Abstract
B-1 cells, which constitute a predominant lymphocyte subset in serosal cavities and produce most of natural antibodies, are subdivided into the CD5+ B-1a and CD5- B-1b cell subpopulations, but the differential roles of B-1a and B-1b cells are not well understood. We report that B-1a cells preferentially migrate out of the peritoneal cavity and upregulate the expression of CXCR4 with heightened sensitivity to CXCL12 and CXCL13 upon LPS treatment compared to B-1b and B-2 cells. Whereas B-1a cells were slightly more abundant than B-1b and B-2 cells in the homeostatic condition, the number of B-1a cells preferentially decreased 48 hr after LPS treatment. The decrease in the peritoneal B-1a cell number was accompanied with increased migration of B-1a cells toward CXCL-12 and CXCL-13 in in vitro transmigration assay using peritoneal B cells from LPS treated mice. The expression level of CXCR4, but not of CXCR5, was also more prominently increased in B-1a cells upon LPS stimulation. LPS-stimulated B-1a cells did not accumulate in omental milky spots in contrast to B-2 cells. These results suggest that B-1a cells actively migrate out of the peritoneal cavity through the regulation of the migratory responsiveness to chemokines and actively participate in systemic immune responses.
Keywords: B Lymphocyte Subsets; Chemokine CXCL12; Chemokine CXCL13; Chemotaxis; Receptor, CXCR4; Lipopolysaccharides
INTRODUCTION
B-1 cells are innate B cells that are distinguished from follicular B-2 cells by their cell surface phenotype, anatomical localization, self-renewal, and functional properties (1, 2). Although B-1 cells are a minor population of B cells, they produce more than 80% of circulating natural IgM that play a critical role in host defense against common infections. Characteristically, B-1 cells are found in the peritoneal and other serosal cavities and express a high level of IgM, a low level of IgD, and CD11b on their cell surface. B-1 cells are divided into CD5-bearing B-1a cells and CD5-negative B-1b cells, which develop from distinct progenitor cells. While follicular B-2 cells and B-1b cells develop from adult hematopoietic progenitors in bone marrow, B-1a cells develop early in the neonate from fetal hematopoietic progenitors in fetal liver (3). Functionally, B-1a cells are thought to be responsible for spontaneously generated natural antibodies and B-1b cells were reported to constitute a T cell-independent humoral memory (4), but differential functions of B-1a and B-1b cells are not clearly understood.
Besides the natural antibody production, B-1a cells are also reported to be involved in systemic immune responses such as delayed type hypersensitivity or alloimmune response (5, 6). B-1a cells were constantly found in blood compartment and required the spleen for their maintenance (7). These findings suggest that B-1a cells are constantly circulating in the body at a low speed although most of B-1a cells reside in the serosal cavity and that B-1a cells are dynamically mobilized upon systemic inflammatory events. However, it is not known well how B-1a cells are localized in the peritoneal cavity and migrate from the peritoneal cavity and enter the peripheral sites and whether B-1b cells behave similarly or not.
Chemokines are a family of small cytokines or proteins secreted by various types of cells and defined by their ability to trigger the tissue-specific targeting of leukocytes (8). Chemokine and chemokine receptors are participating in the recruitment of cells into the lymphoid organs and in the egress of cells from the lymphoid tissues (9). Homing of B cells to lymphoid follicles principally depends on expression of CXCR5 by B cells and production of CXCL13 (B lymphocyte chemoattractant), the ligand for CXCR5, by radiation-resistant follicular stromal cells (10). CXCR5 is upregulated during B cell development and is expressed by all mature B cells including B-1 cells (11). For B-1 cell localization, CXCR5 and CXCL13 does not explain why B-1 cells are not localized in the lymphoid follicle, but retained in the peritoneal cavity. Another important chemokine for B cell function is CXCL12 (stromal cell-derived factor-1), which binds to CXCR4. CXCL12 has functions other than the chemotaxis, in that it supports survival of precursor B cells and B cell development (12, 13). The studies on CXCR4 signaling implicates that CXCR4 has differential signaling pathways leading to cell survival or chemotaxis, suggesting that different effects of CXCL12 depend on developmental or activation status (14). Since CXCL12 is produced by peritoneal mesothelial cells, it is likely that the CXCL12-CXCR4 pathway is responsible for the survival and localization of B-1 cells (15, 16).
In the current study, we investigated whether the innate immune stimulation of B-1 cells with lipopolysaccharide (LPS) change their migratory behaviors and found that B-1 cells enhanced the capability to migrate in response to CXCL12 and CXCL13 upon LPS stimulation. Unexpectedly, we also observed that B-1a cells were characteristically different from B-1b and B-2 cells in their migratory behavioral changes upon LPS stimulation.
MATERIALS AND METHODS
Animals and agents
C57BL/6 mice were purchased from Orient Bio (Sungnam, Korea), maintained, and used according to institutional guidelines in a pathogen-free facility. This study was reviewed and approved by the institutional animal care and use committee (IACUC) of Sungkyunkwan University School of Medicine (Permission No. 10-31), which is an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) accredited facility and abide by the Institute of Laboratory Animal Resources (ILAR) guide.
Mouse recombinant CXCL12, CXCL13, and anti-mouse CXCL12 antibody were purchased from the R&D systems (Minneapolis, MN, USA).
LPS treatment and collection of peritoneal and omental cells
Eight to ten week old C57BL/6 mice were injected intraperitoneally with 100 µg of LPS (Sigma Aldrich, St. Louis, MO, USA). After 48 hr, peritoneal cells were isolated by flushing the peritoneal cavity with 8 mL of phosphate buffered saline (PBS). Omental cells were isolated after mincing through nylon mesh. Cells were washed twice in PBS with 5% bovine calf serum (BCS), 0.05% sodium azide for flow cytometric analysis. The exact numbers of cells were determined by calculating the total number based on the number of cells per ml corrected for the volume injected and volume recovered and analyzing of flow cytomertic data.
For in vitro studies, peritoneal cells of C57BL/6 mice were suspended in serum-free RPMI 1640 culture medium, supplemented with 25 mM sodium bicarbonate, 2 mM glutamine, 50 U/mL of penicillin, 50 µg/mL of streptomycin, and 10 mM HEPES (all reagents are from Invitogen Gibco, Carlsbad, CA). Then, peritoneal cells were incubated for 24 hr with or without 10 µg/mL LPS and then were used for flow cytometric analysis.
Flow cytometric analysis
For analysis of B cell subsets, peritoneal cells and omental cells were stained on ice for 15 min with combinations of following fluorochrome-conjugated antibodies in PBS with 5% BCS, 0.05% sodium azide. Antibodies against B220 (RA3-6B2), CD19 (1D3), and CXCR5 (2G8) were from BD Biosciences (San Jose, CA, USA); antibodies against IgM (II/41) and CXCR4 (2B11) from eBioscience (San Diego, CA, USA); and antibodies against CD5 (53-7.3) and CD11b (M1/70) from BioLegend (San Diego, CA, USA). After washing with PBS, the stained cells were analyzed on a FACSCantoII (BD Biosciences). In all cases, doublets were excluded based on forward scatter area versus height gating.
Transwell migration assay
Peritoneal B cells labelled with anti-CD19 magnetic beads were purified with the use of MACS (Miltenyi Biotec, Auburn, CA, USA) and used for transwell migration assay. Purified B cells were resuspended in serum-free RPMI 1640 culture medium. 0.5-1 × 106 cells in 100 µL medium were loaded into the upper chamber of 5 µM pore size transwell (Corning Costar, Cambridge, MA, USA). The lower wells were filled with 600 µL of medium either with or without 100 ng/mL CXCL12 or CXCL13. Cells that migrated into the lower chambers were harvested after 2 hr of incubation and counted by flow cytometry.
ELISA
Plates were coated with purified anti-CXCL12 and blocked with PBS containing 1% BSA. Bound CXCL12 was detected biotinylated anti-mouse CXCL12 antibody, followed by horseradish peroxidase-Streptavidin (BD Biosciences) and subsequent development with substrate (eBioscience).
Statistical analysis
Student t test was used to assess statistical significance and P value of < 0.05 was considered statistically significant for all tests. Histogram was plotted with GraphPad Prism 4.0 program (GraphPad Software, San Diego, CA, USA).
RESULTS
Preferential migration of B-1a cells out of peritoneal cavity upon LPS stimulation
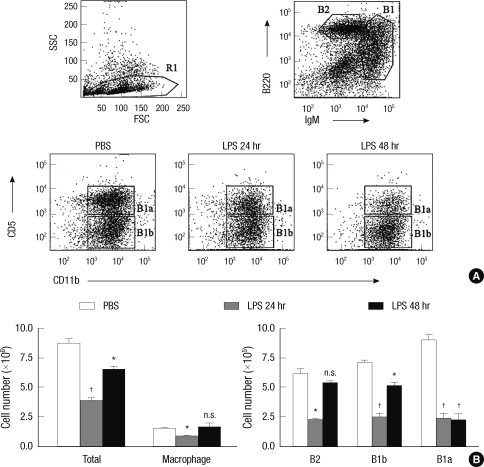
B-1a and B-1b cells are mainly localized in the peritoneal cavity, but B-2 cells are also found in this compartment. To investigate whether LPS stimulation changes the proportion of B cell subsets in the peritoneal cavity, we accurately enumerated the numbers of B cell subsets in the resting state and 24 or 48 hr after intraperitoneal LPS injection. In the homeostatic condition, B-1a cells outnumbered B-1b and B-2 cells (Fig. 1). LPS stimulation led to transient decrease in numbers of all B cell subsets in 24 hr, but the numbers of peritoneal B-2 and B-1b cells recovered nearly to those in the homeostatic condition in 48 hr. Characteristically, the number of peritoneal B-1a cells did not recover to that in the homeostatic condition in 48 hr (Fig. 1B). The decrease in the B-1a cell number upon LPS stimulation was statistically significant in 24 and 48 hr. Reflecting differential migration of B-1a and B-1b cells, the relative ratio of B-1a cells versus B-1b cells was reversed in 48 hr after LPS injection (Fig. 1A). These findings suggest that the peritoneal B-1a cells have migratory characteristics distinct from those of B-1b cells or B-2 cells.
Fig. 1.
LPS induces the preferential egress of peritoneal B-1a cells. (A) Peritoneal cells obtained from 10 week-old C57BL/6 mice at the indicated time after intraperitioneal injection of LPS were stained with fluorescence labeled anti-IgM, -B220, -CD11b, and -CD5 and were analyzed by flow cytometry. In all cases, doublets were excluded by forward scatter area vs height histogram. B-2 cells were gated based on B220high and IgMlow expression and B-1 cells were gated based on and IgMhigh expression. (B) At the indicated times after LPS injection, the absolute numbers of indicated cells in the peritoneal cavity were calculated from the total cell number and flow cytometric data. Representative flow cytometric data from three mice of each treatment group were shown in (A). Statistical analysis was performed by Student's t test in comparison with the numbers of PBS treatment group. n.s., differences not statistically significant. *P = 0.01-0.05; †P = 0.001-0.01.
Preferential increase of the migratory capability of LPS-stimulated B-1a cells toward CXCL12
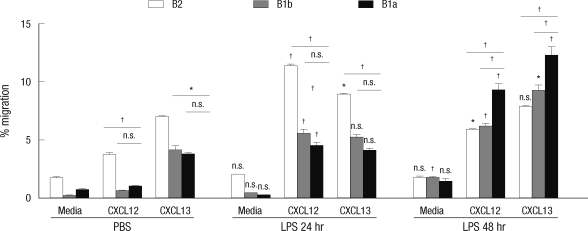
To prove into the mechanism of differential migration of peritoneal B cell subpopulations, we checked migratory responsiveness of resting or LPS-activated B cells to CXCL12 and CXCL13, representative B cell-attracting chemokines, and the expression of their receptors. Peritoneal B cells were purified from mice treated with PBS or LPS for 24 hr or 48 hr and then were used for transwell migration assay. We performed flow cytometric analysis to determine the ratios of B cell subsets in input and transmigrated cells and calculated the percentage of migration for each B cell subset (Fig. 2). B-2 cells from PBS-treated mice transmigrated efficiently toward CXCL12 and CXCL13, but B-1a and B-1b cells migrated poorly toward CXCL12 with showing relatively fine migration toward CXCL13, suggesting that the capability of migration toward CXCL12 is differentially regulated in B cell subsets. The migratory responses to CXCL12 and CXCL13 were all greatly increased in all B cell subsets from LPS-treated mice, but the time-dependent changes of migratory responses for three B cell subsets were different. For B-2 cells, the migration of B-2 cells toward CXCL-12 and CXCL-13 increased in 24 hr upon LPS treatment, but B-2 cells from 48 hr LPS treatment showed lower migration capability to a level of that of homeostatic B-2 cells. Noticeably, B-1a cell migratory responsiveness to CXCL12 and CXCL13 increased significantly after 48 hr LPS treatment and B-1b cells from LPS-treated mice migrated to an intermediate level between those of B-1a and B-2 cells.
Fig. 2.
Heightened migratory response of peritoneal B cells obtained from LPS-treated mice in response to CXCL12 or CXCL13. In vitro transwell migration assay was performed with peritoneal B cells isolated from 10 week-old C57BL/6 mice at the indicated time after intraperitioneal injection of LPS. Peritoneal B cells were added to the upper chamber of a transwell plate in the presence of CXCL12 or CXCL13 in the lower chamber. Two hour later, the numbers of B-1a, B-1b, and B-2 cells in the lower chambers were counted. Results represent the percentage of the cell number in the lower chamber over the input number in response to medium alone, CXCL12, or CXCL13. Data were collected from three mice each experimental group. Statistical analyses were performed by Student's t test for comparison between groups of treatment with PBS and LPS (24 and 48 hr) (above each bar) and also between B-1a cells and B-2 cells or B-1b cells (above each drawn line). n.s., differences not statistically significant. *P = 0.01-0.05; †P = 0.001-0.01.
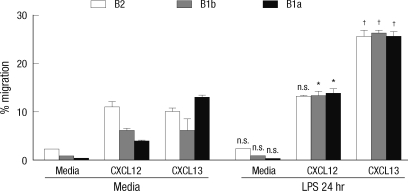
The changes of B-1 cell responses to CXCL12 and CXCL13 were also observed in in vitro LPS stimulation (Fig. 3). Here B cells were purified and then stimulated in vitro with LPS and transwell migration assays were performed with B cells with or without stimulation. Here again the responses of resting B-1a cells to CXCL-12 were lower than that of resting B-2 cells although responses to CXCL-13 were comparable. Consistent with in vivo LPS stimulation results, LPS stimulation increased the migratory response of B-1a cells toward CXCL-12 to a level of that of B-2 cells. 48 hr LPS stimulation experiments could not be performed since the surface expression of Mac-1 was lost in B-1 cells and thus B-1 cells could not be defined in in vitro culture. These findings suggest that the switch of B-1a cell responses to CXCL-12 and CXCL13 upon LPS treatment may be responsible for the selective migration of B-1a cells.
Fig. 3.
Increased chemotactic response of LPS-stimulated peritoneal B cells in response to CXCL12 or CXCL13. In vitro migration assay was performed with purified peritoneal B cells stimulated for 24 hr with or without LPS in vitro. Peritoneal B cells were added to the upper chamber of transwell plate in the presence of CXCL12 or CXCL13 in the lower chamber. 2 hr later, the numbers of B-1a, B-1b, and B-2 cells in the lower chambers were counted. Results represent the percentage of the cell number in the lower chamber over the input number in response to medium alone, CXCL12, or CXCL13. Statistical analyses from three mice of each experimental group were performed by Student's t test in comparison with the group of media treatment. n.s., differences not statistically significant. *P = 0.01-0.05; †P = 0.001-0.01.
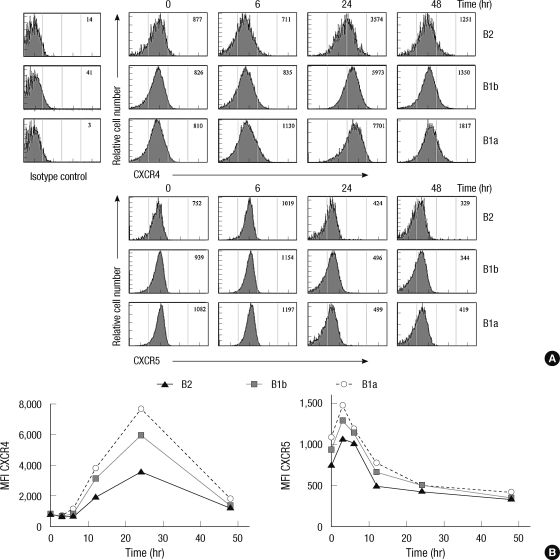
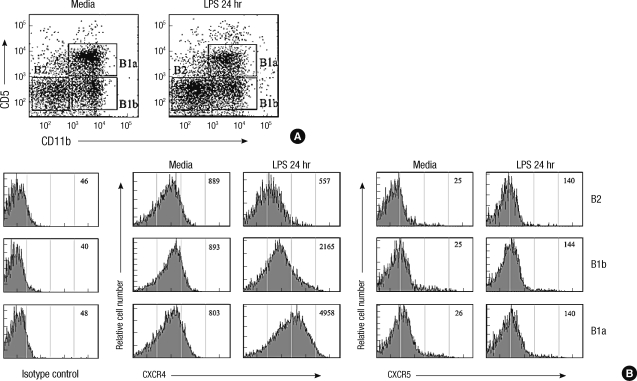
Greater upregulation of CXCR4 in LPS-stimulated B-1a cells than in B-1b and B-2 cells
We next investigated whether the enhancement of the B cell migration toward CXCL12 and CXCL13 upon LPS stimulation was accompanied by the upregulated expression of CXCR4 and CXCR5 (Fig. 4). The expression levels of CXCR4 and CXCR5 were comparable among three B cell subsets in the resting condition. Upon LPS treatment, the expression of CXCR4 increased greatly with time and reached to a peak level in 24 hr and then returned to the basal expression level in 48 hr. The upregulation of the CXCR4 expression upon LPS treatment was most prominent in B-1a cells and the lowest in B-2 cells. In contrast, the the expression of CXCR5 was upregulated very rapidly in 3 hr upon LPS treatment and declined to a level even lower than that in the resting condition in 48 hr. The expression levels of S1P receptors in B-1 cells were not altered by LPS stimulation (data not shown). The results suggest that the increased migratory responsiveness of B-1a cells to CXCL12 upon LPS stimulation may be due to the increased expression of CXCR4, but this cannot explain the increased responsiveness to CXCL13 since the responsiveness to CXCL13 was not corresponding to the expression level of its receptor, CXCR5.
Fig. 4.
Time-course expression of CXCR4 and CXCR5 in peritoneal B cell subsets upon LPS treatment. (A) Peritoneal cells isolated from 10 week-old C57BL/6 mice at the indicated time after intraperitioneal injection of LPS were stained with fluorescence labeled anti-IgM, -CD11b, -CD5, -CXCR4, and -CXCR5 and and were analyzed by flow cytometry. Histograms show mean fluorescence intensities (MFI) of surface CXCR4 (upper panel) and CXCR5 (lower panel). (B) Kinetics of the time-dependent expression of surface CXCR4 (left) or CXCR5 (right) on given peritoneal B cell subsets from C57BL/6 mice treated with LPS for given time duration.
The change of expression of chemokine receptors was also investigated in in vitro LPS-stimulated B cells. Here the proportion of B-1a cells over B-1b cells did not change by 24 hr in vitro culture with LPS (Fig. 5A). The increased expression of CXCR4 by in vitro culture with LPS was most prominent in B-1a cells, whereas upregulation of CXCR5 expression upon LPS stimulation was not significantly different among B cell subsets (Fig. 5B). These results suggest that B-1a cells respond more sensitively to LPS by the increased chemotaxis toward CXCL12 and the upregulated expression of CXCR4 than B-1b and B-2 cells.
Fig. 5.
CXCR4, but not CXCR5, is differentially regulated in peritoneal B cell subsets upon LPS stimulation in vitro. (A) Purified peritoneal B cells were incubated for 24 hr with or without LPS and then were stained with fluorescence labeled anti-IgM, -CD11b, -CD5, -CXCR4, and -CXCR5 and were analyzed by flow cytometry. (B) Representative flow cytometric histograms of B cells are shown from three independent experiments. Numbers within the histograms are mean fluorescence intensities of surface CXCR4 or CXCR5 in given B cell subsets that are identified in (A).
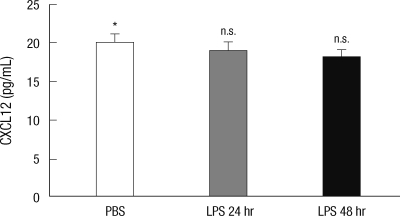
No change of peritoneal CXCL12 production upon LPS treatment
Previously, it was reported that CXCL12 concentration was significantly produced by steady-state mesothelial cells (15) and, therefore, it is likely that B-1 cells stay in the peritoneal cavity through the CXCL12-CXCR4 pathway in the homeostatic condition. To test whether the decreased production of CXCL12 by mesothelial cells upon LPS treatment explain for the migration of peritoneal B cells, we measured concentrations of CXCL12 in peritoneal fluids from mice with or without LPS treatment by ELISA.
Concentrations of CXCL12 did not significantly change upon LPS treatment (Fig. 6). These findings suggest that B-1a cells migrate out of the peritoneal cavity through the intrinsic regulation of their responsiveness to CXCL12, not through the B cell-extrinsic regulation of CXCL12 production.
Fig. 6.
The concentration of CXCL12 in the peritoneal fluids from LPS-injected mice. Peritoneal fluids isolated from 10 week-old C57BL/6 mice at the indicated time after intraperitioneal injection of LPS were measured for the CXCL12 concentrations by ELISA. Statistical analysis from three mice of each group was performed by Student's t test in comparison with the concentration of PBS treatment group. n.s., differences not statistically significant.
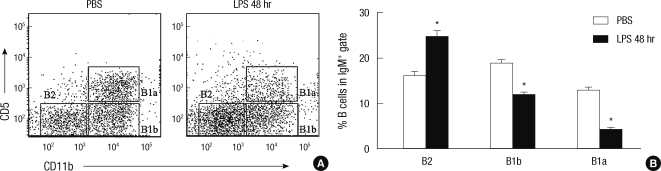
B-1a cells do not migrate into the omental milky spots upon LPS treatment
The omental milky spots (OMSs) are the lymphoid compartment connecting between the systemic circulation and the peritoneal cavity, where the interaction among lymphoid cells occur during peritoneal inflammation (17). Therefore, we tested whether B cell subpopulations migrated to this compartment upon LPS treatment. When we analyzed B cell subsets in OMSs by flow cytometry, we found that the number of B-1a as well as B-1b cells in OMSs were significantly decreased upon LPS treatment, whereas the number of B-2 cells in this compartment increased upon LPS treatment (Fig. 7). The results suggest that B-1a cells do not stay in the OMSs upon LPS stimulation, but enter other systemic sites, whereas B-2 cells are accumulated in the OMSs for the subsequent T-B cell interaction.
Fig. 7.
B-1a cells do not migrate into the omental milky spots upon the LPS treatment in contrast to B-2 cells. (A) Omental cells obtained from 10 week-old C57BL/6 mice at 48 hr after intraperitioneal injection of LPS were stained with fluorescence labeled anti-IgM, -CD11b, and C-D5 and were analyzed by flow cytometry. (B) Percentages of given B cell subsets among total IgM+ B cells from the omental milky spots 48 hr after i.p. injection of LPS or PBS. Statistical analysis from three mice of each experimental group was performed by Student's t test in comparison with the percentage of PBS treatment group. *P = 0.01-0.05.
DISCUSSION
The localization of lymphocytes is determined by multiple factors including chemokines and adhesion receptors. B-1 cells are mainly retained in the peritoneal cavity, but they do recirculate, although to a lesser extent than B-2 cells, and require the spleen for their development and maintenance (7). Among many chemokines, CXCL13 and CXCL12 are especially important for B cell development and functions. CXCL13 is well established to be essential for the recruitment of B cells into follicular dendritic cells and the formation of B cell follicles (10, 18). Meanwhile, CXCL12 is rich in dark zone of the germinal center and the segregation of CXCL12- and CXCL13-rich regions are responsible for the germinal center organization (19). Germinal center B cells are either CXCR4-positive or CXCR4-negative and the expression of CXCR4 determine the localization of germinal center B cells in dark or light zones (20). These studies suggest that the dynamic regulation of B cell localization critically depends on the expression of CXCR4 and its signaling as well as those of CXCR5. For B-1 cells, it is interesting to ask whether CXCL12, CXCL13, and other chemokines are responsible for the peritoneal localization of B-1 cells. Interestingly, both CXCL12 and CXCL13 were reported to be produced in the peritoneal cavity. CXCL13 was shown to be produced by omental cells and peritoneal macrophages and CXCL12 was by mesothelial cells (15, 21). Therefore, both chemokines seem to have roles in the B-1 cell localization, but so far the differential functional roles of these chemokines are not well understood. It is intriguing that both B-1a and B-1b cells in the homeostatic condition showed a poor migratory response to CXCL12 with a good response to CXCL13. We interpreted that both B-1 cell types may be retained in the peritoneal cavity by prolonged CXCL12 stimulation, which does not induce the migration in chronic stimulatory status, but acts as a pro-adhesive and survival signal (22). In contrast, we thought that the responsiveness to CXCL13 may be maintained since the sufficient amount of CXCL13 is not present in the cavity or the CXCL13 signaling is not exhaustive.
The principal function of B-1 cells is the production of natural antibodies for the protection against common pathogens especially early in life, but more complex functions of B-1 cells are being recognized such as regulation of immune responses by secreting IL-10 and elicitation of delayed type hypersensitivity (6, 23). To perform these systemic functions, B-1a cell are required to leave the peritoneal cavity and enter into other peripheral sites. The systemic functions of B-1a cells are thought to be pro-inflammatory in one aspect and anti-inflammatory in other aspect. The locally produced natural antibody-antigen complex may activate the complement cascade and enhance the overall antigen presentation process, as exemplified in the role of B-1a cells in the elicitation of delayed type hypersensitivity (5). On the other hand, IL-10 produced in peripheral inflammatory sites by B-1a cells exerts a negative immune regulation (23). In contrast, B-1b cells were reported to perform a major role in the T cell-independent humoral IgM memory against microbial antigens, but other functions of B-1b cells are not so well known (4). In our experiments, B-1b cells showed a migratory pattern that was intermediate between those of B-1a and B-2 cells, which may reflect differential functions of B-1b cells. We thought that the relationship between chemokines and their receptors should be differentially altered in B cell subsets when systemic inflammatory signals were present. The observation that B-1a cells migrated out of the peritoneal cavity upon LPS treatment to a larger extent than B-1b and B-2 cells may be explained by a time requirement to re-establish the steeply unequal distribution of B-1a cells after peritoneal B-1a cells were once released from the cavity and dispersed. B-2 and B-1b cells may re-enter the peritoneal cavity rapidly since these cells are more abundant in systemic sites than B-1a cells. However, the concurrent changes in the migratory response of B-1a cells to CXCL12 as well as CXCL13 and the surface expression of CXCR4 led us to presume that the migratory change may at least partially be mediated by the switch of chemokine-induced migratory responsiveness in B-1a cells upon LPS stimulation, which is more prominent than in B-1b and B-2 cells.
Distinct from other chemokines, CXCL12 perform important functions other than the migratory regulation of leukocytes. The CXCR4-CXCL12 pathway was reported to play essential roles in the maintenance of hematopoietic stem cells and long-lived plasma cells in bone marrow (24, 25). For developing B lineage cells, CXCL12 exerts different outcomes depending on the expression of SOCS3, which was low in progenitor B cells and the highest in mature B cells (22). CXCL12 induced prolonged focal adhesion kinase phosphorylation and sustained proadhesive responses in progenitor bone marrow B cells, but not in mature B cells (26). With regard to our results, we think that B-1a cells have some characteristics similar to progenitor B cells, which are chronically activated by pre-B cell receptor and CXCL12. We hypothesized that B-1a cells also responded to prolonged CXCL12 stimulation by adhesion to CXCL12-producing mesothelial cells, not by migration, in the homeostatic condition. Since B cell receptors (BCRs) of B-1a cells are basically autoreactive, constitutive BCR-mediated signaling is also likely to contribute to the peritoneal localization of B-1a cells in the homeostatic condition (27, 28). According to our results, the interaction between B-1a cells and mesothelial cells appears to be greatly weakened by LPS stimulation. At present, we do not know how LPS signaling breaks this interaction, but we think that the LPS effect may somehow turn off the CXCR4 signaling pathway for adhesion and release B-1a cells from the peritoneal cavity with a heightened migratory sensitivity to chemokines for systemic migration.
The omentum is a bilayered sheet of mesothelial cells connecting the spleen, pancreas, stomach, and transverse colon and contains milky spots, which are clusters of macrophages, B-1 cells, and a few T cells embedded in the omental tissue (29). The number and size of OMSs increase with surgery and inflammation and, therefore, the OMSs are thought to be sites for lymphocyte interaction and immune responses against peritoneal antigens (17, 30). Here we could observe that the number of B-1a cells in OMSs decrease upon LPS stimulation, which suggest that B-1a cells are not primarily participating in the T-B cell interaction in this lymphoid tissue. However, B-2 cells were thought to participate in the local antibody responses since the number of B-2 cells increased upon LPS stimulation.
In summary, we here addressed how B-1a cells migrate to the systemic sites in inflammatory situations. Using LPS-treated mice, we showed that B-1a cells preferentially migrated out of the peritoneal cavity compared to B-1b and B-2 cells. Mechanistically, B-1 cells showed a greatly increased migratory responsiveness to CXCL12 and CXCL13 after intraperitoneal injection of LPS. Since B-1a cells did not stay in the omentum upon the LPS injection, we interpreted that B-1a cells were actively migrating out of peritoneal cavity upon LPS stimulation and participate in the systemic immune responses. It is suggested that the intricate regulation of CXCR4 in B-1a cells may be a key to understand the dynamic behaviors of B-1a cells.
Footnotes
This work was supported by a grant from (01-PJ3-PG6-01GN12-0001) from the 2001 Good Health R & D Project, Ministry of Health and Welfare, Republic of Korea.
References
- 1.Montecino-Rodriguez E, Dorshkind K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006;27:428–433. doi: 10.1016/j.it.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006;18:547–555. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 4.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Szczepanik M, Akahira-Azuma M, Bryniarski K, Tsuji RF, Kawikova I, Ptak W, Kiener C, Campos RA, Askenase PW. B-1 B cells mediate required early T cell recruitment to elicit protein-induced delayed-type hypersensitivity. J Immunol. 2003;171:6225–6235. doi: 10.4049/jimmunol.171.11.6225. [DOI] [PubMed] [Google Scholar]
- 6.Nogueira-Martins MF, Mariano M. B-1 cell participation in T-cell-mediated alloimmune response. Immunobiology. 2010;215:264–274. doi: 10.1016/j.imbio.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195:771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 9.Sallusto F, Mackay CR. Chemoattractants and their receptors in homeostasis and inflammation. Curr Opin Immunol. 2004;16:724–731. doi: 10.1016/j.coi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Förster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 11.Bowman EP, Campbell JJ, Soler D, Dong Z, Manlongat N, Picarella D, Hardy RR, Butcher EC. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J Exp Med. 2000;191:1303–1318. doi: 10.1084/jem.191.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 13.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmesino E, Moepps B, Gierschik P, Thelen M. Differences in CXCR4-mediated signaling in B cells. Immunobiology. 2006;211:377–389. doi: 10.1016/j.imbio.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Foussat A, Balabanian K, Amara A, Bouchet-Delbos L, Durand-Gasselin I, Baleux F, Couderc J, Galanaud P, Emilie D. Production of stromal cell-derived factor 1 by mesothelial cells and effects of this chemokine on peritoneal B lymphocytes. Eur J Immunol. 2001;31:350–359. doi: 10.1002/1521-4141(200102)31:2<350::aid-immu350>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Yun HJ, Jo DY. Production of stromal cell-derived factor-1 (SDF-1)and expression of CXCR4 in human bone marrow endothelial cells. J Korean Med Sci. 2003;18:679–685. doi: 10.3346/jkms.2003.18.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, Kusser K, Hartson L, Moquin A, Randall TD. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30:731–743. doi: 10.1016/j.immuni.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansel KM, Ngo VN, Hyman PL, Luther SA, Förster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 19.Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 20.Caron G, Le Gallou S, Lamy T, Tarte K, Fest T. CXCR4 expression functionally discriminates centroblasts versus centrocytes within human germinal center B cells. J Immunol. 2009;182:7595–7602. doi: 10.4049/jimmunol.0804272. [DOI] [PubMed] [Google Scholar]
- 21.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 22.Le Y, Zhu BM, Harley B, Park SY, Kobayashi T, Manis JP, Luo HR, Yoshimura A, Hennighausen L, Silberstein LE. SOCS3 protein developmentally regulates the chemokine receptor CXCR4-FAK signaling pathway during B lymphopoiesis. Immunity. 2007;27:811–823. doi: 10.1016/j.immuni.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima H, Hamaguchi Y, Watanabe R, Ishiura N, Kuwano Y, Okochi H, Takahashi Y, Tamaki K, Sato S, Tedder TF, Fujimoto M. CD22 expression mediates the regulatory functions of peritoneal B-1a cells during the remission phase of contact hypersensitivity reactions. J Immunol. 2010;184:4637–4645. doi: 10.4049/jimmunol.0901719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Tarlinton D, Radbruch A, Hiepe F, Dörner T. Plasma cell differentiation and survival. Curr Opin Immunol. 2008;20:162–169. doi: 10.1016/j.coi.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Glodek AM, Honczarenko M, Le Y, Campbell JJ, Silberstein LE. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. J Exp Med. 2003;197:461–473. doi: 10.1084/jem.20021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 28.Chumley MJ, Dal Porto JM, Cambier JC. The unique antigen receptor signaling phenotype of B-1 cells is influenced by locale but induced by antigen. J Immunol. 2002;169:1735–1743. doi: 10.4049/jimmunol.169.4.1735. [DOI] [PubMed] [Google Scholar]
- 29.Krist LF, Eestermans IL, Steenbergen JJ, Hoefsmit EC, Cuesta MA, Meyer S, Beelen RH. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995;241:163–174. doi: 10.1002/ar.1092410204. [DOI] [PubMed] [Google Scholar]
- 30.Carlow DA, Gold MR, Ziltener HJ. Lymphocytes in the peritoneum home to the omentum and are activated by resident dendritic cells. J Immunol. 2009;183:1155–1165. doi: 10.4049/jimmunol.0900409. [DOI] [PubMed] [Google Scholar]