Abstract
AIM: To shed some light on the relationship between anti-apoptotic serum Bcl-2 concentrations and metabolic status, anthropometric parameters, inflammation indices, and non-alcoholic fatty liver disease severity were investigated in 43 young individuals with fatty liver (FL) and 41 with nonalcoholic steatohepatitis (NASH).
METHODS: Circulating levels of Bcl-2 were detected in 84 patients with ultrasonographic findings of “bright liver” and/or hyper-transaminasemia of unknown origin and/or increase in γ-glutamyl-transpeptidase (γ-GT) strictly in the absence of other acute or chronic liver disease, whose age was not advanced, who gave consent to liver biopsy and were then divided on the basis of the histological results into two groups (43 with FL and 41 with NASH). Twenty lean subjects, apparently healthy and young, were chosen as controls.
RESULTS: Serum Bcl-2 concentrations were significantly higher in the FL group than in the NASH group. Insulin resistance and γ-GT activity were significantly higher in NASH subjects. Apoptotic hepatocytes were significantly more numerous in NASH patients. NASH patients presented with larger spleens and augmented C-reactive protein (CRP) concentrations than healthy subjects. Steatosis grade at histology was similar in both NASH and FL populations. The number of apoptotic cells was significantly related to anti-apoptotic Bcl-2 protein values in FL patients. Bcl-2 serum levels positively correlated to body mass index (BMI) values (P ≤ 0.0001) but not to age of the population. Triglycerides/HDL ratio correlated well to waist circumference in males (P = 0.0008). γ-GT activity was associated with homeostatic metabolic assessment (HOMA) (P = 0.0003) and with serum ferritin (P = 0.02). Bcl-2 concentrations were not related to either spleen size or CRP values. NASH patients presented a weak negative correlation between lobular inflammation and Bcl-2 levels. A prediction by low values of serum Bcl-2 towards a greater presence of metabolically unhealthy overweight/obese patients (MUOs) was evidenced. HOMA, BMI and uric acid, in that sequence, best predicted serum Bcl-2 concentrations.
CONCLUSION: MUOs could be detected by Bcl-2 levels. By favoring the life span of hepatocytes, and enhancing triglyceride formation, the anti-apoptotic process inhibits free fatty acids toxicity in FL.
Keywords: Bcl-2, Nonalcoholic fatty liver disease, Metabolically unhealthy overweight/obese
INTRODUCTION
High serum levels of triglycerides/free fatty acids (FFA) and insulin resistance (IR) are features of non-alcoholic fatty liver disease (NAFLD), an additional manifestation of the metabolic syndrome[1]; the former is extremely common in patients suffering from overweight/obesity (O/O), and ranges from fatty liver (FL) to nonalcoholic steatohepatitis (NASH) and liver cirrhosis. In determining NASH, a role is thought to be played by FFAs that directly engage the core apoptotic machinery by activating the proapoptotic protein Bax, in a c-jun N-terminal kinase-dependent manner[2]. Moreover, increased apoptosis in liver specimens from NASH patients is associated with iron overload[3], which clearly correlates with IR and inflammation. Indeed, interesting approaches to study pro-apoptotic and anti-apoptotic processes at the level of the liver are the use of in vitro models to explore the molecular events, tissue expression and circulating levels of the single factors. The first procedure partially limits the translation to the human scenario, mainly when moving from FL to NASH, because NAFLD reflects the long-lasting, complex dynamics orchestrated by various cells, such as adipocytes, β cells, muscle cells, macrophages and natural killer T cells. Apoptosis being a composite system regulated in a complex fashion with the contribution of many different factors, the expression of a single protein in liver specimens gives only a snapshot of a process that is hardly static. In favor of the serum concentrations stands the fact that either the stimulatory or the counter-regulatory circuits might eventually determine the net global effect of a given component. In conditions of chronic inflammation or oxidative stress, as for metabolic syndrome (MS), it is conceivable that the entire network is in a long-term persistent state of activation leading to increased circulating levels. In the serum of NAFLD patients, cytochrome c, an indicator of apoptosis-related mitochondrial damage, has been scarcely detected[4]. Among the anti-apoptotic factors, circulating levels of soluble Fas have been studied in some acute and chronic liver diseases[5,6]. The expression of Bcl-2 in the liver tissue of NAFLD patients has been evaluated by real-time PCR or, alternatively, by immuno-histochemical staining, highlighting surprisingly different patterns. In fact, in patients with NASH the expression of the Bcl-2 protein in liver specimens is mildly increased[7]. In contrast, in rats with high-fat diet-induced NASH, the hepatic expression of Bcl-2 did not differ from that of animals on control diet[8]. Similarly, no significant difference in the expression of Bcl-2 in NASH and biopsy-proven FL patients was observed[2]. Other authors have shown that the tissue expression of this anti-apoptotic protein diminished with the advancement of liver steatosis[9]. Recent data have shown that NAFLD patients with elevated gamma glutamyl-transpeptidase (γ-GT) activity have significantly higher Bcl-2 staining levels compared to patients evidencing normal γ-GT[10]. We firstly hypothesized that serum Bcl-2 levels reflect the steady state of this anti-apoptotic protein. A recent study, showing that urinary Bcl-2 concentrations are elevated in females suffering from ovarian cancer regardless of their creatinine levels or age[11], supports this hypothesis. A further confirmation is given by the fact that decreased apoptosis is in part associated with increased serum Bcl-2 levels in patients with melanoma[12]. Secondly, novel evidence suggests that low-grade chronic inflammation, in which spleen volume plays a key role[13], is the cause of IR in O/O patients, whose serum levels of soluble Fas interfere with the apoptotic pathway[14]. Finally, serum levels of Bcl-2 and cellular oxidative stress have already been studied in patients with viral hepatitis, showing a close link[15]. On the other hand, a “metabolically benign obesity” which is not accompanied by IR has recently been postulated to exist in humans[16-18], although this hypothesis has been challenged by some authors[19]. As a consequence, some researchers have started considering NAFLD, independent of its severity, as a divide between metabolically healthy O/O and metabolically unhealthy O/O (MUO)[20] individuals. Against this background, we felt the need to shed some light on the relationship between anti-apoptotic serum Bcl-2 concentrations and metabolic status, anthropometric parameters, inflammation indices, such as C-reactive protein (CRP) and spleen volume[13], as well as NAFLD severity in the vast complex of O/O and MS, in young adults and middle aged individuals.
MATERIALS AND METHODS
This research was performed by initially screening 178 consecutive subjects referred to our out-patient Metabolic Unit with established (at least 4 years) O/O, from February 2006 to December 2009. The study was carried out according to the principles of the Declaration of Helsinki and informed written consent was obtained from each patient.
Enrollment criteria
Out of the 178 initial participants 15 were excluded due to marked intestinal meteorism which made it difficult to perform abdominal ultrasound (first step to screening NAFLD), 16 patients because they had undergone steroid therapy (seven for bronchial asthma, five for rheumatoid arthritis, two for neuritis and two for inflammatory bowel disease), and 28 because they had received one or more drugs (i.e., aspirin, metformin, statins and fibrates) that may have altered their laboratory data. Fourteen others were excluded because of the presence of hepatic co-morbidities in their medical history (HCV infection, alcohol abuse), and finally twenty subjects were considered drop-outs, because they refused to undergo full laboratory-instrumental examinations. Eighty-four of the 119 patients, with ultrasound findings of “bright liver” and/or hyper-transaminasemia of unknown origin and/or increase in γ-GT strictly in the absence of other acute or chronic liver disease, whose age was not advanced (Table 1), gave consent to liver biopsy and were then divided on the basis of the histological results (see below) into two groups (43 with FL and 41 with NASH) that formed the final study population. Twenty lean subjects, apparently healthy and young, were chosen as controls to avoid any confounding factor with circulating levels of Bcl-2 (see below).
Table 1.
Clinical characteristics and laboratory data of the study population (mean ± SD)
| Diagnosis | ||||
| H | FL | NASH | P value | |
| Subjects (n) | 21 | 43 | 41 | |
| Females | 11 | 30 | 24 | 0.951 |
| Age (yr) | 19.1 ± 3.8 | 31.4 ± 13.9 | 35.6 ± 13 | < 0.0012 |
| Overweight | 0 | 3 | 3 | 0.71 |
| Obesity 1st grade | 0 | 13 | 9 | 0.51 |
| Obesity 2nd grade | 0 | 9 | 6 | 0.61 |
| Obesity 3rd grade | 0 | 18 | 24 | 0.851 |
| MS | 0 | 24 | 36 | 0.0015 |
| MUO | 0 | 34 | 39 | 0.061 |
| BMI | ||||
| F | 23.8 ± 1.13 | 38.8 ± 8.24 | 37.8 ± 6.6 | < 0.0012 |
| M | 23.7 ± 0.6 | 38.6 ± 4.4 | 45.7 ± 10.5 | 0.013 |
| WC (cm) | ||||
| F | 86.3 ± 1.4 | 114.8 ± 15.7 | 117.3 ± 17.7 | 0.0012 |
| M | 93.4 ± 2.3 | 121.2 ± 11.3 | 131.1 ± 15.1 | < 0.0013 |
| HOMA [median (range)] | 1.77 (1.03-6) | 2.3 (1.4-7.3) | 4.1 (0.6-10.2) | < 0.00013 |
| γ-GT U/L [median (range)] | 33.1 (23.4-44.8) | 40.5 (26-80) | 54 (22.5-397) | < 0.00019 |
| ALT U/L [median (range)] | 27 (18-34) | 36 (14-133) | 40 (11-153) | 0.0046 |
| Uric acid | 3.5 ± 0.4 | 5.1 ± 1.45 | 5.4 ± 1.4 | < 0.0012 |
| Ferritin | ||||
| F | 84.6 ± 10.3 | 139.6 ± 76. | 149.2 ± 69 | 0.0012 |
| M | 166.8 ± 37.4 | 218.5 ± 121. | 280 ± 103 | 0.014 |
| Triglycerides | 85 (66-121) | 96 (51-290) | 117 (51-386) | 0.1610 |
| HDL (mg/mL) | ||||
| F | 63.6 ± 7.97 | 51.8 ± 9.38 | 50 ± 11.15 | 0.0012 |
| M | 55.3 ± 8.4 | 50.8 ± 7.9 | 44 ± 4.4 | < 0.0018 |
| Triglycerides/HDL [median (range)] | ||||
| F | 1.32 (1.16-1.49) | 2.19 (1.18-6.5) | 1.51 (1.07-7.6) | 0.0046 |
| M | 1.635 (1.39-1.75) | 1.67 (1.25-2.7) | 2.65 (1.25-8.8) | 0.037 |
χ2 test between fatty liver (FL) and non alcoholic steato hepatitis (NASH);
analysis of variance (ANOVA) [significance between healthy (H) and FL and between H and NASH];
ANOVA (significance between H and FL, between H and NASH and also between FL and NASH);
ANOVA (significance between H and NASH);
Pearson χ2 (significance between FL and NASH);
Kruskal-Wallis test (significance between H and FL and between H and NASH);
Kruskal-Wallis test (significance between NASH and FL and between NASH and H);
ANOVA (significance between NASH and FL and between NASH and H);
Kruskal-Wallis test (significance between H and FL, between H and NASH and also between FL and NASH);
Kruskal-Wallis test. F: Females; M: Males; n: Number; H: Healthy; FL: Fatty liver; NASH: Non alcoholic steato hepatitis; ALT: Alaninamino transferase; γ-GT: γ-glutamil transferase; HDL: High density lipoprotein; HOMA: Homeostatic metabolic assessment; BMI: Body mass index; MS: Metabolic syndrome; MUO: Metabolically unhealthy overweight/obese; WC: Waist circumference.
Exclusion criteria
Any viral, autoimmune, metabolic liver disease (Wilson disease, hemochromatosis or antitrypsin deficiency) was ruled out by using appropriate testing, following the accepted diagnostic guidelines. Celiac disease was excluded by estimates of IgA anti-tissue transglutaminase antibodies. Alcohol abuse was ruled out, according to the DSM-IV diagnostic criteria, by means of screening tests such as MAST (Michigan alcohol screening test) and CAGE (Cut down, Annoyed, Guilty, and Eye opener), as well as random tests for blood alcohol concentration and the use of a surrogate marker, e.g., Mean Corpuscular Volume. Patients on antihypertensive drugs maintained a balanced therapeutic regimen throughout the study.
Metabolic profile
The degrees of obesity was established on the basis of BMI cut-off points of 25-29.9, 30-34.9, 35-39.9 and > 40 kg/m2, respectively. Visceral obesity was identified by measuring waist circumference (WC) at the midpoint between the lower border of the rib cage and the iliac crest. MS was defined according to the revised Adults Treatment Panel III (2001), and three or more criteria were considered: plasma glucose concentration of at least 100 mg/dL, WC > 88 cm, serum HDL concentration < 50 mg/dL for women and < 40 mg/dL for men, blood pressure of at least 130/85 mm Hg, and serum triglyceride concentration of at least 150 mg/dL. IR status was determined by the Homeostatic metabolic assessment (HOMA), which was assessed by the formula: fasting insulin (μU/mL) × fasting glucose (mg/dL)/405[21]. As a stringent measure of IR, a value of HOMA > 2 was introduced, according to the cut-off value of surrogate measures of IR for MS in non-diabetic adults[22]. Moreover, as the repeated HOMA measurements presented high within-person variability in O/O patients, HOMA values were averaged on the basis of at least five determinations to avoid misclassification. When reporting triglycerides values, only the data of examinees who had fasted a minimum of 9 h before the morning examination were included to avoid false increases, averaging the results of at least two determinations made on different days. To avoid the high intra-individual variability the triglyceride/HDL cholesterol ratio was evaluated (see below). MUO subjects were categorized in such a way in the presence of IR, MS, high levels of serum uric acid (UA),[23] and if their triglyceride/HDL cholesterol ratio was ≥ 1.65 (men) or ≥ 1.32 (women)[24].
Ultrasound evaluation
Ultrasonographic (US) measurements were performed by two operators, using a general electric vivid system. Spleen longitudinal diameter (SLD) was chosen to evaluate spleen volume and was carried out by postero-lateral scanning. Maximum length (the optically greatest overall longitudinal dimension obtained from one of the two poles) and cranio-caudal length (the optically maximal transversal dimension intercepting one of the two poles) were measured; the resulting values were then averaged, since the two measurements do not always coincide. The classification of “bright liver” or hepatic steatosis (HS) was based on the following scale of hyper-echogenicity: 0 = absent, 1 = light, 2 = moderate, 3 = severe, pointing out the difference between the densities of the liver and the right kidney. Technically, echo intensity can be influenced by many factors, particularly by gain intensity. To avoid confounding factors that could modify echo intensity and thus bias comparisons, the mean brightness levels of both the liver and the right kidney cortex were obtained on the same longitudinal sonographic plane. The levels of brightness of the liver and the right kidney were calculated three times directly from the frozen images.
Blood pressure measurements
Systolic/diastolic blood pressure was the average of three consecutive readings taken by the physician during the day, during the usual practice hours, after subjects had rested for 5 min in the sitting position.
Laboratory data
Serum triglycerides, HDL, basal insulin, ALT, γ-GT, UA, glycemia and ferritin were performed by in-house standard procedures. Hs-CRP values were determined by ELISA test, with reference values between 0.3 and 8.6 mg/L in healthy men and between 0.2 and 9.1 mg/L in healthy women (BioCheck, Inc CA, United States). Bcl-2 was determined using a Human Bcl-2 ELISA Kit (producer Bender MedSystems, Austria, EU) with a coefficient of variation ranging from 5.1 to 17.7, a sensitivity of 2.5 ng/mL, an overall intra-assay coefficient of 8.6 and a reference interval calculated on 21 healthy subjects (Table 1) by the non-parametric percentile method (CLSI C28-A3) of 7.4-22.6 ng/mL.
Liver histology
The diagnosis of NASH was made when three of the following five criteria were proven by liver biopsy: steatosis, hepatocyte ballooning, lobular inflammation, portal inflammation and mallory bodies. Data on Mallory bodies were collected as inclusion criteria to pinpoint the accuracy of diagnosis, but they were not used for evaluation. A 4-point scale for each of the four following criteria resulted in a sum score ranging from 0 to 12. Specifically, the scores were: Steatosis: 0 = None; 1 = Up to 33% of acini, mainly macrovesicular; 2 = 34%-66% of acini, commonly mixed steatosis; 3 = Over 66% of acini (panacinar), commonly mixed steatosis; Hepatocyte ballooning: 0 = None; 1 = Occasional in zone III; 2 = Obvious in zone III; 3 = Marked, predominantly in zone III; Lobular inflammation: 0 = None; 1 = Scattered neutrophils, occasional mononuclear cells, 1 or 2 foci per 20× objective; 2 = Neutrophils associated with ballooned hepatocytes, mild chronic inflammation, 3 or 4 foci per 20 × objective; 3 = Acute and chronic inflammation, neutrophils may concentrate in zone III, over 4 foci per 20 × objective. Portal inflammation: 0 = None; 1 = Mild, some portal areas; 2 = Mild to moderate, most portal areas; 3 = Moderate to severe, most portal areas. Fibrosis was staged as follows: Stage 0 = None; Stage 1 = Zone III perivenular, perisinusoidal (pericellular); Stage 2 = Stage 1 changes plus periportal fibrosis; Stage 3 = Bridging fibrosis; Stage 4 = Cirrhosis. Biopsy samples were taken within eight weeks prior to inclusion. FL was diagnosed when only steatosis was present, using the same grades previously reported[1-3]. The biopsy sample had to be at least 1.5 cm long with a minimum diameter of 0.8 mm. Inclusion of a NASH patient in the study required a sum score of at least 6 points.
Apoptosis detection
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) is a common method for detecting DNA fragmentation that results from apoptotic signaling cascades. We used the Click-iT® TUNEL Alexa Fluor® 647 Imaging Assay (Invitrogen). This method utilizes a dUTP modified with an alkyne, a small, bio-orthogonal functional group that enables the nucleotide to be more readily incorporated by TdT than other modified nucleotides, including BrdUTP, biotin-dUTP or fluorescein-dUTP. Detection is based on a click reaction, i.e., a copper catalyzed reaction between an azide and alkyne.
Cells displaying TUNEL-labeled fluorescent nuclei were quantified by counting the number of positive cells per high-power field. A total of 10 high-power fields were analyzed for each patient with excitation and emission wavelengths of 380 and 430 nm, respectively, using an inverted laser scanning confocal microscope (Carl Zeiss) equipped with a ×40 NA 1.4 lens. Data are expressed as the number/mm2 of TUNEL-positive cells.
Statistical analysis
Age data, derived from a normally distributed population [Kolmogorov-Smirnov test (K-S), P = 0.15], ferritin (K-S, P = 0.6), BMI (K-S, P = 0.2), WC (K-S, P = 0.18), SLD (K-S, P = 0.52), UA (K-S, P = 0.105) are given as mean plus SD. Variables not normally distributed, such as triglycerides (K-S, P = 0.0013), triglycerides/HDL (K-S, P ≤ 0.0001), HOMA (K-S, P = 0.0016), ALT (K-S, P = 0.0001), CRP (S-W, P = 0.001) are expressed as median (range). Grades at histology and US were considered ordinals and managed in the same way. The difference in medians was assessed by the Mann-Whitney test for independent samples. One-way analysis of variance (ANOVA) was used to test the difference between the means of several subgroups of a variable (multiple testing). If the ANOVA test was positive (P < 0.05) then a Student-Newman-Keuls test for pairwise comparison of subgroups was performed. The ANOVA K-W test was used to calculate the differences between the medians of several subgroups of a variable. If K-W was positive (P < 0.05) then a test for pairwise comparisons of subgroups according to Conover was adopted. When adjusted for a covariate the ANOVA was transformed into ANCOVA and the significance was expressed as F-ratio. The Two-Way Tables cross-tabulated one categorical row variable with one categorical column variable and the significance was set by the Pearson χ2. When cross-tabulation was stratified for another dichotomous variable, the Mantel-Haenszel χ2 was carried out. Tracking the degree of association between single parameters, Spearman’s coefficient of rank correlation (rho) for non uniform intervals was used. The Pearson’s coefficient (r) was employed to analyze the correlation between data derived from a normally distributed population. To predict a binary variable, the logistic regression (Enter Method), with relative Odds ratios and 95% confidence intervals (CI), was employed utilizing data from an independent variable. To assess the independent effect of a quantitative variable on the prediction of the values of another variable, the linear regression analysis (least squares) was used, evaluating the standardized coefficient β (β) and t, which is a measure of the precision with which the regression coefficient is measured. A tolerance of less than 0.20 and/or a variance inflation factor of 5 and above indicated a multi-colinearity problem. When confronted with the question of how accurate a parameter was in identifying MUO cases, the discrimination was evaluated using receiver operating characteristic curve analysis (ROC), expressed as area under the ROC (AUC). A criterion or cut-off was set and then sensitivity, specificity and positive likelihood ratio were estimated. Negative predictive value with the Bayes method was also calculated. The factor analysis was applied to detect the structure in the relationships among variables, selecting a subset of variables having the highest correlations with the principal component factors. The Cattell Scree plot, with relative eigen values, was performed to screen the real factors. Extraction of the main components amounted to a variance maximizing (varimax) the rotation of the original variable space. The critical value was calculated by doubling Pearson’s correlation coefficient for 1% level of significance (5.152)/square root of patients (84) minus 2, i.e., 0.568. The concordance correlation coefficient (ρc), which measures precision and accuracy, was adopted to evaluate the degree of pair observations at US. Statistical analysis was performed operating on Systat 13 (Richmond, CA, United States) and MedCalc Version 11.4® (Frank Schoonjans) software packages.
RESULTS
Prevalence
The number of individuals affected by being overweight, or by 1st, 2nd and 3rd grade obesity was comparable in NASH and FL populations and was well-balanced for gender. NASH patients were characterized by older age and a larger percentage of MUO cases, having the largest number of MS among males (Mantel-Haenszel Chi-Square P = 0.005), a greater BMI and a larger WC in males (ANOVA, F = 55.5, P = 0.0001 and F = 59.5, P = 0.0001, respectively). Both insulin resistance and γ-GT activity were significantly higher in subjects diagnosed with NASH. Raised values of ALT activity were hardly detected in NASH and FL patients. Men with NASH were affected by hyperferritinemia and had the lowest levels of HDL and the highest triglycerides/HDL ratio. Serum uric acid was increased in FL and NASH patients without any difference between the two groups (Table 1). Apoptotic hepatocytes were significantly more numerous in NASH patients. NASH patients presented with larger spleens and augmented CRP concentrations compared with healthy subjects. In both populations of NASH and FL the steatosis grade at histology was similar, so was the steatosis severity at US. Serum concentrations of Bcl-2 were significantly higher in the FL group than in the NASH group and patients of both groups showed increased values compared to healthy subjects (Table 2 and Figure 1). The significance did not change after adjusting the values of Bcl-2 for γ-GT activity, with only the diagnosis being significant (ANCOVA, F = 14. 9, P = 0.0001).
Table 2.
Serum concentrations of the anti-apoptotic protein Bcl-2, histology features and inflammation parameters of the patients enrolled
| Diagnosis | ||||
| H | FL | NASH | P value | |
| Subjects (n) | 21 | 43 | 41 | |
| Bcl-2 (ng/mL) | 2.5 (1.4-13) | 25 (2.5-168) | 14 (2.5-89) | < 0.0013 |
| Hepatocyte ballooning grade | NA | NA | 2 (1-3) | NA |
| Steatosis score | NA | 2 (1-3) | 2 (1-3) | 0.492 |
| Lobular inflammation grade | NA | NA | 2 (1-3) | NA |
| Portal inflammation grade | NA | NA | 1 (1-3) | NA |
| Fibrosis score | NA | NA | 1 (1-2) | NA |
| Apoptotic cells (n/mm2) | NA | 290 (120-900) | 780 (110-1650) | < 0.00012 |
| HS at US grade | NA | 3 (0-3) | 3 (0-3) | 0.0642 |
| CRP (mg/L) | 0.9 (0.4-5.4) | 3.9 (0.1-21.1) | 5 (0.1-27) | 0.00011 |
Kruskal-Wallis test [significance between healthy (H) and fatty liver (FL) and between H and non alcoholic steato hepatitis (NASH)];
Mann-Whitney test;
Kruskal-Wallis test (significance between H and FL, between H and NASH and also between FL and NASH). n: Number; H: Healthy; FL: Fatty liver; NASH: Non alcoholic steato hepatitis; NA: Not applicable; HS: Hepatic steatosis at ultrasound; US: Ultrasound; SLD: Spleen longitudinal diameter at ultrasound; CRP: C-reactive protein.
Figure 1.
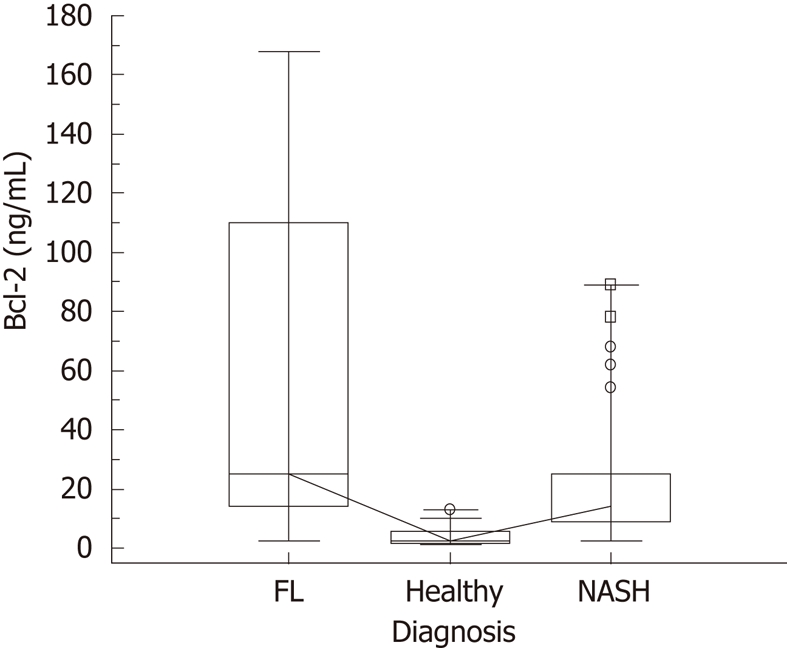
Serum concentrations of Bcl-2 in the whole population. Kruskal-Wallis test (significance between healthy and fatty liver (FL), between healthy and non alcoholic steato hepatitis (NASH) and also between FL and NASH); the highest median value was found in the FL group, P ≤ 0.001.
Associations and prediction
The number of apoptotic cells was significantly related to the anti-apoptotic Bcl-2 protein values (rh0 = 0.43, P = 0.003) in FL patients but not in NASH ones (rh0 = 0.17, P = 0.27). Bcl-2 serum levels correlated well with the BMI values of the whole population (rh0 = 0.55, P = < 0.0001). The correlation between serum concentrations of Bcl-2 and age was not significant (rh0 = 0.14, P = 0.15). Triglycerides/HDL ratio correlated well with WC in males (Pearson’s r = 0.51, P= 0.0008). γ-GT activity was associated with HOMA values (rh0 = 0.345, P = 0.0003), as was γ-GT with serum ferritin (rh0 = 0.22, P = 0.02). Bcl-2 concentrations were not related with either SLD or CRP values (rh0 = 0.10 and 0.13 with P = 0.3 and P = 0.2, respectively). A non significant correlation was found between lobular inflammation and Bcl-2 levels in NASH patients (rh0 = -0.21, P = 0.2).
As to age, at linear regression, advancing years did not completely predict Bcl-2, β = -0.19, t = -2, P = 0.053 (Figure 2A). UA predicted Bcl-2 values well, β = 0.35, t = 3.8, P = 0.0002 (Figure 2B).
Figure 2.
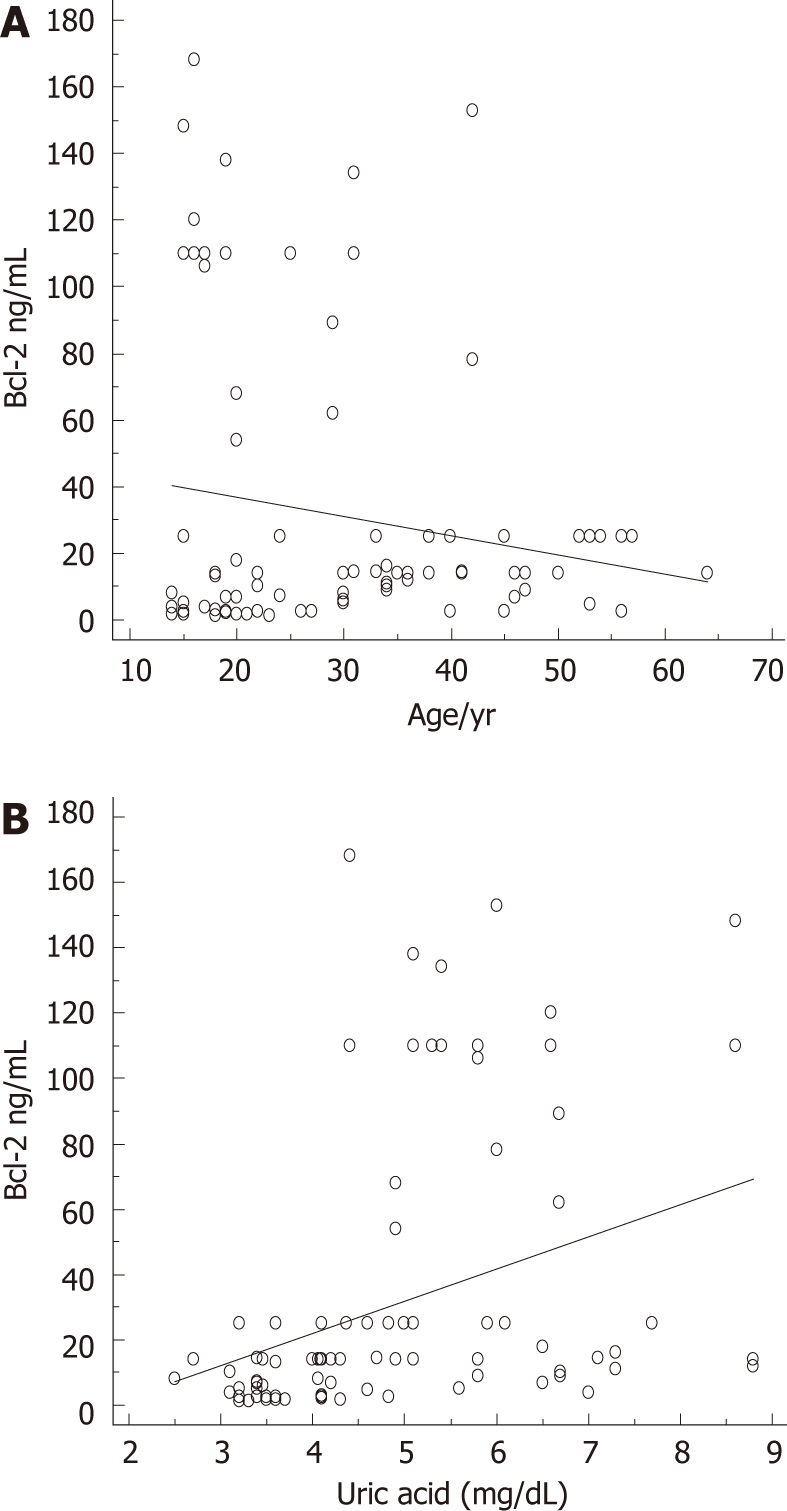
Prediction of Bcl-2 serum levels by age and uric acid. A: Prediction of antiapoptotic Bcl-2 protein serum concentrations by age, β = -0.19, t = -2, P = 0.0527; B: Prediction of antiapoptotic Bcl-2 protein serum concentrations by uric acid, β = 0.35, t = 3.8, P = 0.0002.
At multiple regression analysis, HOMA, BMI and UA, in this sequence, best predicted Bcl-2 serum concentrations, as higher IR corresponded to lower Bcl-2 values, whereas considerable BMI and elevated amount of UA matched with higher Bcl-2 serum concentrations (Table 3). A sufficient prediction by low serum Bcl-2 values towards a higher rate of MUOs was evidenced in logistic regression, OR = 0.98, CI 0.97-0.995, P = 0.004. Obviously, HOMA alone did not predict the status of MUO, OR 1.38, CI 0.87-2.2.
Table 3.
Prediction of anthropometric parameters, apoptosis detection and laboratory data by Bcl-2 concentrations
| Effect | β | t | P value | Tolerance |
| HOMA | -0.505 | -4.27 | 0.000 | 0.440 |
| HDL | 0.22 | 1.4 | 0.16 | 0.242 |
| WC | 0.25 | 1.7 | 0.09 | 0.288 |
| BMI | 0.35 | 2.65 | 0.009 | 0.344 |
| Triglycerides | -0.07 | -0.17 | 0.86 | 0.040 |
| Triglycerides/HDL | 0.22 | 0.5 | 0.63 | 0.029 |
| Uric acid | 0.24 | 2.1 | 0.03 | 0.509 |
| Ferritin | -0.15 | -1.8 | 0.08 | 0.853 |
| Apoptosis in NASH | 0.013 | 0.13 | 0.9 | 1 |
NASH: Nonalcoholic steatohepatitis; BMI: Body mass index; HOMA: Homeostatic metabolic assessment; HDL: High density lipoprotein; triglycerides and triglycerides/HDL were considered variables affected by collinearity; WC: Waist circumference; β: Beta, standardized coefficient; t: A measure of the precision with which the regression coefficient is measured.
Insulin resistance was closely related to inflammation and lipid asset; apoptosis did not seem related to age (Table 4 and Figure 3). The concentrations of serum Bcl-2 were not predicted by the number/mm2 of TUNEL-positive cells in NASH patients (β = 0.013, t = 0.13, P = 0.9, Figure 4).
Table 4.
Hidden relationships highlighted by factor analysis
| Factors | 1 | 2 |
| Bcl-2 | -0.105 | 0.672 |
| Age | -0.348 | -0.424 |
| Apoptosis | 0.293 | -0.425 |
| MS | 0.606 | 0.011 |
| BMI | 0.564 | 0.528 |
| WC | 0.604 | 0.467 |
| HOMA | 0.781 | -0.211 |
| HDL | -0.715 | 0.107 |
| Triglycerides | 0.521 | -0.471 |
| Triglycerides /HDL | 0.698 | -0.468 |
| Uric Acid | 0.554 | 0.520 |
| Ferritin | 0.279 | 0.064 |
| ALT | 0.545 | 0.097 |
| Γ-GT | 0.074 | -0.384 |
| CRP | 0.717 | -0.274 |
| SLD | -0.202 | 0.282 |
| HS at US | 0.471 | 0.255 |
| Steatosis | 0.293 | 0.265 |
Insulin resistance is closely related to inflammation and lipid asset; apoptosis does not seem related to age. Percent of total variance explained for factor 1:26; for factor 2:14; the critical value is 0.568. HS: Hepatic steatosis; US: Ultrasound; SLD: Spleen longitudinal diameter at ultrasound; CRP: C-reactive protein; BMI: Body mass index; MS: Metabolic syndrome; ALT: Alaninamino transferase; γ-GT: γ-glutamyl transferase; HDL: High density lipoprotein; HOMA: Homeostatic metabolic assessment; WC: Waist circumference.
Figure 3.
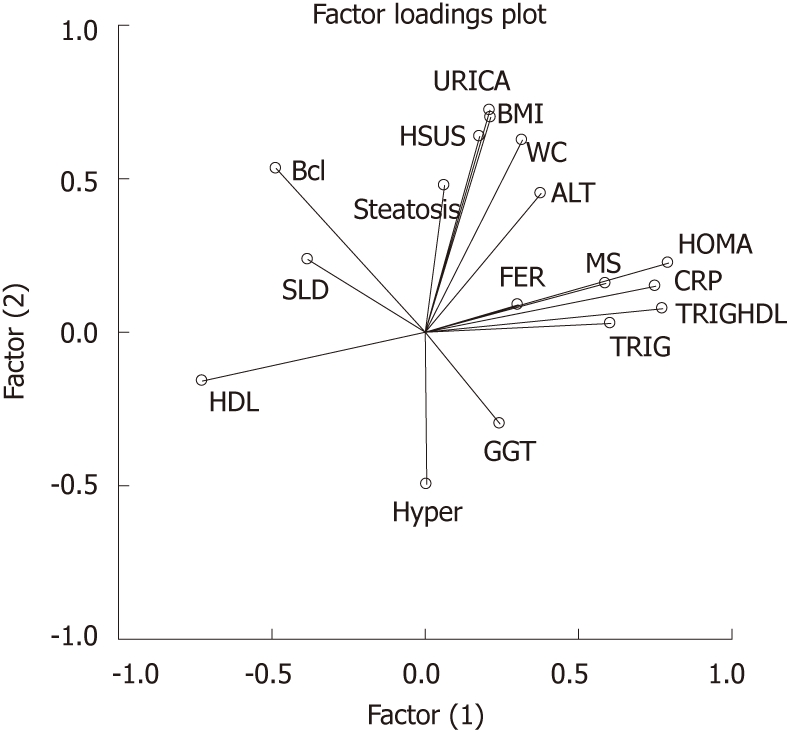
Variables clustered around factors. Homeostatic metabolic assessment, C-reactive protein, triglycerides, triglycerides/high density lipoprotein ratio, ferritin and metabolic syndrome clustered around factor 1. HDL: High density lipoprotein. WC: Waist circumference; BMI: Body mass index; HOMA: Homeostatic metabolic assessment; HDL: High density lipoprotein; GGT: γ-glutamyl-transpeptidase; TRIG:Triglycerides; TRIGHDL: Triglycerides/high density lipoprotein; CRP: C-reactive protein; MS: Metabolic syndrome; FER: Ferritin; ALT: Alaninamino transferase; BMI: Body mass index; URICA: Uric acid; HSUS: Hepatic steatosis at ultrasound; SLD: Spleen longitudinal diameter.
Figure 4.
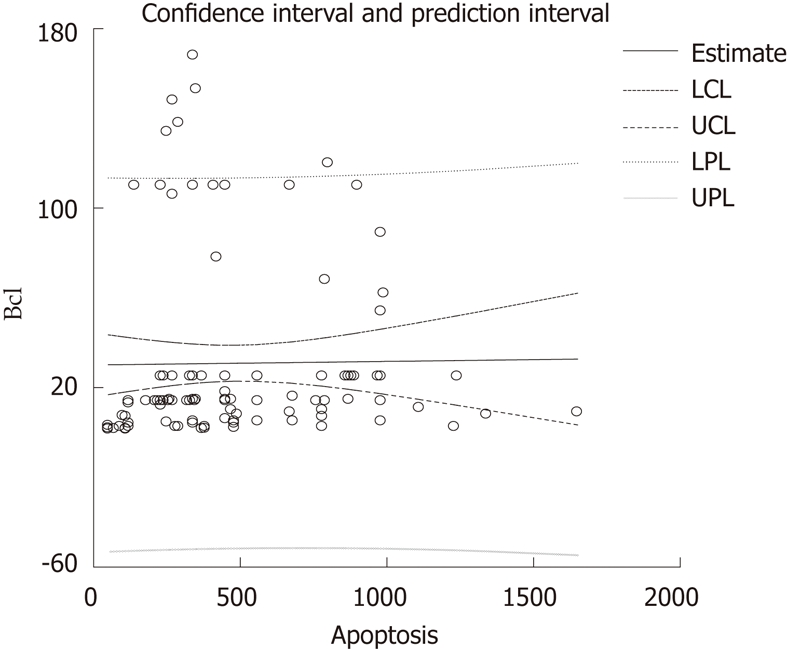
The concentration of serum Bcl-2 was not predicted by the number/mm2 of terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling-positive cells. LCL: Low confidence limit; UCL: Upper confidence limit; LPL: Low prediction limit; UPL: Upper prediction Limit.
Reliability
Grades of hepatic steatosis at US correlated with those at histology (rh0 = 0.45, P ≤ 0.0001). The AUC of serum Bcl-2 only modestly predicted MUOs, i.e., 0.65%, CI 0.53 to 0.75, with a sensitivity of 87% and a specificity of 50%, a positive likehood ratio of 1.73 using as cut-off 106 ng/mL. The negative predictive value was 94% on the basis of a disease (MS) prevalence in the population of 20%. The intra-inter-observational reproducibility of the sonographic estimations for bright liver was high, with a ρχ of 0.93 and 0.89, respectively.
DISCUSSION
Medical reports emphasize that MS, of which NAFLD is a further expression, is a major health problem in western countries. This research was conducted to establish whether serum Bcl-2 concentration could help clarify the role of the anti-apoptotic process in this common disease, not to detect a further marker to diagnose NASH. The main finding was that low HOMA values predicted high Bcl-2 levels. Furthermore, high levels of this anti-apoptotic protein were found in patients with FL, which is also characterized by fewer apoptotic cells than the more severe form, i.e. NASH. First of all, to avoid a possible bias[25], the authors stress that the population’s age did not affect anti-apoptotc protein Bcl-2 concentrations, as confirmed by regression equation and factor analysis. UA levels modestly prognosticate serum Bcl-2 concentrations according to previous research on serum soluble Fas concentrations, which correlated significantly with UA levels[26]. These findings indicate that the antioxidant properties of UA[27,28] are of biological importance in vivo. Comparing the present results with relevant findings from other studies dealing with NASH, having found lower Bcl-2 concentrations, we support a low liver expression of Bcl-2[28]. To reinforce this finding, apoptosis is recognized as common in liver injury and may also contribute to tissue inflammation, fibrogenesis, and development of cirrhosis. The intensification of inflammation in NAFLD is accompanied by an inhibition of antiapoptotic Bcl-2[9]. Accordingly, in our study anti-apoptotic Bcl-2 protein concentrations showed an inverse trend towards lobular inflammation grades.
Contrary to current theory, hepatic steatosis appears to be a detoxification process, as FFAs are directly cytotoxic for the hepatocyte. The anti-apoptotic process, favoring the life span of hepatocytes, and enhancing triglyceride formation, inhibits FFAs toxicity. Probably this is the reason for the major concentrations of Bcl-2 in FL. On the other hand, our laboratory findings do not necessarily mirror the processes happening in liver due to the lack of correlation between apoptosis as well as inflammation and Bcl-2 levels in NASH patients. The strict association between IR, as well as BMI and Bcl-2 levels, could at least partially be explained as follows. All individuals possess a maximum capacity for adipose expansion which is determined by both genetic and environmental factors. Once the limit of adipose tissue expansion is reached, this ceases to store energy efficiently, with the subsequent accumulation of lipids in other tissues. Ectopic lipid accumulation in non-adipocyte cells causes lipotoxic insults including IR, apoptosis and inflammation. The adipose tissue expandability hypothesis states that a failure in the capacity of adipose tissue expansion, rather than obesity per se, is the key factor linking positive energy balance and MS[29]. MUOs could represent subjects who are unable to sustain the expandability of adipose tissue but are burdened by an abundant extra adipose tissue localization, for instance in liver. An alternative hypothesis suggests that a hyperleptinemic status, generally present in obese patients, might be involved. Now, since leptin reduces apoptosis, possibly via its ability to increase Bcl-2 and decrease Bax, altering the Bcl-2/Bax ratio, this status could explain the elevated concentrations of Bcl-2[30]. Some results show that IGF-1 increases mRNA levels and protein expression of antiapoptotic Bcl-2[31-33] even though, in obese subjects all the main components of the GH/IGF-1 axis might be widely variable[34]. In fact, serum IGF-1 levels inversely vary with severity of hepatic steatosis[35]. Coming back to serum concentrations of Bcl-2, a shift towards an antiapoptotic process could be protective in other areas[14]. By which mechanisms is Bcl-2 supposed to act? The endoplasmic reticulum (ER) is the main site for lipid biosynthesis in the cell. Disturbances of this critical cellular function lead to ER stress. Several recent observations suggest a role for Bcl-2 at the ER. Bcl-2 located at the ER was shown to interfere with apoptosis induction. In fact, Bcl-2 at the ER may regulate calcium flow between the ER and the mitochondria. In addition, Bcl-2 is able to interact with the endoplasmic protein Bap31, thus avoiding caspase activation at the ER. Bcl-2 may also hinder the function of ER located pro-apoptotic Bcl-2[36].
Limitations to this study are having detected a single protein in the apoptosis universe, and the use of US as first screening method for O/O. In fact, although US has acceptable sensitivity and specificity, nevertheless, it has drawbacks that include its inaccuracy in exact quantification of fat accumulation, possibly excluding patients with light hepatic steatosis. Furthermore, the sample size is apparently too small (the minimal required sample size per group with a type I error of 0.05 and a Type II error of 0.05 for Bcl-2-analyzed as mean ± SD was calculated at 42 patients). Finally, in our study there is some overlap in the Bcl-2 values seen in NASH and FL patients, the minimum in both groups being 2.5 ng/mL(detection limit).
In conclusion, IR is strictly linked to serum Bcl-2 values. Those were higher in FL than in NASH patients suggesting a protective role of the anti-apoptotic process in liver and perhaps in other areas[37].
COMMENTS
Background
High serum levels of triglycerides/free fatty acids and insulin resistance are features of non-alcoholic fatty liver disease, an additional manifestation of the metabolic syndrome; the former is extremely common in patients suffering from overweight/obesity, and ranges from fatty liver to nonalcoholic steatohepatitis and liver cirrhosis. In determining nonalcoholic steatohepatitis, a role is thought to be played by free fatty acids that directly engage the core apoptotic machinery. Moreover, increased apoptosis in liver specimens from nonalcoholic steatohepatitis patients is associated with iron overload, which clearly correlates with insulin resistance and inflammation. Obviously, there is a certain balance between apoptosis and anti-apoptosis in determining the net effect on hepatocytes survival.
Research frontiers
The authors hypothesized that serum BCL-2 levels reflect the steady state of this anti-apoptotic protein.
Innovations and breakthroughs
The expression of Bcl-2 in the liver tissue of non-alcoholic fatty liver disease patients has previously been evaluated by real-time PCR or, alternatively, by immuno-histochemical staining, highlighting surprisingly different patterns. Serum Bcl-2 increase is a likely response to the apoptotic process to improve survival of hepatocytes. If the response to the metabolic injury was good (increased serum levels of Bcl-2) probably the more severe form. i.e., non-alcoholic steatohepatitis would not develop.
Applications
Metabolically unhealthy obese/overweight subjects could be further detected by Bcl-2 levels. By favoring the life span of hepatocytes, and enhancing triglyceride formation, the anti-apoptotic process probably inhibits free fatty acids toxicity in fatty liver.
Terminology
Bcl-2 (B-cell lymphoma 2) is the founding member of the Bcl-2 family of apoptosis regulator proteins encoded by the BCL2 gene.
Peer review
This is a very well carried out study and well written paper. However it needs a few clarifications from the authors.
Footnotes
Peer reviewer: Andrew Seng Boon Chua, MD, Department of Gastroenterology, Gastro Centre Ipoh, 1, lorong Rani, 31, lebuhraya Tmn Ipoh, Ipoh Garden South, IPOH 30350, Malaysia
S- Editor Tian L L- Editor O’Neill M E- Editor Xiong L
References
- 1.Tarantino G, Saldalamacchia G, Conca P, Arena A. Non-alcoholic fatty liver disease: further expression of the metabolic syndrome. J Gastroenterol Hepatol. 2007;22:293–303. doi: 10.1111/j.1440-1746.2007.04824.x. [DOI] [PubMed] [Google Scholar]
- 2.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imeryuz N, Tahan V, Sonsuz A, Eren F, Uraz S, Yuksel M, Akpulat S, Ozcelik D, Haklar G, Celikel C, et al. Iron preloading aggravates nutritional steatohepatitis in rats by increasing apoptotic cell death. J Hepatol. 2007;47:851–859. doi: 10.1016/j.jhep.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Tarantino G, Colao A, Capone D, Conca P, Tarantino M, Grimaldi E, Chianese D, Finelli C, Contaldo F, Scopacasa F, et al. Circulating levels of cytochrome C, gamma-glutamyl transferase, triglycerides and unconjugated bilirubin in overweight/obese patients with non-alcoholic fatty liver disease. J Biol Regul Homeost Agents. 2011;25:47–56. [PubMed] [Google Scholar]
- 5.Elsing C, Harenberg S, Stremmel W, Herrmann T. Serum levels of soluble Fas, nitric oxide and cytokines in acute decompensated cirrhotic patients. World J Gastroenterol. 2007;13:421–425. doi: 10.3748/wjg.v13.i3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghuraman S, Abraham P, Daniel HD, Ramakrishna BS, Sridharan G. Characterization of soluble FAS, FAS ligand and tumour necrosis factor-alpha in patients with chronic HCV infection. J Clin Virol. 2005;34:63–70. doi: 10.1016/j.jcv.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro PS, Cortez-Pinto H, Solá S, Castro RE, Ramalho RM, Baptista A, Moura MC, Camilo ME, Rodrigues CM. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708–1717. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Ausman LM, Russell RM, Greenberg AS, Wang XD. Increased apoptosis in high-fat diet-induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. J Nutr. 2008;138:1866–1871. doi: 10.1093/jn/138.10.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panasiuk A, Dzieciol J, Panasiuk B, Prokopowicz D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:6198–6202. doi: 10.3748/wjg.v12.i38.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tahan V, Canbakan B, Balci H, Dane F, Akin H, Can G, Hatemi I, Olgac V, Sonsuz A, Ozbay G, et al. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterology. 2008;55:1433–1438. [PubMed] [Google Scholar]
- 11.Anderson NS, Bermudez Y, Badgwell D, Chen R, Nicosia SV, Bast RC, Kruk PA. Urinary levels of Bcl-2 are elevated in ovarian cancer patients. Gynecol Oncol. 2009;112:60–67. doi: 10.1016/j.ygyno.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tas F, Duranyildiz D, Argon A, Oguz H, Camlica H, Yasasever V, Topuz E. Serum bcl-2 and survivin levels in melanoma. Melanoma Res. 2004;14:543–546. doi: 10.1097/00008390-200412000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Tarantino G, Conca P, Pasanisi F, Ariello M, Mastrolia M, Arena A, Tarantino M, Scopacasa F, Vecchione R. Could inflammatory markers help diagnose nonalcoholic steatohepatitis? Eur J Gastroenterol Hepatol. 2009;21:504–511. doi: 10.1097/MEG.0b013e3283229b40. [DOI] [PubMed] [Google Scholar]
- 14.Tamakoshi A, Suzuki K, Lin Y, Ito Y, Yagyu K, Kikuchi S, Watanabe Y, Inaba Y, Tajima K, Nakachi K. Relationship of sFas with metabolic risk factors and their clusters. Eur J Clin Invest. 2010;40:527–533. doi: 10.1111/j.1365-2362.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 15.Osman HG, Gabr OM, Lotfy S, Gabr S. Serum levels of bcl-2 and cellular oxidative stress in patients with viral hepatitis. Indian J Med Microbiol. 2007;25:323–329. doi: 10.4103/0255-0857.37333. [DOI] [PubMed] [Google Scholar]
- 16.Uretsky S, Messerli FH, Bangalore S, Champion A, Cooper-Dehoff RM, Zhou Q, Pepine CJ. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120:863–870. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 18.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 19.Arnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- 20.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Choi S, Kim HJ, Chung YS, Lee KW, Lee HC, Huh KB, Kim DJ. Cutoff values of surrogate measures of insulin resistance for metabolic syndrome in Korean non-diabetic adults. J Korean Med Sci. 2006;21:695–700. doi: 10.3346/jkms.2006.21.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sui X, Church TS, Meriwether RA, Lobelo F, Blair SN. Uric acid and the development of metabolic syndrome in women and men. Metabolism. 2008;57:845–852. doi: 10.1016/j.metabol.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connell J, Lynch L, Cawood TJ, Kwasnik A, Nolan N, Geoghegan J, McCormick A, O’Farrelly C, O’Shea D. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS One. 2010;5:e9997. doi: 10.1371/journal.pone.0009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phaneuf S, Leeuwenburgh C. Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol. 2002;282:R423–R430. doi: 10.1152/ajpregu.00296.2001. [DOI] [PubMed] [Google Scholar]
- 26.Choi JW. Associations of Fas (CD95), tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), and biochemical manifestations in elderly persons. Clin Chim Acta. 2006;365:113–118. doi: 10.1016/j.cca.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin Sci (Lond) 2003;105:425–430. doi: 10.1042/CS20030149. [DOI] [PubMed] [Google Scholar]
- 28.Torer N, Ozenirler S, Yucel A, Bukan N, Erdem O. Importance of cytokines, oxidative stress and expression of BCL-2 in the pathogenesis of non-alcoholic steatohepatitis. Scand J Gastroenterol. 2007;42:1095–1101. doi: 10.1080/00365520701286680. [DOI] [PubMed] [Google Scholar]
- 29.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Brown JE, Dunmore SJ. Leptin decreases apoptosis and alters BCL-2 : Bax ratio in clonal rodent pancreatic beta-cells. Diabetes Metab Res Rev. 2007;23:497–502. doi: 10.1002/dmrr.726. [DOI] [PubMed] [Google Scholar]
- 31.Hilmi C, Larribere L, Giuliano S, Bille K, Ortonne JP, Ballotti R, Bertolotto C. IGF1 promotes resistance to apoptosis in melanoma cells through an increased expression of BCL2, BCL-X(L), and survivin. J Invest Dermatol. 2008;128:1499–1505. doi: 10.1038/sj.jid.5701185. [DOI] [PubMed] [Google Scholar]
- 32.Tseng YH, Ueki K, Kriauciunas KM, Kahn CR. Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J Biol Chem. 2002;277:31601–31611. doi: 10.1074/jbc.M202932200. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Wu H, Khardori R, Song YH, Lu YW, Geng YJ. Insulin-like growth factor-1 receptor activation prevents high glucose-induced mitochondrial dysfunction, cytochrome-c release and apoptosis. Biochem Biophys Res Commun. 2009;384:259–264. doi: 10.1016/j.bbrc.2009.04.113. [DOI] [PubMed] [Google Scholar]
- 34.Frystyk J, Brick DJ, Gerweck AV, Utz AL, Miller KK: Bioactive insulin-like growth factor-I in obesity. J Clin Endocrinol Metab. 2009;94:3093–3097. doi: 10.1210/jc.2009-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savastano S, Di Somma C, Pizza G, De Rosa A, Nedi V, Rossi A, Orio F, Lombardi G, Colao A, Tarantino G. Liver-spleen axis and Insulin-like Growth Factor-(IGF)-1 levels in overweight/obese females. J Transl Med. 2011;9:136. doi: 10.1186/1479-5876-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szegezdi E, Macdonald DC, Ní Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296:C941–C953. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- 37.Tarantino G. Should nonalcoholic fatty liver disease be regarded as a hepatic illness only? World J Gastroenterol. 2007;13:4669–4672. doi: 10.3748/wjg.v13.i35.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]


