Abstract
Purpose
The purpose of this study is to determine whether a Protected Graft Copolymer (PGC) containing fatty acid can be used as a stabilizing excipient for GLP-1 and whether PGC/GLP-1 given once a week can be an effective treatment for diabetes.
Methods
To create a PGC excipient, polylysine was grafted with methoxypolyethyleneglycol and fatty acid at the epsilon amino groups. We performed evaluation of 1) the binding of excipient to GLP-1, 2) the DPP IV sensitivity of GLP-1 formulated with PGC as the excipient, 3) the in vitro bio-activity of excipient-formulated GLP-1, 4) the in vivo pharmacokinetics of excipient-formulated GLP-1, and 5) the efficacy of the excipient-formulated GLP-1 in diabetic rats.
Results
We showed reproducible synthesis of PGC excipient, showed high affinity binding of PGC to GLP-1, slowed protease degradation of excipient-formulated GLP-1, and showed that excipient-formulated GLP-1 induced calcium influx in INS cells. Excipient-formulated GLP-1 stays in the blood for at least 4 days. When excipient-formulated GLP-1 was given subcutaneously once a week to diabetic ZDF rats, a significant reduction of HbA1c compared to control was observed. The reduction is similar to diabetic ZDF rats given exendin twice a day.
Conclusions
PGC can be an ideal in vivo stabilizing excipient for biologically labile peptides.
Keywords: Glucagon like peptide, peptide excipient, Protected Graft Copolymer, Diabetes
INTRODUCTION
Incretins such as Glucagon-like peptide-1 and its mimetics are considered to be efficient type 2 diabetic therapeutics (1–4). The Glucagon-like peptide-1 (GLP-1) possesses many glucoregulatory functions and ameliorates endocrine pancreatic functions by: stimulating glucose-dependent insulin secretion, suppressing glucagon secretion, reducing gastric mobility and food intake, and stimulating pancreatic β-cell proliferation and/or neogenesis (3, 4). The short biological half-life of GLP-1, due to enzymatic degradation by dipeptidyl peptidase IV (DPP IV), encouraged numerous researchers to focus on the development of DPP IV resistant GLP-1 analogs and these efforts resulted in the successful clinical application of exendin-4 from lizard venom (5–7). However, being a foreign peptide, antibody production was observed in a significant portion of patients using exendin-4 (8). Although the co-administration of exendin-4 with traditional hypoglycemic agents improved the anti-diabetic effects of treatment, such as decreased glycosylated hemoglobin (HbA1c) levels and body weights of type 2 diabetic patients, a strong demand exists for long acting GLP-1 with improved therapeutic effects from extending the duration of actions (7, 9, 10). Furthermore, low molecular weight peptide incretins and their mimetics are eliminated by glomerular filtration and enzymatic degradation, for example exendin-4 has an elimination half-life of only 2.4 h (11, 12). To address this issue, researchers have focused on the development of long-acting incretins, their mimetics, or sustained delivery systems. Specific approaches target the prevention of rapid renal clearance by increasing the molecular size (13–17). The strategies adopted to date include modifications of GLP-1 receptor agonists by poly(ethylene glycol) conjugation, known as PEGylation (15–17), or chemical or genetic modifications with serum albumin (13, 14). Other approaches involve the acylation of incretin (18), and its mimetics (9, 19–21), allowing for albumin binding and preventing rapid kidney elimination. An approach using a sustained-release formulation of GLP-1 receptor agonist based on biodegradable microsphere technology is also being investigated in the context of long-acting anti-diabetics (10). A new approach is formulation of native GLP-1 in an affinity based polymeric carrier such as the protected graft copolymer (PGC) with an affinity for GLP-1 that is greater than the affinity of DPP IV for GLP-1, but less than the affinity of GLP-1 for its receptor.
In this study, we designed long-acting native GLP-1 for type 2 diabetic therapy. Specifically, we developed a PGC with a hydrophobic region made up of stearic acid covalently linked to a polymer backbone. This PGC has a dissociation constant (Kd) of 250nM which is lower than the Michaelis-Menten constant (Km) of DPP IV for GLP-1 (1–3uM) but greater than the Kd of GLP-1 to its receptor (1–2nM). Because the formulation is affinity based, a decrease in free GLP-1 resulting from binding to its receptor or normal metabolism will result in the release of more GLP-1 from PGC. The PGC is made up of a 20 kDa polylysine backbone in which 50–55% of the epsilon amino groups are derivatized with 5kDa PEG and the remaining amino groups are derivatized with stearic acid. The overall hydrodynamic diameter of this PGC is 20nm which allows trafficking from a subcutaneous site to the blood. We present data relating to the binding of native GLP-1 to PGC, protection of PGC-GLP-1 from DPP IV degradation, in vitro biological activity of PGC-GLP-1, extension of blood circulation half-life of PGC-GLP-1, and the ability of the PGC-GLP-1 formulation, administered once a week, to lower HbA1c levels in ZDF rats similar to the action of exendin-4 given twice a day.
MATERIALS AND METHODS
Materials and Animals
GLP-1, exendin-4 and N-hydroxysulfosuccinimide (NHSS) were purchased from ChemPep, Inc (Miami, FL). The following were from Sigma-Aldrich (Saint Louis, MO) irrespective of the original manufacturer: 20 kDa Polylysine (20PL; SAFC catalog #Q4926), N-hydroxysuccinimide (NHS); 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide Hydrochloride (EDC); Dichloromethane (DCM), N,N-Diisopropylethylamine (DIPEA), and Porcine kidney DPP IV. Morpholino ethane sulfonate (MES) buffer and Dicyclohexylcarbodiamide (DCC) were from Thermo Scientific/Pierce (Rockford, IL). Filter papers, Chloroform, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer, 10×PBS (1x is 137mM NaCl, 2.7mM KCl, 10mM Phosphate) buffer and Stearic acid (C18; Alfa Aesar) were from Fisher Scientific (Pittsburgh, PA). Methoxy-polyetheleneglycol-succinate (MPEG) was from Laysan Bio (Arab, AL). All chemical reagents for PGC synthesis, unless otherwise indicated, were of 95% purity or greater. The ELISA Kit for biologically active GLP-1 was purchased from Linco/Millipore Cat. # EGLP-35K (Billerica, MA). Microcentrifuge filters with 100 kDa molecular weight cut-off (regenerated cellulose filter, YM-100) for separation of PGC bound and free GLP-1 were from Millipore (Billerica, MA). HPLC Gel Permeation Columns (GPC) for hydrodynamic size determination of PGC (0.78×30cm; G4000PWXL) were from Tosoh Bioscience (King of Prussia, PA). Globular protein standards of known hydrodynamic diameters were from Sigma-Aldrich (Saint Louis, MO). An additional HPLC Gel Permeation Column for determination of PGC binding to GLP-1 (0.78 × 30 cm; BioSEP S2000 column) plus a reverse phase column (Synergi 2.5um Max, 0.4×2cm) for quantification of GLP-1 were from Phenomenex (Torrance, CA). An Ultrafiltration cartridge (100kDa MWCO; UFP-100-E-5A) was from GE Healthcare Life Sciences (Piscataway, NJ). Assay kits for blood glucose levels and Hemoglobin A1c (HbA1c) were from Bayer (Sunnyvale, CA). The Fura-2AM for Calcium influx assay was from Invitrogen (Carlsbad, CA). A stock of INS-1 cells was a gift from Dr. Paul Robertson (President of ADA). ZDF rats were from Charles River Laboratories (Wilmington, MA). All animals were cared for in accordance with the National Institute of Health (NIH) guidelines for the care and use of laboratory animals (NIH publication 85–23, revised 1985).
Preparation and purification of PGC and PGC-C18
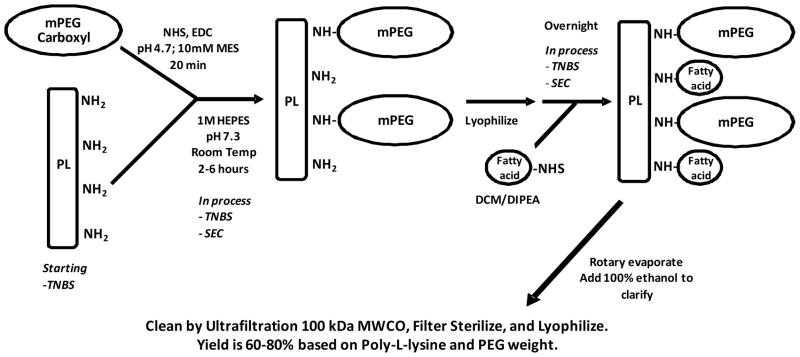
Poly-L-lysine (20PL), hydrobromide (21μmol or 1g; Sigma, Average Mw = 26kDa; d.p.126) was dissolved in 25 ml of 1M HEPES, pH 7.4. An aliquot of this solution was removed for NH2-groups determination by TNBS titration (22) and found to be 2.6mmol. Methoxy polyethylene glycol carboxymethyl (MPEG-CM; 10g; Mw=5kDa; 2 mmol; Laysan Bio) was dissolved in 25 ml of 10mM MES pH=4.7 with 4mmol NHSS, and, once dissolved, 6mmol EDC was added while stirring. Activation was allowed to proceed for 20 minutes and the activated MPEG-CM was added directly to the 20PL solution. The pH of the solution was adjusted to 7.7 using NaOH and stirred for 2 hours at room temperature. An aliquot was taken and amino groups were measured by TNBS and found to be 1.2 mmol, indicating 54% MPEG-CM saturation. The hydrodynamic diameter was determined by Size Exclusion chromatography using TosohG4000WXL (0.78 × 30cm) and found to be 14.4 nm. The crude product (crude PGC) was lyophilized. The synthetic scheme for the preparation of PGC is presented in Figure 1.
Fig. 1. Schematic illustration of synthetic scheme for PGC as described in the methods.
Stearyl-NHS (C18-NHS) was prepared by dissolving stearic acid (20g or 71mmol) in 115ml chloroform at 55°C. NHS (8.3g or 72mmol) was dissolved in 42ml THF and added to the stearic acid solution. DCC (11.6ml or 72mmol) was added and stirred for 2 hours. The solution was cooled down on ice and the precipitate (DCC-urea, un-reacted fatty acid and NHS) was removed by filtration (Fisher filter paper P2). The filtrate containing C18-NHS was rotary evaporated and dissolved in 70°C isopropanol (500ml), followed immediately by filtration (Fisher filter paper Q8). The filtrate remained clear initially and was allowed to crystallize overnight. The next day, C18-NHS crystals were collected by filtration (Fisher filter paper Q8) and dried.
Crude PGC-C18 was dissolved in ~100ml DCM. The solution was vortexed and centrifuged for 12 min at max speed to remove precipitates. The precipitate was further washed with ~50ml DCM. The supernatants were pooled and the solid was discarded. C18-NHS (3.6mmol; 3x molar excess over amino groups) in 20ml DCM was added to the pooled supernatant with magnetic stirring. DIPEA (6mmol; 5x molar excess over amino groups) was added and allowed to react for 4 hours. Additional C18-NHS (3.6mmol) was added and allowed to react overnight. An aliquot was taken, dried, and dissolved in water for amino group analysis and a trace amount (0.06mmol) of total amino groups was indicated by TNBS assay. The reaction mixture was concentrated by rotary evaporation (bath temp = 41°C) under vacuum to remove DCM/DIPEA until it formed a thick oil. The oil was dissolved in ethanol (~100ml) followed by the addition of ~10ml of water. The solution was filtered through glass-fiber filter (Fisher G6). The filtrate loaded in a QuixStand™ benchtop system with a 100kDa MWCO ultrafiltration cartridge (UFP-100-E-5A), concentrated to 100 ml, washed with 80% ethanol (13 volume changes) followed by water (10 volume changes). The retentate (PGC-C18) was collected, 0.2 um filtered (polysulfone filter, Nalgene, Rochester, NY) and lyophilized, yielding ~8 grams. One mg/ml was analyzed and found to contain 7nmol amino groups/mg with GPC (0.78×30cm; G4000PWXL) retention time of 12.2 min (or approximately 18.6nm diameter). The GPC column was calibrated using globular protein standards of known hydrodynamic diameters: bovine thyroglobulin, horse apoferritin, Catalase, Yeast alcohol dehydrogenase, bovine serum albumin, and ribonuclease with hydrodynamic diameters of 17.10, 15.60, 10.44, 9.20, 7.11, and 3.28nm, respectively. A regression line equation of the plot of square root of negative logKav values against known hydrodynamic diameters was used to obtain hydrodynamic diameters of the PGC.
Binding of GLP-1 to PGC-C18
Binding experiments were performed using a GPC column (0.78×30cm; BioSEP S2000, Phenomenex) eluted with PBS at a flow rate of 1.5ml/min. Under these conditions, PGC-C18 alone eluted at the Void volume (Vo) and GLP-1 alone eluted at the Total volume (Vt). To qualitatively determine the GLP-1 bound to PGC-C18, varying concentrations of PGC-C18 (5, 10, 20 mg/ml; 50ul injection volume) alone were injected onto the column as blanks and the same concentrations were injected in the presence of 2mg/ml GLP-1. The chromatograms of the PGC-C18 blanks at 220nm were subtracted from the chromatograms of the PGC-C18/GLP-1 mixture to accurately track the binding of GLP-1 to the carrier as GLP-1 moved to the Vo.
To determine the dissociation constant of GLP-1 to the PGC-C18 carrier, 5 mg of PGC-C18/tube was mixed with 0.50, 0.40, 0.30, 0.25, 0.20, 0.15, 0.10 mg of GLP-1 in 250ul of 100mM HEPES buffer, pH 7.4. The samples were filtered using a 5nm diameter cutoff centrifugal filter (100kDa MWCO, regenerated cellulose filter, YM-100) and the filtrate containing free (unbound) GLP-1 (3297.7Da; 1.6nm diameter) was quantified by reverse phase HPLC (Synergi 2.5um Max, 0.4×2cm) at a flow rate of 1.5ml/min using a gradient of 0%B for 1min and 25–50% B from 1–5min, where A is 5% acetonitrile with 0.1%TFA and B is 100% acetonitrile with 0.1% TFA. The filter retentate containing bound GLP-1 was eluted twice with 100 ul of 70% acetonitrile and diluted with water for reverse phase HPLC quantitation as above. The filter used does not bind GLP-1 (data not shown) and thus all GLP-1 retained by the filter are bound to the 350kDa PGC-C18. The bound/free GLP-1 was plotted along the y-axis against bound GLP-1 (nM) along the x-axis. The slope of the graph (Scatchard plot) of bound/free on the y-axis and bound (nM) x-axis was taken as −1/Kd, where Kd is the dissociation constant. The capacity per PGC-C18 was taken as the x-intercept divided by molar concentration of the PGC-C18 used.
DPP IV degradation of GLP-1 in the presence or absence of PGC or PGC-C18
To test the ability of PGC-C18 to protect GLP-1 from DPP IV degradation, 1mg/ml of GLP-1 in PBS buffer, pH 7.4 was incubated with 1.25mU/ml of DPP IV for 24 hour at 37°C in the presence of 10mg/ml PGC-C18 or 10mg/ml PGC (Polylysine with PEG but without C18). Next, an equal volume of isopropyl alcohol was added to dissociate the GLP-1 from the PGC and the solutions were filtered through the 100 kDa MWCO filter as above. The filtrates containing degraded and un-degraded GLP-1 were analyzed by reverse phase HPLC as above. DPP IV removes the N-terminus of GLP-1 containing histidine and, under the acidic conditions of the HPLC column, the degradation product is more hydrophobic and is retained longer by the column (seen as a later eluting peak, 2.82min vs. 2.70min for intact GLP-1).
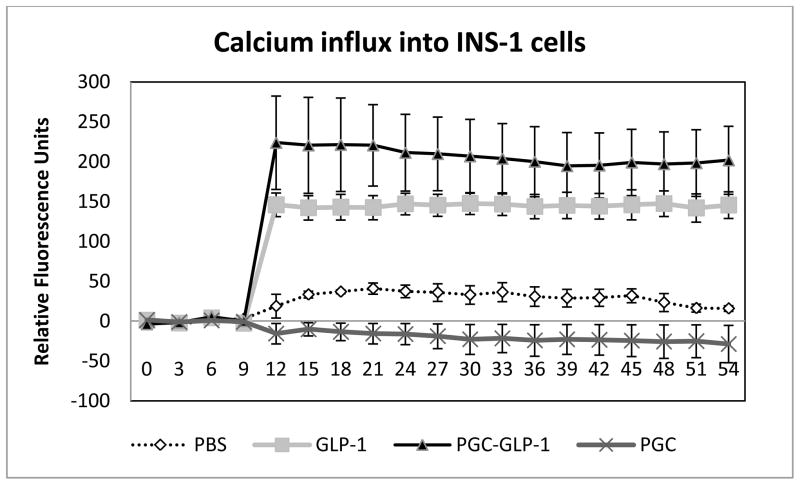
PGC-C18- GLP-1 formulation stimulated Ca influx in INS-1 cells
To determine whether formulated GLP-1 is still active, we performed a Ca influx assay in INS-1 cells. INS-1 cells are insulinoma cells expressing GLP-1 receptors. A calcium influx assay was used to confirm that the bound GLP-1 maintained its activity after formulation. For this assay, INS-1 cells were seeded in black 96 well plates at 200,000 cells per well in a buffered medium containing RPMI/10%FBS/11.1mM glucose/10mM HEPES, pH7.4/1mM pyruvate/50uM 2-Mercaptoethanol. Cells were allowed to adhere overnight and then labeled by replacing the medium with 20uL of 5 ug/ml Fura2 in PBS pH7.4/2%FBS/11.1mM glucose; incubation for two hours was followed by the addition of 180 uL PBS pH7.4/2%FBS/11.1mM glucose. Fluorescence (340ex/510em) was monitored using a BioScan Chameleon (Washington, DC) and, after 10 seconds, free GLP-1 and PGC-C18-GLP-1 (n=6 each) was added to a final GLP-1 concentration of 30 nM and monitoring was continued for 1min. Prior to testing, ionomycin was used as a positive control to ensure that the cells were properly loaded with Fura2 and the Calcium influx could be properly detected under these conditions (not shown). For a control, an equivalent amount of PGC-C18 or PBS (n=6 each) was analyzed. The influx of calcium into the cells is reflected by an increase in fluorescence. The average of 6 tracings from each group was obtained.
Pharmacokinetic profiles of GLP-1 and GLP-1 in PGC-C18
GLP-1 was formulated with or without 50x weight of PGC-C18 (2% loading) in saline and given subcutaneously at a dose of 1mg GLP-1/kg in Sprague-Dawley rats (n=10). The rats were bled at 0, 1, 20min, 1, 2, 6, 24, 48, 72, 96, and 144h. To prevent further degradation of GLP-1, blood samples were collected in tubes containing DPP IV inhibitor that comes with the GLP-1 ELISA kit. The assay for blood GLP-1 was done using a sandwich-type GLP-1 ELISA kit that uses alkaline phosphatase and the MUP (methyl umbelliferyl phosphate) fluorogenic substrate. The assay was done according to the kit manufacturer’s instructions.
Efficacy of PGC-C18 GLP-1 formulation in the treatment of a type 2 diabetes animal model
Zucker diabetic fatty (ZDF) rats were treated for 7 weeks (n=10/group) with saline (daily), GLP-1 (1mg/kg, 3x/week), exendin (3ug/kg, 2x/day), or PGC-C18-GLP-1 (1mg/kg, 1x/week or 2x/week). The average starting HbA1c (Average +/− standard deviation; n=10) at the beginning of the treatment (week 0) for saline group, exendin-4 group, PGC-C18 GLP-1 group 1x/week, and PGC-C18 GLP-1 group 2x/week were 7.7+/− 0.91, 7.5+/− 0.72, 9.5+/− 0.72, and 9.5+/− 0.94, respectively. The PGC-C18-GLP-1 formulation contained 1mg GLP-1 per 50mg of PGC-C18 (2% loading) in saline. The dosing of GLP-1 compared to exendin-4 was based on the fact that exendin-4 is 5000x more potent than GLP-1 (24) therefore the total weekly dose for exendin of 42ug would have the equivalent biological activity of 210mg GLP-1. When combined with PGC-C18, however, the GLP-1 dose to achieve the same biological activity was reduced by 210-fold. All treatments were given subcutaneously at the dorsal shoulder. HbA1c is a proxy for the average blood glucose level over the past 3–5 weeks and was measured using a HbA1c kit (A1Cnow) at 7 weeks.
Stability of PGC-C18-GLP-1 formulation
To get a sense of the stability of GLP-1 formulation as a potential commercial product, we incubated lyophilized GLP-1 mixed with PGC-C18 at 1 to 50 w/w (GLP-1 to PGC-C18) ratio and GLP-1 alone. These were lyophilized in sterile water and there was no salt or buffer in these lyophilized powders. We also incubated similar vials after reconstitution in sterile 100mM glycine, pH 6.8, to obtain a final GLP-1 concentration of 2mg/ml. These lyophilized powders and liquid solutions were incubated at 37°C and aliquots were taken monthly for analysis of free GLP-1 and PGC-C18 bound GLP-1 by reverse phase HPLC as above. An increase in free GLP-1 (not bound to PGC-C18) without corresponding degradation with time is an indication of either a loss of GLP-1 secondary structure responsible for binding to PGC-C18 or a breakdown of the carrier. The stability study was stopped when a significant degradation (10%) of GLP-1 was observed based on the total area of GLP-1 peak (both PGC-C18 bound GLP-1 and free GLP-1).
RESULTS
Reproducibility of PGC-C18 Synthesis
Synthesis of several batches of PGC-C18 indicates good reproducibility. In Table I below are the in-process quality control parameters that are measured to ensure reproducibility of PGC-C18 from batch to batch. This indicates that the synthesis of the carrier is reproducible at two batch sizes and the synthetic process is robust and can easily be transferred to a cGMP facility to produce sufficient material for clinical trials.
Table I.
Reproducibility of PGC-C18 carriers
| PGC-C18 Lot # | 70510A | 70510B | 70613C |
|---|---|---|---|
| Polylysine degree of pofymerization (average) (Polydispersity Mn/Mw) | 115 (1.2) | 115 (1.2) | 115 (1.2) |
| % PEG Sat | 54% | 54% | 55% |
| Carrier Retention Time Before Fatty acid Addition (min) using Tosoh GX4000XL (0.78×30 cm) | 12.9 | 12.9 | 12.9 |
| Size Diameter of PGC | 14 nm | 14 nm | 14 nm |
| Carrier Retention Time After Fatty acid Addition (min) using Tosoh GX4000XL (0.78×30 cm) | 12.16 | 12.22 | 12.03 |
| Carrier Diameter of PGC-C18 | 19 nm | 18 nm | 19 nm |
| Yield (based on starting PEG plus Polylysine weight as 100%) | 79% | 81% | 81% |
| Amount | 8.9 g | 9.2 g | 4.5g |
| NH2 left in Carrier (started at 3umol/mg PL; % +/− STD) | 6 nmol/mg (4+/−2%) | 8 nmol/mg (5.2+/−2%) | 10 nmol/mg (6.6 +/− 2%) |
| GLP-1 loading test (%loaded +/− STD when GLP-1 at 2 % of carrier weight was loaded in 10mg/ml Carrier) | 95.8 +/− 0.05 | 96.1 +/− 0.43 | 95.9 +/− 0.09 |
| Lysine content by weight after complete acid digestion | 4.96% | 4.87% | 4.82% |
Binding of GLP-1 to PGC-C18
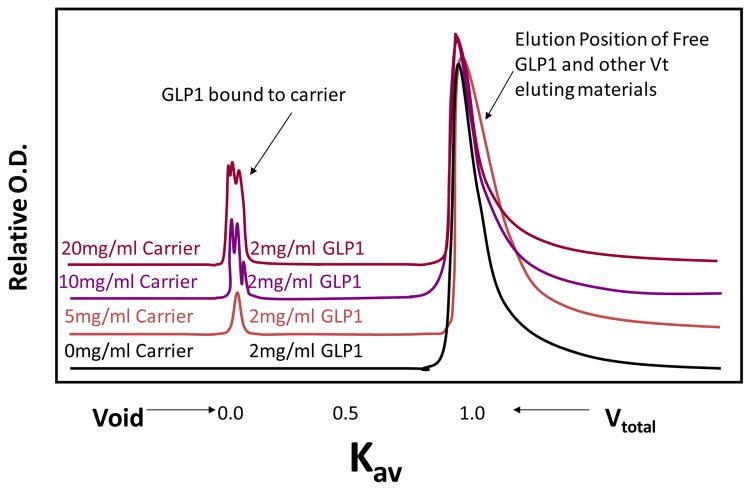
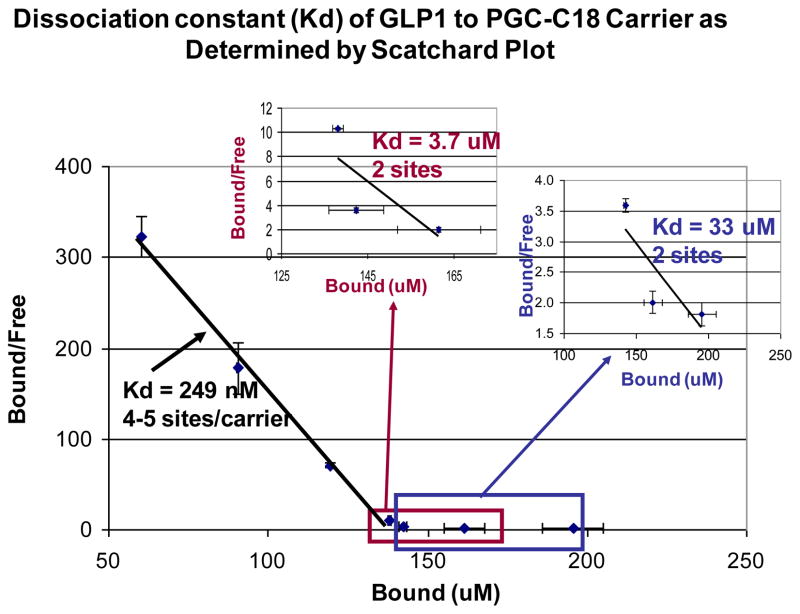
We used a gel permeation column (0.78 × 30 cm; BioSEP S2000 column) to initially determine the ability of PGC-C18 to bind GLP-1. Shown in Figure 2 are chromatograms of GLP-1 samples in the absence or presence of various concentrations of PGC-C18. In this particular column, GLP-1 (mw=3kDa) alone elutes at the column total volume (Vt) and the PGC-C18 (Mw=350kDa) alone elutes at the void volume (Vo); the peaks for PGC-C18 alone were quantitatively subtracted from each of the chromatograms. As can be seen, as the GLP-1 weight decreases relative to PGC-C18 weight (40%, 20%, and 10% of PGC-C18 weight) or as the GLP-1 loading of the PGC-C18 decreases, more GLP-1 is seen at Vo indicating that GLP-1 binds to the carrier. This was confirmed by determination of Kd using a Scatchard plot. Figure 3 shows high affinity binding of GLP-1 to the PGC-C18 with a Kd of 248nM and a capacity of 4–5 GLP-1 molecules per PGC-C18 molecule. In addition there are some low affinity binding sites that can be observed with apparent Kds of 3.7 and 33uM and capacities of 2 GLP-1 molecules per PGC-C18 molecule in both cases. It is possible that the overall GLP-1 binding observed in PGC-C18 carrier is an average of many heterogeneous sites that range from very high affinity to low affinity, rather than a very distinct population of sites present in a polymeric structure. In both the gel permeation column binding analysis and the filtration assay for bound and free GLP-1, no binding was observed with PGC without C18. This indicates that C18 is essential for GLP-1 binding to PGC-C18.
Fig. 2. Gel Permeation HPLC chromatograms showing the extent of binding of GLP-1 to various concentrations of PGC-C18 carrier.
Four chromatograms monitored at 220nm are shown. The corresponding PGC-C18 signal was already subtracted from each of the chromatograms of the PGC-C18-GLP-1 mixtures, leaving only the signal due to GLP-1. As the amount of PGC-C18 in the mixture was increased from 0 mg/mL through 5, 10, and 20 mg/ml, the relative amount of GLP-1 carried by PGC-C18 from the total column volume (Vtotal) to the Void volume (Vo) increases. Chromatograms also demonstrate the stability of the complex between GLP-1 and PGC-C18; during the passage of the PGC-C18-GLP-1 complex through the column (0.78 × 30 cm; BioSEP S2000 column), the complex undergoes several thousand re-equilibrations (equal to the number of theoretical plates) driven by the thermodynamic equilibrium defined by Kd. If the interaction is weak, no GLP-1 should be present in the column void volume. The fact that GLP-1 co-elutes with the carrier at the Vo indicates strong interaction.
Fig. 3. Scatchard plot of GLP-1 binding to PGC-C18 shows various affinities.
In this particular experiment three Kd values (249 nM, 3.7 uM, and 33 uM) were calculated over three ranges of bound GLP-1 concentration. The % of GLP-1 bound to the carrier varies based on the available GLP-1. It is evident from this data that there is a range of interaction from high affinity (Kd of nM) to lower affinity interactions (Kd of uM) between the carrier and PGC-C18, and that a PGC-C18 capacity for GLP-1 of up to 9 is attainable. Those sites with average Kd of 249 nM have a capacity of 4–5 GLP-1(3kDa) molecules per molecule of PGC-C18 (350kDa). Those sites with Kd of 3.7uM have a capacity of 2 GLP-1 molecules per molecule of PGC-C18. Those sites with Kd of 33uM have a capacity of 2 GLP-1 molecules per molecule of PGC-C18. The Kd of the sites with the least affinity is very similar to the Km of DPP IV to GLP-1 (36uM), indicating that this site may not be a good sequestering site to protect GLP-1 from the DPP IV enzyme. In this particular experiment, 500uL samples were prepared in triplicate containing 0 or 5.0 mg carrier with 0.10, 0.15, 0.20, 0.25, 0.30, 0.40, and 0.50 mg of GLP-1 in PBS, pH 7.35. These correspond to 2%, 3%, 4%, 5%, 6%, 8% and 10% loading relative to carrier weight, respectively.
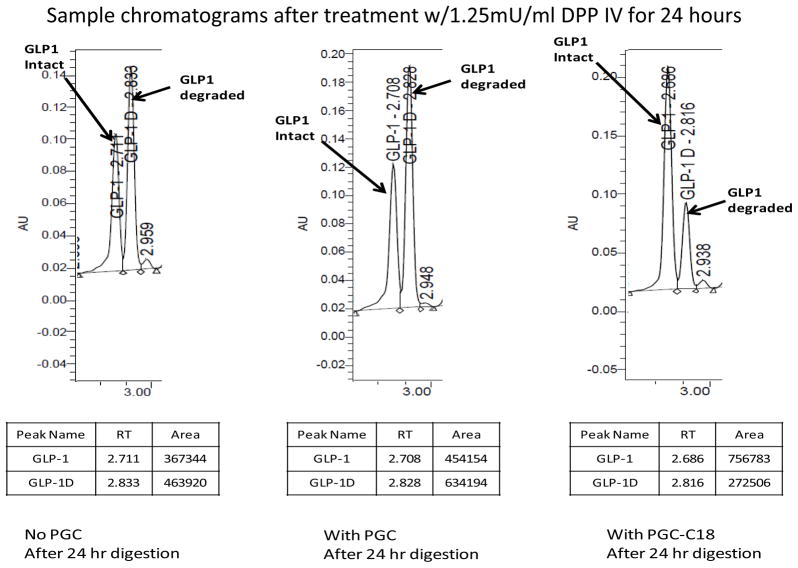
PGC-Hydrophobic Core (PGC-HC) protects GLP-1 from rapid DPP IV digestion
Only the carrier containing C18 (which is essential for binding) would protect the GLP-1 from DPP IV degradation. This also indicates that binding of GLP-1 to PGC-C18 is essential for protection of GLP-1 from DPP IV digestion. In vivo, DPP IV is involved in glucose metabolism, with GLP-1 being one of its substrates. Figure 4 illustrates the ability of the carrier to protect GLP-1 from DPP IV degradation. DPP IV removes the N-terminus of GLP-1 containing histidine and, under the acidic conditions of the HPLC column, the degradation product is more hydrophobic and retained longer by the column (seen as a later eluting peak, 2.82min vs. 2.70min for intact GLP-1). The weight ratio of GLP-1 to PGC-C18 or PGC used in this experiment was 1:10, indicating that the PGC-C18 is fully saturated at high and low affinity sites. Without any carrier, 58+/−5% (n=6; presented as mean +/−SD) GLP-1 was degraded by 1.25mU/mL DPP IV in 24 hours. In the presence of the carrier PGC-C18, only 26+/−2% (n=6) of GLP-1 was degraded. A PGC without C18 fatty acid was used as a carrier control and showed similar results to the GLP-1 control with 58+/−8% (n=6) degradation. The Km of DPP IV for GLP-1 is 36uM (25) and the high affinity Kd of GLP-1 for the PGC-C18 carrier is 249nM. In order for the DPP IV to function at maximum efficiency, the accessible or free GLP-1 concentrations needs to be in the uM range, close to Km. The capacity of the PGC-C18 for GLP-1 at the high affinity site is 4–5, indicating that if 100mg PGC-C18 is mixed with 4–5mg of GLP-1, the concentration of free GLP-1 on which DPP IV could act is in the 249nM range which will significantly decrease the efficiency of DPP IV to degrade GLP-1. Since all sites are saturated with GLP-1, including the low affinity sites, it is expected that free GLP-1 under these conditions will be higher than 249nM and the low affinity site Kd (3.7 and 33uM) will initially determine the amount of free GLP-1. Once the amount of GLP-1 decreases such that the low affinity sites are empty, the concentration of free GLP-1 will be determined by the high affinity sites. The results presented in this figure show a certain level of degradation consistent with the presence of free GLP-1 on which the DPP IV can act, though not in a very efficient way. The presence of a low level of free GLP-1 is necessary and sufficient to saturate the GLP-1 receptors with Kd of 1–2nM (26).
Fig. 4. PGC with C18 protects GLP-1 from rapid DPP IV digestion but not PGC without C18.
HPLC profiles of GLP-1 after a 24-hour digestion with 1.25mU/ml of DPP IV at 37°C. It is clear that only the carrier containing C18 (which is essential for binding) protects GLP-1 from dipeptidyl peptidase-IV (DPP IV) degradation compared to the control without PGC. Without the carrier 58+/−5% (n=6) GLP-1 was degraded in 24 hours. In the presence of the carrier PGC-C18, only 26+/−2% (n=6) of GLP-1 was degraded. A PGC without C18 fatty acid was used as a carrier control and showed similar results to the GLP-1 control with 58+/−8% (n=6) degradation. A 10x excess of PGC-C18 or PGC over GLP-1 by mass was used for this experiment. Typical formulation has 50-fold excess PGC over GLP-1 which will push equilibrium (Fig. 8) to much less free GLP-1, and thus much less accessible to proteases.
PGC-C18- GLP-1 formulation has sufficient free GLP-1 to stimulate Ca influx in INS-1 cells
In order to determine if the PGC-C18-GLP-1 formulation has sufficient free GLP-1 to be biologically active, we used INS-1 cells that express a GLP-1 receptor. A calcium influx assay was used to confirm that PGC-C18 formulated GLP-1 maintained its activity after formulation. Figure 5 shows that PGC-C18 formulated GLP-1 has a sufficient amount of free and conformationally active GLP-1 that can bind and stimulate GLP-1 receptors as evident from stimulation of calcium influx similar to unformulated GLP-1. Both PBS and PGC-C18 alone do not result in a significant increase in calcium influx. This clearly shows that the PGC-C18-GLP-1 formulation can stimulate GLP-1 receptors.
Fig. 5. Calcium influx experiment in INS-1 cells indicates that GLP-1 formulated in PGC-C18 is biologically active.
PBS, GLP-1, GLP-1 in PGC-C18, and PGC-C18 alone were applied to INS-1 cells at the 10 second time point and the increase in fluorescence (mean +/− SEM) resulting from calcium influx into the cells was monitored. In this assay, INS-1 cells were seeded in black 96 well plates at 200,000 cells per well in a buffered medium containing RPMI/10%FBS/11.1mM glucose/10mM HEPES, pH7.4/1mM pyruvate/50uM 2-Mercaptoethanol. Cells were allowed to adhere overnight and were then labeled by replacing the medium with 20ul of 5 ug/ml Fura2AM in PBS pH7.4/2%FBS/11.1mM glucose. Cells were exposed to this Fura2AM solution for two hours followed by the addition of 180 uL PBS solution (pH7.4/2%FBS/11.1mM glucose) to dilute the Fura2AM that was not absorbed into the cell cytoplasm. Fluorescence (340ex/510em) was monitored and after 10 seconds free GLP-1, and GLP-1 formulated in PGC-C18 (n=6 each) were added to a final GLP-1 concentration of 30nM (0.1ug/mL). The PGC-C18 formulated GLP-1 has a PGC-C18:GLP-1 weight ratio of 50:1, similar to that used in the animal efficacy study. For controls, PBS or an equivalent amount of PGC-C18 (n=6 each) was added instead of GLP-1. The influx of calcium into the cells is reflected by the increase of fluorescence, along the y-axis. There was no statistical significant difference in the fluorescence between the PBS and PGC-C18 groups, or between GLP-1 and PGC-C18:GLP-1 groups. When the PBS group was compared to PGC-C18:GLP-1 and GLP-1 alone group for the fluorescence measured after the 12sec time point (2 sec after addition of the compounds), the p-values of t-tests were p<0.01 and <0.001, respectively, indicating that both GLP-1 alone and GLP-1 formulated in PGC-C18 were biologically active.
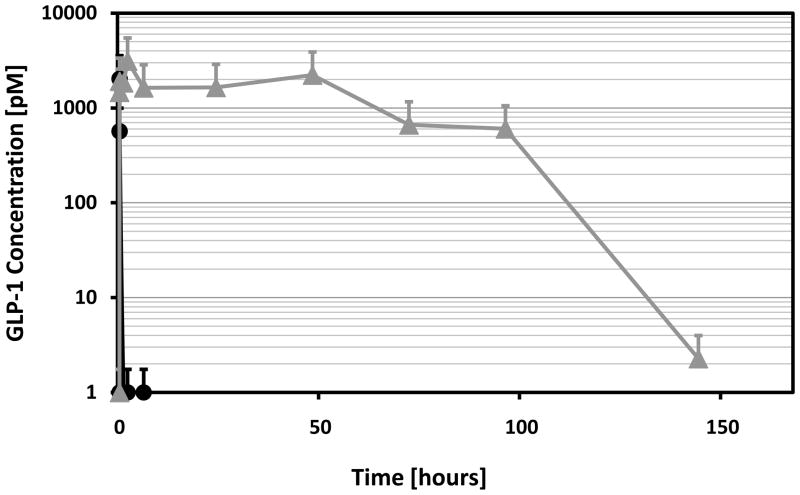
PGC-C18-GLP-1 formulation has a much longer blood circulation time
Due to the size of PGC-C18 (19nm), GLP-1 bound to it will have an apparent size exceeding the glomerular filtration cut off of 4 nm and therefore be expected to stay in circulation for a longer period of time. In addition, the PEG shielding the GLP-1 bound to PGC-C18 will allow a significant reduction in the ability of the reticuloendothelial system to clear the complex, contributing to the increase in blood circulation time of the complex. By administering the formulation with a hydrodynamic diameter of 19 nm subcutaneously, the Tmax in the blood will be delayed, further prolonging the blood circulation time of the complex. Because the complex acts as reservoir that releases GLP-1 based on the Kd, a more controlled exposure of the body to free GLP-1 is expected. Figure 6 is a representative profile of many pharmacokinetic (PK) studies of PGC-C18 formulations that we have done over the past four years, showing the total GLP-1 in the blood (both PGC-C18 bound and free). The ELISA kit has sufficient immobilized antibody to pull all the GLP-1 (bound and free) towards the antibody bound to the plate because the amount of PGC-C18 is quite low despite its high affinity. While unformulated GLP-1 is gone from the blood in a matter of a few minutes, GLP-1 formulated in PGC-C18 stays in the blood for at least 100 hours, an ideal formulation for twice a week administration in rats. Because the nature of the formulation has two compartments (bound GLP-1 and free GLP-1) as well as another two compartments due to the subcutaneous administration (subcutaneous site and blood), the half-life expected for GLP-1 measured as total (as presented in Figure 6) will not be first order and thus a linear regression of the graph will not be meaningful even if started at the Cmax.
Fig. 6. GLP-1 formulated in PGC-C18 has extended blood circulation time compared to GLP-1 alone.
Shown are the levels of total serum GLP-1 as measured using an ELISA specific for active GLP-1 (mean +/− SD). Sprague-Dawley rats (n=10) were given subcutaneous injections of 1 mg/kg of either GLP-1 alone or GLP-1 formulated in PGC-C18. The GLP-1 formulated in PGC-C18 is at 2% loading or the ratio of GLP-1:PGC-C18 by weight is 1:50. The GLP-1 ELISA kit is a sandwich-type that uses alkaline phosphatase and the fluorogenic substrate MUP (methyl umbelliferyl phosphate).
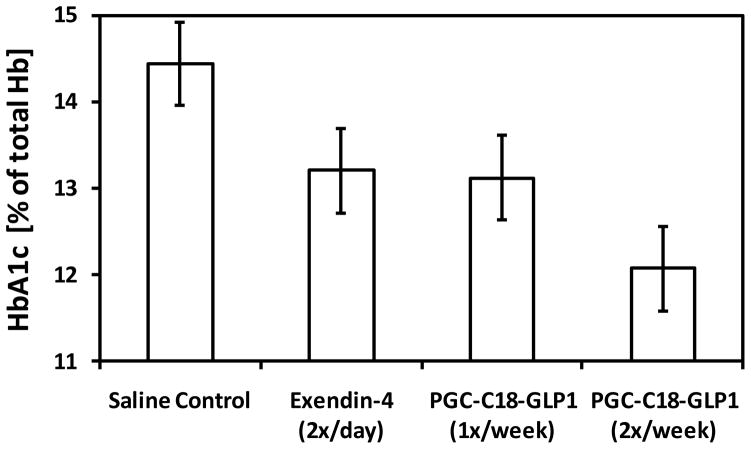
PGC-C18 GLP-1 formulation given twice in the treatment type 2 diabetes animal model
Zucker diabetic fatty (ZDF) rats were treated in three different experiments for up to 7 weeks with PGC-C18 formulated GLP-1 at 2% loading (1:50 w/w; GLP-1:PGC-C18). Figure 7 shows HbA1c values from week seven in ZDF rats (n=10) using 1mg/kg dose and various frequencies. All treatments were given subcutaneously. Free GLP-1 was given at 1 mg/kg three times/week and exendin-4 BID 3 ug/kg twice daily. All treatment groups are significantly different (P<0.05) from the control group. It is interesting that the group given unformulated GLP-1 at dose 1mg/kg 3x a week is not significantly less effective in reducing HbA1c than those given exendin-4 twice a day. This may be because the frequency and dose estimation based on potency of exendin-4 (24) relative to GLP-1 may not have been properly matched. It is clear, however, that once a week administration of GLP-1 in PGC-C18 is as effective as twice a day administration of exendin-4. There were 98 injections of exendin-4 compared to 7 injections of GLP-1 over 49 days, giving the GLP-1 in PGC-C18 a potential advantage in terms of convenience. Further, twice a week administration of GLP-1 in PGC-C18 showed a significantly lower HbA1c at week seven than the group that received the twice a day injection of exendin-4 (P<0.005). HbA1c is a proxy for the average blood glucose level over the previous 3–5 weeks.
Fig. 7. GLP-1 formulated in PGC-C18 is effective in improving HbA1c level in ZDF rats.
Shown are HbA1c values (+ or − standard deviation) in ZDF rats (n=10) after 7 weeks of treatment. The Saline control group received once a day subcutaneous injections over 7 weeks. Exendin-4 2x/day group received twice a day subcutaneous injection, or 98 injections of exendin-4, at 3ug/kg over 7 weeks. The 1x/week GLP-1 group received once a week subcutaneous injections of 1mg/Kg of GLP-1 formulated in PGC-C18 or a total of 7 injections over 7 weeks. The 2x/week GLP-1 group received twice a week subcutaneous injections of 1mg/Kg of GLP-1 formulated in PGC-C18 or a total of 14 injections over 7 weeks. The GLP-1 formulated in PGC-C18 was at 2% loading or the ratio of GLP-1:PGC-C18 by weight is 1:50. The unformulated GLP-1 group received three times a week subcutaneous injections over 7 weeks (data not shown, not statistically significant from exendin-4 2x/day). HbA1c is a proxy for the average blood glucose level over the previous 3–5 weeks.
Stability PGC-C18 GLP-1 formulation at 37°C
In order to determine the potential commercial use of the PGC-C18 formulation, we determined the stability of PGC-C18 formulation at 37°C. We choose 37°C to force a rapid degradation of the formulation under worse conditions than the sample would likely encounter, to ensure that, under mild conditions such as room temperature or refrigeration at 4°C, we will be confident that the formulation will be stable. The stability was measured based on the total amount of intact GLP-1 and the amount of free and PGC-C18 bound GLP-1 by reversed phase HPLC. A 10% decrease in total GLP-1 in the formulation was considered degraded. Without PGC-C18, greater than 10% of GLP-1 was broken down within a month while the formulation PGC/GLP-1 was stable for 1 month at 37°C, both in liquid. Analysis at two months of the liquid formulations showed a decrease in the total GLP-1 peak (bound and unbound) of greater than 10% and an increase in the free GLP-1 from less than 5% to 50%. In contrast, the lyophilized formulation was stable for 10 months at 37°C: analysis at 11 months showed a decrease in total GLP-1 peak (bound and unbound) of greater than 10% and an increase in the free GLP-1 from less than 5% to 10% and at 12 months the free GLP-1 was 18.5%. This study indicates that a relatively stable formulation of GLP-1 in PGC-C18 is possible.
DISCUSSION
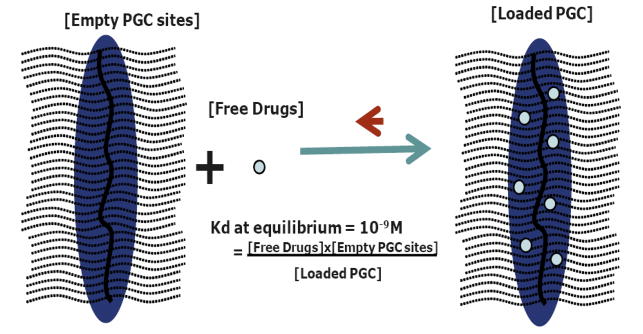
Type 2 diabetes is a chronic and progressive metabolic disease characterized by progressive hyperglycemia followed by dysfunctional insulin secretion and/or its impaired utilization. Although many commercially available hypoglycemic agents have been widely utilized to achieve glycemic control, strong demand still exists for therapies that improve glycemic control and maintain or improve pancreatic endocrine function (4, 6). Enhanced GLP-1 receptor activation is one of various therapeutic targets that are considered to be most effective because of the multiple antidiabetic effects of GLP-1 and its receptor agonists (1–4). Because of a very short blood half-life of native GLP-1, a search for a more stable agonist of GLP-1 resulted in the successful clinical application of exendin-4 for antidiabetic therapy (6, 7, 27). Because exendin-4 is administered twice a day, further efforts to develop longer-acting GLP-1 receptor agonists with improved therapeutic efficacies continue, using various chemical conjugations such as PEGylation or acylation, physical entrapment into sustained delivery systems, and genetic approaches such as albumin-based fusion protein (13–17, 19–21). All these approaches to increase blood half-life and decrease frequency of administration involved chemical modification of native GLP-1 or used analogs of GLP-1 (such as exendin-4) that may potentially induce a neutralizing antibody response upon chronic use by diabetic patients (12). In this study, we developed an excipient of native GLP-1 by simply conjugating stearic acid to PGC resulting in PGC-C18 that has high affinity for native unmodified GLP-1. This PGC-C18 binds GLP-1 in an affinity-based manner and maintains a small amount of free GLP-1. When free GLP-1 binds to a receptor or is metabolized, the resulting decrease in free GLP-1 will trigger release of more GLP-1 from the excipient in a manner driven by Kd. Such a mechanism will, in principle, maintain a relatively constant level of free GLP-1 in the blood until the GLP-1 in the PGC-C18 reservoir is depleted. This is illustrated in Figure 8.
Fig. 8. Schematic illustration of the equilibrium of free and PGC-C18 bound drugs.
PGC-C18 is comprised of a polylysine backbone (dark wavy line at the center) where approximately every other epsilon amino groups are derivatized with methoxypolyethylene glycol or MPEG (dotted wavy lines) and the remaining epsilon amino groups are derivatized with stearic acid (dark blue oval shading) that provides sites, perhaps together with MPEG, for GLP-1 (small light blue circles, not to scale) to interact. The interaction can be defined by a thermodynamic equilibrium where free GLP-1 [free drug] is determined by experimentally measurable dissociation constant or Kd. In the present study, the PGC-C18 was used as an excipient for native GLP-1 such that it maintains a small amount of free GLP-1. When free GLP-1 binds to receptors or is metabolized, the resulting decrease in free GLP-1 will trigger release of more GLP-1 from the excipient in a manner driven by Kd. In principle, such a mechanism will maintain a relatively constant level of free GLP-1 in the blood until the GLP-1 in the PGC-C18 reservoir is depleted.
Because the PGC used in this study has fatty acids and PEG, one would suspect that this structure would form a supramolecular structure, such as a micelle, in a water environment. Although this is true if the weight ratio of PEG to fatty acid in the molecule is below 15, it is not necessarily true when the PEG fatty acid ratio is above 15 (data not shown). The PEG to fatty acid ratio of the PGC used here is 21 and this composition does not elute at the void volume when run on gel permeation chromatography with aqueous solvent as mobile phase (see method section), indicating no micelle or other supramolecular structures formation. In addition, the solution of PGC appears clear even at 100mg/ml, prior to a concentration dependent increase in viscosity. However, PGC with a PEG to fatty acid ratio below 15 not only shows cloudiness at 100mg/ml when dissolved in water but also elutes at the void volume on the gel permeation column with a molecular weight cut off of greater than one million (data not shown). This is the result of our investigation of various PGC compositions that range in PEG to fatty acid weight ratio from 1.76 to 43. In fact, elution at the void volume on gel permeation chromatography only occurs at a PEG to fatty acid ratio of 15 and below. The design of PGC specifically avoids supramolecular structure formation by the absence of a hydrophobic “end” in the 3-dimentional structure of the molecule. Without this hydrophobic end, formation of a supramolecular structure in water would theoretically be avoided. The step that prevents formation of the hydrophobic end is the addition of PEG where a statistical reaction puts PEG in a random order that allows an average distribution of PEG along the polylysine chain with no clustering of PEG at either end of the polylysine. Because PEG provides steric hindrance between the core of PGC and large molecules, such as very large proteins and other PGC molecules, such supramolecular structure formation was avoided despite the addition of hydrophobic moieties at the core of PGC. This can only be possible if there are sufficient PEG molecules on the polylysine backbone and this limit was determined to be a ratio of PEG to fatty acid of greater than 15.
This technology is applicable to peptides that have significant hydrophobic domains or can assume a more hydrophobic alpha-helical conformation such as GLP-1 (28). When heated to 50°C, GLP-1 at 1mg/ml in neutral solution is quite clear, but turns cloudy and less soluble as it cools down to room temperature. We believe this change is associated with the formation of the alpha helix and is also responsible for efficient binding of GLP-1 to the PGC-C18 excipient (Figures 2 and 3). The Scatchard plot indicated that when PGC-C18 is fully saturated it can bind up to 9 GLP-1 molecules with four of those being low affinity sites that have Kd of 3–33uM. Because the Km of the DPP IV for GLP-1 (36uM) is only slightly above the low affinity site range, fully saturating the PGC-C18 will offer limited protection to half of the GLP-1 loaded onto PGC-C18. Therefore the ideal formulation will be 4–5 GLP-1 molecules per PGC-C18 molecule to ensure that mainly high affinity sites are occupied. Because each PGC-C18 molecule has an average of 65 units of 5kDa MPEG molecules linked to the epsilon amino groups of polylysine in a random or statistical distribution, there will be significant shielding of the C18 moieties that are also linked to the remaining epsilon amino groups of polylysine. The C18 in PGC is a necessary component for binding of GLP-1, so the GLP-1 must interact close to the polylysine backbone where the C18 is attached, allowing for the MPEG moiety (3.5nm hydrodynamic diameter) to partially shield the GLP-1 (2nm hydrodynamic diameter) from its surroundings including DPP IV (Figure 4). Since the bound GLP-1 will not be expected to bind to GLP-1 receptors because of the shielding, the question of whether the free GLP-1 concentration, provided by the Kd force of the equilibrium, is sufficient to bind and stimulate to GLP-1 receptor. Figure 5 indicated that the free GLP-1 in PGC-C18 formulation is active and can stimulate GLP-1 receptors. In principle, for the affinity based GLP-1 delivery system to work as intended, the Kd of PGC-C18 for GLP-1 must be smaller that the Km of DPP IV for GLP-1 but greater than the Kd of GLP-1 for its target receptor. If this is satisfied then the concentration of free GLP-1 will be too low for DPP IV to function efficiently, but more than enough to saturate the GLP-1 receptors.
There are other features of PGC-C18 that make it an ideal peptide drug delivery system. In this particular example PGC-C18 has a hydrodynamic radius of 20nm. The filtration cut off of the kidney glomerulus is 4nm therefore anything bound to the PGC-C18 will not be immediately flushed out of the kidneys. In addition, the exterior portion of PGC-C18 is mostly MPEG which significantly slows down the ability of the reticuloendothelial system (29) to clear the complex from the circulation. This is consistent with the blood circulation half-life of FITC labeled PGC-C18 in rats of 39 hours (not shown) and, when administered subcutaneously, a Tmax of approximately 50 hours. Therefore when administered subcutaneously, the PGC-C18 will have a blood residence time of at least 89 hours (Tmax + half-life). The PK data presented in Figure 6 indicates that the blood residence time of GLP-1 from the PGC-C18 formulation is consistent with a PGC-C18-GLP-1 complex present in the blood because without PGC-C18 such GLP-1 level in the blood is not observed. Further, we know that PGC-C18 travels from the subcutaneous site to the blood based on a study of FITC labeled PGC-C18 administered subcutaneously. In addition, filtration of serum using 100kDa cut-off membrane (see methods) indicated that 90% of GLP-1 observed in serum is associated with high molecular weight molecules (not shown), presumably the PGC-C18 because this was not observed in serum from animals given only GLP-1. Another feature of PGC is its ability to passively accumulate in areas of increased vascular permeability such as inflammation and cancer. This is known as the enhanced permeability and retention (EPR) effect and is a function of the size of the nanocarrier (ideally 15–100nm). Passive accumulation of PGC using EPR has been shown in an inflamed pancreas in a diabetes model (30), thus allowing for an increase in local concentrations of GLP-1 at one of the sites of GLP-1 action.
Because Type 2 diabetes will require chronic administration of GLP-1, ideally we would want the PGC-C18 to be cleared from circulation prior to the next administration. The PGC-C18-GLP-1 can be administered in diabetic ZDF rats once a week or twice a week and be effective in improving HbA1c, comparable to or even more effective than exendin-4 given twice a day (Figure 7). Because the blood circulation time of the PGC-C18 excipient is about 4 days in rats, a twice a week administration is possible without accumulation of PGC-C18 over time. Because humans have a different metabolic rate, dosing in humans needs to be investigated in the future. In addition to accumulation, the question of PGC-C18 toxicity must be clarified for any formulation that will use the PGC-C18 excipient. To this end, we have given mice (n=10) PGC-C18 (0.5 g/Kg) every other day for 8 weeks and we did not observe any behavioral and physical (weight) abnormalities over the test period. After 8 weeks, no histological abnormalities were observed upon examination of H&E stained sections of liver, kidney and spleen (not shown). When mice were given 1 g/kg every other day, the normal weight gain stopped, compared to control, indicating that 1g/kg is not tolerated in mice. When the 1g/kg treatment was stopped weight gain resumed within 3 days, indicating that PGC-C18 can be metabolized rapidly. The expected amount of PGC-C18 that will be given to a human patient is likely to be less than 10mg/kg every other week or even once a week. Typical pegylation of protein uses molecular weights of PEG of 20kDa or greater to overcome rapid kidney elimination and this has been documented to accumulate over time (23). In contrast, PGC-C18 has 5kDa PEG that can be flushed out of the kidney once hydrolyzed by enzymes from the PGC. The fatty acid is likely to be hydrolyzed by fatty acyl amide hydrolase and the product fatty acid can be utilized by the body for fuel. The backbone polylysine can be hydrolyzed into individual amino acids. Although polylysine is toxic, due to its polycationic property that is disruptive to cell membranes and lysosomal pH, the polylysine makes up only 5% by weight of the PGC so it is not likely to present as a highly concentrated polycation because the metabolism is expected to be gradual and non-localized to a single organ.
The synthesis of PGC-C18 is straightforward and begins with conjugation of MPEG to polylysine in a water based reaction, making the complex soluble in both water and organic solvents. In order to add fatty acid, the reaction mixture was lyophilized followed by acylation in organic solvent. No chemical modification of native GLP-1 is needed to make the final formulation. The process is reproducible with a yield of 70–80% of the starting PEG and polylysine weight (Table I). In summary, the present study suggests PGC-C18 is a powerful excipient that can prolong the half-life of GLP-1 by at least 1000-fold, potentially allowing for twice a week or even once a week administration in humans. The native GLP-1 formulated in PGC-C18 has a well-preserved biological activity as well as protracted pharmacokinetic and anti-diabetic characteristics similar to more frequently administered exendin-4.
CONCLUSION
Protected Graft Copolymer can prolong the blood residence time of GLP-1 by at least 1000-fold and can be an ideal in vivo stabilizing excipient for other biologically labile peptides.
Acknowledgments
This work was supported by the SBIR Grant #DK069727 from National Institute of Diabetes and Digestive and Kidney diseases of the National Institute of Health. We are grateful to Dr. Alexei Bogdanov, Jr (University of Massachusetts Medical School) for providing consultations regarding the synthesis and purification of hydrophobic core PGC carrier. The authors thank Ms. Cynthia Jones, Ms. Akiko Nishimoto-Ashfield, and Ms. Marisa Robinson for assistance with the manuscript.
ABBREVIATIONS
- DPP IV
dipeptidyl peptidase IV
- GLP-1
Glucagon-like peptide-1
- PEGylation
poly(ethylene glycol) conjugation
- PGC
protected graft copolymer
- HbA1c
glycosylated hemoglobin
References
- 1.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007 Jan;117(1):24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999 Dec;20(6):876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 3.Meier JJ, Nauck MA. Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev. 2005 Mar–Apr;21(2):91–117. doi: 10.1002/dmrr.538. [DOI] [PubMed] [Google Scholar]
- 4.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006 Nov 11;368(9548):1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 5.Eng J, Yu J, Rattan S, Yalow RS. Isolation and amino acid sequences of opossum vasoactive intestinal polypeptide and cholecystokinin octapeptide. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1809–11. doi: 10.1073/pnas.89.5.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995 Aug;136(8):3585–96. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 7.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005 May;28(5):1083–91. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 8.Amylin Pharmaceuticals. 2008 http://www.rxlist.com/cgi/generic4/byetta_ad.htm.
- 9.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009 Jul 4;374(9683):39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 10.Kim D, MacConell L, Zhuang D, Kothare PA, Trautmann M, Fineman M, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007 Jun;30(6):1487–93. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 11.Simonsen L, Holst JJ, Deacon CF. Exendin-4, but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetised pigs. Diabetologia. 2006 Apr;49(4):706–12. doi: 10.1007/s00125-005-0128-9. [DOI] [PubMed] [Google Scholar]
- 12.BYETTA. prescribing information. [article online, revised june 2008], URL: http://pi.lilly.com/us/byetta-pi.pdf.
- 13.Huang YS, Chen Z, Chen YQ, Ma GC, Shan JF, Liu W, et al. Preparation and characterization of a novel exendin-4 human serum albumin fusion protein expressed in Pichia pastoris. J Pept Sci. 2008 May;14(5):588–95. doi: 10.1002/psc.942. [DOI] [PubMed] [Google Scholar]
- 14.Kim JG, Baggio LL, Bridon DP, Castaigne JP, Robitaille MF, Jette L, et al. Development and characterization of a glucagon-like peptide 1-albumin conjugate: the ability to activate the glucagon-like peptide 1 receptor in vivo. Diabetes. 2003 Mar;52(3):751–9. doi: 10.2337/diabetes.52.3.751. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Youn YS, Lee SH, Byun Y, Lee KC. PEGylated glucagon-like peptide-1 displays preserved effects on insulin release in isolated pancreatic islets and improved biological activity in db/db mice. Diabetologia. 2006 Jul;49(7):1608–11. doi: 10.1007/s00125-006-0234-3. [DOI] [PubMed] [Google Scholar]
- 16.Pan CQ, Buxton JM, Yung SL, Tom I, Yang L, Chen H, et al. Design of a long acting peptide functioning as both a glucagon-like peptide-1 receptor agonist and a glucagon receptor antagonist. J Biol Chem. 2006 May 5;281(18):12506–15. doi: 10.1074/jbc.M600127200. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Cai ZH, Li L, Kou C, Gao YF. Preparation and PEGylation of exendin-4 peptide secreted from yeast Pichia pastoris. Eur J Pharm Biopharm. 2009 Jun;72(2):412–7. doi: 10.1016/j.ejpb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Chae SY, Choi YG, Son S, Jung SY, Lee DS, Lee KC. The fatty acid conjugated exendin-4 analogs for type 2 antidiabetic therapeutics. J Control Release. 2010 May 21;144(1):10–6. doi: 10.1016/j.jconrel.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Irwin N, O’Harte FP, Gault VA, Green BD, Greer B, Harriott P, et al. GIP(Lys16PAL) and GIP(Lys37PAL): novel long-acting acylated analogues of glucose-dependent insulinotropic polypeptide with improved antidiabetic potential. J Med Chem. 2006 Feb 9;49(3):1047–54. doi: 10.1021/jm0509997. [DOI] [PubMed] [Google Scholar]
- 20.Madsen K, Knudsen LB, Agersoe H, Nielsen PF, Thogersen H, Wilken M, et al. Structure-activity and protraction relationship of long-acting glucagon-like peptide-1 derivatives: importance of fatty acid length, polarity, and bulkiness. J Med Chem. 2007 Nov 29;50(24):6126–32. doi: 10.1021/jm070861j. [DOI] [PubMed] [Google Scholar]
- 21.Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, et al. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002 Oct;283(4):E745–52. doi: 10.1152/ajpendo.00030.2002. [DOI] [PubMed] [Google Scholar]
- 22.Spadaro AC, Draghetta W, Del Lamma SN, Camargo AC, Greene LJ. A convenient manual trinitrobenzenesulfonic acid method for monitoring amino acids and peptides in chromatographic column effluents. Anal Biochem. 1979 Jul 15;96(2):317–21. doi: 10.1016/0003-2697(79)90587-6. [DOI] [PubMed] [Google Scholar]
- 23.Bendele A, Seely J, Richey C, Sennello G, Shopp G. Short communication: renal tubular vacuolation in animals treated with polyethylene-glycol-conjugated proteins. Toxicol Sci. 1998 Apr;42(2):152–7. doi: 10.1006/toxs.1997.2396. [DOI] [PubMed] [Google Scholar]
- 24.Young AA, Gedulin BR, Bhavsar S, Bodkin N, Jodka C, Hansen B, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta) Diabetes. 1999 May;48(5):1026–34. doi: 10.2337/diabetes.48.5.1026. [DOI] [PubMed] [Google Scholar]
- 25.Lambeir AM, Proost P, Scharpe S, De Meester I. A kinetic study of glucagon-like peptide-1 and glucagon-like peptide-2 truncation by dipeptidyl peptidase IV, in vitro. Biochem Pharmacol. 2002 Dec 15;64(12):1753–6. doi: 10.1016/s0006-2952(02)01415-6. [DOI] [PubMed] [Google Scholar]
- 26.Crespel A, De Boisvilliers F, Gros L, Kervran A. Effects of glucagon and glucagon-like peptide-1-(7–36) amide on C cells from rat thyroid and medullary thyroid carcinoma CA-77 cell line. Endocrinology. 1996 Sep;137(9):3674–80. doi: 10.1210/endo.137.9.8756532. [DOI] [PubMed] [Google Scholar]
- 27.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992 Apr 15;267(11):7402–5. [PubMed] [Google Scholar]
- 28.Al-Sabah S, Donnelly D. The primary ligand-binding interaction at the GLP-1 receptor is via the putative helix of the peptide agonists. Protein Pept Lett. 2004 Feb;11(1):9–14. doi: 10.2174/0929866043478365. [DOI] [PubMed] [Google Scholar]
- 29.Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev. 2002 Jun 17;54(4):459–76. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 30.Medarova Z, Castillo G, Dai G, Bolotin E, Bogdanov A, Moore A. Noninvasive magnetic resonance imaging of microvascular changes in type 1 diabetes. Diabetes. 2007 Nov;56(11):2677–82. doi: 10.2337/db07-0822. [DOI] [PubMed] [Google Scholar]