Abstract
Background
The specialized cardiac conduction system (CCS) expresses a unique complement of ion channels that confer a specific electrophysiological profile. ATP sensitive potassium (KATP) channels in these myocytes have not been systemically investigated.
Methods and Results
We recorded KATP channels in isolated CCS myocytes using Cntn2-EGFP reporter mice. The CCS KATP channels were less sensitive to inhibitory cytosolic ATP compared to ventricular channels and more strongly activated by MgADP. They also had a smaller slope conductance. The two types of channels had similar intraburst open and closed times, but the CCS KATP channel had a prolonged interburst closed time. CCS KATP channels were strongly activated by diazoxide and less by levcromakalim, whereas the ventricular KATP channel had a reverse pharmacological profile. CCS myocytes express elevated levels of Kir6.1, but reduced Kir6.2 and SUR2A mRNA compared to ventricular myocytes (SUR1 expression was negligible). SUR2B mRNA expression was higher in CCS myocytes relative to SUR2A. Canine Purkinje fibers expressed higher levels of Kir6.1 and SUR2B protein relative to the ventricle. Numerical simulation predicts a high sensitivity of the Purkinje action potential to changes in ATP:ADP ratio. Cardiac conduction time was prolonged by low-flow ischemia in isolated, perfused mouse hearts, which was prevented by glibenclamide.
Conclusions
These data imply a differential electrophysiological response (and possible contribution to arrhythmias) of the ventricular CCS to KATP channel opening during periods of ischemia.
Keywords: K+ Channels, KATP channel, Purkinje fiber, conduction, numerical simulation, ischemia
Introduction
The electrophysiological diversity of the heart is best illustrated by the unique action potential profiles within the specialized pacemaker and cardiac conduction systems. For example, myocytes from the sino-atrial (SA) node, atrio-ventricular (AV) node, bundle of His, and Purkinje network all display various degrees of pacemaking activity, and distinct levels of maximum diastolic potential and action potential waveform profiles1. Before the advent of patch clamping, the electrophysiological properties of Purkinje strands have been studied extensively because their cable-like morphological structure was well suited for the prevailing recording techniques (sucrose gap and two-electrode voltage clamping techniques). Recent patch clamp data have further clarified this picture and we now know that Purkinje myocytes express a unique complement of ion channels (several types of Na+ channels, including a large non-inactivating component, L- and T-type Ca2+ channels, a variety of K+ channels and a robust pacemaker current)2. The interaction of these various ion channels, exchangers and pumps is responsible for the characteristic electrophysiological properties of the Purkinje myocyte, including a rapid upstroke, action potential notch, long duration, negative maximum diastolic potential and automaticity. Little data are available regarding the properties of the KATP channel in the specialized conduction system, which comes to play during ischemic events. Given the contribution of the ventricular conduction system and Purkinje-muscle junctions in the generation of ischemia-induced arrhythmias3, it is important to understand the biophysical, regulatory and pharmacological properties of KATP channels in this tissue.
KATP channels are ubiquitously expressed in the heart. Since their original description in cardiac ventricular myocytes4, they have also been identified in the atrium5, as well as in the specialized cardiac pacemaker/conduction system, including the sino-atrial (SA) node6, atrio-ventricular (AV) node7 and myocytes from Purkinje fibers8. Structurally, KATP channels are octaheteromeric proteins that are composed of four inwardly rectifying pore-forming subunits (Kir6.1 or Kir6.2) and four regulatory, sulfonylurea receptor subunits (SUR1, SUR2A and SUR2B)9. Their potassium selectivity, inward rectification, and unitary conductance are determined primarily by the Kir6.x subunit, whereas the nucleotide sensitivity and pharmacology of the channel depend largely on the type of SURx subunit present in the channel protein9. In this study, we characterized the biophysical and pharmacological properties, regulation by cytosolic nucleotides, and the molecular composition of KATP channels in the mouse ventricular cardiac conduction system (CCS) and compared these to the better characterized ventricular KATP channel. We also investigated the pathological consequences of CCS KATP channels in conduction slowing during ischemia and our results suggest that when KATP channels open during ischemia, electrophysiological effects in the CCS may be especially severe. These studies were facilitated by the use of Cntn2-EGFP reporter mice in which EGFP expression is restricted to the cardiac conduction system10.
Methods
A complete description of the methods used is available on the online supplemental information.
Cardiac myocyte isolation
All animal procedures were in accordance with NIH guidelines and were approved by the Institutional Animal Care and Use Committees of New York University School of Medicine. The methods used were described previously11.
Patch clamp recording techniques
Single-channel recordings were performed in the inside-out configuration at room temperature using standard patch-clamp techniques. Patch pipettes (2–4 MΩ) were filled with (in mmol/L) 150 KCl, 2 CaCl2, 1 MgCl2, and 10 HEPES, pH 7.4. The bath solution consisted of (mmol/L) 150 KCl, 1 EGTA, 10 HEPES, 1.2 MgCl2 and pH 7.2. Currents were filtered (−3 dB at 1 kHz) and digitized (5 kHz) for computer storage and analysis. Unless otherwise indicated, the pipette potential was +80mV. If needed, rundown was corrected. Dwell times and burst analysis were estimated using patches containing a single active channel. Following baseline subtraction, event detection was performed with 50% single channel current amplitude threshold. A minimum resolution was imposed by ignoring events shorter than 0.18 ms12.
Real-time and conventional RT-PCR
Real-time semi-quantitative RT-PCR (qRT-PCR) was used to quantify mRNA expression, as described previously13. EGFP-positive myocytes (originating from the CCS) or non-GFP expressing cells (ventricular myocytes) were enzymatically isolated from the ventricles of the Cntn2-EGFP reporter mouse strain10. Individual cells were manually selected by epifluorescence microscopy and RNA was isolated from 80–100 pooled cells (PicoPure™, Arcturus). Reverse transcription (RT; Superscript III, Invitrogen) was performed using random hexamer primers. The PCR threshold cycle (Ct) values were determined and data were analyzed using custom routines written in the R programming language (available upon request). Gene expression was calculated relative to the following reference genes: cyclophilin B (PPIB) and hydroxymethylbilane synthase (HMBS). Conventional RT-PCR was performed using the same RT reactions described above. Primer sequences are given as Supplementary Information
Membrane preparation and Western blotting
Membrane fractions were prepared as described11 with modifications. Free running canine Purkinje strands and left ventricular free wall tissue were snap-frozen, pulverized in liquid nitrogen and homogenized using 30 strokes of a glass-glass homogenizer followed by 30 strokes in a Dounce homogenizer in (in mmol/L) 250 sucrose, 1 EDTA, 10 HEPES, 1 DTT and pH 7.4 supplemented with protease inhibitor cocktail (Roche Applied Science). After a brief centrifugation (1,000 g for 5 min at 4°C), the supernatant was loaded on top of an 18% Optiprep (Sigma-Aldrich) layer (with in mmol/L: 150 sucrose, 1 EDTA, 10 HEPES, 1 DTT and pH 7.4 supplemented with protease inhibitor cocktail) and subjected to ultracentrifugation (200,000 g for 1 h at 4°C). The membrane fraction was collected from the interphase and membranes were recovered by centrifugation (after dilution with 10mM Tris HCl, 1 mM EDTA, pH 7.5). After overnight solubilization at 4°C in 20 mmol/L HEPES, pH 7.4 and 0.5% v/v Triton X100, unboiled proteins were resolved by 10% SDS-PAGE and immunoblotted using standard techniques11. Antibodies used were: rabbit anti-Kir6.1 (NAF-1), chicken anti-Kir6.2 (C-62), goat anti-SUR2B (C-15, Santa Cruz) and GAPDH (Millipore). The specificity of these antibodies is illustrated in Supplemental Figure S1.
Isolated, perfused hearts: low-flow ischemia
Following sacrifice, mouse hearts were excised and the right atrial free wall was removed. Hearts were cannulated by the aorta and retrogradedly perfused at 74 mmHg with Tyrode's solution (in mM: 1.8 CaCl2, 1.0 MgCl2, 1.2 KH2PO4, 130 NaCl, 4.7 KCl, 11.1 glucose, 24 NaHCO3, 0.52% albumin w/v, pH 7.4, equilibrated with 95% O2/5% CO2). Throughout the experiment, the left atrium was electrically paced (ms stimuli 1.5× above threshold) at a cycle length of 300 ms, while the volume conducted local cardiac electrical response (‘electrocardiogram’ or ECG) was recorded using Ag-AgCl electrodes flanking the heart. The time from the end of atrial depolarization to the beginning of ventricular depolarization (PR segment) was measured as an index of overall conduction time. The experimental protocol consisted of a stabilization time of 20 min before the hearts were divided into two groups. For experimental group, hearts were perfused with the KATP channel blocker, glibenclamide (2 µM), for 5 minutes followed by 20 minute low-flow ischemia, which was induced by decreasing the perfusion pressure to 25% of the pre-ischemic period. The control group was identical, except that glibenclamide was substituted by the solvent (DMSO; 0.017% v/v final concentration). In other experiments, rat hearts (Sprague-Dawley, 250–300g) were perfused in Langendorff as described above and the coronary artery flow was monitored (Transonic Systems). The perfusion pressure was decreased to achieve low-flow ischemia. The volume conducted ECG was recorded and the time from the beginning of ventricular depolarization to partial ventricular repolarization (QRS duration) was measured as an index of distal conduction time.
Numerical model of the KATP channel
We developed numerical models of the ventricular and CCS KATP channels, based upon our experimental data and a previously described model14. Full details of the models are available in the Supplementary information. In brief, the KATP channel current is expressed as
| (1) |
where N represents the channel density (channels/µm2), Po the intrinsic open probability in the absence of modulation by Na+, Mg2+, γ the unitary conductance (pS), and nucleotides, fK,ATP the fraction of open channels, Em the membrane potential (mV) and EK the equilibrium potential for K+ ions (mV). The number of functional KATP channels and intrinsic open probabilities were estimated from our patch clamp data. Inhibition by ATP and stimulation by MgADP were modeled by experimental data obtained. To simulate effects of KATP channel on the action potential duration, we used published models of either the human ventricular15 or the human Purkinje2 myocyte (see online supplement for full information).
Statistical analysis
The Kolmogorov-Smirnov test was used to test whether data followed a normal distribution. Assumption of equal variance per group was confirmed with the use of Bartlett's test. Data are given as mean ± SEM (where n indicates number of patches or number of animals per group). Statistical tests used included unpaired or paired Student’s t-test. With small sample sizes or when the normality or equal variance test failed, the non-parametric Mann-Whitney rank sum test was used. Statistical significance was assumed at a p-value <0.05.
Results
Given the importance of the specialized cardiac conduction system in electrical propagation and arrhythmogenesis, we set out to characterize the KATP channel of the mouse cardiac conduction system (CCS). This study was facilitated by the use of a Cntn2-EGFP BAC transgenic reporter mouse strain10, which allowed us to visualize isolated cells of the CCS by their EGFP epifluorescence. Atria were removed before cell isolation and EGFP-positive myocytes may originate from both proximal and distal elements of the CCS (the ventricular Purkinje network). We examined the biophysical and regulatory properties, as well as the molecular composition, of ventricular CCS KATP channels in comparison with non-EGFP expressing ventricular myocytes. We also investigated the role of KATP channels during ischemia-induced conduction slowing.
Unitary conductance
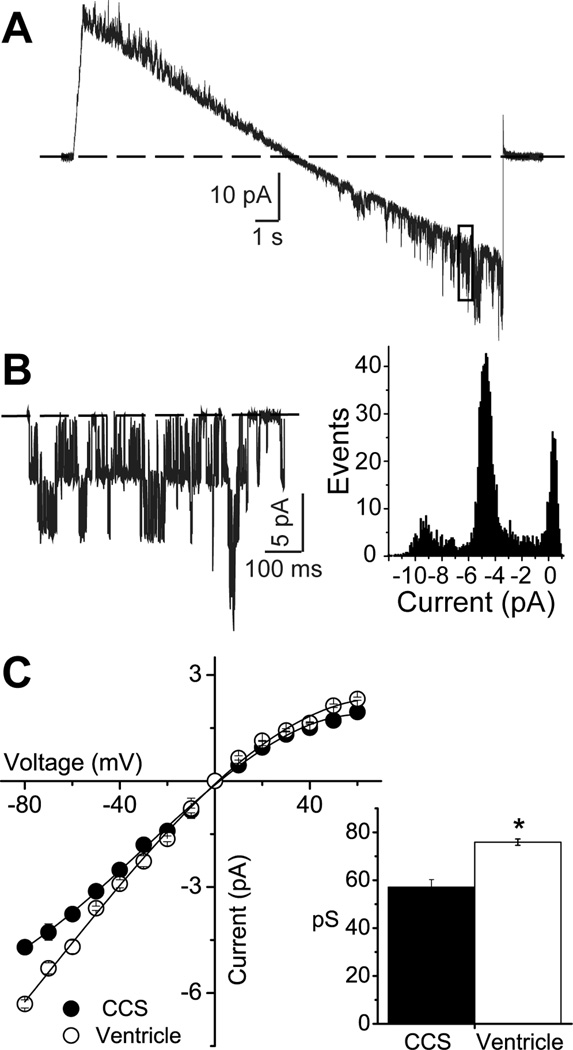
The unitary conductance was determined using patches with only a few KATP channels per patch, using a ramp voltage step (Figure 1). After baseline subtraction, an all-points histogram was constructed to obtain the unitary current at different voltages (Figure 1B). The current-voltage relationships of CCS and ventricular KATP channels were both weakly inward rectifying in the presence of ‘intracellular’ Mg2+. The unitary conductance was respectively 57.1±3.20 pS (n=8) and 75.9±1.32 pS (n=6; p=0.001, Mann-Whitney rank sum test) for CCS and ventricular KATP channels (Figure 1C). Most patches recorded in the inside-out patch clamp configuration of CCS or ventricular myocytes contained a large number of channels (pipette resistance 2.4±0.04 MΩ, n=57, Student’s t-test, p = 0.572)). Although the number of channels per patch was variable, the data suggested a similar channel density for the two types of myocytes. The estimated median KATP channel number was 28.6 (n=28) and 31.7 channels/patch (n=29) respectively for CCS and ventricular myocytes (Mann-Whitney rank sum test, p = 0.549).
Figure 1.
The unitary conductance of KATP channels recorded from mouse cardiomyocytes originating from the cardiac conduction system (CCS) and the ventricle. A) A representative trace of CCS KATP channel unitary events recorded during a voltage clamp ramp (111 mV/s) from −100 to 100 mV. B) Following baseline subtraction, the region of the current marked by the box (membrane potential around −80mV) is shown. Also shown is an all-points histogram for this current section. C) Current-voltage relationship of the unitary current amplitude for KATP channels recorded from CCS or ventricular myocytes. Inset: slope conductances (mean±SEM), measured between −80 and −20 mV. *p<0.05; Student’s t-test.
Single channel kinetics
Superficially, CCS and ventricular KATP channels displayed similar dwell characteristics in the absence of nucleotides. Dwell times were difficult to analyze given the high channel densities. We were able to obtain recordings with a single active channel in a few patches when the pipette resistance was increased to ~10 MΩ. We analyzed the mean open and closed times within bursts over a 30 s period, using recordings from patches containing a single active channel (Figure 2). Open and closed time histograms were constructed and data were subjected to curve fitting with a single exponential function (long closing events were ignored). The mean open times were respectively 2.2±0.16 ms (n=6) and 2.9±0.95 ms (n=5; Student’s t-test, p = 0.931) for CCS and ventricular KATP channels, whereas the mean closed times were respectively 0.4±0.02 ms (n=6) and 0.4±0.02 ms (n=5; Mann-Whitney rank sum test, p = 0.208). Both channel types displayed bursting behavior. The intraburst and interburst kinetics were similar for the two types of KATP channels, with the exception of a longer interburst closed time observed with the CCS KATP channels (Table 1).
Figure 2.
Dwell time kinetics of CCS and ventricular KATP channels. Shown are representative traces of KATP channel activity. Dwell time histograms were constructed (bin size of 0.2 ms) to obtain the mean open (left) and closed (right) times, obtained by exponential curve fitting. Channel activity was recorded for 30 s at −80 mV.
Table 1.
Properties of KATP channels recorded from mouse ventricular myocytes or from myocytes isolated from the specialized conduction system.
| Ventricle | Conduction system | P value | |
|---|---|---|---|
| Single channel conductance (pS) | 75.8±1.32 (n=6) | 57.1±3.20 (n=8) | 0.001* |
| Intrinsic open probability | 0.153±0.06 (n=4) | 0.167±0.06 (n=5) | 0.690 |
| Bursting kinetics | |||
| Mean intraburst open time (ms) | 2.2±0.95 | 2.2±0.16 | 0.931 |
| Mean intraburst closed time (ms) | 0.4±0.02 | 0.4±0.02 | 0.208 |
| Number of openings per burst | 34±3.1 | 41±5.8 | 0.324 |
| Burst duration | 115±43.7 | 139+49.1 | 0.320 |
| Interburst duration (ms) | 16±6.1 | 27±9.5 | 0.027 |
| 207±43.1 | 594±230.7 | 0.063* |
The unitary conductance was obtained between −80 and 0 mV. The intrinsic open probability reflects the average behavior of the channel (including interburst closed times). Bursting kinetics were calculated from n=5 and n=4 patches respectively from CCS and ventricular myocytes.
The data marked with * are compared with Mann-Whitney rank sum test, other data are compared with Student’s t-test.
Nucleotide sensitivity
We next examined the sensitivity of KATP channels to intracellular nucleotides. A concentration-response curve for ATP inhibition was constructed by exposing patches sequentially to ATP between 1 and 1000 µM (Figure 3A). The concentration of ATP producing half-maximal inhibition (IC50) was 59±12.4 µM (Hill coefficient 1.5±0.14; n=12) for ventricular KATP channels, which was significantly lower than that of the CCS KATP channel (116±22.8 µM, Hill coefficient 1.4±0.17; n = 12, p=0.04; Figure 3B). Thus, in addition to having a smaller unitary conductance, the CCS KATP channel is less sensitive to the inhibitory effect of cytosolic ATP. We also examined the degree of stimulation of KATP channel activity by MgADP. For these experiments, channels were half-maximally inhibited by ATP (50 or 100 µM respectively for ventricular or CCS KATP channels). ADP was then applied at a ADP:ATP ratio of 0.1–10 (Figure 3C). Consistent with previous reports, ventricular KATP channels were stimulated by lower MgADP concentrations16, but inhibited as the ADP concentration was increased17. By contrast, the CCS KATP channels were stimulated by MgADP, but not inhibited within the concentration range examined (Figure 3D). Half-maximal activation occurred at 84.±3.7 µM MgADP (n=13), which also reasonably describes the MgADP activation phase of ventricular KATP channel. A major difference between these channel types appears to be their differential sensitivities to inhibitory ADP.
Figure 3.
Nucleotide sensitivities of CCS and ventricular KATP channels. A) Representative traces of the mean patch current (patches contained many active channels). ATP (1 µmol/L to 1 mmol/L) was applied until steady state block occurred. B): Current (normalized to the maximal channel current in the absence of ATP; Io) is plotted as a function of the ATP concentration. Shown are mean±SEM of cumulative data (n=12 for CCS and ventricle). C) Representative experimental recordings demonstrating the effect of MgADP on KATP channels. D) The degree of activation was normalized as (I-I½)/(Io-I½), with I is the mean patch current, I½ the current recorded in the presence of 50 or 100 µM ATP, and Io the current measured in the absence of nucleotides (n=13 for CCS and n=12 for ventricle). The solid lines were produced by a KATP channel numerical model (see Supplemental Information). The dashed lines represent zero current.
Pharmacological properties
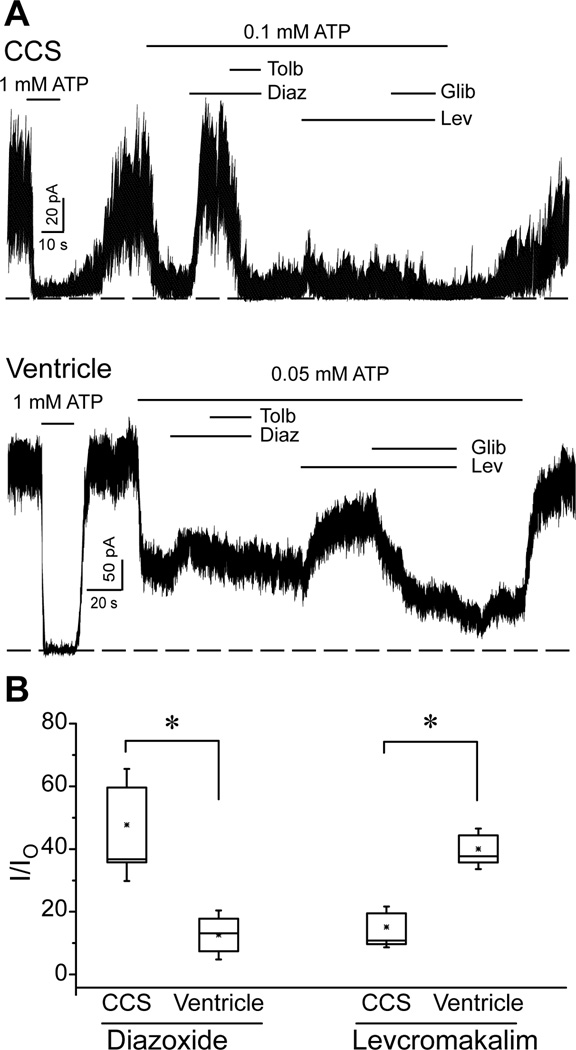
KATP channels from diverse tissues have unique pharmacological profiles. For example, the ventricular KATP channel is strongly activated by levcromakalim18, but is less sensitive to diazoxide19. We examined the effects of these compounds on ventricular and CCS KATP channels (Figure 4). Experiments were performed in the absence of ADP and in the presence of an ATP concentration close to the respective IC50 values (see above) to avoid confounding influences of nucleotides. Diazoxide (200µM) had a relatively small stimulatory effect on ventricular KATP channels (16±4.4%; n=12). By contrast, diazoxide induced a significantly larger increase in the CCS KATP channel activity (48±11.9%; n=7; p = 0.011) (Figure 4B). The activation of the CCS KATP channel by diazoxide was readily reversed by tolbutamide (200 µM). The opposite result was observed with levcromakalim (30 µM), which activated the ventricular KATP channel more readily (40±5.8%; n=12) than the CCS KATP channel (15±4.3%; n=7; p = 0.001; Figure 4B). The effect of levcromakalim was reversed by glibenclamide (2 µM) in both cell types. These data demonstrate that mouse CCS KATP channels have a different pharmacological sensitivity compared to the ventricular KATP channel.
Figure 4.
Pharmacological profiles of CCS and ventricular KATP channels recorded in the inside-out patch clamp configuration. A) Representative recordings of a CCS or ventricular KATP channel demonstrating the effects of diazoxide (Diaz, 200 µM), diazoxide plus tolbultamide (Tolb, 200µM), levcromakalim (Lev, 30 µM) and levcromakalim plus glibenclamide (Glib, 2 µM). B) The fractional currents (relative to the maximum current in the absence of ATP; I0) in the presence of diazoxide or levcromakalim are depicted as box and whisker plots. *p<0.05; Student’s t-test.
KATP channel subunit mRNA expression levels
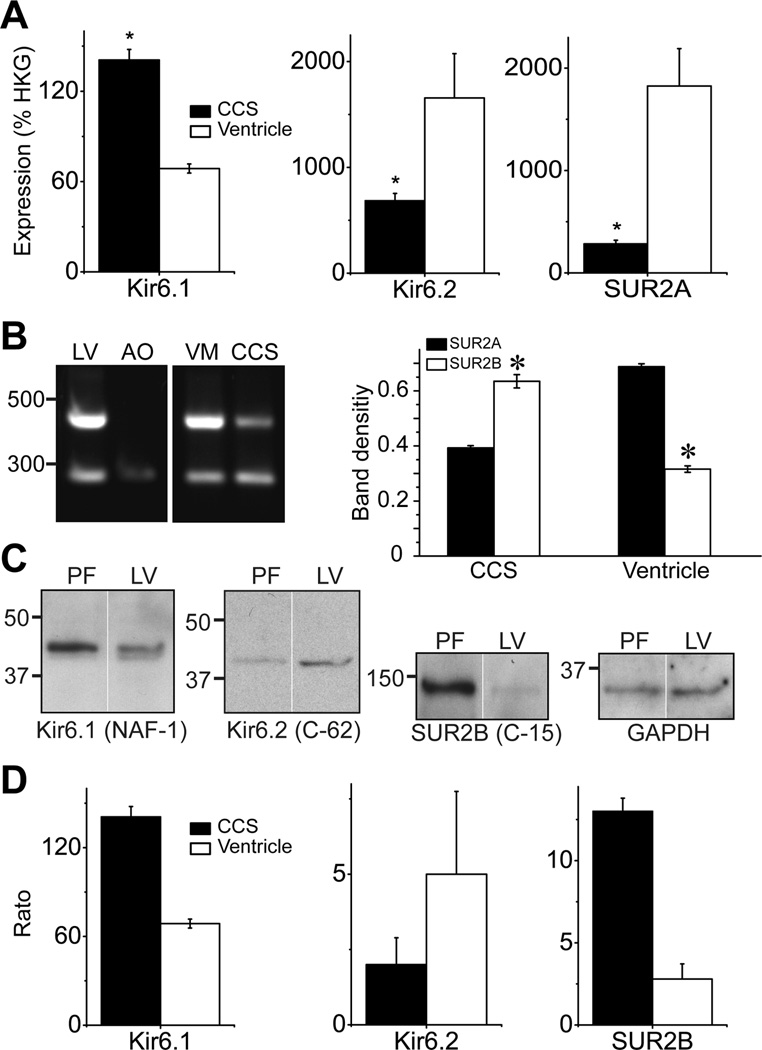
We next evaluated KATP channel subunit mRNA expression by real-time qRT-PCR. As expected, Kir6.2 and SUR2A subunit mRNA expression was robust in mouse ventricular myocytes (Figure 5A). The Kir6.2 and SUR2A mRNA expression levels were lower in the CCS myocytes. The Kir6.1 mRNA expression was low, but was double in the CCS myocytes relative to the ventricular myocytes. SUR1 mRNA expression was negligible in both cell types. Due to primer design considerations, it was not possible to discriminate between the major SUR2 splice variants by qRT-PCR. We therefore used conventional RT-PCR with primers that can discriminate between SUR2A and SUR2B20. As expected, these primers only amplified the SUR2B isoform in mouse aortic smooth muscle, whereas both SUR2A and SUR2B amplicons were observed in mouse left ventricle (Figure 5B). Both CCS and ventricular myocytes expressed SUR2A and SUR2B mRNA. However, CCS myocytes contained higher levels of SUR2B (Mann-Whitney rank sum test, p = 0.005 for SUR2A vs. SUR2B), whereas SUR2A was expressed at elevated levels in ventricular myocytes (p = 0.001 SUR2A vs. SUR2B, Figure 5B). These data suggest that the molecular composition of CCS KATP channels may be heterogeneous and include contributions from Kir6.1, Kir6.2 and SUR2B.
Figure 5.
KATP channel subunit expression in the CCS and ventricle. A) Real-time semi-quantitative RT-PCR (qRT-PCR) analysis of indicted KATP channel subunit mRNA expression in mouse CCS and ventricular myocytes. Data are expressed relative to reference genes (HKG) and represent mean±SEM of 3 experiments (*p<0.05; paired t-test). B) Conventional RT-PCR discriminates between SUR2 spice variants. A primer pair was used that resulted in a 471 bp amplicon for SUR2A and a 276 bp band for SUR2B. Shown are reactions performed with RNA isolated from mouse left ventricle (LV), aorta (AO), enzymatically isolated ventricular myocytes (VM) and CCS myocytes (CCS). The PCR products were sequenced to confirm the identity of the amplicons. The relative expression of SUR2A/SUR2B is plotted as a bar graph (mean±SEM; n=3 per group; *p<0.05). C) KATP channel subunit protein expression in canine cardiac Purkinje fibers and left ventricle. Western blotting was performed using membrane fractions and antibodies against Kir6.1 (NAF-1, 1:500), Kir6.2 (C-62, 1:200), SUR2B (C-15, 1:200) subunits and GAPDH (1:5000). D) Bar graphs represent KATP channel subunit expression levels, normalized by GAPDH expression. Data are mean±SEM (n=2–5).
KATP channel subunit protein expression levels
Due to limitations in obtaining sufficient quantities of CCS tissue from the mouse heart, we performed Western blotting using free-running Purkinje strands from canine hearts. The Kir6.2 subunit was lower (albeit not statistically significant) in Purkinje fibers, whereas both Kir6.1 and SUR2B subunits were expressed at significantly elevated levels in Purkinje fibers relative to the canine left ventricle (Figure 5), consistent with the measurements of mRNA levels. Immunohistochemistry performed with cryosections from Cntn-2 EGFP mouse hearts confirmed the expression of Kir6.1, Kir6.2 and SUR2B protein in CCS myocytes (Supplemental Figure S4).
Simulation of KATP channel activity: effects on the action potential
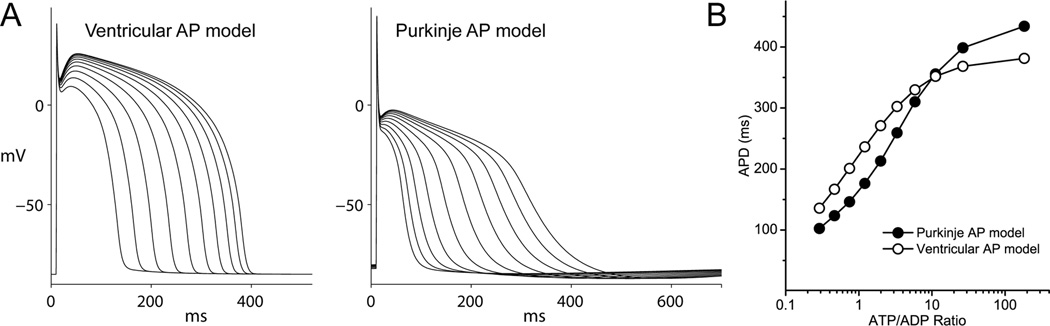
We adapted an empirical model of the cardiac KATP channel14 by incorporating data obtained in our study to produce KATP channel models for the CCS and ventricular KATP channels. Minor changes to the model included alterations in the parameters for channel density, unitary conductance, intrinsic open probability and inhibitory ATP. We adapted the model to account for the degree of MgADP activation (especially pronounced for the CCS KATP channel) and inhibition (most pronounced for the ventricular KATP channel). The solid lines in Figure 6 are produced by these KATP channel models, which approximate the experimental data. Some of the characteristics of the CCS KATP channel (such as the reduced unitary conductance) predict a smaller net effect on the action potential compared to the ventricle, whereas others (reduced ATP sensitivity and increased ADP sensitivity) suggest the opposite. Since it may be difficult to address this issue experimentally (e.g. obtaining equivalent degrees of nucleotide alterations in different tissue types), we incorporated the CCS and ventricular KATP channel models respectively into action potential simulations of the human Purkinje fiber2 and human ventricle15. To simulate ischemia, we progressively decreased intracellular [ATP] while increasing [ADP], thereby keeping total nucleotide concentration constant (see Supplement for details). Each level of ATP:ADP ratio produced a different degree of KATP channel activation, and the steady-state effects on action potentials (1 Hz pacing) were computed. The analysis predicts that KATP channel activation, caused by alterations in cytosolic nucleotide levels, confers greater sensitivity in CCS compared to the ventricle (Figure 6). A systematic analysis of changes in ATP and ADP concentrations on action potential duration in the two tissue types is shown in Supplemental Figures S2 and S3.
Figure 6.
Simulation of the effect of KATP channel opening on the action potential duration. A) A numerical model was developed to represent the measured activities of CCS or ventricular KATP channels. Tissue-specific KATP channel models were incorporated into an action potential model of the human ventricle or Purkinje fibers. Shown are effects on the action potential durations caused by KATP channel activation, induced by changing the ATP: ADP ratio (ATP was decreased from 6.4 to 1 mM and ADP was increased from 35 to 180 µM in 10 steps). B) Plot of the action potential duration (measured at −90% repolarization) of the data in Panel A against the ATP:ADP ratio.
CCS KATP channels contribute to conduction slowing during ischemia
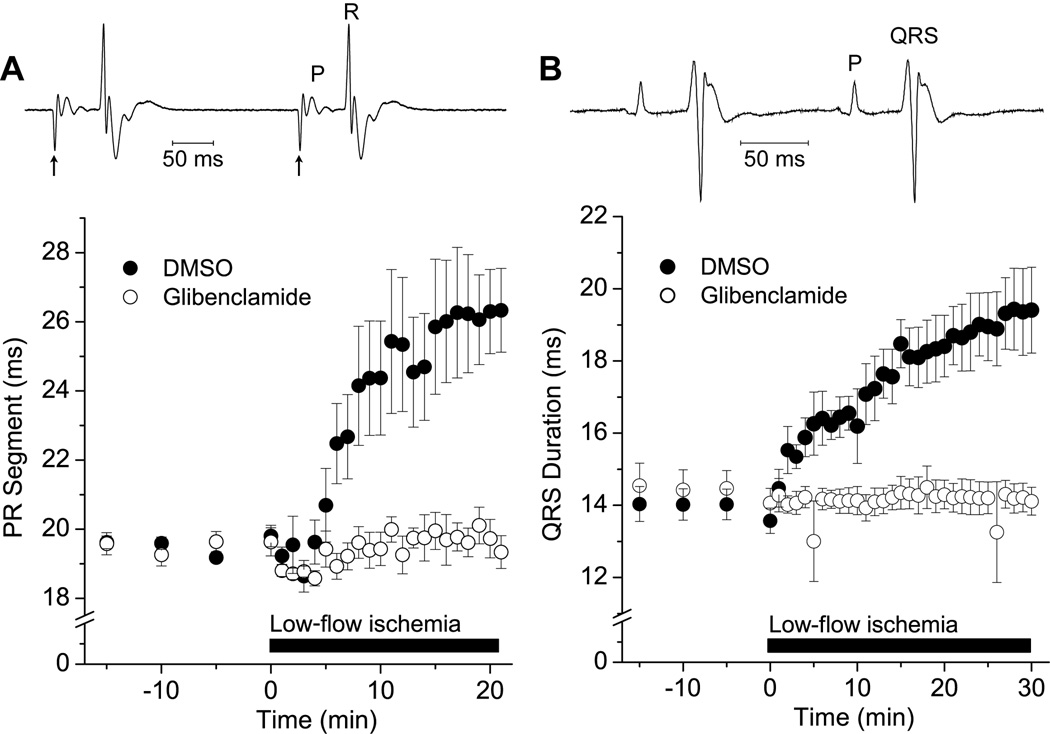
We investigated the potential role of CCS KATP channels in electrical conduction during cardiac ischemia, during which these channels are expected to open. During low-flow ischemia of isolated, Langendorff perfused mouse hearts (the perfusion pressure was decreased to 25% of the pre-ischemic level) the PR segment progressively prolonged from 19.8±3.12 ms to 26.3±1.22 ms at 20 minutes ischemia (n=4; p=0.045; Figure 7A). In the presence of the KATP channel blocker, glibenclamide (2 µM), the PR segment was unchanged during ischemia (19.6±0.41 ms and 19.7±0.56 ms respectively at the beginning and at 20 minutes of ischemia, n=5; p=0.574, Figure 7A). Hearts developing AV conduction block (n=3 and n=4 respectively in control and treatment groups) were excluded from this analysis. In separate experiments using perfused rat hearts, the QRS duration progressively prolonged during low-flow ischemia (n=4; p=0.019; Figure 7B). This QRS widening was not observed in the presence of glibenclamide (n=4; p=0.327; Figure 7B), suggesting a contribution of KATP channels within the distal Purkinje fibers and the Purkinje-ventricular junctions in electrical propagation and conduction disturbances during ischemia. No AV conduction block occurred in this group of experiments.
Figure 7.
Blocking KATP channels improves cardiac conduction during ischemia. A) The ECG was measured in isolated, perfused mouse hearts (top), paced at the right atrium (arrows depict stimulation artifacts). The PR segment is plotted as a function of time (bottom). The solid horizontal bar represents low-flow ischemia (25 cm H2O perfusion pressure). Shown are data with glibenclamide (2 µM, open symbols, n=5) or vehicle (DMSO, filled symbols, n=4). B) The volume-conducted ECG was also recorded from isolated rat hearts (top) and the QRS duration was measured as an index of distal Purkinje fiber function (bottom). Prior to the introduction of ischemia, hearts were perfused with glibenclamide (2 µM; n=4) or vehicle (DMSO; n=4). Ischemia was introduced by lowering the perfusion height, which caused the flow rate to decrease from 9.6±0.39 to 1.9±0.29ml/min (7.4±0.45 to 1.5±0.15 in the experimental group).
Discussion
Our data demonstrate that KATP channels in the ventricular CCS share certain properties with their ventricular counterparts (including the open probability and rapid dwell time kinetics). However, the CCS KATP channel is also conferred with a unique set of biophysical, regulatory and pharmacological properties. These include a lower unitary conductance, an altered sensitivity to intracellular nucleotides, and a different pharmacological profile. Data obtained using RT-PCR, Western blotting and immunohistochemistry demonstrate that Purkinje myocytes express Kir6.2, Kir6.1, SUR2B and SUR2A subunits, with little evidence of SUR1 expression. We found that ischemia-induced cardiac conduction delay is prevented by blocking KATP channels. Numerical simulation of the properties of CCS KATP channels suggests that the consequences of KATP channel opening may be more severe in the Purkinje system than in the ventricle.
Biophysical properties of Purkinje KATP channels
The unitary conductance of mouse CCS KATP channel was ~57pS (in symmetrically high K+ concentrations), which is close to the 60pS value reported for rabbit Purkinje KATP channels8, but is smaller than that of the mouse ventricular channel under identical experimental conditions (~76pS). This is a potentially revealing finding, since the unitary conductance is largely dependent on the Kir6.x isoform present. For example, both Kir6.2/SUR1 and Kir6.2/SUR2A channels have unitary conductances of around 75–80pS21–22, whereas corresponding values for Kir6.1-containing channels are around 35pS23–24. The conductance of heteromeric Kir6.1/Kir6.2 channels is intermediate between these two values24–25 and the difference between Kir6.1 and Kir6.2 conductance is determined to a large extent by specific amino acid residues present in the outer pore region23. Since we observed expression of both Kir6.1 and Kir6.2 in CCS myocytes, the possibility is raised that the CCS KATP channel might be composed of both of these subunits (similar to our report for heteromeric KATP channels in coronary endothelial cells26). It is also of interest to note that Kir6.1 channels are normally silent after patch excision (unless activated by NDPs) whereas Kir6.2-containing channels (or heteromeric Kir6.1/Kir6.2 channels) exhibit spontaneous openings24, 27, which further suggests that CCS KATP channels may be heteromeric Kir6.1/Kir6.2 channels. We were not able to investigate this possibility formally with co-immunoprecipitation approaches due to limited mouse CCS tissue availability.
We found a similar open probability, mean open time and mean closed time when comparing CCS and ventricular KATP channels. When excluding long closing events, the rapid dwell times represent the intraburst open/closed events, which were similar for the two types of KATP channels. The mean open time of ~2.2 ms, and the mean closed time of ~0.4 ms (at −80mV) of the CCS KATP channel were similar to those recorded for the channel in the ventricle [this study; see also21, 28], atrium29, skeletal muscle30, pancreatic β-cells31, and heterologously expressed Kir6.2/SUR131 or Kir6.2/SUR2A channels21. Both types of channels displayed bursting behavior, with the CCS KATP channel having a prolonged interburst closed time relative to the ventricular channel. It is unclear at present to what extent these kinetic differences are due to the diverse molecular composition, which is known to influence bursting behavior31.
Nucleotide regulation
The CCS KATP channels behaved in many respects the same as ventricular channels. They opened spontaneously upon patch excision in ATP-free solutions and their activity ran down over long periods of recording. They were blocked by cytosolic ATP, but differed from ventricular KATP channels in their ATP-sensitivity. The CCS KATP channels had an IC50 for inhibitory ATP similar to the value of 119 µmol/L previously reported for rabbit Purkinje KATP channels8. In contrast, the ventricular KATP channel was approximately twice as sensitive to inhibitory ATP. The molecular mechanisms for the differences in ATP sensitivity may be complex and the differences in ATP-sensitivity are not straightforward to interpret. Kir6.1 and Kir6.2 channels have been described to have a similar sensitivity to inhibitory ATP32, but clear differences exist in terms of their regulation by other NDPs, such as UDP27. Moreover, their combination with SURx subunits may alter the nucleotide sensitivity9. It is conceivable, for example, that the presence of SUR2B in the CCS confers the higher MgADP sensitivity33 (see also Suppl Figure S3). Further experiments are underway to fully address this issue.
Pharmacological differences between CCS and ventricular KATP channels
A prior report demonstrated that rabbit Purkinje KATP channels are activated by 10 µmol/L levcromakalim and are blocked by glibenclamide8. Although levcromakalim activated the mouse CCS KATP channels, we found diazoxide to be a much more effective opener of these channels. The ventricular KATP channel had a reverse pharmacological profile with levcromakalim being a more effective KATP channel opener and diazoxide having little effect on channel activity. This profile is similar to that of the corresponding channel subtypes found in the pancreatic β-cell34, the vascular endothelium35–36, smooth muscle37, atrium38–39 or post-infarcted ventricular myocytes40. The pharmacological profile is determined largely by KATP channel molecular composition and the metabolic state of the cell. In particular, SUR1 and SUR2B subunits confer a high sensitivity to diazoxide9, 41. In contrast to atrial KATP channels, in which the SUR1 subunit is expressed39, we found little SUR1 expression but robust SUR2B mRNA and protein expression in CCS myocytes. Collectively, these data are in support of the concept that dog and mouse CCS KATP channels are SUR2B-based. Assuming that CCS KATP channels in other species have similar pharmacological properties, these data may have important implications for pharmacotherapy (e.g. heart disorders in diabetic patients treated with sulfonylureas).
Pathophysiological and clinical relevance
Myocytes in the cardiac conduction system have a high membrane resistance with a long action potential duration and it is therefore unlikely that KATP channels are constitutively active in these cells under normoxic conditions. Rather, a role for KATP channels is visualized under conditions of metabolic stress, such as during high heart rates, hypoxia or cardiac ischemia. Our numerical simulation data are supportive of the notion that KATP channel opening contributes to action potential duration shortening in the CCS. We introduced a KATP channel model into a numerical simulation of the human ventricle or Purkinje fiber. Interestingly, the Purkinje action potential was more sensitive to alterations in KATP channel opening (induced by changing the ATP:ADP ratio) when compared to the ventricle. This was due in part to the inherent differences in properties of the two action potentials (e.g. the total membrane conductance during the action potential). However, there was also a greater contribution of the CCS KATP channel itself to action potential shortening, as evidenced by simulations in which CCS KATP channels were incorporated into ventricular myocytes, and vise-versa (Supplemental Figure S2). Thus, the specialized cardiac conduction system may be particularly prone to the effects of KATP channel opening.
It is likely that KATP channel opening in the CCS contribute to electrophysiological responses during ischemia. Free-running Purkinje strands may be oxygenated directly from circulating blood and are probably less susceptible to ischemic events. In contrast, the sub-endocardial Purkinje strands and Purkinje-muscle junctions are strongly affected by myocardial perfusion and local ischemia42. Within minutes following the onset of acute myocardial ischemia, conduction velocity is depressed43. This conduction delay coincides with the occurrence of ventricular tachycardia and ventricular fibrillation3. We directly investigated the role of KATP channels in ischemia-induced conduction delay. The PR interval, which under non-ischemic conditions is due mainly to conduction delay within the AV node, was lengthened by low-flow ischemia. Without His recordings, it is not possible to determine to what extent the His-Purkinje network contributed to the increased conduction delay during ischemia. Since glibenclamide effectively prevented ischemia-induced PR segment lengthening, our data support the concept that CCS KATP channels (AV node and/or His-Purkinje) contribute to ischemia-induced conduction abnormalities under these conditions. The fact that the Purkinje system plays a pronounced role in the development of ventricular fibrillation during myocardial ischemia44 further underscores the relevance of our findings with ischemia-induced arrhythmias. This notion is supported by the inhibitory effect of KATP channel blockers on ischemia-induced arrhythmias45. The sensitivity of Purkinje KATP channels to “non-cardiac” pharmacological compounds may therefore offer anti-arrhythmic therapeutic potential. It may be possible that preventing KATP channels from opening in the CCS may confer antiarrhythmic properties, but allowing opening in the ventricles may conserve energy and prevent cell death. Our data suggest that a SUR2B-specific KATP channel blocker may fulfill these criteria (if one becomes available), with the caveat that this drug may also impair blood flow (by blocking smooth muscle KATP channels).
Conclusions
We characterized mouse Purkinje KATP channels and found them to have biophysical and pharmacological properties that differ from those of the ventricular KATP channel. The molecular composition is also different, with a potential contribution by Kir6.1, Kir6.2 and SUR2B subunits. These properties might confer different electrophysiological responses of Purkinje fibers during metabolic stress conditions, such as hypoxia or ischemia.
Cardiovascular ATP-sensitive K+ channels have both protective and deleterious roles in the heart. A clear beneficial role for these channels in cardiac pathophysiological states has long been identified; opening of KATP channels protects against ischemic events by reducing the infarct size and participates as one of the triggers for ischemic preconditioning. However, KATP channel opening may also be detrimental. For example, their opening during myocardial ischemia may promote K+ efflux and reduce the action potential duration. The ensuing electrical heterogeneity creates a substrate for re-entrant arrhythmias. Our present study characterizes the KATP channel in the specialized conduction system and found them to have biophysical and pharmacological properties that differ from those of the ventricular KATP channel. Their molecular composition is also different and they express subunits normally abundant in non-cardiac cells. Numerical simulation studies predict these channels to be more sensitive to metabolic stress than their ventricular counterparts, suggesting that they may preferentially open during the early phases of cardiac ischemia. We demonstrate that KATP channel blockade mitigates electrical conduction slowing during ischemia, suggesting a contribution of the cardiac conduction system KATP channels to ischemia-induced conduction disturbances and arrhythmias.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by National Institutes of Health grants HL105046 to LB, HL105983 & HL82727 and a NYSTEM award to GIF, HL076751 to GEM, a Glorney-Raisbeck Fellowship from the NY Academy of Medicine to JML and HL085820 & HL093563 to WAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Noble D. The initiation of the heart beat. Oxford: Oxford University Press; 1975. [Google Scholar]
- 2.Sampson KJ, Iyer V, Marks AR, Kass RS. A computational model of Purkinje fibre single cell electrophysiology: implications for the long QT syndrome. J Physiol. 2010;588:2643–2655. doi: 10.1113/jphysiol.2010.187328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janse MJ, Kleber AG. Electrophysiological changes and ventricular arrhythmias in the early phase of regional myocardial ischemia. Circulation Research. 1981;49:1069–1081. doi: 10.1161/01.res.49.5.1069. [DOI] [PubMed] [Google Scholar]
- 4.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 5.Ito H, Nakajima T, Takikawa R, Hamada E, Iguchi M, Sugimoto T, Kurachi Y. Coenzyme Q10 attenuates cyanide-activation of the ATP-sensitive K+ channel current in single cardiac myocytes of the guinea-pig. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:133–136. doi: 10.1007/BF00167394. [DOI] [PubMed] [Google Scholar]
- 6.Satoh H. Effects of ATP-sensitive k+ channel openers on pacemaker activity in isolated single rabbit sino-atrial node cells. J.Pharm.Pharmacol. 1993;22:863–868. doi: 10.1097/00005344-199312000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Kakei M, Noma A. Adenosine-5'-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. Journal of Physiology (London) 1984;352:265–284. doi: 10.1113/jphysiol.1984.sp015290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Light PE, Cordeiro JM, French RJ. Identification and properties of ATP-sensitive potassium channels in myocytes from rabbit Purkinje fibres. Cardiovascular Research. 1999;44:356–369. doi: 10.1016/s0008-6363(99)00218-7. [DOI] [PubMed] [Google Scholar]
- 9.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 10.Pallante BA, Giovannone S, Fang-Yu L, Zhang J, Liu N, Kang G, Dun W, Boyden PA, Fishman GI. Contactin-2 expression in the cardiac Purkinje fiber network. Circ Arrhythm Electrophysiol. 2010;3:186–194. doi: 10.1161/CIRCEP.109.928820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao L, Hadjiolova K, Coetzee WA, Rindler MJ. Endosomal KATP channels as a reservoir after myocardial ischemia: a role for SUR2 subunits. Am J Physiol Heart Circ Physiol. 2011;300:H262–H270. doi: 10.1152/ajpheart.00857.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colquhoun D, Hawkes AG. A note on correlations in single ion channel records. Proc R Soc Lond B Biol Sci. 1987;230:15–52. doi: 10.1098/rspb.1987.0008. [DOI] [PubMed] [Google Scholar]
- 13.Harrell MD, Harbi S, Hoffman JF, Zavadil J, Coetzee WA. Large-scale analysis of ion channel gene expression in the mouse heart during perinatal development. Physiol Genomics. 2007;28:273–283. doi: 10.1152/physiolgenomics.00163.2006. [DOI] [PubMed] [Google Scholar]
- 14.Ferrero JM, Jr, S iz J, Ferrero JM, Thakor NV. Simulation of action potentials from metabolically impaired cardiac myocytes - Role of ATP-sensitive K + current. Circulation Research. 1996;79:208–221. doi: 10.1161/01.res.79.2.208. [DOI] [PubMed] [Google Scholar]
- 15.ten Tusscher KH, Noble D, Noble PJ, Panfilov AV. A model for human ventricular tissue. Am J Physiol Heart Circ Physiol. 2004;286:H1573–H1589. doi: 10.1152/ajpheart.00794.2003. [DOI] [PubMed] [Google Scholar]
- 16.Findlay I. Effects of ADP on upon the ATP-sensitive K+ channel in rat ventricular myocytes. Journal of Membrane Biology. 1988;101:83–92. doi: 10.1007/BF01872823. [DOI] [PubMed] [Google Scholar]
- 17.Kakei M, Noma A, Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. Journal of Physiology (London) 1985;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanguinetti MC, Scott AL, Zingaro GJ, Siegl PK. BRL 34915 (cromakalim) activates ATP-sensitive K+ current in cardiac muscle. Proc Natl Acad Sci U S A. 1988;85:8360–8364. doi: 10.1073/pnas.85.21.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faivre JF, Findlay I. Effects of tolbutamide, glibenclamide and diazoxide upon action potentials recorded from rat ventricular muscle. Biochimica et biophysica acta. 1989;984:1–5. doi: 10.1016/0005-2736(89)90334-9. [DOI] [PubMed] [Google Scholar]
- 20.Morrissey A, Parachuru L, Leung M, Lopez G, Nakamura TY, Tong X, Yoshida H, Srivastiva S, Chowdhury PD, Artman M, Coetzee WA. Expression of ATP-sensitive K+ channel subunits during perinatal maturation in the mouse heart. Pediatric Research. 2005;58:185–192. doi: 10.1203/01.PDR.0000169967.83576.CB. [DOI] [PubMed] [Google Scholar]
- 21.Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac KATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circulation Research. 1998;83:1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki N, Gonoi T, Seino S. Subunit stoichiometry of the pancreatic β-cell ATP-sensitive K+ channel. FEBS letters. 1997;409:232–236. doi: 10.1016/s0014-5793(97)00488-2. [DOI] [PubMed] [Google Scholar]
- 23.Repunte VP, Nakamura H, Fujita A, Horio Y, Findlay I, Pott L, Kurachi Y. Extracellular links in Kir subunits control the unitary conductance of SUR/Kir6.0 ion channels. EMBO Journal. 1999;18:3317–3324. doi: 10.1093/emboj/18.12.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondo C, Repunte VP, Satoh E, Yamada M, Horio Y, Matsuzawa Y, Pott L, Kurachi Y. Chimeras of Kir6.1 and Kir6.2 reveal structural elements involved in spontaneous opening and unitary conductance of the ATP-sensitive K+ channels. Receptors.Channels. 1998;6:129–140. [PubMed] [Google Scholar]
- 25.Babenko AP, Gonzalez GC, Bryan J. Hetero-concatemeric KIR6.X4/SUR1 channels display distinct conductivities but uniform ATP inhibition. J Biol.Chem. 2000;275:31563–31566. doi: 10.1074/jbc.C000553200. [DOI] [PubMed] [Google Scholar]
- 26.Pountney DJ, Sun ZQ, Porter LM, Nitabach MN, Nakamura TY, Holmes D, Rosner E, Kaneko M, Manaris T, Holmes TC, Coetzee WA. Is the molecular composition of K(ATP) channels more complex than originally thought? Biochemical and electrophysiological evidence for heteromultimeric assembly of the K ATP channel subunits Kir6.1 and Kir6.2. Journal of Molecular and Cellular Cardiology. 2001;33:1541–1546. doi: 10.1006/jmcc.2001.1407. [DOI] [PubMed] [Google Scholar]
- 27.Takano M, Xie LH, Otani H, Horie M. Cytoplasmic terminus domains of Kir6.x confer different nucleotide-dependent gating on the ATP-sensitive K + channel. Journal of Physiology (London) 1998;512:395–406. doi: 10.1111/j.1469-7793.1998.395be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zilberter Y, Burnashev NA, Papin A, Portnov V, Khodorov B. Gating kinetics of ATP-sensitive single potassium channels in myocardial cells depends on electromotive force. Pflugers Archives-European Journal of Physiology. 1988;411:584–589. doi: 10.1007/BF00582382. [DOI] [PubMed] [Google Scholar]
- 29.Baron A, van Bever L, Monnier D, Roatti A, Baertschi AJ. A novel K(ATP) current in cultured neonatal rat atrial appendage cardiomyocytes. Circulation Research. 1999;85:707–715. doi: 10.1161/01.res.85.8.707. [DOI] [PubMed] [Google Scholar]
- 30.Spruce AK, Standen NB, Stanfield PR. Studies on the unitary properties of adenosine-5'-triphosphate- regulated potassium channels of frog skeletal muscle. Journal of Physiology (London) 1987;382:213–236. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alekseev AE, Kennedy ME, Navarro B, Terzic A. Burst kinetics of co-expressed Kir6.2/SUR1 clones: Comparison of recombinant with native ATP-sensitive K+ channel behavior. Journal of Membrane Biology. 1997;159:161–168. doi: 10.1007/s002329900279. [DOI] [PubMed] [Google Scholar]
- 32.Babenko AP, Bryan J. A conserved inhibitory and differential stimulatory action of nucleotides on K(IR)6.0/SUR complexes is essential for excitation-metabolism coupling by K(ATP) channels. J.Biol.Chem. 2001;276:49083–49092. doi: 10.1074/jbc.M108763200. [DOI] [PubMed] [Google Scholar]
- 33.Matsuoka T, Matsushita K, Katayama Y, Fujita A, Inageda K, Tanemoto M, Inanobe A, Yamashita S, Matsuzawa Y, Kurachi Y. C-terminal tails of sulfonylurea receptors control ADP-induced activation and diazoxide modulation of ATP-sensitive K(+) channels. Circ Res. 2000;87:873–880. doi: 10.1161/01.res.87.10.873. [DOI] [PubMed] [Google Scholar]
- 34.Trube G, Rorsman P, Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Archives-European Journal of Physiology. 1986;407:493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- 35.Katnik C, Adams DJ. An ATP-sensitive potassium conductance in rabbit arterial endothelial cells. J.Physiol. 1995;485:595–606. doi: 10.1113/jphysiol.1995.sp020755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langheinrich U, Daut J. Hyperpolarization of isolated capillaries from guinea-pig heart induced by K+ channel openers and glucose deprivation. J.Physiol. 1997;502:397–408. doi: 10.1111/j.1469-7793.1997.397bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quayle JM, Bonev AD, Brayden JE, Nelson MT. Pharmacology of ATP-sensitive K+ currents in smooth muscle cells from rabbit mesenteric artery. Am.J Physiol. 1995;269:C1112–C1118. doi: 10.1152/ajpcell.1995.269.5.C1112. [DOI] [PubMed] [Google Scholar]
- 38.Poitry S, van Bever L, Coppex F, Roatti A, Baertschi AJ. Differential sensitivity of atrial and ventricular K(ATP) channels to metabolic inhibition. Cardiovascular Research. 2003;57:468–476. doi: 10.1016/s0008-6363(02)00715-0. [DOI] [PubMed] [Google Scholar]
- 39.Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, Coetzee WA, Nichols CG. Differential Structure of Atrial and Ventricular KATP: Atrial KATP Channels Require SUR1. Circulation Research. 2008;103:1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isidoro Tavares N, Philip-Couderc P, Papageorgiou I, Baertschi AJ, Lerch R, Montessuit C. Expression and function of ATP-dependent potassium channels in late post-infarction remodeling. J Mol Cell Cardiol. 2007;42:1016–1025. doi: 10.1016/j.yjmcc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J.Biol.Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 42.Lazzara R, El-Sherif N, Scherlag BJ. Disorders of cellular electrophysiology produced by ischemia of the canine His bundle. Circ Res. 1975;36:444–454. doi: 10.1161/01.res.36.3.444. [DOI] [PubMed] [Google Scholar]
- 43.Kleber AG, Janse MJ, Wilms-Schopmann FJG, Wilde AAM, Coronel R. Changes in conduction velocity during acute ischemia in ventricular myocardium of the isolated porcine heart. Circulation. 1986;73:189–198. doi: 10.1161/01.cir.73.1.189. [DOI] [PubMed] [Google Scholar]
- 44.Janse MJ, Kleber AG, Capucci A, Coronel R, Wilms-Schopman F. Electrophysiological basis for arrhythmias caused by acute ischemia. Role of the subendocardium. J Mol Cell Cardiol. 1986;18:339–355. doi: 10.1016/s0022-2828(86)80898-7. [DOI] [PubMed] [Google Scholar]
- 45.Gross GJ, Auchampach JA. Role of ATP dependent potassium channels in myocardial ischaemia. Cardiovascular Research. 1992;26:1011–1016. doi: 10.1093/cvr/26.11.1011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.