Abstract
Objective
To explore the prevalence of pregnancy related nausea (PN) and vomiting (PV), and hyperemesis gravidarum (HG), in women with bulimia nervosa (BN) and EDNOS purging subtype (EDNOS-P).
Method
38,038 pregnant women enrolled in the Norwegian Mother and Child Cohort Study had questionnaire-based information on eating disorder diagnosis and PN, PV, and HG. We estimated the odds for PN, PV, and HG using logistic regression.
Results
Women with BN, purging subtype, but not women with BN, nonpurging subtype, had statistically significant higher odds of PN and PV compared to women without eating disorders. The EDNOS-P group showed significantly higher odds of PV. The odds of HG did not differ significantly between those with and without an eating disorder, or across eating disorder subtypes.
Conclusion
Our results suggest that eating disorders marked by the symptom of purging are associated with increased odds of PN and PV.
Keywords: Pregnancy complications, Hyperemesis Gravidarum, nausea and vomiting of pregnancy, bulimia nervosa
Nausea and vomiting of pregnancy encompasses mild symptoms of nausea and vomiting during the first trimester of pregnancy (1). It is unknown the extent to which individuals with eating disorders experience nausea and vomiting of pregnancy. Indeed, the majority of individuals with bulimia nervosa (BN) use self-induce vomiting as a compensatory method (2). Another eating disorder subtype characterized by self-induced vomiting is a form of eating disorder not otherwise specified (EDNOS) characterized by purging in the absence of binge eating (EDNOS-P) (3). Women with BN report being readily able to distinguish between pregnancy-related vomiting episodes and the self-induced vomiting associated with their eating disorder (4). What remains unknown is whether the frequent vomiting associated with the eating disorder renders it more likely for a woman to experience nausea and vomiting of pregnancy either due to underlying neurobiological, behavioral, or psychological factors. This question is of clinical importance as women with eating disorders have been reported to have elevated maternal and fetal complications (5) and pregnancy has been highlighted as a time for increased risk for both remission from and re-emergence of eating disorders symptoms (6–8).
Relatively rarely, 1–5/1000 pregnancies, the symptoms of nausea and vomiting of pregnancy are prolonged and can escalate into hyperemesis gravidarum (HG) (9;10). Characterized by symptoms of dehydration, electrolyte imbalance, and weight loss of greater than 5% of body weight, HG may require hospitalization and/or extensive outpatient management (11). Women with a prior history of BN have been reported to have a higher than expected prevalence of HG (5;12). Previous studies on pregnancy in eating disorders, however, have mainly focused on clinical samples and employed retrospective methods (6;12). The goal of the present paper was to extend the previously published preliminary observations by exploring the incidence of nausea and vomiting of pregnancy and HG in women with and without BN and EDNOS-P in a large sample of 38,038 women.
Methods
Participants
The data collection was conducted as part of the Norwegian Mother and Child Cohort Study (MoBa) at the Norwegian Institute of Public Health (13). The study has been approved by the appropriate regional committees for ethics in medical research and the Norwegian National Data Inspectorate. The study is described in more detail elsewhere (13;14). In brief, MoBa is a longitudinal prospective pregnancy cohort study. Participants are recruited at 17 week’s gestation. To date, 42% of invited mothers have agreed to participate in MoBa. The present study is based on Questionnaire 1 (gestational week 17) and Questionnaire 3 (gestational week 30). The MoBa cohort is linked to the Medical Birth Registry of Norway (MBRN) (15) to capture pregnancy outcome variables.
The analysis population for this report included MoBa participants who: a) had information from the MoBa Questionnaires and the MBRN on all variables included in this study, and b) had a singleton birth. If a woman was enrolled in MoBa more than once, due to additional pregnancies, only the first pregnancy was included. Of the initial 54,714 pregnancies enrolled in MoBa, the resulting net sample was 38,038 for the analyses based on Questionnaire 1 and 35,580 for the analyses based on Questionnaire 3.
Measures
Questionnaire 1 included items on eating disorders and disordered eating behaviors designed in accordance with the DSM-IV criteria (16), and previously used for studies of eating disorders in the Norwegian Institute of Public Health Twin Panel (17–20). Diagnostic algorithms were constructed from the questionnaire items to define the eating disorder categories used. Broadly defined bulimia nervosa (BN), with at least weekly frequency of binge eating and compensatory behaviors, were divided into three subtypes: BN purging type (BN-purge), BN nonpurging type (BN-nonpurge), and BN-other, which includes individuals who responded to questions that defined BN-nonpurge (i.e. fasting and exercise), but had missing values for the questions that defined BN-purge (i.e. laxatives and vomiting). Broadly defined EDNOS-P required purging at least weekly in the absence of binge eating. Criteria for binge eating included both eating an unusually large amount of food and feeling out of control. BN and EDNOS-P were assessed for the 6 month period prior to pregnancy (retrospective assessment). According to the DSM-IV criteria, EDNOS-P and BN are mutually exclusive diagnoses as are BN-purge and BN-non-purge.
In Questionnaire 3 (gestational Week 30), the respondents were asked to complete a checklist reflecting pregnancy-related symptoms, covering eight different epochs during gestation between weeks 1 and weeks 30. In this study we included pregnancy related nausea (PN) and pregnancy related vomiting (PV) (i.e. not self-induced). Participants were also asked whether they had been hospitalized during pregnancy because of pregnancy-related vomiting. This item was used to identify HG, thereby including only cases that were severe enough to require hospital admission. Thus, our estimates should be considered conservative, but comparable to other studies on HG (10).
Demographic data were obtained both from the MoBa questionnaires and the MBRN, including maternal age, marital status, education, and income. Marital status, education and income were not associated with PN, PV, and HG, and were therefore not included as covariates in the regression model.
Data Analysis
SAS® software for Windows and for Solaris (v9.1.3) was used for all the analyses (21). SAS/STAT® PROC GENMOD was used to estimate the logistic regression models. These models produced estimated odds of PN, PV, and the odds of HG by eating disorder status before pregnancy. Age and epoch of pregnancy were included in each model as covariates. Model estimation included generalized estimating equations GEE (22) with an autoregressive working correlation matrix that accounted for correlation between a mother’s responses over time. Statistical tests of eating disorder effects for PN, PV, and HG were conducted to determine associations between eating disorder subtypes and the referent eating disorder group at the baseline time of 0–4 weeks of pregnancy. The absence of HG events at certain epochs precluded any time-specific test for this outcome, thus odds of HG across eating disorder subtypes were tested without a time interaction effect.
Additionally, time effects of PN and PV were tested for each eating disorder subtype. Instead of testing each time effect separately, a type 3 analysis score statistic was used to simultaneously test the equivalence of all time effects to zero. Specifically, this analysis involved the computation of a score statistic for the time and eating disorder interaction term in each of the two models examining PN, or PV during pregnancy. This test was considered preferable due to the unbalanced counts across eating disorder subtypes and to conserve type I error.
Results
Sample demographics
Forty-seven percent of the 38,038 women represented here were younger than 30 years, 97% were married or cohabiting and 52% had one or more previous live births. In addition, the sample was relatively highly educated with 58% attending some form of college. A more detailed presentation of the sample is given elsewhere (13;14).
Prevalence of eating disorders
Two hundred and ninety women (0.8%) reported broadly defined BN during the six month period prior to pregnancy. Of these women, 118 women (0.3%) reported BN purging subtype, 109 women (0.3%) reported BN non-purging subtype, and 63 women (0.2%) reported BN but could not reliably be categorized as purging or non-purging due to missing data on these questions. EDNOS-P was reported by 42 (0.1%) of the women. Ninety-two percent of BN-purge group and 60 percent of EDNOS-P group used vomiting and/or laxatives as their purging behavior.
Pregnancy related nausea and eating disorders
Seventy percent of all women reported PN. Table 1 presents the proportion of women who reported PN by eating disorder subgroup. The highest rates of PN occurred in weeks 5 to 12. Approximately half of all women reported PN during this period. Of the women with BN-other, 31.7% reported PN in the first month in contrast to only 20.8% among the women with no eating disorder. The largest point differences between all eating disorder subtypes and the referent occurred in the first month of gestation with positive point differences of 11, 8, 8 and 5 for BN-other, BN purge, EDNOS-P and BN non-purge.
Table 1.
Percent with pregnancy-related nausea (PN) by gestational week and eating disorder
| Gestational Weeks | BN purge | BN non-purge | BN other | EDNOS-P | No ED |
|---|---|---|---|---|---|
| Entire pregnancy | 76.3 | 68.8 | 71.4 | 73.8 | 69.7 |
|
| |||||
| 1–4 | 28.8 | 25.7 | 31.7 | 28.6 | 20.8 |
| 5–8 | 60.2 | 49.5 | 49.2 | 52.4 | 52.6 |
| 9–12 | 50.0 | 44.0 | 47.6 | 52.4 | 47.5 |
| 13–16 | 27.1 | 25.7 | 27.0 | 19.0 | 25.4 |
| 17–20 | 16.1 | 11.0 | 12.7 | 11.9 | 11.5 |
| 21–24 | 5.9 | 8.3 | 9.5 | 7.1 | 6.3 |
| 25–28 | 9.3 | 8.3 | 11.1 | 7.1 | 5.8 |
| 29+ | 9.3 | 5.5 | 7.9 | 4.8 | 5.2 |
|
| |||||
| n* | 118/104 | 109/100 | 63/54 | 42/38 | 37706/35284 |
Note:
Questionnaire 1 at gestational week 18/Questionnaire 3 at gestational week 30
Table 2 presents the odds ratios (ORs) for PN for the eating disorder groups compared to the referent group. In the first gestational month, the OR for PN in the BN- purge and BN-other groups were 1.55 (p=0.04) and 1.76 (p=0.04), respectively. Further, a type 3 score statistic simultaneously testing time effects at the seven epochs following the first month of pregnancy for each of the ED subtypes versus the referent group indicates no significant variation in differences over time from the baseline value (χ2(28)=31, p>0.1).
Table 2.
Odds Ratios of pregnancy-related nausea (PN) for ED groups’ vs. non-ED group by time1. 95% CI in paragraphs.
| ED groups | Time (gestational weeks)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1–4 | 5–8 | 9–12 | 13–16 | 17–20 | 21–24 | 25–28 | 29+ | |
| BN purge | 1.55 (1.04–2.30) | 1.37 (0.95–1.98) | 1.11 (0.78–1.60) | 1.10 (0.73–1.65) | 1.48 (0.91–2.42) | 0.95 (0.44–2.04) | 1.68 (0.90–3.13) | 1.89 (1.01–3.52) |
| BN non-purge | 1.32 (0.86–2.03) | 0.89 (0.61–1.29) | 0.87 (0.60–1.28) | 1.02 (0.66–1.57) | 0.95 (0.52–1.74) | 1.35 (0.68–2.68) | 1.47 (0.74–2.91) | 1.07 (0.47–2.43) |
| BN other | 1.76 (1.03–2.99) | 0.87 (0.53–1.43) | 1.00 (0.61–1.65) | 1.09 (0.62–1.89) | 1.11 (0.53–2.34) | 1.57 (0.68–3.65) | 2.03 (0.92–4.45) | 1.57 (0.63–3.92) |
| EDNOS-P | 1.53 (0.78–2.98) | 1.00 (0.54–1.83) | 1.22 (0.67–2.24) | 0.70 (0.32–1.50) | 1.04 (0.41–2.65) | 1.16 (0.36–3.75) | 1.26 (0.39–4.07) | 0.92 (0.22–3.80) |
Adjusted for mother’s age.
Pregnancy related vomiting and eating disorders
As can be seen in Table 3, thirty-five percent of all women reported PV. The largest point differences between eating disorder subtype groups and the referent occurred in the first month of gestation with positive point differences of 11, 9 and 8 for EDNOS-P, BN- purge and BN-other. The BN non-purge group was the exception with a maximum point difference from the referent at a different time, namely, weeks 13 to 16.
Table 3.
Percent with pregnancy-related nausea with vomiting (PV) by gestational week and eating disorder
| Gestational Weeks | BN purge | BN non-purge | BN other | EDNOS-P | No ED |
|---|---|---|---|---|---|
| Entire pregnancy | 50.0 | 42.2 | 38.1 | 45.2 | 34.7 |
|
| |||||
| 1–4 | 16.9 | 11.0 | 15.9 | 19.0 | 7.8 |
| 5–8 | 29.7 | 27.5 | 27.0 | 31.0 | 24.2 |
| 9–12 | 31.4 | 27.5 | 25.4 | 26.2 | 25.8 |
| 13–16 | 16.3 | 13.0 | 11.1 | 10.5 | 9.4 |
| 17–20 | 11.5 | 7.0 | 9.3 | 7.9 | 5.5 |
| 21–24 | 7.7 | 4.0 | 9.3 | 5.3 | 2.8 |
| 25–28 | 4.8 | 3.0 | 5.6 | 5.3 | 1.8 |
| 29+ | 4.8 | 2.0 | 5.6 | 5.3 | 1.4 |
|
| |||||
| n* | 118/104 | 109/100 | 63/54 | 42/38 | 37706/35284 |
Note
Questionnaire 1 at gestational week 18/Questionnaire 3 at gestational week 30
Table 4 presents the ORs for PV during pregnancy for the eating disorder groups versus the referent. The BN-purge and BN-other groups had an OR of 2.3 for PV in the first month of pregnancy (p=0.001 and p=0.02) while the OR for PV in the EDNOS-P group was 2.6 (p=0.01). A type 3 score statistic simultaneously testing all seven time effects across the four ED subgroups versus the referent was χ2(28)=38, p>0.09. The BN non-purge group is the exception to a trend of increasing odds of PV versus the referent over time. There is therefore weak evidence to support the hypothesis that women with BN or EDNOS-P vary in their difference of reported PV over the duration of pregnancy compared to the referent group.
Table 4.
Odds Ratios of pregnancy-related nausea with vomiting (PV) for ED groups’ vs. non-ED group by time1. 95% CI in paragraphs.
| ED group | Time (gestational weeks)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1–4 | 5–8 | 9–12 | 13–16 | 17–20 | 21–24 | 25–28 | 29+ | |
| BN purge | 2.28 (1.41–3.69) | 1.25 (0.84–1.87) | 1.24 (0.83–1.85) | 1.97 (1.19–3.24) | 2.28 (1.28–4.08) | 2.89 (1.43–5.87) | 2.67 (1.11–6.42) | 3.39 (1.39–8.27) |
| BN non-purge | 1.41 (0.77–2.57) | 1.16 (0.76–1.77) | 1.06 (0.69–1.63) | 1.44 (0.81–2.53) | 1.29 (0.61–2.73) | 1.43 (0.54–3.80) | 1.62 (0.52–4.99) | 1.37 (0.35–5.41) |
| BN other | 2.27 (1.15–4.51) | 1.18 (0.67–2.08) | 0.99 (0.56–1.75) | 1.10 (0.43–2.81) | 1.69 (0.64–4.51) | 3.53 (1.35–9.23) | 3.17 (0.94–10.64) | 4.14 (1.25–13.67) |
| EDNOS-P | 2.62 (1.22–5.66) | 1.33 (0.68–2.59) | 0.95 (0.47–1.90) | 0.93 (0.29–3.05) | 1.28 (0.35–4.69) | 1.73 (0.38–7.98) | 2.74 (0.63–11.86) | 3.59 (0.84–15.28) |
Adjusted for mother’s age.
Hyperemesis Gravidarum
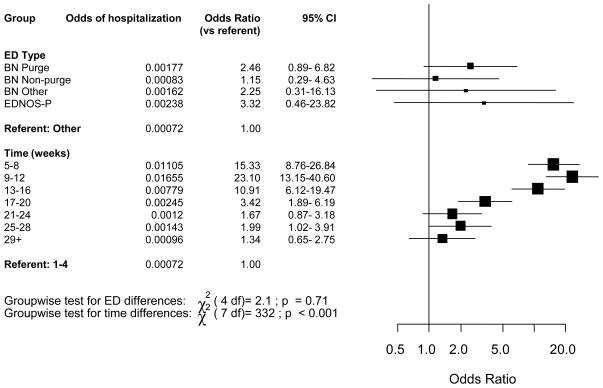
A total of 1.3 % (469 of 35580) of all women reported that they had been hospitalized for vomiting during pregnancy and thus fulfilled our criterion for HG. Women with BN-purge and EDNOS-P had the highest prevalence of HG. The proportion of women with HG by eating disorder subtype were 3.8 % for BN- purge (4/104), 2.6 % for EDNOS-P (1/38), 2.0 % for BN non-purge (2/100), 1.9 % for BN- other (1/54), and 1.3 % for women without eating disorders (461/35,284). Although these numbers were numerically different, due to considerable variability, they were not significantly different. The small number of women with HG in certain eating disorder subgroups did not allow for an estimation of ORs at every time point.
The OR for hospitalization for HG do not differ significantly by eating disorder subtype while controlling for epoch of gestation (χ2 (4) =2.0, p>0.1) (figure 1). However, the same analysis shows that ORs for HG vary significantly over gestation and peak at 9 to 12 weeks (p<0.001) with OR for hospitalization 29 times higher at weeks 9 to 12 than the first month of pregnancy, which is the referent time period. The ORs for HG by gestational week remain statistically significantly elevated until 20 weeks of pregnancy with odds 4 times higher than at gestational weeks 1–4. However, these risk estimates contain wide confidence intervals suggesting imprecision.
Figure 1.
Odds ratios of hospitalization for vomiting by ED status and time1.
1 Adjusted for mother’s age.
Discussion
To our knowledge, this is the first investigation documenting the prevalence of pregnancy related nausea and vomiting among women with BN and EDNOS-P in a large cohort of pregnant women. We found that in gestational week one to four the BN- purge and BN-other groups showed over twice the odds of PN and PV compared to the women without any eating disorder. The EDNOS-P group showed significantly higher odds of PV in the same period. Women with BN and EDNOS-P did not have an increased odds for HG, compared to women without any eating disorder.
Seventy percent of all women reported PN and 35% of all women reported PV. Similar prevalence have been reported in other studies (74% and 38%) (23). Our results suggest that women with an eating disorder that includes the behavior of self-induced purging have higher odds for PN and PV compared to women without eating disorders. We did not find this association in women with the non-purging type of BN. The difference is most salient in early pregnancy before the typical onset of PN and PV in women without eating disorders.
There are several possible explanations for the higher odds of PN and PV among women with eating disorders involving purging behavior. First, unknown shared neurobiological mechanisms could underlie both PV and self-induced vomiting in BN. Self-induced vomiting is heritable (24), and has proven to be an informative covariate in linkage studies of BN (25) which could suggest a biological origin. Despite some research, the etiology of PN and PV in pregnancy remains unclear (26). Some of the explanations include hormonal imbalances, vitamin B deficiencies, metabolic disturbances, and undefined pregnancy induced factors. Second, individuals with a history of prolonged self-induced vomiting may have lower thresholds for vomiting in response to pregnancy-related nausea. Although a qualitative study of pregnant women with BN reported that the women could easily distinguish PV from self-induced vomiting (4), it is possible that the likelihood of vomiting in response to nausea increases with repeated exposure to self-induced vomiting. The vomiting reflex may become more automatic over time.
We found no statistically significant elevated odds of HG among women with BN or EDNOS-P, compared to women without eating disorders. The few studies examining the effect of eating disorders on HG have reported conflicting findings (5;6;12). Retrospective study design, modest group size, and lack of a control group have limited the generalizability of the findings in the studies published to date. Only one study not based on a clinical sample examined whether women with eating disorders are at increased risk for HG. Kouba and colleagues (2005) conducted a study on pregnancy and neonatal outcomes, based on women recruited from prenatal clinics in early pregnancy. Women previously diagnosed with eating disorder (49 women with anorexia nervosa or BN) were at increased risk for HG during pregnancy, compared with 68 controls. HG was evaluated based on the information reported in the medical records. The prevalence of HG (67 % among women with ED and 13 % among controls) indicates however that Kouba used a less conservative definition of HG. Notably, our conservative definition based on hospitalization yielded a prevalence estimate (1.2%) that is in line with previously published reports of HG using the same definition (0.3% – 1.1%) (10;27). Interpretations of studies on HG should consider definitional issues carefully.
The findings of the current study should be interpreted with the following limitations in mind. First, the answers were based on self-report and targeted broadly defined disorders. However, given the magnitude of the sample, diagnostic interviews were not practical. Second, although our frequency criteria for purging differed from current DSM criteria, it is important to note that the established criteria have not been empirically supported (28). Third, 42% of invited women agreed to participate in MoBa. This response rate is typical for large epidemiologic studies, and it does not necessarily imply a biased sample (29). Even if the prevalence estimates of both eating disorders and nausea and vomiting during pregnancy differ from the general population, this would not invalidate the observations of the relations between the syndromes. Finally, not all women with EDNOS-P and BN-purge subtype used self-induced vomiting as a compensatory behavior. It was however not possible to identify all women who used only vomiting, due to missing values for some question. We know that women with purging subtypes are at risk for pregnancy related vomiting, but we do not know if it is specifically related to symptom of vomiting, or other characteristics associated with the purging subtypes of BN and EDNOS.
Future studies will explore the impact of PN, PV, and HG in the context of eating disorders on pregnancy outcome and fetal development. Clinicians should remain mindful of the impact of eating disorders on PN and PV and consider screening for eating disorders during prenatal visits—especially when PN and PV are reported.
Acknowledgments
This research was supported by the National Institutes of Health Grants (HD047186) to CMB and the MoBa study is supported by the Norwegian Ministry of Health, NIH/NIEHS (grant no. N01 - ES – 85433), NIH/NINDS (grant no. 1 UO1 NS 047537-01) and Norwegian Research Council/FUGE (grant no. 151918/S10). The donations of questionnaire data and biological material from MoBa participants is gratefully acknowledged.
Reference List
- 1.Deuchar N. Nausea and vomiting in pregnancy: a review of the problem with particular regard to psychological and social aspects. British Journal of Obstetrics & Gynaecology. 1995;102(1):6–8. doi: 10.1111/j.1471-0528.1995.tb09017.x. [DOI] [PubMed] [Google Scholar]
- 2.Reba L, Thornton L, Tozzi F, Klump KL, Brandt H, Crawford S, et al. Relationships between features associated with vomiting in purging-type eating disorders. International Journal of Eating Disorders. 2005;38(4):287–94. doi: 10.1002/eat.20189. [DOI] [PubMed] [Google Scholar]
- 3.Wade TD. A retrospective comparison of purging type disorders: eating disorder not otherwise specified and bulimia nervosa. International Journal of Eating Disorders. 2007;40(1):1–6. doi: 10.1002/eat.20314. [DOI] [PubMed] [Google Scholar]
- 4.Lacey JH, Smith G. Bulimia nervosa: The impact of pregnancy on mother and baby. British Journal of Psychiatry. 1987 Jun;150:777–781. doi: 10.1192/bjp.150.6.777. [DOI] [PubMed] [Google Scholar]; Royal College of Psychiatrists. :777–81. [Google Scholar]
- 5.Kouba S, Hallstrom T, Lindholm C, Hirschberg AL. Pregnancy and neonatal outcomes in women with eating disorders. Obstetrics & Gynecology. 2005;105(2):255–60. doi: 10.1097/01.AOG.0000148265.90984.c3. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell JE, Seim HC, Glotter D, Soll EA, Pyle RL. A retrospective study of pregnancy in bulimia nervosa. International Journal of Eating Disorders. 1991;10(2):209–14. [Google Scholar]
- 7.Morgan JF, Lacey JH, Sedgwick PM. Impact of pregnancy on bulimia nervosa. British Journal of Psychiatry. 1999;174:135–40. doi: 10.1192/bjp.174.2.135. [DOI] [PubMed] [Google Scholar]
- 8.Conrad R, Schablewski J, Schilling G, Liedtke R. Worsening of symptoms of bulimia nervosa during pregnancy. Psychosomatics. 2003;44(1):76–8. doi: 10.1176/appi.psy.44.1.76. [DOI] [PubMed] [Google Scholar]
- 9.Charlin V, Borghesi L, Hasbun J, Von Mulenbrock R, Moreno MI. Parenteral nutrition in hyperemesis gravidarum. Nutrition. 1992;9(1):29–32. [PubMed] [Google Scholar]
- 10.Bailit JL. Hyperemesis gravidarium: Epidemiologic findings from a large cohort. American Journal of Obstetrics & Gynecology. 2005;193(3:Pt:1):t-4. doi: 10.1016/j.ajog.2005.02.132. [DOI] [PubMed] [Google Scholar]
- 11.Abell TL, Riely CA. Hyperemesis gravidarum. Gastroenterology Clinics of North America. 1992;21(4):835–49. [PubMed] [Google Scholar]
- 12.Abraham S. Sexuality and reproduction in bulimia nervosa patients over 10 years. Journal of Psychosomatic Research. 1998;44(3–4):491–502. doi: 10.1016/s0022-3999(97)00272-9. [DOI] [PubMed] [Google Scholar]
- 13.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C, et al. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) International Journal of Epidemiology. 2006;35(5):1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 14.Bulik CM, Von Holle A, Hamer R, Berg CK, Torgersen L, Magnus P, et al. Patterns of remission, continuation, and incidence of eating disorders during early pregnancy in the Norwegian Mother and Child Cohort Study (MoBa) Psychological Medicine. 2007 doi: 10.1017/S0033291707000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstetricia et Gynecologica Scandinavica. 2000;79(6):435–9. [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington D.C: American Psychiatric Association Press; 1994. [Google Scholar]
- 17.Harris JR, Magnus P, Tambs K. The Norwegian Institute of Public Health Twin Panel: a description of the sample and program of research. Twin Research. 2002;5(5):415–23. doi: 10.1375/136905202320906192. [DOI] [PubMed] [Google Scholar]
- 18.Reichborn-Kjennerud T, Bulik CM, Kendler KS, Roysamb E, Maes H, Tambs K, et al. Gender differences in binge-eating: a population-based twin study. Acta Psychiatrica Scandinavica. 2003;108(3):196–202. doi: 10.1034/j.1600-0447.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 19.Reichborn-Kjennerud T, Bulik CM, Tambs K, Harris JR. Genetic and environmental influences on binge eating in the absence of compensatory behaviors: a population-based twin study. International Journal of Eating Disorders. 2004;36(3):307–14. doi: 10.1002/eat.20047. [DOI] [PubMed] [Google Scholar]
- 20.Reichborn-Kjennerud T, Bulik CM, Sullivan PF, Tambs K, Harris JR. Psychiatric and medical symptoms in binge eating in the absence of compensatory behaviors. Obesity Research. 2004;12(9):1445–54. doi: 10.1038/oby.2004.181. [DOI] [PubMed] [Google Scholar]
- 21.Cary N. SAS/STAT® Software: Version 9. SAS Institute, Inc; 2004. [Google Scholar]
- 22.Liang KY, Zeger SL. Longitudinal Data-Analysis Using Generalized Linear-Models. Biometrica. 1986;73(1):13–22. [Google Scholar]
- 23.Lacroix R, Eason E, Melzack R. Nausea and vomiting during pregnancy: A prospective study of its frequency, intensity, and patterns of change. American Journal of Obstetrics & Gynecology. 2000;182(4):931–7. doi: 10.1016/s0002-9378(00)70349-8. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan PF, Bulik CM, Kendler KS. Genetic epidemiology of binging and vomiting. British Journal of Psychiatry. 1998;173:75–9. doi: 10.1192/bjp.173.1.75. [DOI] [PubMed] [Google Scholar]
- 25.Bulik CM, Devlin B, Bacanu SA, Thornton L, Klump KL, Fichter MM, et al. Significant linkage on chromosome 10p in families with bulimia nervosa. American Journal of Human Genetics. 2003;72(1):200–7. doi: 10.1086/345801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin TM. Nausea and vomiting of pregnancy: an obstetric syndrome. American Journal of Obstetrics & Gynecology. 2002;186(5:Suppl:Understanding):Suppl-9. doi: 10.1067/mob.2002.122592. [DOI] [PubMed] [Google Scholar]
- 27.Bennett TA, Kotelchuck M, Cox CE, Tucker MJ, Nadeau DA. Pregnancy-associated hospitalizations in the United States in 1991 and 1992: a comprehensive view of maternal morbidity. American Journal of Obstetrics & Gynecology. 1998;178(2):346–54. doi: 10.1016/s0002-9378(98)80024-0. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan PF, Bulik CM, Kendler KS. The epidemiology and classification of bulimia nervosa. Psychological Medicine. 1998;28(3):599–610. doi: 10.1017/s0033291798006576. [DOI] [PubMed] [Google Scholar]
- 29.Hartge P. Participation in population studies. Epidemiology. 2006;17(3):252–4. doi: 10.1097/01.ede.0000209441.24307.92. [DOI] [PubMed] [Google Scholar]