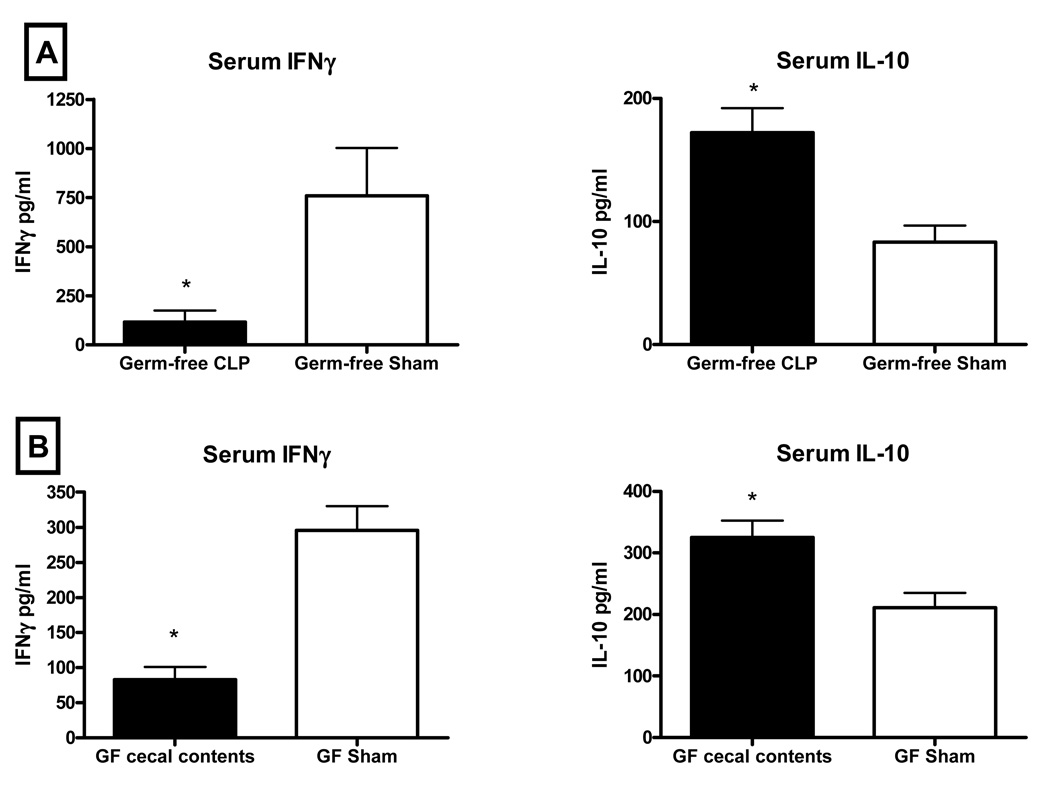
3.6. CLP or abdominal contamination with germ-free cecal luminal contents caused an increase in serum IL-10 and a decrease in IFNγ in response to the Pseudomonas challenge
Because neither CLP in germ-free mice nor abdominal contamination with germ-free cecal contents was associated with impairment of innate immune function, we did not expect that either had an effect on the IFNγ or IL-10 response to the Pseudomonas challenge. However, as in the same models in normobiotic mice, serum IFNγ was depressed and serum IL-10 was elevated in germ-free mice subjected to those manipulations compared to sham controls. (Figure 4)
Figure 4. Abdominal contamination with germ-free cecal contents was associated with lower serum concentrations of IFNγ and higher serum concentrations of IL-10.
Serum IFNγ was lower and IL-10 was higher in germ-free mice subjected to CLP prior to Pseudomonas challenge when compared to sham controls (4A). Abdominal contamination with cecal contents collected from germ-free donor mice resulted in a similar IL-10/IFNγ profile (4B). (* = p<0.05, N= 4–6/group).
3.7. Abdominal contamination with cecal luminal contents caused impaired clearance of a subsequent Pseudomonas challenge in mice lacking the TLR4 signaling pathway
To determine if LPS was the microbial-related factor that contributed to the decline in innate immune function after abdominal contamination, TLR4-deficient mice were subjected to abdominal contamination with cecal contents harvested from wild-type mice, or a similar volume of saline as a control. TLR4−/− mice in this group had impaired clearance of the Pseudomonas challenge as demonstrated by the increased number of Pseudomonas colonies on culture of their spleens when compared to control TLR4−/− mice. (Figure 5)
Figure 5. Abdominal contamination of TLR4-deficient mice with cecal contents was associated with impaired clearance of a subsequent Pseudomonas challenge.
Intraperitoneal injection of a solution containing cecal contents into TLR4-deficient mice impaired clearance of a subsequent Pseudomonas challenge as these mice had higher numbers of Pseudomonas colonies in their spleen homogenates when compared to those from sham control mice. (* = p<0.05, N = 4–5/group)
3.8. IFNγ and IL-10 responses to the Pseudomonas challenge in TLR4-deficient mice were not altered by prior abdominal contamination with cecal contents
To determine if TLR4 signaling played a role in the reversal of the IFNγ and IL-10 serum responses to Pseudomonas challenge after polymicrobial sepsis, plasma concentrations of IFNγ and IL-10 were measured 6hrs after Pseudomonas challenge in TLR4-deficient mice previously subjected to abdominal contamination with cecal contents harvested from wild-type mice, or saline control. Unlike wild-type mice subjected to models of abdominal contamination, which had suppressed IFNγ and elevated IL-10 plasma concentrations compared to sham controls, abdominal contamination in TLR4-deficient mice did not affect the IFNγ and IL-10 response to the Pseudomonas challenge. (Figure 6)
Figure 6. Abdominal contamination of TLR4-deficient mice with cecal contents did not affect IFNγ and IL-10 responses to a subsequent Pseudomonas challenge.
Plasma concentrations of IFNγ and IL-10 after the Pseudomonas challenge were similar in TLR4−/− mice previously subjected to either abdominal injection of cecal contents or saline control. (* = p<0.05, N= 4–5/group).
4. Discussion
Sepsis is not typically encountered clinically as a primary lesion but rather as occurring secondarily to a major illness or injury. It is commonly believed that these primary events are followed by a period of depressed immune function that predisposes the patient to the development of infections. To model this scenario in a clinically-relevant manner, we have utilized a ‘two-hit’ model system in which mice are subjected to a minimally lethal of CLP as the primary injury. Typically, abscess formation has walled off the affected portion of the cecum in the abdominal cavity by the time of the bacterial challenge 5 days later. As in patients who have suffered a major traumatic injury, mice have depressed immune function following the CLP injury and this is demonstrated by impaired clearance of a live bacterial challenge and alteration of the serum IFNγ/IL-10 balance when compared to non-injured mice [7,8]. CLP is a complex injury, however, and it was not clear whether impairment of immune function was attributable to the surgical trauma, the tissue ischemia/necrosis of the ligated cecum, the exposure to cecal luminal contents, or all 3 components together.
The present work indicates that anesthesia and surgical trauma in this model did not have an effect on the response to a subsequent bacterial challenge. Sham mice were subjected to those components and had no difference in bacterial clearance when compared to normal mice with no prior manipulations. Furthermore, the serum IFNγ and IL-10 responses were similar in both groups. These suggest that anesthesia and the surgical trauma associated with sham CLP had minimal effects on innate immune function. Another component of CLP involves ligation of the blood supply to the blind apex of the cecum, thus inducing tissue ischemia and ultimately tissue necrosis of the affected area. Prior investigators have reported that cecal ligation alone (ie, without puncture) was associated with an immunosuppressive phenotype based upon higher macrophage concentrations of IL-10, but did not see a higher rate of mortality in that model [9]. In this study, mice implanted with amputated cecal tissue also had a predominantly anti-inflammatory response to a subsequent Pseudomonas challenge, with lower serum IFNγ and higher IL-10 responses than seen in uninjured control animals. However, cecal ischemia/necrosis did not appear to have an adverse effect on subsequent innate immune function as mice implanted with cecal tissue implants did not have impairment of the ability to clear the subsequent bacterial challenge when compared to control mice.
Conversely, exposure to cecal luminal contents was associated with an adverse effect on subsequent innate immune function since mice subjected to abdominal contamination with cecal luminal contents had impaired clearance of a subsequent Pseudomonas challenge when compared to sham control mice. Factors within the luminal contents of the cecum are multiple and include dietary molecules including lipids and amino acids; gastrointestinal secretions including mucus, bile acids, and various enzymes; and a polymicrobial population. Exposure to some of these components may affect immune function. For example, bilirubin has been shown to impair bacteriocidal function in neutrophils [10]. However, our results suggest that CLP-induced immunosuppression is likely to be due to exposure to microbial-related factors as CLP in germ-free mice was not associated with subsequent impairment of bacterial clearance. Likewise, mice exposed to cecal contents from germ-free mice did not have impairment of bacterial clearance. Immunosuppression after exposure to cecal contents from normobiotic mice was not caused by live bacteria as the cecal contents were heat-killed, suggesting that induction of impaired innate immunity was due to microbial-associated molecules rather than microbial viability and function. Many of the bacterial-associated molecules are common to many species of bacteria and initiate an immune and/or inflammatory response after host recognition by innate immune receptors, including toll-like receptors (TLRs) or NOD-like receptors (NLRs). We have shown that exposure to some of these bacterial ligands can have prolonged effects on the efficacy of the innate immune response to subsequent bacterial challenges. For example, LPS is a cell wall molecule common to gram-negative bacteria and is a strong inducer of acute inflammatory and immune responses through TLR4. The prolonged effect after exposure to non-lethal amounts of LPS, however, is a depression of proinflammatory responses and an increase in anti-inflammatory responses to subsequent inflammatory challenges. We have shown that mice exposed to small doses of LPS also have an enhanced ability during the next few days to clear a subsequent bacterial challenge [11,12]. Similarly, we have also shown the improved clearance of a bacterial challenge in mice pre-exposed to TLR2 or NOD ligands [13,14]. The results from the TLR4-deficient mice in this study were consistent with those reports in that TLR4 activation/signaling appeared important in the development of inflammatory tolerance after abdominal contamination but did not contribute to impairment of bacterial clearance. We have observed that injection of mice with ligands for TLR5 or TLR9 had an adverse effect on clearance of a subsequent bacterial challenge (unpublished observations), so it is a possibility that microbial factors such as flagellin or bacterial DNA contributed to the impairment of bacterial clearance in our models.
While cytokine balances have been used frequently as surrogate assessments of the state of immune function, we did not see any relation between the serum balance of the IFNγ/IL-10 response and the efficacy of innate immune function after the bacterial challenge. The IL-10 response was predominant in the immunosuppressed CLP and cecal contents groups of mice compared to the IFNγ predominant response in the normal mice. However, the same IL-10 predominance was observed in groups of mice that did not display any change in innate immune function. This change of balance from Th1 IFNγ to the anti-inflammatory cytokine IL-10 was likely due to a development of inflammatory tolerance which may be induced by many TLR ligands and a myriad of other factors and is apparently independent of immune function. It was particularly interesting that the same change in the IFNγ/IL-10 balance occurred in the experiments in which mice were subjected to abdominal contamination with germ-free cecal contents, suggesting that inflammatory tolerance in these animals developed in the absence of microbe-related molecules.
Clinically, sepsis rarely occurs as a primary event and a recent clinical study showed that patients with sepsis have on average 1.07 co-morbid conditions [15]. Two-hit models have been suggested to be more clinically relevant for investigations [16]. CLP followed by bacterial challenge has been used by us and others to replicate the clinical scenario of sepsis occurring as a sequelae to a primary illness or injury. Steinhauser, et al, performed CLP followed by intratracheal instillation of Pseudomonas [17]. Muenzer, et al, also used CLP prior to induction of pneumonia with either Pseudomonas or Streptococcus [18]. Other clinically relevant two-hit models include bacterial challenge after hemorrhage or after burn injury. Suzuki, et al, used CLP as a polymicrobial challenge after the primary injury of controlled hemorrhage [19]. Thermal injury has been used in many studies of sepsis. Sepsis can clearly be established in burned mice by topical application of bacteria to the burn wound, but whether thermal injury causes immunosuppression in mice is not clear and there is really not a good control for topical application of bacteria in nonburned animals. Our experience has been that systemic bacterial challenges (i.v. or i.p.) are cleared as well, if not more rapidly, in mice several days after burn injury (Murphey, unpublished observations) when compared to non-burned control mice. Burned mice were also more resistant than controls to mortality caused by E.coli challenge [20]. Many other studies using two-hit models report depression of surrogate markers of immune function, including IFNγ response, depressed T cell proliferation, etc, in response to the second hit but, as we have shown, it is not always clear how well that these surrogates correspond to antimicrobial defense and clearance. Two-hit models have also been used to study immune function within specific organs injured by the first hit and include acid aspiration for study of the lung [21] and renal toxicity induced by administration of folic acid [22].
This study details the use of CLP not as a primary model of sepsis, but rather as a first hit that induces a period of impaired innate immune function that is manifested by diminished ability to clear a second hit of live bacteria. Exposure to microbial related factors in the intestinal lumen contributes to the decline in bacterial clearance capacity, as neither surgical trauma nor cecal ischemia/necrosis alone had the same effect. Further, it appeared that the injury to immune function was not dependent upon bacterial function because abdominal injection of heat-killed cecal contents impaired clearance of a subsequent Pseudomonas challenge. From a practical standpoint, cecal contents can be standardized based upon LPS content or some other factor and intra-abdominal instillation of cecal contents could be an easier and less variable model to achieve the same effect as CLP. Further work is needed to identify the molecule(s) within the intestinal lumen that lead to the decrease in innate immune function.
Acknowledgments
This work was supported by a grant from the NIH-NIGMS (K08 GM072857)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J. Surg. Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 2.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun. 1999;67:6603–6610. doi: 10.1128/iai.67.12.6603-6610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbull IR, Javadi P, Buchman TG, Hotchkiss RS, Karl IE, Coopersmith CM. Antibiotics improve survival in sepsis independent of injury severity but do not change mortality in mice with markedly elevated interleukin 6 levels. Shock. 2004;21:121–125. doi: 10.1097/01.shk.0000108399.56565.e7. [DOI] [PubMed] [Google Scholar]
- 4.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin. Microbiol. Rev. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat. Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphey ED, Lin CY, McGuire RW, Toliver-Kinsky T, Herndon DN, Sherwood ER. Diminished bacterial clearance is associated with decreased IL-12 and interferon-gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock. 2004;21:415–425. doi: 10.1097/00024382-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Murphey ED, Sherwood ER. Bacterial clearance and mortality are not improved by a combination of IL-10 neutralization and IFN-gamma administration in a murine model of post-CLP immunosuppression. Shock. 2006;26:417–424. doi: 10.1097/01.shk.0000226343.70904.4f. [DOI] [PubMed] [Google Scholar]
- 9.Ayala A, Song AGY, Chung CS, Redmond KM, Chaudry IH. Immune depression in polymicrobial sepsis: the role of necrotic (injured) tissue and endotoxin. Crit Care Med. 2000;28:2949–2955. doi: 10.1097/00003246-200008000-00044. [DOI] [PubMed] [Google Scholar]
- 10.Arai T, Yoshikai Y, Kamiya J, Nagino M, Uesaka K, Yuasa N, Oda K, Sano T, Nimura Y. Bilirubin impairs bactericidal activity of neutrophils through an antioxidant mechanism in vitro. J. Surg. Res. 2001;96:107–113. doi: 10.1006/jsre.2000.6061. [DOI] [PubMed] [Google Scholar]
- 11.Murphey ED, Fang G, Varma TK, Sherwood ER. Improved bacterial clearance and decreased mortality can be induced by LPS tolerance and is not dependent upon IFN-gamma. Shock. 2007;27:289–295. doi: 10.1097/01.shk.0000245024.93740.28. [DOI] [PubMed] [Google Scholar]
- 12.Murphey ED, Fang G, Sherwood ER. Endotoxin pretreatment improves bacterial clearance and decreases mortality in mice challenged with Staphylococcus aureus. Shock. 2008;29:512–518. doi: 10.1097/shk.0b013e318150776f. [DOI] [PubMed] [Google Scholar]
- 13.Murphey ED, Fang G, Sherwood ER. Pretreatment with the Gram-positive bacterial cell wall molecule peptidoglycan improves bacterial clearance and decreases inflammation and mortality in mice challenged with Staphylococcus aureus. Crit Care Med. 2008;36:3067–3073. doi: 10.1097/CCM.0b013e31818c6fb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphey ED, Sherwood ER. Pretreatment with the Gram-positive bacterial cell wall molecule peptidoglycan improves bacterial clearance and decreases inflammation and mortality in mice challenged with Pseudomonas aeruginosa. Microbes. Infect. 2008;10:1244–1250. doi: 10.1016/j.micinf.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit Care Med. 2006;34:2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998.;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J. Immunol. 1999;162:392–399. [PubMed] [Google Scholar]
- 18.Muenzer JT, Davis CG, Dunne BS, Unsinger J, Dunne WM, Hotchkiss RS. Pneumonia after cecal ligation and puncture: a clinically relevant "two-hit" model of sepsis. Shock. 2006;26:565–570. doi: 10.1097/01.shk.0000235130.82363.ed. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T, Shimizu T, Szalay L, Choudhry MA, Rue LW, III, Bland KI, Chaudry IH. Androstenediol ameliorates alterations in immune cells cytokine production capacity in a two-hit model of trauma-hemorrhage and sepsis. Cytokine. 2006;34:76–84. doi: 10.1016/j.cyto.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Maung AA, Fujimi S, MacConmara MP, Tajima G, McKenna AM, Delisle AJ, Stallwood C, Onderdonk AB, Mannick JA, Lederer JA. Injury enhances resistance to Escherichia coli infection by boosting innate immune system function. J. Immunol. 2008;180:2450–2458. doi: 10.4049/jimmunol.180.4.2450. [DOI] [PubMed] [Google Scholar]
- 21.Nemzek JA, Call DR, Ebong SJ, Newcomb DE, Bolgos GL, Remick DG. Immunopathology of a two-hit murine model of acid aspiration lung injury. Am. J. Physiol Lung Cell Mol. Physiol. 2000;278:L512–L520. doi: 10.1152/ajplung.2000.278.3.L512. [DOI] [PubMed] [Google Scholar]
- 22.Doi K, Leelahavanichkul A, Hu X, Sidransky KL, Zhou H, Qin Y, Eisner C, Schnermann J, Yuen PS, Star RA. Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome. Kidney Int. 2008;74:1017–1025. doi: 10.1038/ki.2008.346. [DOI] [PMC free article] [PubMed] [Google Scholar]