Abstract
The association of Chlamydia pneumoniae and atherosclerosis has been well documented. Recently, it has been demonstrated that C. pneumoniae upregulates expression of the lectin-like ox-LDL receptor (LOX-1) in endothelial cells. Many of the pro-atherogenic effects of ox-LDL occur through its activation of and uptake by LOX-1. This class E scavenger receptor contains a carbohydrate recognition domain common to the C type lectin family. Previously, we have demonstrated that the major outer membrane protein of the chlamydiae is glycosylated and glycan removal abrogates infectivity of C. pneumoniae for endothelial cells. In this study, we investigated whether C. pneumoniae binds to LOX-1. The results show that 1) infection of endothelial cells by C. pneumoniae is inhibited by ligands that bind to the LOX-1 receptor, but not by ligands binding to other scavenger receptors; 2) anti-LOX-1 antibody inhibits C. pneumoniae infectivity, while antibodies against other scavenger receptors do not; 3) anti-LOX-1 antibody inhibits attachment of C. pneumoniae to endothelial cells; and 4) C. pneumoniae co-localizes with LOX-1. These effects were not observed for Chlamydia trachomatis. In conclusion, C. pneumoniae binds to the LOX-1 receptor, which is known to promote atherosclerosis.
Keywords: Chlamydia pneumoniae, LOX-1 receptor, Atherosclerosis
1. Introduction
The association of Chlamydia pneumoniae and atherosclerosis has been documented by the detection of the organism in atheromatous lesions by immunohistochemistry and PCR and by isolation of viable organisms from the plaques [1]. Subsequently, a pathogenic role of C. pneumoniae in atherosclerosis has been demonstrated experimentally in animal models of atherosclerosis, in which infection of hyperlipidemic [2], but not normolipidemic animals [3], accelerates the development of atherosclerosis. This atherogenic property is unique to C. pneumoniae because Chlamydia trachomatis, another human chlamydial pathogen, has not been detected in atheromas [4], nor does infection of hyperlipidemic mice with C. trachomatis accelerate plaque development [5].
In 1997, Sawamura et al. identified a novel receptor for oxidized-low density lipoprotein (ox-LDL) in vascular endothelial cells which he designated LOX-1 for lectin-like ox-LDL receptor [6]. LOX-1 expression is increased in hyperlipidemia and atherosclerotic lesions and many of the pro-atherogenic effects of ox-LDL occur through its binding to and uptake by LOX-1 [7]. Activation of LOX-1 induces a plethora of events including up-regulation of pro-atherogenic factors including adhesion molecules, matrix metalloproteinases, and monoctye chemoattractant protein-1 (MCP-1) [8–10]. LOX-1 is a class E scavenger receptor (SR), which is distinct from class A and B SRs. This receptor was found also in macrophages and smooth muscle cells [11]. LOX-1 has a broad ligand specificity including polyinosinic acid, polysaccharides, phospholipids and bacteria. The latter has led to the suggestion that SRs may provide a mechanism for recognition and internalization of pathogens [11].
We have previously determined that the major outer membrane protein (MOMP) of C. trachomatis is glycosylated, the structure of the carbohydrate is an N-linked high mannose type oligosaccharide, and the high mannose oligosaccharide mediates attachment and infectivity of C. trachomatis, C. psittaci and C. pneumoniae [12]. We further determined that the mannose-oligosaccharide of C. pneumoniae, but not C. trachomatis, is phosphorylated, which may account for differences in receptor usage and pathogenesis [13, 14]. Specifically, we demonstrated that C. pneumoniae uses the mannose 6-phosphate receptor on endothelial cells. Removal of the chlamydial glycan abrogates infectivity of C. pneumoniae for endothelial cells. However, pre-incubation of cells with ligands that bind to the mannose 6-phosphate receptor or antibodies against this receptor significantly decreases infectivity, but does not completely inhibit infection, suggesting that an additional receptor may also bind the C. pneumoniae glycan. Recently, Yoshida et al. [15] demonstrated that C. pneumoniae up-regulates LOX-1 expression on endothelial cells and promotes uptake of ox-LDL. Because the LOX-1 receptor has a carbohydrate binding domain and multifactorial effects in atherogenesis, we evaluated whether C. pneumoniae binds to the LOX-1 receptor on human endothelial cells.
2. Materials and methods
2.1. Chlamydial organisms and cell lines
C. pneumoniae AR-39 and C. trachomatis E/UW-5/Cx and L2/434/Bu were grown in HL (human line) cells and HeLa cells, respectively. Organisms were purified by Hypaque gradient centrifugation [16]. Purified organisms were suspended in sucrose-phosphate-glutamic acid (SPG) buffer and stored at −70°C in aliquots until used. Human micro-vascular endothelial cells (HMEC-1) were originally obtained from E.W. Ades (Centers for Disease Control and Prevention, Atlanta).
2.2. Reagents
The following reagents were used: low density lipoproteins (LDL) including oxidized LDL (ox-LDL), acetylated LDL (ac-LDL) and native LDL (n-LDL), polyinosinic acid (poly I), polycytidylic acid (poly C), phosphatidyl choline and phosphatidyl serine. Ox-LDL, poly I and phosphatidyl serine are known to bind to LOX-1 while poly-C and phosphatidyl choline do not (11). Poly I, poly C, phosphatidyl serine, and phospatidyl choline were obtained from Sigma (Sigma, Corp., St. Louis, MO). LDL was obtained from Dr. Alan Chait (University of Washington School of Medicine, Seattle WA), which had been isolated from healthy, normolipidemic donors by ultracentrifugation as described previously [17]. LDL was dialyzed agains 0.15 M NaCl and 0.05% EDTA and stored under nitrogen. LDL was used within one week of isolation. Ox-LDL was generated by incubating LDL (300 µg/ml) in the presence of 5 µmol/litter copper sulfate for 18 hr at 37°C (18). Acetylated LDL (ac-LDL) was made by adding acetic anhydride to LDL at a ratio of 1.5 ml per milligram of LDL protein over a 1 hour period followed by dialysis in normal saline containing 1.0 mMol/L EDTA for 8 hrs [19]. Alternatively, LDLs were obtained from Sigma.
2.3. Anti-receptor antibodies
Antibodies tested were anti-human monoclonal antibodies (MAbs) against scavenger receptors including MARCO, a member of the type A family (anti-MARCO, Cell Sciences, Inc., Canton, MA), CD36 (Cell Sciences, Inc., Canton, MA), a member of the type B family, LOX-1 (anti-SR-E1, R&D Systems, Inc., Minneapolis, MN) and anti-human polyclonal antibody against SREC-1, a member of the type F family (SR-F) (anti-SREC-I, Hycult Biotechnology, Inc., the Netherlands).
2.4. Infectivity inhibition assay
Endothelial cells were plated in 24 well plates to a confluency of 4 × 105 cells. Cells were incubated with 0.2 ml of the appropriate antibody dilution or PBS for 1 hr before inoculation. Alternatively, cells were treated with 0.2 ml of ligands of scavenger receptors or PBS for 1 hr prior to infection. Infected cells were cultured for 3 days. Infectivity titers were determined by inclusion counts following fluorescence antibody stain with the Chlamydia genus specific antibody CF-2 [20].
2.5 Binding assays
For binding assays, organisms were metabolically labeled with [35S] methionine as described previously [21]. Endothelial cells were treated with antibody or PBS for 1 hr, inoculated with organisms, and adsorbed for 2 hrs at 4°C (organisms attach, but do not enter host cells) with gentle rocking. After being washed with PBS to remove unbound radiolabel, cells were harvested, briefly sonicated in a water bath sonicator, and transferred to a scintillation vial and scintillation fluid added (Biodegradable counting scintillant, Amersham, Arlington Heights, Ill). Radioactivity was counted in a scintillation counter (Analyzer Model 6000, Beckman Coulter, Inc., Brea, CA).
2.6. Co-localization of C. pneumoniae with LOX-1 receptor
Co-localization of C. pneumoniae and the LOX-1 receptor was performed by incubation of C. pneumoniae with endothelial cells at 4°C for 30 min to permit attachment, but not internalization. Following fixation of the cells with paraformaldehyde, double immunofluorescence labeling of infected cells was done by staining the LOX-1 receptor with anti-LOX-1 as the primary antibody followed by goat anti-mouse IgG conjugated to Texas Red (red fluorescence). After multiple washes of slides with PBS, Chlamydia were stained with FITC conjugated monoclonal antibody (green fluorescence). For experiments using anti-SR-A and SR-F as primary antibodies, Alex-Fluor 549 conjugated to rabbit anti-mouse IgG and Alex-Fluor 647 conjugated to rabbit anti-goat IgG (Life Technologies, Carlsbad, CA), respectively, were used. Co-localization was determined by confocal fluorescence microscopy using a LSM5 PASCAL Zeiss or a LSM 510 Zeiss confocal microscope.
3. Results
3.1. Infectivity of C. pneumoniae is inhibited by ligands that bind to the LOX-1 receptor
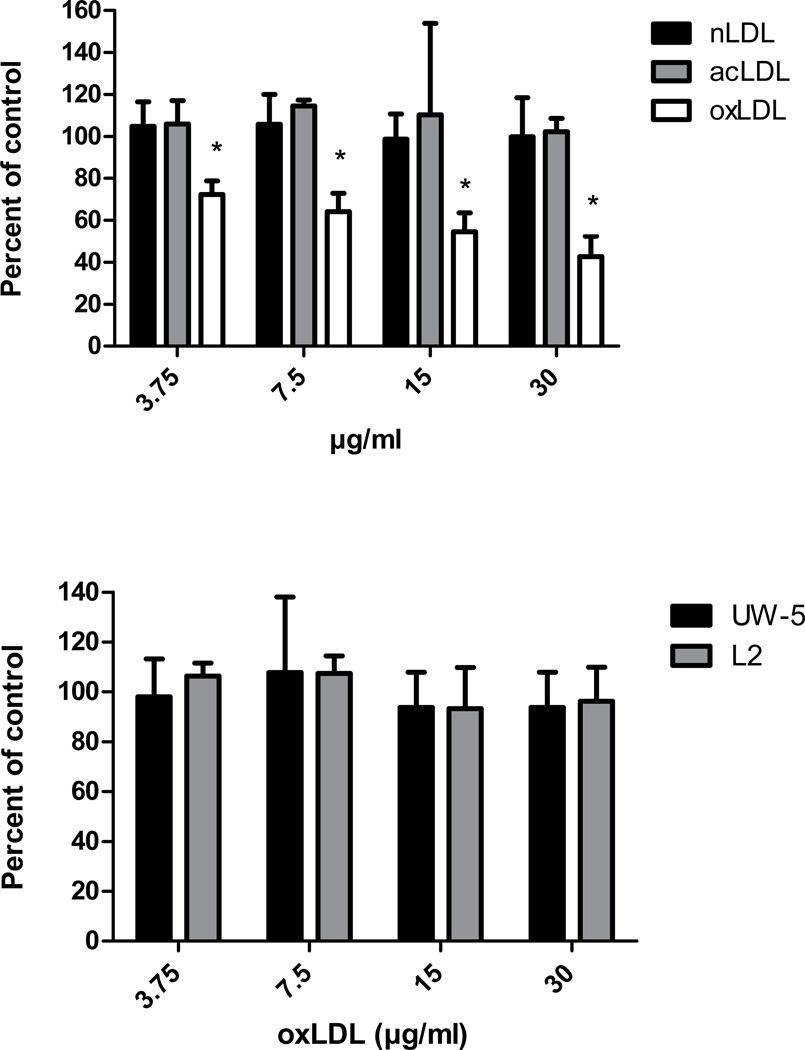
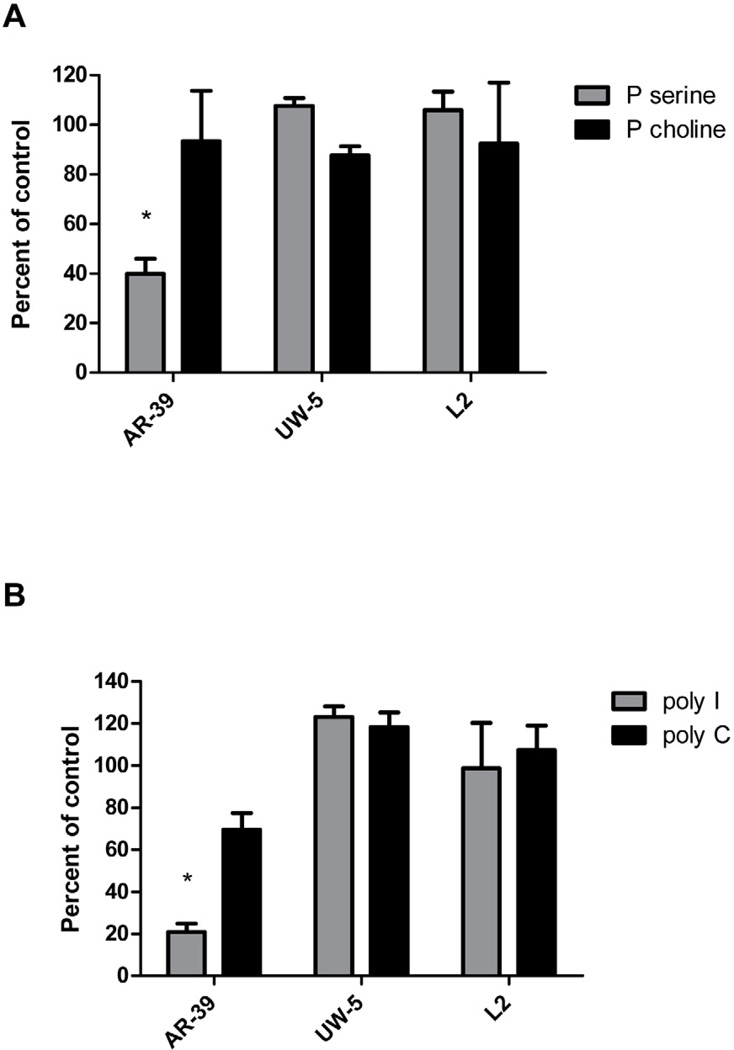
Of the scavenger receptors that have been tested, all bind ox-LDL but differ in other ligand-binding properties. For example, Class A, B, C, and F SRs bind ac-LDL, while the LOX-1 receptor does not [11]. LOX-1 also does not bind n-LDL [11]. Poly I and phosphatidyl serine are ligands for LOX-1, while LOX-1 does not bind poly C or phosphatidyl choline [6, 11, 22, 23, 24]. The other SRs differ in binding one or more of these ligands (see reference 11). Thus, hapten inhibition assays were conducted to determine whether ligands that bind to the LOX-1 receptor affect C. pneumoniae infection of endothelial cells. As shown in Fig. 1, infectivity of AR-39 was significantly inhibited in a dose dependent manner by ox-LDL at concentrations ranging from 3.75 to 30 µg/ml, but not by n-LDL or ac-LDL. Higher concentrations of n-LDL and ac-LDL (tested up to 125 µg/ml) also had no effect on C. pneumoniae infection of endothelial cells (data not shown). In addition, phosphatidyl serine and poly I, which bind to LOX-1, also significantly inhibited C. pneumoniae infection (60% inhibition at 31.25 ug/ml and 79 % inhibition at 125 ug/ml, respectively) while phosphatidyl choline and poly C had no effect at these concentrations (Figs. 2A and 2B). In contrast, none of these ligands inhibited infectivity of C. trachomatis serovars E or L2 (Figs. 1B, 2A and 2B).
Fig. 1.
Effects of LDLs on Chlamydia pneumoniae infectivity of endothelial cells. Endothelial cells (HMEC-1) were incubated with 0.2 ml of the indicated LDL concentrations or PBS for 1 hr prior to infection. Infectivity titers were determined by inclusion counts following staining with the Chlamydia genus specific antibody CF-2. Infectivity titers (inclusion counts) of LDL treated cells were compared with PBS treated cells (control) and are denoted as percent of control on the y axis. Panel A - effect of native LDL (n-LDL), acetylated LDL (ac-LDL) and oxidized LDL (ox-LDL) on C. pneumoniae infectivity (three coverslips were counted per concentration tested). Panel B - effect of ox-LDL on C. trachomatis serovars E (UW-5) and L2 (three coverslips were counted per concentration tested). A representative experiment is shown.
Fig. 2.
Effect of ligands that bind to different scavenger receptors on C. pneumoniae infectivity. Endothelial cells (HMEC-1) were incubated with 0.2 ml of the indicated ligand concentrations or PBS for 1 hour prior to infection. Infectivity titers were determined by inclusion counts following staining with the Chlamydia genus specific antibody CF-2. Infectivity titers of ligand treated cells were compared with PBS treated cells (control) and are denoted as percent of the control on the y axis. Three coverslips were counted for each ligand concentration tested. Panel A - effect of phosphatidyl serine (P serine and phosphatidyl choline (P choline) on C. pneumoniae (AR-39) and C. trachomatis serovars E (UW-5) and L2. Panel B - Effects of polyinosinic acid (poly I) and polycytidylic acid (poly C) on C. pneumoniae and C. trachomatis serovars E (UW-5) and L2 (three coverslips were counted per concentration tested. A representative experiment is shown.
3.2. Anti-LOX-1 antibody inhibits C. pneumoniae infectivity while antibodies against other scavenger receptors do not
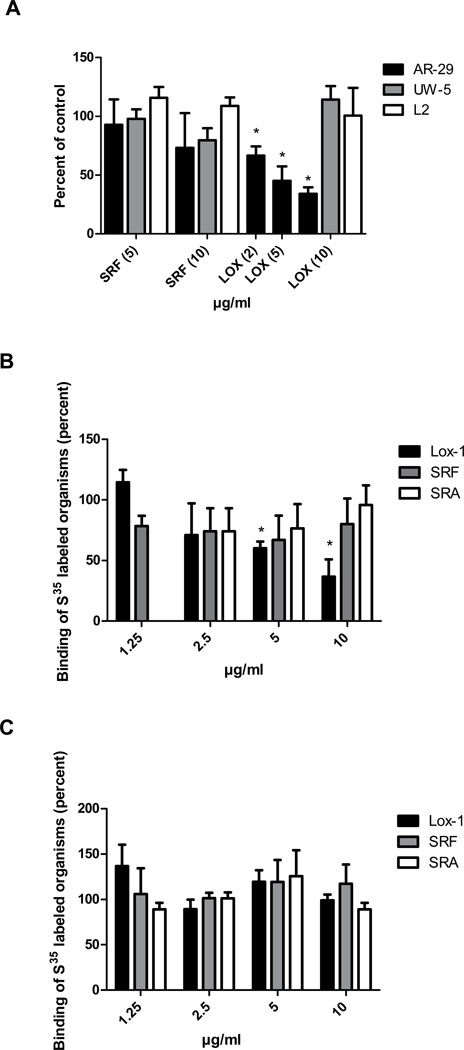
The effect of anti-LOX-1 antibody on infectivity was determined in order to confirm the finding of the hapten inhibition assay. Anti-LOX-1 MAb inhibited the infectivity of C. pneumoniae in a dose dependent manner (Fig. 3A). The inhibition ranged from 33% to 60% at 2 to 10 ug/ml of antibody (p<0.01). In contrast, 10 ug/ml of LOX-1 MAb had no effect on infectivity of C. trachomatis serovars E or L2 (Fig. 3A). To ascertain whether inhibition of C. pneumoniae infectivity by anti-LOX-1 antibody was specific, similar experiments were conducted by using MAbs against MARCO (SR-A family) and CD36 (SR-B family) as negative controls, because there is little or no expression of these receptors on endothelial cells. Neither of these antibodies significantly inhibited infectivity of C. pneumoniae (data not shown).
Fig. 3.
LOX-1 monoclonal antibody inhibits attachment and infectivity of Chlamydia pneumoniae. Panel A. Effects of antibodies against different classes of scavenger receptors on C. pneumoniae infectivity. Endothelial cells (HMEC-1) were incubated with 0.2 ml of the indicated antibody concentrations or PBS for 1 hour prior to infection. Infectivity titers were determined by inclusion counts following staining with the Chlamydia genus specific antibody CF-2. Infectivity titers of antibody treated cells were compared with PBS treated cells (control) and are indicated as percent of the control. Three coverslips were counted for each assay. Panel B. Binding assays using metabolically labeled C. pneumoniae (AR-39) organisms. Endothelial cells were incubated for 1 hr with anti-LOX-1, anti-SRA, anti-SREC-I receptor antibody or PBS. Subsequently, cells were inoculated with radiolabeled organisms at 4°C (organisms attach, but are not internalized). After washing of cells, radioactive counts were determined. Assays were done in triplicate. Panel C. Binding assays using metabolically labeled C. trachomatis (UW-5) organisms. Binding assays were done as described in Panel B. A representative experiment is shown.
To determine whether C. pneumoniae may also use another SR known to be expressed on endothelial cells, antibody against the SREC-I receptor (SR-F family) was tested. The SREC-1 receptor binds oxLDL and acLDL (11). No effect was observed (Fig. 3A).
3.3. Anti-LOX-1 antibody inhibits attachment of C. pneumoniae, but not C. trachomatis, to the endothelial cells
Whether the anti-LOX-1 monoclonal antibody inhibits attachment of C. pneumoniae to the LOX-1 receptor was examined by doing the binding assay at 4°C. At this temperature, the organism attaches to the host cell, but does not enter. Preincubation of HMEC-1 cells with anti-LOX-1 monoclonal antibody inhibited attachment of C. pneumoniae to HMEC-1 cells in a dose response manner (Fig. 3B), but had no effect on C. trachomatis (Fig. 3C). In contrast, antibodies against SR-F or SR-A (as a negative control), had no effect on attachment of C. pneumoniae (Fig. 3B) or C. trachomatis (Fig. 3C).
3.4 C. pneumoniae co-localizes with the LOX-1 receptor
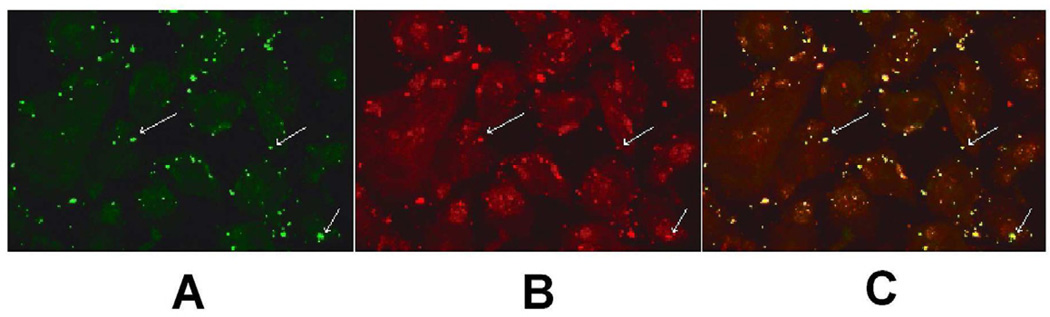
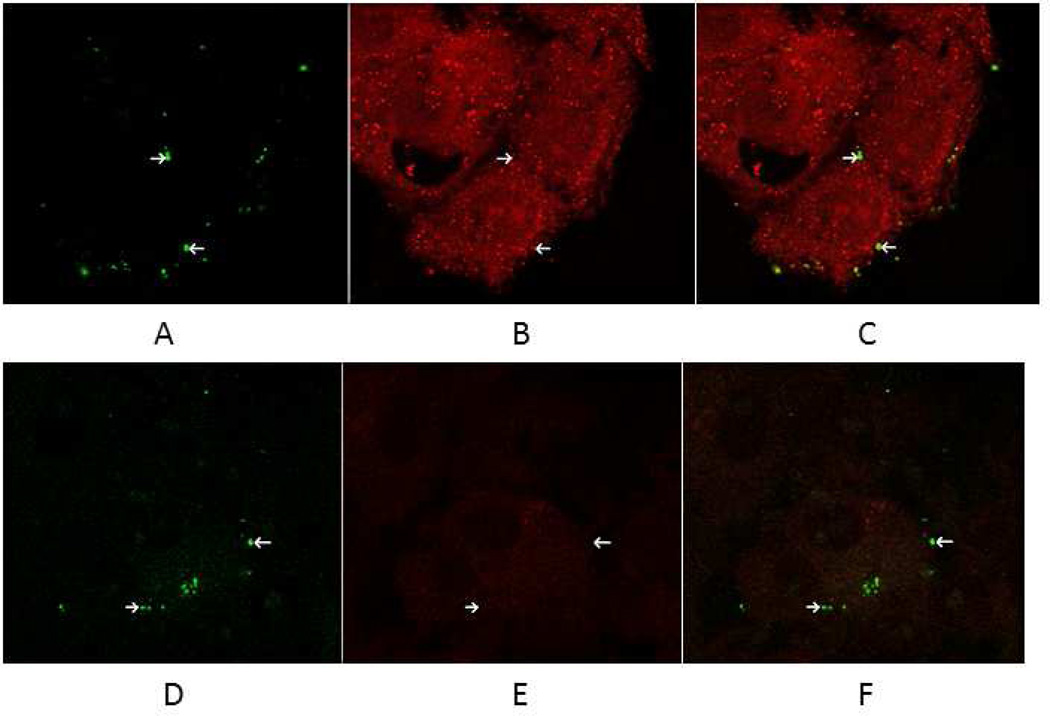
To further confirm that C. pneumoniae binds to the LOX-1 receptor, a co-localization experiment was conducted by double immunofluorescent labeling using green (FITC-conjugated anti-chlamydial antibody) for staining C. pneumoniae (Fig. 4, panel A) and red (anti-LOX-1 antibody and goat anti-mouse conjugated to Texas Red) for staining the LOX-1 receptor (Fig. 4, panel B). The binding assay was conducted at 4oC for 30 min to permit attachment but not internalization. As shown in Fig. 4 panel C, in which panels A and B are superimposed, extensive co-localization was observed with C. pneumoniae and the LOX-1 receptor. In contrast, C. pneumoniae does not co-localize with the SREC-I receptor (Fig. 5, panels A–C). No non-specific staining nor co-localization was observed when staining with antibodies against SR-A, which is not expressed on endothelial cells (Fig. 5, panels D–F)
Fig. 4.
C. pneumonaie colocalizes with the LOX-1 receptor. Endothelial cells were inoculated with C. pneumoniae at 4°C for 30 min to permit attachment, but not internalization. Following fixation of cells, double immunofluorescence labeling of infected cells was done by staining the LOX-1 receptor by incubating with anti-LOX-1 antibody followed by goat anti-mouse IgG coupled to Texas Red and Chlamydia with FITC conjugated monoclonal antibody (green fluorescence). Co-localization was determined by confocal fluorescence microscopy using a LSM5 PASCAL Zeiss confocal microscope. Panel A - Staining of C. pneumoniae with the genus specific FITC conjugated CF2 antibody. Panel B - Staining of endothelial cells with anti-LOX-1 receptor monoclonal antibody. Panel C - Panels A and B are superimposed demonstrating extensive co-localization of C. pneumoniae and the LOX-1 receptor. Arrows highlight stained organism/cells.
Fig. 5.
Chlamydia pneumoniae does not co-localize with the SREC-I receptor. Endothelial cells were inoculated with C. pneumoniae at 4°C for 30 min to permit attachment, but not internalization. Following fixation of cells, double immunofluorescence labeling of infected cells was done by staining the SREC-I receptor by incubating with anti-SREC-I antibody followed by rabbit anti-goat IgG conjugated to Alexa Fluor 647 and C. pneumoniae with FITC conjugated monoclonal antibody CF2 (green fluorescence). As a negative control, infected cells were stained by incubating with anti-SRA antibody followed by rabbit anti-mouse IgG conjugated to Alexa Fluor 549 and C. pneumoniae with FITC conjugated monoclonal antibody CF2 (green fluorescence). Co-localization was determined by confocal fluorescence microscopy using a LSM 510 Zeiss confocal microscope. Panels A and D - Staining of C. pneumoniae with the genus specific FITC conjugated CF2 antibody. Panel B - Staining of endothelial cells with anti-SREC-1 receptor antibody. Panel C - Panels A and B are superimposed demonstrating that C. pneumoniae does not co-localize with the SREC-I (SR-F) receptor. Panel E - Absence of staining of endothelial cells with anti-SR-A receptor antibody. Panel F - Panels D and E are superimposed. Arrows highlight stained organism/cells.
4. Discussion
We have previously shown that the MOMP of C. trachomatis is glycosylated and the structure of carbohydrate on the MOMP is an N-linked high-mannose type oligosaccharide [12]. Importantly, glycopeptides containing high-mannose oligosaccharide inhibited attachment of C. trachomatis, C. psittaci, and C. pneumoniae to HeLa cells [12]. Because of the sugar binding activity of lectins, which have carbohydrate-recognition domains [25], we proposed that one mechanism of pathogen/host interaction involved attachment of the high mannose oligosaccharide glycan to lectin-like receptors on the host cell. By hapten inhibition experiments using ligands that are recognized by the mannose receptor or mannose 6-phosphate receptor (M6PR), we demonstrated that C. trachomatis uses the mannose receptor while C. pneumoniae uses the M6PR for infection [14, 26]. In addition we have now presented data suggesting that C. pneumoniae can also bind to the LOX-1 receptor, the major scavenger receptor on endothelial cells.
A recent study by Wang et al. used a systems biology approach to identify network and modules involved in C. pneumoniae entry followed by gene knockdown experiments targeting critical network components (27). These included proteins known to serve as receptors (CXCR7, integrin beta chain 2, and VCAM-1) in entry of other pathogens. While individual gene knockdowns significantly inhibited C. pneumoniae entry in endothelial cells and Hep-2 cells, none completely blocked infectivity. Knockdown of combinations of 3 or 6 of these proteins increased inhibition of infectivity to >80%, but did not completely ablate infectivity. Similarly, in our studies using either hapten inhibition or antibody blocking of the M6PR (14) or the LOX-1 receptor, only a partial reduction in infectivity was observed. While combined use of antibodies against both the M6PR and LOX-1 receptor resulted in increased inhibition in comparison to either alone, infectivity of endothelial cells was not abrogated (unpublished data). Cumulatively, these studies suggest that C. pneumoniae can utilize more than one receptor for cell entry.
The finding that C. pneumoniae binds to the LOX-1 receptor suggests a possible mechanism for C. pneumoniae atherogenesis, because LOX-1 is a receptor for ox-LDL and binding of ox-LDL to LOX-1 is known to induce endothelial dysfunction. In 2006, Yoshida et al [15] reported that infection of HUVEC cells with C. pneumoniae enhances uptake of ox-LDL and expression of LOX-1 mRNA. In addition, preliminary results suggest that intranasal inoculation of mice with C. pneumoniae induces LOX-1 expression in both the lung and aorta (Campbell et al. Proceedings of the 12th International Meeting on Human Chlamydial Infections).
In this current study, hapten inhibition and antibody neutralization assays were used to demonstrate that C. pneumoniae uses LOX-1 receptor for infection of human endothelial cells. The patterns of those ligands that inhibit C. pneumoniae infectivity are consistent with the binding specificities of LOX-1 receptor, which differ from other SRs [11]. Antibody neutralization assays using antibodies against SR representative of other SR families further showed that C. pneumoniae binds to the LOX-1 receptor, but not SR-A, SR-B, and SR-F, which are major scavenger receptors for modified LDL. In addition, these studies demonstrate that the inhibition of C. pneumoniae infectivity was specific as antibodies against the other SRs that were tested had no effect, and anti-LOX-1 antibody had no effect on infectivity of C. trachomatis for endothelial cells. We have previously shown that primary peritoneal macrophages from SR-A knockout mice were as susceptible to C. pneumoniae infection as macrophages isolated from wild type mice [28]. Consistent with evidence that C. pneumoniae binds to the LOX-1 receptor were the confocal microscopy studies demonstrating that C. pneumoniae co-localizes with the LOX-1 receptor (Fig. 4) and not with SREC-I (SR-F) (Fig. 5). Most importantly, C. trachomatis, which has not been detected in human atheromatous lesions and has been shown not to accelerate atherosclerotic lesion development in an animal model of atherosclerosis, did not bind to LOX-1. This was further demonstrated by the lack of any effect on C. trachomatis infectivity of endothial cells by ligands that bind to LOX-1 or anti-LOX-1 antibody.
The current study is also compatible with the existing knowledge on the effect of C. pneumoniae on atherogenesis. For example, intranasal inoculation of normolipidemic mice induces transient infection of the aorta, but does not induce atherosclerosis [3]. However, intranasal inoculation of hyperlipidemic mice with C. pneumoniae induces prolonged infection of the aorta and accelerates the development of atherosclerosis in C57BL/6J fed an atherogenic diet and ApoE−/− mice, which spontaneously develop atherosclerosis [2, 29]. It has been shown that LOX-1 expression is increased in hyperlipidemia and atherosclerotic lesions [7, 30]. Many of the pro-atherogenic effects of ox-LDL occur by its binding to and uptake by LOX-1. Activation of LOX-1 by ox-LDL binding in endothelial cells induces a plethora of events including the up-regulation of MCP-1, ICAM-1, VCAM-1, E-selectin, P-selectin, MMP-1 (collagenase) and MMP-3 (stromelysin-1) expression [8, 9, 10, 31], which leads to adherence of monocytes to endothelial cells, release of superoxide anion and a reduction in the release of NO [27]. Interestingly, C. pneumoniae infection of endothelial cells has been shown to induce a similar pattern of responses including up-regulation of MCP-1, ICAM-1 and VCAM-1 [32, 33]. MMP-1 and MMP-3 are up-regulated in monocytes and/or smooth muscle cells by C. pneumoniae [34]. Indeed, C. pneumoniae infection increases production of MMP-2 and MMP-9 in LDLR/ApoE double knockout mice and reduces the fibrous cap area, suggesting that C. pneumoniae could contribute to plaque erosion [35].
In conclusion, this study demonstrates that C. pneumoniae binds to the LOX-1 receptor, which plays a key role in atherogenesis.
Acknowledgements
We thank Dr. Alan Chait for preparation of LDL for this study. This study was supported by the USPH grant AI-43060. MP was supported by Sigrid Jusélius Foundation and the Academy of Finland (#217554, #130043).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campbell LA, Kuo C-C. Chlamydia pneumoniae, an infectious risk factor for atherosclerosis? Nature Med. Rev. Microbiol. 2004;2:23–32. doi: 10.1038/nrmicro796. [DOI] [PubMed] [Google Scholar]
- 2.Blessing E, Campbell LA, Rosenfeld ME, Kuo C-C. Chlamydia pneumoniae infection accelerates hyperlipidemia induced atherosclerotic lesion development in C57BL/6J mice. Atherosclerosis. 2001;158:13–17. doi: 10.1016/s0021-9150(00)00758-9. [DOI] [PubMed] [Google Scholar]
- 3.Blessing E, Campbell LA, Rosenfeld ME, Kuo C-C. Chlamydia pneumoniae and hyperlipidemia are co-risk factors for atherosclerosis: Infection prior to induction of hyperlipidemia does not accelerate development of atherosclerotic lesions in C57BL/6J mice. Infect. Immun. 2002;70:5332–5334. doi: 10.1128/IAI.70.9.5332-5334.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo C-C, Gown AM, Benditt EP, Grayston JT. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arteroscler. Thromb. 1993;13:1501–1504. doi: 10.1161/01.atv.13.10.1501. [DOI] [PubMed] [Google Scholar]
- 5.Blessing E, Nagano S, Campbell LA, Rosenfeld ME, Kuo C-C. Effects of Chlamydia trachomatis infection on atherosclerosis in Apolipoprotein E-deficient mice. Infect. Immun. 2000;68:7195–7197. doi: 10.1128/iai.68.12.7195-7197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Kakutani M, Minami M, Kataoka H, Kume N, Narumiya S, Kita T, Masaki T, Sawamura T. Increased expression of lectin-like oxidized low density lipoprotein receptor-1 in initial atherosclerotic lesions of Watanabe heritable hyperlipidemic rabbits. Atheroscler Thromb Vasc Biol. 2000;20:1107–1115. doi: 10.1161/01.atv.20.4.1107. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Mehta J. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889–2895. doi: 10.1161/01.cir.101.25.2889. [DOI] [PubMed] [Google Scholar]
- 9.Li D, Liu L, Chen H, Sawamura T, Ranganathan S, Mehta JL. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinase in human coronary artery endothelial cells. Circulation. 2003;107:612–617. doi: 10.1161/01.cir.0000047276.52039.fb. [DOI] [PubMed] [Google Scholar]
- 10.Zhu H, Xia M, Hou M, Tang Z, Ling W. Ox-LDL plays dual effect in modulating expression of inflammatory molecules through LOX-1 pathway in human umbilical vein endothelial cells. Front. Biosci. 2005;20:2585–2584. doi: 10.2741/1722. [DOI] [PubMed] [Google Scholar]
- 11.Peiser L, Gordon S. The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microb. Infect. 2001;3:149–159. doi: 10.1016/s1286-4579(00)01362-9. [DOI] [PubMed] [Google Scholar]
- 12.Kuo C-C, Takahashi N, Swanson AF, Ozeki Y, Hakomori S-i. An N-linked high-mannose type oligosaccharide, expressed at the major outer membrane protein of Chlamydia trachomatis, mediates attachment and infectivity of the microorganism to HeLa cells. J. Clin. Invest. 1996;98:2813–2818. doi: 10.1172/JCI119109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo C-C, Puolakkainen M, Lin TM, Witte W, Campbell LA. Mannose-receptor positive and negative mouse macrophages differ in their susceptibility to infection to infection by Chlamydia species. Mol. Pathog. 2002;32:43–48. doi: 10.1006/mpat.2001.0479. [DOI] [PubMed] [Google Scholar]
- 14.Puolakkainen M, Kuo C-C, Campbell LA. Chlamydia pneumoniae uses the mannose 6- phosphate/insulin-like growth factor 2 receptor for infection of endothelial cells. Infect. Immun. 2005;73:4620–4625. doi: 10.1128/IAI.73.8.4620-4625.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida T, Koide N, Mori I, Ito H, Yokochi T. 2006. Chlamydia pneumoniae infection enhances lectin-like oxidized low-density lipoprotein receptor (LOX-1) expression on human endothelial cells. FEMS Microbiol. Lett. 2006;260:17–22. doi: 10.1111/j.1574-6968.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuo C-C, Grayston JT. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect. Immun. 1976;13:1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung BH, Wilkinson T, Greer JC, Segrest JP. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J. Lipid Res. 1980;21:284–291. [PubMed] [Google Scholar]
- 18.Heinecke JW, Backer J, Rosen H, Chait A. Super oxide-mediated free radical modification of low density lipoprotein by arterial smooth muscle cells. J. Clin. Invest. 1986;79:757–761. doi: 10.1172/JCI112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtani K, Suzuki Y, Eda S, Kawai T, Kase T, Keshi H, Sakai Y, Fukuoh A, Sakamoto T, Itabe H, Suzutani T, Ogasawara M, Yoshida I, Wakamiya N. The membrane-type collectin CL-P1 is a scavenger receptor on vascular endothelial cells. J. Biol. Chem. 2001;276:44222–44228. doi: 10.1074/jbc.M103942200. [DOI] [PubMed] [Google Scholar]
- 20.Kuo C-C, Chen H-H, Wang S-P, Grayston JT. Identification of a new group of Chlamydia psittaci strains called TWAR. J. Clin. Microbiol. 1986;24:1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez Melgosa M, Kuo C-C, Campbell LA. Outer membrane complex proteins of Chlamydia pneumoniae. FEMS Microbiol. Lett. 1993;112:199–204. doi: 10.1111/j.1574-6968.1993.tb06448.x. [DOI] [PubMed] [Google Scholar]
- 22.Moriwaki H, Kume N, Sawamura T, Aoyama T, Hoshikawa H, Ochi H, Nishi E, Masaki T, Kita T. Ligands specificity of LOX-1, a novel endothelial receptor for oxidized low density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 1998;18:1541–1547. doi: 10.1161/01.atv.18.10.1541. [DOI] [PubMed] [Google Scholar]
- 23.Murphy JE, Tacon D, Tedbury PR, Hadden JM, Knowling S, Sawamura T, Peckham M, Phillips SEV, Walker JH, Ponnambablam S. LOX-1 scavenger receptor mediates calcium-dependent recognition of phosphatidylserine and apoptotic cells. Biochem. J. 2006;393(Pt 1):107–115. doi: 10.1042/BJ20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimaoka T, Kume N, Minami M, Hayashida K, Sawamura T, Kita T, Yonehara S. LOX-1 supports adhesion of gram-positive and gram-negative bacteria. J. Immunol. 2001;166:5108–5114. doi: 10.4049/jimmunol.166.8.5108. [DOI] [PubMed] [Google Scholar]
- 25.Shimaoka T, Kume N, Minami M, Hayashida K, Sawamura T, Kita T, Yonehara S. Lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) supports cell adhesion to fibronectin. FEBS Lett. 2001;504:65–68. doi: 10.1016/s0014-5793(01)02774-0. [DOI] [PubMed] [Google Scholar]
- 26.Kuo C-C, Puolakkainen M, Lin T-M, Witte M, Campbell LA. Mannose-receptor positive and negative mouse macrophages differ in their susceptibility to infection by Chlamydia species. Microb. Pathogen. 2002;32:43–48. doi: 10.1006/mpat.2001.0479. [DOI] [PubMed] [Google Scholar]
- 27.Wang A, Claiborne Johnston S, Chou J, Dean D. systemic network for Chlamydia pneumoniae entry into human cells. J. Bacteriol. 2010;192:2809–2815. doi: 10.1128/JB.01462-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blessing E, Kuo C-C, Lin TM, Campbell LA, Bea F, Chesebro B, Rosenfeld ME. Foam cell formation inhibits growth of Chlamydia pneumoniae but does not attenuate Chlamydia pneumoniae-induced secretions of proinflammatory cytokines. Circulation. 2002;105:1976–1982. doi: 10.1161/01.cir.0000015062.41860.5b. [DOI] [PubMed] [Google Scholar]
- 29.Moazed TC, Campbell LA, Rosenfeld ME, Grayston JT, Kuo C-C. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J. Infect. Dis. 1999;180:238–241. doi: 10.1086/314855. [DOI] [PubMed] [Google Scholar]
- 30.Kataoka H, Kume N, Miamoto S, Minami M, Moriwaki H, Murase T, Swamura T, Masaki T, Hashimoto N, Kita T. Expression of lectin-like oxidized low density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–3117. doi: 10.1161/01.cir.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 31.Kita T, Kume N, Ishi K, Horiuchi H, Arai H, Yokode M. Oxidized LDL and expression of adhesion molecules. Diabetes Res. Clin. Pract. 1999;45:123–126. doi: 10.1016/s0168-8227(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 32.Kim MP, Gaydos CA, Wood BJ, Hardick JP, Zhang Y, Wahl LM. Chlamydia pneumoniae enhances cytokine-stimulated human monocyte matrix metalloproteinases through a prostaglandin E2-dependent mechanism. Infect. Immun. 2005;73:632–634. doi: 10.1128/IAI.73.1.632-634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kol A, Bourcier T, Lichtman AH, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J. Clin. Invest. 1999;103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodel J, Prochnau D, Prager K, Pentcheeva E, Hatmann M, Strabue E. Increased production of matrix metalloproteinases 1 and 3 by smooth muscle cells upon infection with Chlamydia pneumoniae. FEMS Immunol. Med. Microbiol. 2003;38:159–164. doi: 10.1016/S0928-8244(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 35.Ezzahiri R, Stassen FR, Kunvers HA, van Pul MM, Kilslaar PJ, Bruggeman CA. Chlamydia pneumoniae infection induces an unstable atherosclerotic plaque phenotype in LDL receptor, ApoE double knockout mice. Eur. J. Vasc. Endovasc. Surg. 2003;26:86–95. doi: 10.1053/ejvs.2002.1913. [DOI] [PubMed] [Google Scholar]