Abstract
Background
Exercise stress testing has shown diagnostic utility in adult patients with long QT syndrome (LQTS). However, the QT interval adaptation in response to exercise in pediatric patients with LQTS has received little attention.
Methods and Results
One-hundred and fifty eight patients were divided into three groups: LQT1, LQT2 and normal controls without cardiovascular disease. Each patient underwent a uniform exercise protocol employing a cycle ergometer followed by a 9 minute recovery phase with continuous 12-lead electrocardiogram (ECG) monitoring. Each patient underwent a baseline electrocardiogram while resting in the supine position and in a stand still position during continuous ECG recording for determining changes in the QT and RR intervals.
Fifty patients were gene-positive for LQTS (n=29, LQT1 and n=21, LQT2) and the control group consisted of 108 patients. QT interval adaptation was abnormal in the LQT1 patients compared to LQT2 and control patients (P<0.001). A QTc >460 ms in the late recovery phase at 7 minutes predicted LQT1 or LQT2 vs. controls with 96% specificity, 86% sensitivity and 91% positive predictive value. A recovery ΔQTc (7 minute – 1 minute) >30 ms predicted LQT2 vs. LQT1 with 75% sensitivity, 82% specificity and 75% positive predictive value. The postural ΔQT was significantly different between LQTS and control groups (P=0.005).
Conclusions
Genotype specific changes in repolarization response to exercise and recovery exist in the pediatric population and are of diagnostic utility in LQTS. An extended recovery phase is preferable to assess the repolarization response after exercise in the pediatric population.
Keywords: Ion channels or ion channel, Long-QT syndrome, pediatrics, Exercise stress test
Introduction
Congenital long QT syndrome (LQTS) is an inherited channelopathy characterized by a prolonged QT interval, syncope, ventricular arrhythmias and sudden death(1,2). Children and adolescents with LQTS have been shown to be at high risk of a first cardiac event(3,4). The diagnosis of LQTS can be challenging in the presence of a borderline prolonged QT or normal QT interval (“concealed LQTS”) and additional tests are necessary for clinical evaluation(5). Significant advances in the molecular understanding of LQTS have resulted in genetic testing for 13 LQTS susceptibility genes with the majority of mutations involving the LQT1 (KCNQ1) or LQT2 (KCNH2) genes. However, there are several limitations to genetic testing: it is expensive, not universally available and may be negative in one third of clinically diagnosed patients(6). Additional tests that aid in the diagnosis and genetic characterization of this potentially lethal syndrome are necessary. Exercise stress testing (EST) and assessment of the QTc interval with postural changes are provocative tests that can be readily performed and promptly interpreted in outpatient clinical practice.
There are emerging data regarding genotype specific repolarization responses with exercise stress testing pertaining to adult LQTS patients(7-9). However, genotype specific QT adaptation during exercise, recovery and postural changes has received little attention in children and adolescents with LQTS(10). In addition, it has not been determined if the location of gene mutations also influences repolarization responses. The aim of this study was to determine the diagnostic significance of exercise stress testing and QT adaptation during exercise, recovery and postural changes in children with common LQTS genotypes (LQT1 and LQT2) when compared to normal controls.
Methods
Study Population
The study group consisted of patients referred to the Pediatric Arrhythmia Clinic at The Children’s Hospital of Philadelphia for evaluation of suspected LQTS between January 1998 and January 2010. Inclusion criteria for this study were: 1) patients ≤21 years of age 2) genotype-positive patients for LQT1 (KCNQ1) or LQT2 (KCNH2) genes and 3) patients with exercise stress testing (EST) using a uniform protocol that employed a cycle ergometer performed at our institution during their referral evaluation for LQTS. Genetic mutational analysis of 5 LQTS susceptibility genes (KCNQ1, KCNH2, SCN5A, KCNE1 and KCNE2) was performed on all patients through a commercial laboratory (Familion®, PGxHealth, New Haven, CT, USA). Genetic mutations of the KCNQ1 amino acid sequence were categorized into 3 locations: pre-pore region including N-terminus (1-278th amino acid), pore region (279-354th amino acid) and post-pore region including C-terminus (> 354th amino acid)(11,12). Genetic mutations of the KCNH2 amino acid sequence were characterized into a pore and non-pore region. The pore region was defined as the area extending from S5 to the mid portion of S6 involving amino acid residues 550 through 650(13,14). The control group consisted of age and gender comparable controls evaluated for cardiovascular symptoms and with EST at our institution who were dismissed from follow up due to the absence of cardiovascular disease.
In order to obtain an age and gender comparable control group, we subdivided the study group into 4 categories: 1) 4 to 7, 2) 8 to 10, 3) 11 to 14 and 4) 15 to 21 years of age. An equal number of males/females were present in each age category in the study and control groups. Family-reported ethnicity data was also included in our data analysis. Patients excluded from the study were those with clinically suspected LQTS without a positive genetic test. Due to insufficient sample size, patients with less common LQTS genotypes were also excluded.
The study protocol was approved by The Children’s Hospital of Philadelphia’s Institutional Review Board.
Exercise Stress Test Protocol
All patients underwent a uniform exercise protocol that employed a cycle ergometer (SensorMedics®, Yorba Linda, CA). The initial phase consisted of three minutes of pedaling in an unloaded state followed by a ramp increase in work rate (Watts) to maximal exercise. The progression of cycle resistance was determined by subject weight in kilograms and designed to achieve predicted peak work rate in 10 to 12 minutes of cycling time(15). Following the three minute warm-up phase, resistance was increased at one-minute intervals until maximal volition was achieved. Maximal volition was defined as a respiratory exchange quotient of > 1.10 and was an indication that the patient had reached peak exercise capacity. After reaching peak exercise, each patient completed the EST with a 9 minute recovery period. All EST were performed at The Children’s Hospital of Philadelphia.
Exercise Stress Test Cardiac Monitoring
A l2-lead ECG (Marquette Case-8000, Milwaukee, WI) at paper speed of 25 mm/s was recorded at rest in the supine, and standing positions as well as at one-minute intervals during the exercise phase and during the first nine minutes of recovery.
Exercise Stress Test ECG Measurements
ECG measurements were made by two independent investigators (PA and JG) who were both blinded to the patient’s LQTS status. Inter-observer variability was assessed in 40 randomly selected patients which resulted in an intra-class correlation coefficient of 0.51 and a percentage within 20 ms of 69%, indicating a moderate degree of consistency in QTc measurements between two observers. The QT interval was defined as the beginning of the QRS complex to the end of the T wave. In cases in which the T wave end point did not reach the iso-electric line of the ECG, the maximum down slope of the T wave and the intersection with the iso-electric line of the T-P segment was considered to be the end of the T wave(16). U waves less than ½ of the T wave amplitude were not included as a portion of the QT interval(17). QT interval measurements were made in ECG leads II and V5. QT intervals at rest (supine and standing), peak exercise and in recovery (1, 3, 5, 7, and 9 minutes) were measured. QTc was then calculated according to the Bazett formula (QTc=QT/√RR)(18).
“Concealed” LQTS and Recovery ΔQTc
Patients with “concealed” LQTS defined as a supine resting QTc < 460 ms were sub-analyzed. We determined an additional parameter: Recovery ΔQTc(7 min-1 min) defined as the difference in QTc measured at the 7 minute and 1 minute time interval of the recovery periods.
Postural QT Measurements
Resting ECGs were obtained after the patients rested in the supine position for 5 minutes and the QT (ms), RR (ms) and QTc (ms) intervals were calculated. With continuous ECG monitoring, the patients were asked to immediately stand upright and the QT (ms), RR (ms) and QTc (ms) calculations were repeated within one minute. The postural ΔQT was defined as the difference in the QT interval (ms) between standing and supine positions(7). Similarly, the postural ΔRR was defined as the difference in the heart rate (bpm) acceleration between standing and supine. The postural ΔQTc was defined as the difference between the heart rate corrected QT intervals between standing and supine positions. Postural QT and QTc measurements were analyzed only in patients who were in a drug free state as beta blockers could potentially blunt the heart rate response.
Statistical Analysis
Mean QT, RR and QTc measurements were plotted against time in EST and differences between the LQT1, LQT2 and control groups were assessed. The difference in the mean QT and RR intervals during supine and standing positions were also assessed in the LQTS and control patients. Patients were subdivided based on age, gender and beta-blocker therapy to assess for confounding variables. Differences in the EST characteristics between subgroups classified according to mutation location were evaluated using standard statistical methods. At each time point, a linear mixed effects model with age and gender as covariates, genotype as fixed effects and family as random effect were used for analyzing the association between QTc and genotype. Bonferroni correction was used for post hoc paired wise comparison among the three study groups. All analyses were made by a statistician using SAS version 9.1 (SAS Institute Inc, Cary, NC). A P-value <0.05 was considered statistically significant. For paired wise comparison, P-values less than 0.017 (0.05/3) were considered statistically significant.
Results
Patient and Genotype Characteristics
Between 1998 and 2010, 267 patients were referred for LQTS genetic testing. Genetic testing was feasible in 188 (70%) patients; the remaining patients were deferred due to financial or insurance constraints. A disease causing LQTS gene mutation was identified in 76 (40%) of patients. Fifty LQTS patients fulfilled study inclusion criteria and were enrolled in the study. The control group consisted of 108 patients. There were no major ethnic differences between the LQTS and control group with the majority of patients being self-categorized as “white” (92% LQTS vs. 80% controls, P=NS). Clinical presentation of LQTS patients is summarized in Figure 1. Genotype data including mutation location for patients with LQT1 and LQT2 are summarized in Supplemental Table 1. In both the LQT1 and LQT2 groups, trans-membrane mutations were the most common location type (66%, n=19 and 38%, n=8 respectively). The 29 patients with LQT1 comprised of seventeen distinct families and the 21 patients with LQT2 comprised of 16 distinct families. The supine resting QTc intervals were significantly longer in patients with LQTS compared to controls. At peak exercise, only the LQT1 group exhibited abnormal QTc interval prolongation compared to baseline, whereas the LQT2 patients and controls had QTc interval shortening during peak exercise (Table 1).
Figure 1.
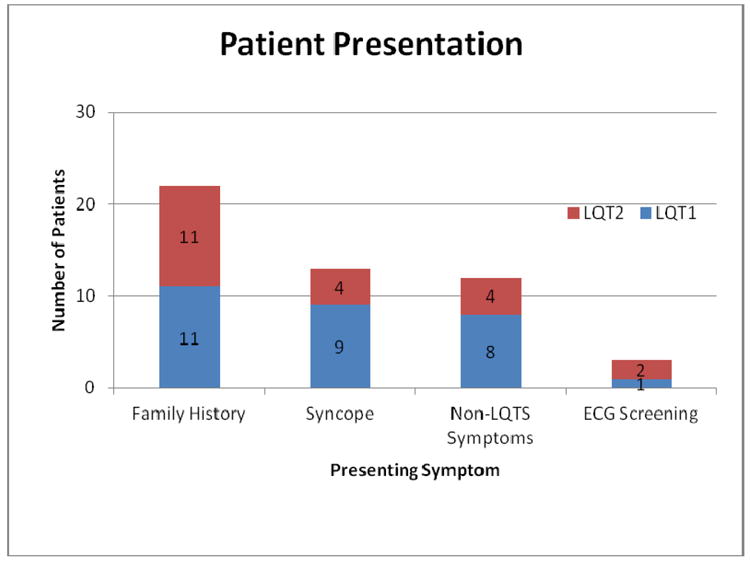
Clinical Presentation. Bar graph demonstrates patient presenting characteristics in the LQT1 and LQT2 groups. The most common presentation was family history followed by syncope. There was no major difference in the presentation distribution in either LQTS group.
Table 1.
Patient Demographic and QTc Intervals (ms) during EST.
| LQT1 (n=29) | LQT2 (n=21) | Control (n=108) | |
|---|---|---|---|
| Mean age±SD | 10.4±4.2 | 11.7±4.7 | 11.8±3.6 |
| Female sex, n (%) | 19(61) | 11(52) | 45(42) |
| Beta-blocker usage, n (%) | 17(59) | 11(52) | |
| Rest (QTc ms±SD) | 452±33† | 475±35†* | 407±21 |
| Peak exercise | 488±35† | 439±32†* | 406±25 |
| 1 min recovery | 481±35† | 434±27†* | 401±22 |
| 3 min recovery | 491±38† | 460±34†* | 408±22 |
| 5 min recovery | 487±32† | 468±22†* | 427±19 |
| 7 min recovery | 484±29† | 478±20† | 431±18 |
| 9 min recovery | 486±32† | 473±23† | 432±18 |
P-value <0.001 LQT1 and LQT2 vs controls
P-value <0.001 LQT1 vs LQT2
QTc Intervals during EST Recovery Phase
QTc intervals plotted against time during EST are depicted in Figure 2. During the entire recovery phase, the QTc intervals remained prolonged in LQT1 patients, similar to peak exercise. The QTc intervals in the LQT2 patients shortened, during peak exercise as well as during the early recovery phase, but prolonged in the late recovery phase (7 and 9 minutes). The heart rate response in the LQTS and control group during the exercise stress test is shown in Figure 3.
Figure 2.

QTc Intervals Plotted Against Time. Shown are the average QTc intervals in ms plotted against time (rest, peak exercise, 1, 3, 5, 7 and 9 minutes of recovery). Standard error bars are also included. Demonstrated in this graph is the abnormal adaptation response to exercise among the LQT1 group. Note the QTc intervals in the LQT2 group shorten with exercise and prolong in late (7 and 9 minutes) recovery, at which time the LQT1 and LQT2 values converge. Both LQT1 and LQT2 were significantly different than controls at all time intervals during the EST.
Figure 3.
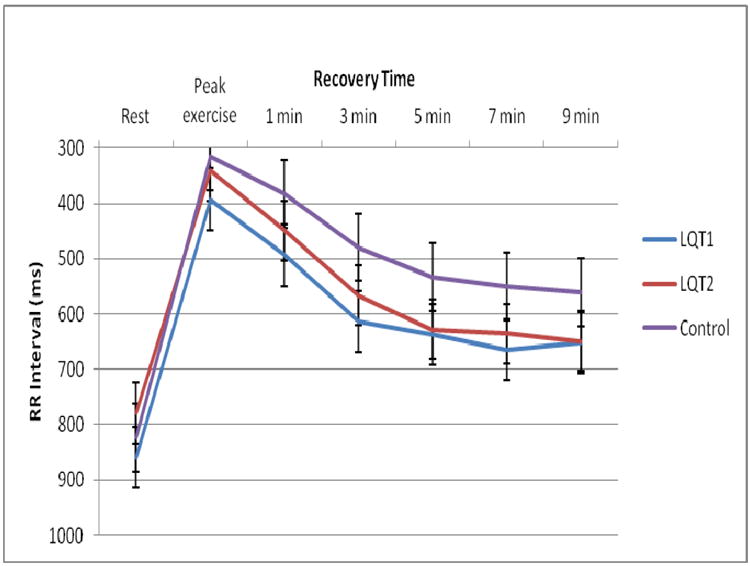
RR Intervals Plotted Against Time. Shown are the average RR intervals (ms) plotted against time (rest, peak exercise, 1, 3, 5, 7 and 9 minutes of recovery). Standard error bars are included. Shown are differences in RR intervals in the LQT1 and LQT2 groups vs controls reflective of beta-blocker therapy. Note, RR intervals do not return to resting values at end recovery (9 minutes).
A QTc > 460 ms at the 7 minute recovery phase provided the best sensitivity in distinguishing LQTS vs. controls without compromising specificity or positive predictive value (Table 2). Beta blocker treatment had no significant effect on the QTc during any stage of the EST (Supplemental Table 2).
Table 2.
Sensitivity, Specificity and Positive Predictive Value Using a QTc of > 460 ms at Different Recovery Intervals.
| 460 (LQTS vs Control) | Sensitivity | Specificity | PPV |
|---|---|---|---|
| 3 min | 64 | 98 | 94 |
| 5 min | 78 | 98 | 95 |
| 7 min | 86 | 96 | 91 |
Recovery ΔQTc(7 min-1 min)
The ΔQTc(7min-1min) was 1.5±28.1 ms and 43.9±31.8 ms (P<0.0001) in the LQT1 and LQT2 groups respectively (Figure 4). A ΔQTc(7min-1min) >30 ms predicted LQT2 vs. LQT1 with 75% sensitivity, 82% specificity and 75% positive predictive value.
Figure 4.
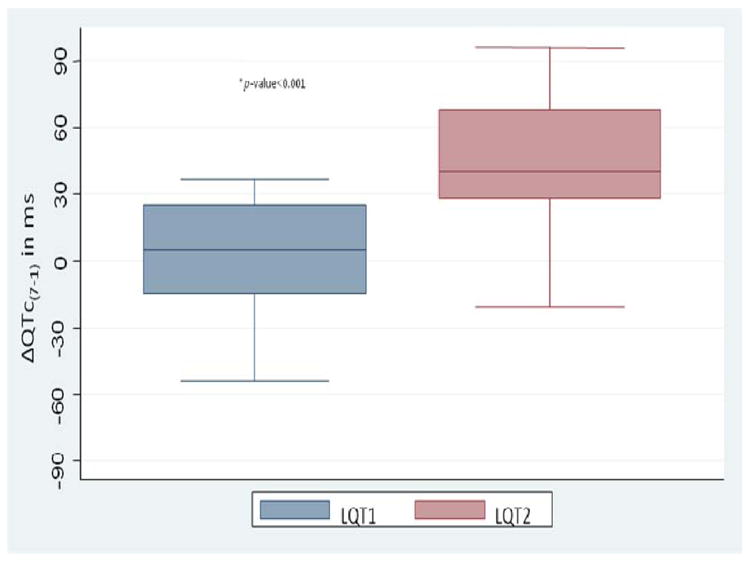
Delta QTc(7 min-1 min). Box plots show the difference in ΔQTc(7 min-1 min) between the LQT1 and LQT2 groups. The ΔQTc(7 min-1 min) is a useful parameter in differentiating LQT1 vs LQT2 as LQT2 patients have a significantly longer ΔQTc.
Concealed LQTS (cLQTS)
Twenty-three (46%) patients (LQT1 and LQT2 subgroups) had concealed LQTS (cLQTS). Their resting QTc was 432±22 ms. ECG patterns are shown in Figure 5. Patients with cLQTS had longer QTc intervals at all time intervals of the EST when compared to control patients (Figure 6). A QTc > 460 ms at the 7 minute recovery phase predicted cLQTS vs. control with 96% specificity, 82% sensitivity and 82% positive predictive value. A QTc > 445 ms at the 7 minute recovery phase yielded a sensitivity of 96%, specificity of 78% and positive predictive value to 67%. Genotype cLQT data is provided in Supplemental Table 3.
Figure 5.
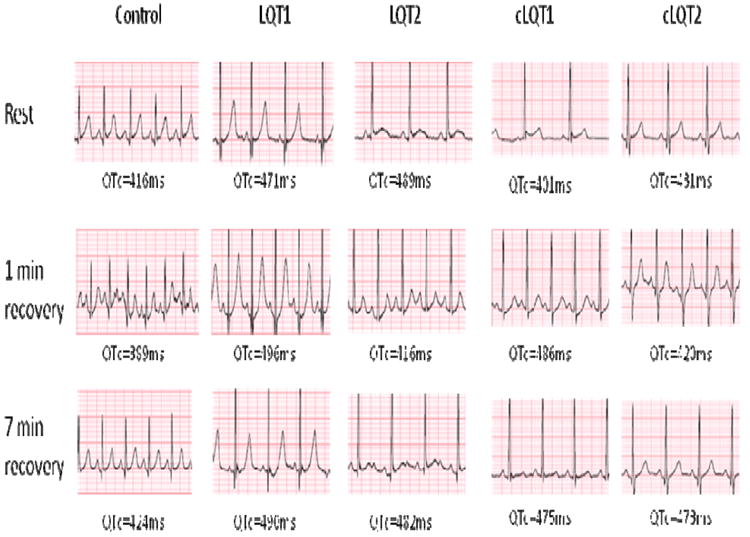
ECG Tracing in LQTS Patients During EST. Examples of ECG tracings reflect gene specific repolarization responses. The control patient had a normal resting QTc (416ms) which shortened in early recovery (389ms) and returned to near baseline at 7 minute recovery (424ms). The LQT1 patient has characteristic QTc prolongation at rest (471ms) which prolonged further in early recovery (496ms) and remained prolonged at 7 minute recovery (490ms). The LQT2 patient also had characteristic QTc prolongation at rest (489ms) which shortened in early recovery (416ms) and prolonged at 7 minute recovery (482ms). The cLQT1 and cLQT2 (concealed LQT) had similar patterns.
Figure 6.
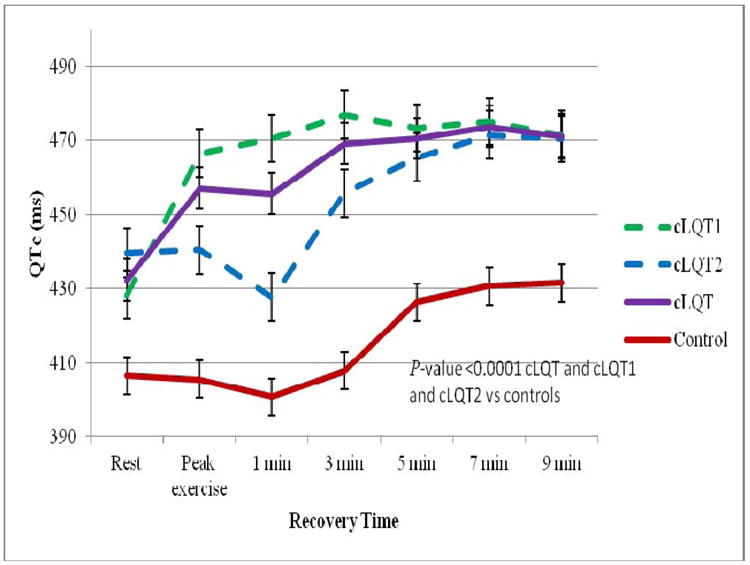
QTc Intervals Plotted Against Time in cLQTS (concealed) Patients. Shown are the average QTc intervals in ms plotted against time (rest, peak exercise, 1, 3, 5, 7 and 9 minutes of recovery) in the patients with resting QTc intervals <460ms (cLQTS). cLQTS (cLQT1 and cLQT2) patients had longer QTc intervals compared to controls at all time intervals of the EST.
Mutation Site Specific Changes of QTc Interval during EST
The QTc intervals at peak exercise and during each time interval of the recovery phase did not differ significantly between the LQT1 patients with pre-pore, pore, and post-pore mutations. Similarly, The QTc intervals at peak exercise and during each time interval of the recovery phase did not differ between the LQT2 patients with pore and non-pore mutations (Supplemental Table 4).
Postural QT and QTc Interval Changes
There were 23 LQTS patients not treated with beta blockers of which 12 (52%) had LQT1 and 11 (48%) had LQT2. In response to standing, LQTS patients had a blunted heart rate acceleration as compared to the control group (an increase of 10.5±15.5 bpm vs. 18.8±15.3 bpm, P=0.02).
The postural ΔQT of the control group was -13.6±16.2ms while the ΔQT of the LQTS patients was -2.2±25.3ms (P=0.003). However, the ΔQTc did not change significantly in the control vs. LQTS patients (26.3±37.8 ms vs. 32±31.0 ms, P=0.55). When comparing patients with LQT1 and LQT2, there was no significant difference in postural ΔQT, postural ΔQTc and postural ΔRR.
Discussion
The primary findings of our study were as follows: 1) Postural changes in the QT interval were useful in distinguishing LQTS patients from controls but were not useful to discriminate between LQT1 and LQT2 genotypes 2) a threshold value of QTc > 460 ms during the late recovery phase (7 minutes) of the EST was useful in distinguishing LQTS children, including the cLQTS sub-cohort, from normal patients 3) LQT1 and LQT2 patients demonstrated unique QT adaptation patterns during exercise and the recovery phase and 4) ΔQTc (7 min-1 min) > 30 ms, which reflects the repolarization difference between the late and early recovery phases, was useful in distinguishing between LQT2 and LQT1 genotypes in children. The latter findings suggest that an extended EST recovery phase may be useful when assessing children and adolescents with LQTS. Exercise stress test protocols for evaluation of LQTS in adult cohorts are limited to a recovery period of 4-5 minutes with QTc measurements performed up to 4 minutes in the recovery phase(8). Children have a more gradual deceleration in their heart rates during the recovery phase of the EST (Figure 3).
The length of the recovery phase becomes crucial as the predominant cellular repolarization currents come into play at critical heart rates. The slow (IKs) component of the delayed rectifying current is enhanced at faster heart rates with resultant adaptation or shortening of the QTc interval. The LQT1 (KCNQ1) gene encodes for the IKs potassium channel, and in the absence of functional IKs, results in paradoxical QTc prolongation at fast heart rates i.e. during peak exercise and the early recovery phase(19). In our study, QTc prolongation was observed to persist throughout the recovery phase of 9 minutes in LQT1 patients, ostensibly because of slower deceleration of the heart rate throughout the recovery phase. This is in variance with the recovery profile of adult LQTS patients reported by Chattha et al., where QTc prolongation was only seen in the early recovery phase with decrease in the QTc interval during late recovery(8). However in that study, the entire recovery phase consisted of a total duration of 4 minutes at which time the heart rate had decreased to baseline values. The time frame of the recovery phase may become even more important in patients with LQT2 who have impaired rapid component of the delayed rectifying current (IKr). IKr is more likely to play a significant role in cardiac repolarization at intermediate heart rates. During peak exercise and phases of recovery when the heart rate remains relatively fast, LQT2 patients will have normal QTc adaptation and minimal QTc prolongation(10). Therefore, in pediatric patients, if the QTc is measured during an abbreviated recovery phase (i.e. 3-5 minutes) when the heart rate still remains relatively fast and has not decreased to intermediate rates, it is possible that the opportunity to capture LQT2 patients may be missed. As shown in Figure 2, in our patient cohort, the significant increase in QTc values in LQT2 patients did not occur until approximately 7 minutes into the recovery phase, a time line when both the LQT1 and LQT2 QTc interval curves seem to merge. For these reasons, a recovery phase of 8-10 minutes or until the heart rate returns to baseline is preferable in pediatric patients in order to maximize the sensitivity of the EST.
Our study found a QTc threshold value of > 460 ms in the late recovery period to be useful in differentiating cLQTS patients from normal controls. In adult cLQTS cohorts, QTc > 445 ms at end recovery has been used to differentiate affected individuals from normal controls(7,8,20). A QTc > 460 ms rather than > 445 ms improved the specificity and positive predictive value of diagnosing LQTS without compromising the sensitivity in our cohort. The recovery ΔQTc(7 min-1 min) is a simple calculation that can be made using the patient as his or her own control. A greater separation in the QTc intervals between the end and early recovery phases (ΔQTc(7 min-1 min) > 30 ms) favors the diagnosis of LQT2. The patterns of QTc response during peak exercise and recovery were not significantly altered secondary to treatment with beta blockers in the current and previous studies(8,21). This observation is helpful in clinical practice as it obviates the need to stop beta-blockers in order to perform the EST. In contrast, other provocative tests such as epinephrine infusion require beta blocker washout prior to performing the test(22,23).
The QT interval predominantly shortened in response to standing in controls but either remained unchanged, minimally shortened or actually increased in LQTS patients in our study. Similar to the observations in adult LQTS patients, the response of the QT interval to a standing position is impaired in children with LQTS. In the study by Viskin et al., LQTS patients and controls had similar heart rate acceleration in response to standing(24). A blunted heart rate acceleration was observed in our LQTS patients as compared to the control group. Sinus rate response in LQTS patients is controversial with some studies demonstrating sinus node impairment especially in LQT1 patients (25-27). Due to the possibility of blunted heart rate acceleration in LQTS patients, postural QT rather than QTc change should be assessed in patients evaluated for LQTS. We did not find added benefit of postural QT changes in differentiating between LQT1 and LQT2 genotypes.
To the best of our knowledge this is the first study to examine if mutations location in the KCNQ1 and KCNH2 genes determines QT adaptation in response to exercise. Moss and colleagues found a markedly increased risk for cardiac events with mutations in the pore region of the KCNH2 gene. Similarly, mutations located in the transmembrane portion were found to be important independent risk factors of clinical events in LQT1 patients. However, neither study investigated the influence of location of mutation on repolarization response to exercise(13,28,29). In our study we did not find a correlation between mutation location and repolarization response to exercise and speculate that additional factors may be responsible for genotype specific repolarization changes.
Our study has several limitations. It is a retrospective study with a modest sample size limited to two common LQTS genotypes limiting the generalization of results. Certain mutations have the likelihood of being overrepresented due to individuals being genetically related but the majority of our study participants came from unrelated families which may strengthen the study. We did not perform gender adjustment for QT thresholds as the majority of our study and control population were children of peri-pubertal age or younger. Zareba et al have shown no gender differences in QTc duration among LQT1 and LQT2 subjects ≤ 15 years of age(4). We attempted to evaluate the influence of specific mutation location to QT adaptation but the results may have been obscured by small sample size. We recognize that the type of QTc response observed during bicycle ergometry EST utilized in our study should be interpreted with caution as they may not carry over to other types of EST protocols. Finally, our study only included patients with confirmed genetic results which could be a source of bias.
Conclusion
Children and adolescents with LQTS have an abnormal QT adaptation response during the recovery phase of exercise stress testing which is genotype specific but not mutation site specific. An extended recovery phase may be preferable to assess the repolarization response after exercise in the pediatric population. A QTc > 460 ms in the late recovery phase can distinguish LQTS from unaffected individuals and a recovery ΔQTc(7 min-1 min) > 30ms is useful in discriminating LQT1 from LQT2 genotypes. These findings are relevant to our cycle ergometer protocol and should not be generalized to other forms of EST. Our findings need to be prospectively validated in a larger cohort prior to implementation as a clinical diagnostic tool.
Supplementary Material
Our study focuses on the repolarization response during exercise stress testing (EST) in pediatric patients with long QT syndrome (LQTS). Employing a cycle ergometer, we examined EST in 50 genotype positive LQTS patients and 108 controls. The primary findings of this study were as follows: 1) Postural changes in the QT interval were useful in distinguishing LQTS patients from controls but were not useful to discriminate between LQT1 and LQT2 genotypes, 2) a threshold QTc value of >460 ms during an extended recovery phase (7 minutes) of EST was useful in distinguishing LQTS children from both control patients and from concealed LQTS children, 3) LQT1 and LQT2 patients demonstrated unique QT interval adaptation patterns during exercise and the recovery phase and 4) the ΔQTc (7min-1min) >30 ms, which reflects the repolarization difference between the late and early recovery phases, was useful in distinguishing between LQT2 and LQT1 genotypes in children. The relative gradual deceleration of heart rate in the recovery phase compared to adults explains the need for an extended recovery phase in the pediatric population. Though this is the first study in genotyped pediatric LQTS patients evaluating repolarization reserve and QT interval adaptation during EST, our results need to be prospectively validated by a larger cohort. Additionally, our results are limited to bicycle ergometry and should not be translated to other exercise protocols.
Acknowledgments
Financial Sources National Heart, Lung and Blood Institute (NHLBI-T32), grant # HL007915
Footnotes
Disclosures none
Bibliography
- 1.Ackerman MJ, Clapham DE. Mechanisms of disease: ion channels: basic science and clinical disease. N Engl J Med. 1997;336:1575–1586. doi: 10.1056/NEJM199705293362207. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. Circ. 1993;88:782–4. doi: 10.1161/01.cir.88.2.782. [DOI] [PubMed] [Google Scholar]
- 3.Liu JF, Jons C, Moss AJ, McNitt S, Peterson DR, Qi M, Zareba W, Robinson JL, Barsheshet A, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin J, Vincent M, Zhang L, Goldenberg I. Risk factors for recurrent syncope and subsequent fatal or near-fatal events in children and adolescents with long QT syndrome. JACC. 2011;57:941–50. doi: 10.1016/j.jacc.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zareba W, Moss AJ, Locati EH, Lehmann MH, Peterson DR, Wall WJ, Schwartz PJ, Vincent GM, Priori SG, Benhorin J, Towbin JA, Robinson JL, Andrews ML, Napolitano C, Timothy K, Zhang L, Medina A. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. JACC. 2003;42:103–9. doi: 10.1016/s0735-1097(03)00554-0. [DOI] [PubMed] [Google Scholar]
- 5.Vincent GM, Timothy K, Leppert M, Keating MT. The spectrum of symptoms and QT intervals in carriers of the gene for the long QT syndrome. N Engl J Med. 1992;327:846–52. doi: 10.1056/NEJM199209173271204. [DOI] [PubMed] [Google Scholar]
- 6.Bai R, Napolitano C, Bloise R, Monteforte N, Priori SG. Yield of genetic screening in inherited cardiac channelopathies: how to prioritize access to genetic testing. Circ Arrhythm Electrophysiol. 2009;2:6–15. doi: 10.1161/CIRCEP.108.782888. [DOI] [PubMed] [Google Scholar]
- 7.Wong JA, Gula LJ, Klein GJ, Yee R, Skanes AC, Krahn AD. Utility of treadmill testing in identification and genotype prediction in long QT syndrome. Circ Arrhythm Electrophysiol. 2010;3:120–5. doi: 10.1161/CIRCEP.109.907865. [DOI] [PubMed] [Google Scholar]
- 8.Chattha IS, Sy RW, Yee R, Gula LJ, Skanes AC, Klein GJ, Bennett MT, Krahn AD. Utility of the recovery electrocardiogram after exercise: a novel indicator for the diagnosis and genotyping of long QT syndrome? Heart Rhythm. 2010;7:906–11. doi: 10.1016/j.hrthm.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Horner JM, Horner MM, Ackerman MJ. The diagnostic utility of treadmill exercise stress testing in the evaluation of congenital long QT syndrome. Heart Rhythm. 2009;7:S45–S87. [Google Scholar]
- 10.Swan H, Toivonen L, Viitasalo M. Rate adaptation of QT intervals during and after exercise in children with congenital long QT syndrome. Eur Heart J. 1998;19:508–13. doi: 10.1053/euhj.1997.0764. [DOI] [PubMed] [Google Scholar]
- 11.Splawski I, Shen J, Timothy K, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circ. 2000;102:1178–85. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 12.Zareba W, Moss AJ, Sheu G, Kaufman ES, Priori S, Vincent GM, Towbin JA, Benhorin J, Schwartz PJ, Napolitano C, Hall WJ, Keating MT, Qi M, Robinson JL, Andrews ML. Location of mutation in the KCNQ1 and phenotypic presentation of long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1149–53. doi: 10.1046/j.1540-8167.2003.03177.x. [DOI] [PubMed] [Google Scholar]
- 13.Moss AJ, Zareba W, Kaufman ES, Gartman E, Peterson DR, Benhorin J, Towbin JA, Keating MT, Priori S, Schwartz PJ, Vincent GM, Robinson JL, Andrews ML, Feng C, Hall WJ, Medina A, Zhang L, Wang Z. Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-a-go-go-related gene potassium channel. Circ. 2002;105:794–9. doi: 10.1161/hc0702.105124. [DOI] [PubMed] [Google Scholar]
- 14.Sanguinetti MC, Curran ME, Spector PS, Keating MT. Spectrum of HERG K+-channel dysfunction in an inherited cardiac arrhythmia. Proc Natl Acad Sci USA. 1996;93:2208–12. doi: 10.1073/pnas.93.5.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowland TW. Pediatric laboratory exercise testing. Human kinetics; Champaign: 1993. [Google Scholar]
- 16.Lepeschkin E, Surawicz B. The measurement of the Q-T interval of the electrocardiogram. Circ. 1952;6:378–388. doi: 10.1161/01.cir.6.3.378. [DOI] [PubMed] [Google Scholar]
- 17.Rautaharju PM, Surawicz B, Gettes LS. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. JACC. 2009;53:982–91. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Bazett H. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 19.Takenaka K, Ai T, Shimizu W, Kobori A, Ninomiya T, Otani H, Kubota T, Takaki H, Kamakura S, Horie M. Exercise stress test amplifies genotype-phenotype correlation in the LQT1 and LQT2 forms of the long-QT syndrome. Circ. 2003;107:838–844. doi: 10.1161/01.cir.0000048142.85076.a2. [DOI] [PubMed] [Google Scholar]
- 20.Walker BD, Krahn AD, Klein GJ, Skanes AC, Yee R. Burst bicycle exercise facilitates diagnosis of latent long QT syndrome. American Heart Journal. 2005;150:1059–1063. doi: 10.1016/j.ahj.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 21.Kaltman JR, Ro PS, Stephens P, McBride MG, Cohen MI, Tanel RE, Vetter VL, Rhodes LA. Effects of beta-adrenergic antagonists on the QT measurements from exercise stress tests in pediatric patients with long QT syndrome. Pediatr Cardiol. 2003;24:553–8. doi: 10.1007/s00246-003-0436-0. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu W, Noda T, Takaki H, Nagaya N, Satomi K, Kurita T, Suyama K, Aihara N, Sunagawa K, Echigo S, Miyamoto Y, Yoshimasa Y, Nakamura K, Ohe T, Towbin JA, Priori SG, Kamakura S. Diagnostic value of epinephrine test for genotyping LQT1, LQT2, and LQT3 forms of congenital long QT syndrome. Heart Rhythm. 2004;1:276–83. doi: 10.1016/j.hrthm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Vyas H, Hejlik J, Ackerman MJ. Epinephrine QT stress testing in the evaluation of congenital long-QT syndrome: diagnostic accuracy of the paradoxical QT response. Circ. 2006;113:1385–92. doi: 10.1161/CIRCULATIONAHA.105.600445. [DOI] [PubMed] [Google Scholar]
- 24.Viskin S, Postema PG, Bhuiyan ZA, Rosso R, Kalman JM, Vohra JK, Guevara-Valdivia ME, Marquez MF, Kogan E, Belhassen B, Glikson M, Strasberg B, Antzelevitch C, Wilde AA. The response of the QT interval to the brief tachycardia provoked by standing: A bedside test for diagnosing long QT syndrome. JACC. 2010;55:1955–61. doi: 10.1016/j.jacc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swan H, Viitasalo M, Piippo K, Laitinen P, Kontula K, Toivonen L. Sinus node function and ventricular repolarization during exercise stress test in long QT syndrome patients with KvLQT1 and HERG potassium channel defects. JACC. 1999;34:823–9. doi: 10.1016/s0735-1097(99)00255-7. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz PJ, Periti M, Malliani A. The long Q-T syndrome. Am Heart J. 1975;89:378–90. doi: 10.1016/0002-8703(75)90089-7. [DOI] [PubMed] [Google Scholar]
- 27.Eggeling T, Osterhues HH, Hoeher M, Gabrielsen FG, Weismueller P, Hombach V. Value of Holter monitoring in patients with the long QT syndrome. Cardiology. 1992;81:107–14. doi: 10.1159/000175784. [DOI] [PubMed] [Google Scholar]
- 28.Splawski I, Shen J, Timothy K, Lehmann MH, Priori SG, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circ. 2000;102:1178–85. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 29.Moss AJ, Shimizu W, Wilde AM, Towbin JA, Zareba W, Robinson JL, Qi M, Vincent GM, Ackerman MJ, Kaufman ES, Hofman N, Seth R, Kamakura S, Miyamoto Y, Goldenberg I, Andrews ML, McNitt S. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circ. 2007;115:2481–9. doi: 10.1161/CIRCULATIONAHA.106.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


