Abstract
The present study evaluated optimal baseline prostate-specific antigen (PSA) level at different ages in order to determine the risk of developing prostate cancer (CaP). We analyzed 6,651 Korean men, aged 40-69 yr. The serum PSA levels for these men were measured at one institute from 2000 to 2004 and were determined to be between 0-4 ng/mL. Patients were divided into 4 groups of 25th-percentile intervals, based on initial PSA level. Of these, the group with an increased risk was selected, and the optimal value was determined by the maximal area under a receiver-operating characteristic curve within the selected group. The risk of CaP diagnosis was evaluated by Cox regression. The mean follow-up period was 8.3 yr. CaP was detected in 27 of the 6,651 subjects. CaP detection rate was increased according to age. The optimal PSA value to distinguish the risk of CaP was 2.0 ng/mL for 50- to 69-yr-olds. Patients with a baseline PSA level greater than the optimal value had a 27.78 fold increase in the prostate cancer risk. Baseline PSA values are useful for determining the risk of developing CaP in Korean men for 50- and 69-yr-old. We suggest that PSA testing intervals be modified based on their baseline PSA levels.
Keywords: Prostate-Specific Antigen, Screening, Prostate Neoplasm, Asian Continental Ancestry Group
INTRODUCTION
The American Urological Association (AUA) and American Cancer Society (ACS) recommend that prostate cancer screening begin at 50 yr of age for men with an average risk of prostate cancer (CaP) and even earlier for men with a higher risk for prostate cancer due to risk factors, such as a positive family history in a first-degree relative or African American heritage (1, 2). Prostate cancer screening is performed for the early detection of curable prostate cancer. Prostate cancer deaths are seen in men as young as 35 to 44 yr of age, according to Surveillance, Epidemiology, and End Results (SEER) data. These deaths indicate that men younger than the recommended screening age of the AUA and ACS for the early detection of prostate cancer can also be affected by the disease. Therefore, depending on the risk for developing prostate cancer, the prevention and detection strategy should be adjusted to include younger men. To assess the risk of prostate cancer, baseline prostate-specific antigen (PSA) levels are used. In 2005, Whittemore and colleagues (3) reported that PSA levels in young adults were useful predictors of CaP detection. Loeb et al. (4), reported that the baseline PSA levels for men in their 40s were a stronger predictor of CaP risk than ethnicity, family history, or digital rectal examination findings. Ultimately, the National Comprehensive Cancer Network (NCCN) recommended a baseline PSA test for all men, starting at 40 yr of age, in order to assess the risk for CaP. It has been determined that PSA level distributions differ based on ethnicity and age (5-7). Therefore, research should be conducted taking into account ethnic differences.
Based on healthy-screened Korean men with PSA levels between 0-4 ng/mL, we assessed the optimal baseline PSA levels for the prediction of the risk of developing prostate cancer in Korean men.
MATERIALS AND METHODS
Study population
Between January 2000 and July 2004, a total of 6,651 men between the ages of 40 and 69 yr with baseline PSA levels between 0-4 ng/mL during routine health checkups were enrolled in the study. Patients who had a previous diagnosis of CaP, a history of prostate surgery, or who had received 5-alpha reductase inhibitors within three months were excluded from the study. In July 2009, cases of CaP in enrolled patients were identified using data available from the Korean National Health Insurance Corporation (NHIC) website, which links to the Korean Severe Patient Registry. We typed in the hospital serial number in the patient information query of order communicating system at the hospital administration office so that their registration in Korean Severe Patient Registry could be confirmed. This registry includes data for over 99% of all cancer cases diagnosed in Korea (8). Of our total study sample of 6,651 men, 17 died due to diseases other than prostate cancer during the study period.
Assessment of risk
Patients were divided into 4 groups at 25th percentile intervals based on initial PSA, as carried out by Whittemore et al. (3). Of these, the group that had an increased risk was selected, and the optimal PSA value was determined using the maximal area under the curve (AUC) of a receiver-operating characteristic curve (ROC) within the selected group. The risk of developing prostate cancer in the enrolled men was studied based on the optimal PSA value. In this analysis, when more than one PSA measurement was available for a subject within an age-decade, the earliest measurement was used as the baseline PSA level for determining risk.
PSA testing
Serum PSA levels were measured using an Elecsys 170 assay (Roche Diagnostics, Mannheim, Germany). Vacutainer tubes were used (Becton Dickinson, Oxford, UK) for blood collection, and the specimen was centrifuged after venipuncture and collection. After centrifugation, the specimens were immediately frozen at -70℃ and were assessed for PSA within three days.
Statistics
All statistical tests were two-sided. A P value < 0.05 was considered statistically significant (SAS software, version 9.1). A Kaplan-Meier survival analysis was performed to estimate prostate cancer-free probability, with time considered as a function of the baseline PSA level. Subjects without cancer were censored at death or July 2009. A log-rank statistical test was used to compare the Kaplan-Meier survival curves among the PSA groups. A Cox proportional hazards regression model was used to examine the long-term relationship between baseline PSA level and prostate cancer risk. Comparison of the performance of each Cox model was calculated by C statistics (area under the ROC curve). The Hazard ratio (HR) and 95% confidence intervals (CI) were estimated using the Cox regression model, with a PSA level lower than the cutoff value treated as the reference group.
Ethics statement
The institutional review board of Gangnam Severance Hospital reviewed summary and full text of the present clinical research proposal, proposal for waiver review, and proposal for waiver informed consent. The board approved this clinical study (3-2010-0033) and waived informed consent due to its nature of retrospective research.
RESULTS
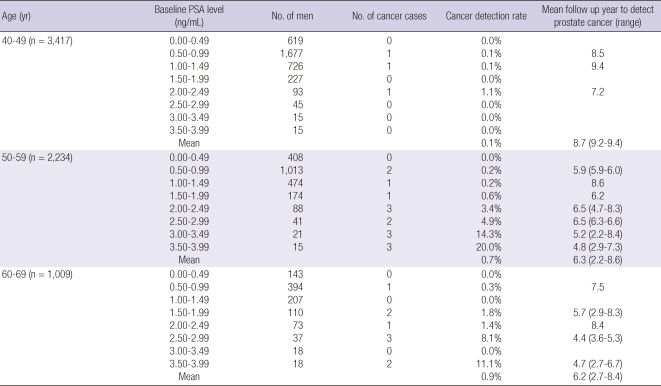
At baseline, the patients had a mean age of 50 yr and a mean PSA value of 0.83 ng/mL. The median PSA levels were 0.72, 0.81, and 0.93 ng/mL, and the third quartile PSA levels were 1.08, 1.23, and 1.51 ng/mL for men in their 40s, 50s, and 60s, respectively. The mean follow-up period was 8.3 yr. CaP was detected in 27 of the 6,651 subjects. After initial PSA measurement, prostate cancer was discovered after at least 2.2 yr in men in their 50s and 2.7 yr in men in their 60s. CaP detection rates increased according to the baseline PSA from their 50s (Table 1).
Table 1.
Characteristics of baseline PSA of study participants
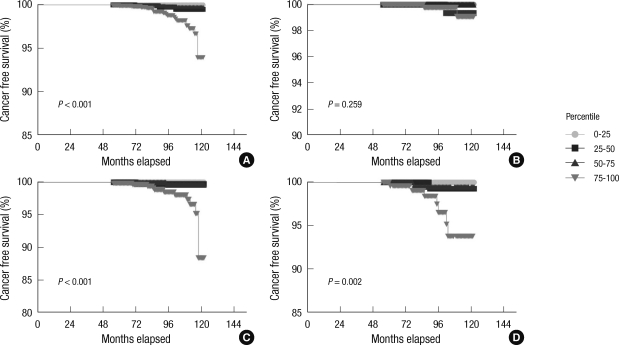
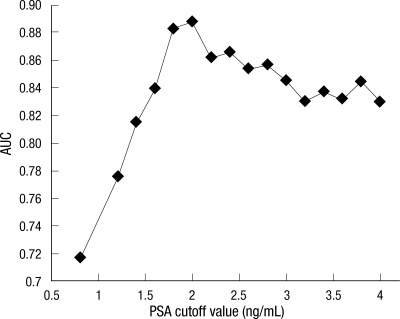
When all subjects were classified into 4 groups based on 25th percentile intervals of baseline PSA level, the risk of prostate cancer significantly increased in men with a baseline PSA level greater than the 75th percentile. However, men in their 40s did not have a significantly increased risk of prostate cancer (Fig. 1). Above the 75th percentile of the baseline PSA level, the cut-off value with the greatest sensitivity (92%) and specificity (80%) was 2.0 ng/mL (93 percentile) for men in their 50s and 60s (Fig. 2).
Fig. 1.
Cumulative prostate cancer-free survival according to the age and baseline PSA percentile. Survival curves for men aged (A) 40 to 69 yr, (B) 40 to 49 yr, (C) 50 to 59 yr and (D) 60 to 69 yr. Markers represent prostate cancer and censored cases. With time, prostate cancer incidence increased significantly in men with a baseline PSA more than the 75th percentile (reverse triangle), except for men in their 40s (log-rank test for trend).
Fig. 2.
Trend of the area under the curve according to a baseline PSA cut-off value greater than the median PSA level in men 50 to 69 yr old. AUC, area under the curve; PSA, prostate-specific antigen.
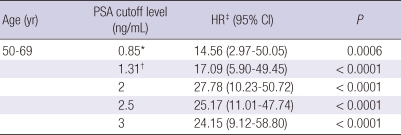
As compared with the group with a baseline PSA level less than the cut-off value, the group with a PSA level higher than the cut-off value had a 27.78-fold increased risk for diagnosis of CaP in their 50s and 60s (P < 0.001). The age-adjusted CaP hazard ratio was also significantly increased 25.76-fold for men in their 50s and 60s.
However, when the cut-off value was set at the median baseline PSA, the risk of CaP was a 13.9-fold increase for men in their 50s and 60s (P = 0.01).
DISCUSSION
Based on our results, the median baseline PSA levels were 0.72 ng/mL, 0.81 ng/mL, and 0.93 ng/mL for men in their 40s, 50s, and 60s, respectively. When the ages of the subjects were restricted to between 50 and 69 yr old, the median baseline PSA of the selected subjects was 0.85 ng/mL. These results are similar to those of previous studies (5, 6). Men in their 40s did not have a significant baseline PSA level that would determine their risk of prostate cancer. However, for those in their 50s and 60s, the group with a baseline PSA higher than the 75th percentile had a significantly increased risk, as compared to their age-specific lowest quartile baseline PSA level. Of these, the baseline PSA level 2.0 ng/mL had a maximal discriminative value according to the receiver-operating characteristic curve, when the subjects who were older than 50 yr old were divided into two groups based on their baseline PSA level (cutoff level of 2.0 ng/mL). As compared with the group with a baseline PSA level less than the cut-off value of 2.0 ng/mL, the group with a PSA level higher than the cut-off value had a 27.78-fold increased risk for diagnosis of CaP in the mean 8.3 yr of follow up. However, despite a relatively high risk for diagnosis of CaP, the median baseline PSA value (0.85 ng/mL) did not suggest a more efficient discriminative value for determining the risk for diagnosis of CaP than the baseline PSA value, 2.0 ng/mL. We believe that PSA screening every one year in men older than 50 yr old with more than 2.0 ng/mL of baseline PSA value would lead to a considerable decrease in the number of screening visits, associated costs and stress, while carrying minimal risk for missing aggressive cancer at a curable stage.
In men over the age of 50 yr, the group with a baseline PSA higher than the 75th percentile had a significantly increased risk, as compared to their age-specific lowest quartile baseline PSA level (Fig. 1). All values of last quartile (from 1.2 ng/mL to 4.0 ng/mL) had an increased risk as compared with lowest quartile values however, of these 2.0 ng/mL had a maximal AUC.
PSA level is also used as a screening tool to detect prostate cancer. Most diagnostic tools have cut-off points for maximal specificity and sensitivity. Therefore, it was important to determine the PSA value with the maximal AUC according to the receiver-operating characteristic curve. When the data were evaluated for men older than 50 yr old, and in regards to the median baseline PSA, there was not sufficient area under the curve to provide a cut-off value. At this point, the AUC was approximately 0.7.
Ultimately, the optimal baseline cut-off PSA value for determining the risk of prostate cancer was 2.0 ng/mL for men in their 50s and 60s. The cut-off value for men in their 50s and 60s was slightly higher than those of western populations (9, 10). This difference is likely due to the lower rate of developing CaP in Korean men in this study. If the overall incidence of CaP increases, the cut-off value of baseline PSA may decrease, as has been observed in statistic results based on Western populations (11).
Upon reviewing the results of similar studies, we found that several authors reported that the median baseline PSA was used as a cut-off value for risk stratification in Western populations. They reported a dozen-fold increased risk of CaP diagnosis (12, 13). However, the authors did not evaluate the lowest and highest quartile value of baseline PSA. Therefore, it is unknown which quartile had the greater affect.
Tang et al. (14) recently reported a 1.5 ng/mL cut-off value for estimating the risk of developing prostate cancer, which was not the median PSA value. However, their study included white and black men.
Schroder et al. (15) studied the probability of progression of PSA in the Netherlands. PSA progression to more than 3.0 ng/mL occurred in 0.9%, 9.3%, and 48.6% of men who presented PSA values less than 1.0, 1 to 1.9 and 2 to 2.9 ng/mL, respectively. They concluded that men who had a baseline PSA level less than 2.0 ng/mL rarely had a chance to progress CaP. Ito et al. (16) also wanted to know the probability of developing CaP in Japanese compared to the Netherlands. However, they did not find any difference of developing prostate cancer over a 4-yr study period. The authors investigated the relationship between baseline PSA and the cumulative probability of increased PSA above 4.0 ng/mL to find the probability of developing prostate cancer indirectly. However, the cumulative probability of increased PSA above 4.0 ng/mL may overestimate the risk of developing screen detectable prostate cancer, because some men may have a non-cancer-related PSA increase.
Wright et al. (17) also reported that baseline PSA levels reflected a long-term risk of prostate enlargement. However, the sample size of that study was too small to produce a meaningful cut-off level.
Unlike other studies, our study did not reveal a significant difference in the risk of prostate cancer among the 4 quartile groups at ages in the 40s. This is probably because of the low incidence of prostate cancer in Korean men.
According to the Surveillance, Epidemiology, and End Results data, CaP deaths occur in North American men between the ages of 35 and 44 yr (18). Similarly, CaP deaths are also beginning to occur in Korean men younger than 50 yr, and there is an increasing trend toward even younger men dying from CaP (19). The age-adjusted incidence rate is 159.3 per 100,000 men per year in the US population (18) as opposed to 20.0 in the Korean population (20). This ethnicity-based difference may be attributed to two factors discussed below. First, a relatively low overall CaP incidence rate in Korean men may lead to a lower incidence at the 40s, as prostate cancer requires time to manifest. Therefore, if the overall incidence is reduced, prostate cancer occurrences may be reduced in younger age groups. Second, Korean men ultimately may not have a risk of CaP due to a lack genetic history for familial prostate cancer and/or lack of African heritage, as compared to individuals in Western populations (21), both of which are factors associated with the development of prostate cancer at earlier ages. Based on these results, baseline PSA testing beginning at age 40 yr is not yet an effective screening strategy for Korean men. We suggest that baseline screening should begin at age 50 for Korean men. We believe that annual screening in men with more than 2.0 ng/mL of baseline PSA level is a reasonable cutoff value.
There were some limitations to this study. First, subjects of the study were participants of a routine health check-up. Therefore, these results might not be the same as those seen in the normal population. Second, digital rectal examination (DRE) was not included in the screening procedure. When PSA values are low, a positive DRE result is associated with CaP in only 3% to 5% of the cases (22, 23). Vis et al. (24) calculated that one would have to perform 289 DREs to find one case of clinically significant CaP in patients with a PSA level less than 3.0 ng/mL. Therefore, we assumed that the omission of DRE did not greatly affect our results.
Third, the presence of CaP was not confirmed by a pathologic report based on prostate biopsy, but rather from data from a cancer registry maintained by NHIC. Therefore, we were unable to access complete pathologic information for all study participants. Finally, we did not know exactly the compliance of prostate biopsy in participants. In a prostate cancer prevention trial (PCPT), 2,950 of 3,568 men with a prostate-specific antigen level < or = 4.0 ng/mL per underwent prostate biopsy at the end of the study (25). According to the PCPT, 14.6% of subjects did not agree to prostate biopsy. We believe that our study might have a similar compliance rate. These should be considered in interpreting our results. Therefore, we believe that more long term and full pathologic data based results will be necessary to reveal the efficacy of baseline PSA testing in Korean men.
Baseline PSA values are useful for determining the risk of developing prostate cancer in Korean men between 50 and 69 yr old. We recommend that men between 50 and 69 yr old should have their baseline PSA levels measured. Moreover, we believe that a baseline PSA cutoff value of 2.0 ng/mL is a discriminative value for determining the risk of developing prostate cancer in Korean men 50 yr old or older. Therefore, we suggest that PSA testing intervals be modified for men older than 50 yr based on their baseline PSA levels.
Table 2.
A comparison of risk of developing prostate cancer in Korean men with baseline PSA values by age
*median baseline PSA value; †75th percentile of baseline PSA value; ‡HR estimated by Cox regression model for comparisons with the reference group (lower than cut-off). PSA, prostate-specific antigen; HR, hazard ratio; CI, confidence interval.
References
- 1.Wolf AM, Wender RC, Etzioni RB, Thompson IM, D'Amico AV, Volk RJ, Brooks DD, Dash C, Guessous I, Andrews K, DeSantis C, Smith RA American Cancer Society Prostate Cancer Advisory Committee. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 2.Greene KL, Albertsen PC, Babaian RJ, Carter HB, Gann PH, Han M, Kuban DA, Sartor AO, Stanford JL, Zietman A, Carroll P. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009;182:2232–2241. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 3.Whittemore AS, Cirillo PM, Feldman D, Cohn BA. Prostate specific antigen levels in young adulthood predict prostate cancer risk: results from a cohort of Black and White Americans. J Urol. 2005;174:872–876. doi: 10.1097/01.ju.0000169262.18000.8a. [DOI] [PubMed] [Google Scholar]
- 4.Loeb S, Roehl KA, Antenor JA, Catalona WJ, Suarez BK, Nadler RB. Baseline prostate-specific antigen compared with median prostate-specific antigen for age group as predictor of prostate cancer risk in men younger than 60 years old. Urology. 2006;67:316–320. doi: 10.1016/j.urology.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 5.Choi YD, Kang DR, Nam CM, Kim YS, Cho SY, Kim SJ, Cho IR, Cho JS, Hong SJ, Ham WS. Age-specific prostate-specific antigen reference ranges in Korean men. Urology. 2007;70:1113–1116. doi: 10.1016/j.urology.2007.07.063. [DOI] [PubMed] [Google Scholar]
- 6.Ku JH, Ahn JO, Lee CH, Lee NK, Park YH, Byun SS, Kwak C, Lee SE. Distribution of serum prostate-specific antigen in healthy Korean men: influence of ethnicity. Urology. 2002;60:475–479. doi: 10.1016/s0090-4295(02)01807-1. [DOI] [PubMed] [Google Scholar]
- 7.Lee SE, Kwak C, Park MS, Lee CH, Kang W, Oh SJ. Ethnic differences in the age-related distribution of serum prostate-specific antigen values: a study in a healthy Korean male population. Urology. 2000;56:1007–1010. doi: 10.1016/s0090-4295(00)00837-2. [DOI] [PubMed] [Google Scholar]
- 8.Ahn YO. Cancer registration in Korea: the present and furtherance. J Prev Med Public Health. 2007;40:265–272. doi: 10.3961/jpmph.2007.40.4.265. [DOI] [PubMed] [Google Scholar]
- 9.Loeb S, Carter HB, Catalona WJ, Moul JW, Schroder FH. Baseline prostate-specific antigen testing at a young age. Eur Urol. 2012;61:1–7. doi: 10.1016/j.eururo.2011.07.067. [DOI] [PubMed] [Google Scholar]
- 10.Crawford ED, Moul JW, Rove KO, Pettaway CA, Lamerato LE, Hughes A. Prostate-specific antigen 1.5-4.0 ng/mL: a diagnostic challenge and danger zone. BJU Int. 2011;108:1743–1749. doi: 10.1111/j.1464-410X.2011.10224.x. [DOI] [PubMed] [Google Scholar]
- 11.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 12.Fang J, Metter EJ, Landis P, Chan DW, Morrell CH, Carter HB. Low levels of prostate-specific antigen predict long-term risk of prostate cancer: results from the Baltimore Longitudinal Study of Aging. Urology. 2001;58:411–416. doi: 10.1016/s0090-4295(01)01304-8. [DOI] [PubMed] [Google Scholar]
- 13.Loeb S, Nadler RB, Roehl KA, Antenor JA, Catalona WJ. Risk of prostate cancer for young men with a prostate specific antigen less than their age specific median. J Urol. 2007;177:1745–1748. doi: 10.1016/j.juro.2007.01.068. [DOI] [PubMed] [Google Scholar]
- 14.Tang P, Sun L, Uhlman MA, Robertson CN, Polascik TJ, Albala DM, Donatucci CF, Moul JW. Initial prostate specific antigen 1.5 ng/mL or greater in men 50 years old or younger predicts higher prostate cancer risk. J Urol. 2010;183:946–950. doi: 10.1016/j.juro.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Schröder FH, Raaijmakers R, Postma R, van der Kwast TH, Roobol MJ. 4-year prostate specific antigen progression and diagnosis of prostate cancer in the European Randomized Study of Screening for Prostate Cancer, section Rotterdam. J Urol. 2005;174:489–494. doi: 10.1097/01.ju.0000165568.76908.5c. [DOI] [PubMed] [Google Scholar]
- 16.Ito K, Raaijmakers R, Roobol M, Wildhagen M, Yamanaka H, Schröder FH. Prostate carcinoma detection and increased prostate-specific antigen levels after 4 years in Dutch and Japanese males who had no evidence of disease at initial screening. Cancer. 2005;103:242–250. doi: 10.1002/cncr.20739. [DOI] [PubMed] [Google Scholar]
- 17.Wright EJ, Fang J, Metter EJ, Partin AW, Landis P, Chan DW, Carter HB. Prostate specific antigen predicts the long-term risk of prostate enlargement: results from the Baltimore Longitudinal Study of Aging. J Urol. 2002;167:2484–2487. doi: 10.1016/s0022-5347(05)65010-0. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute, Division of Cancer Control and Population Science, Surveillance Research Program, Cancer statistics Branch, Surveilance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: mortality-all cause of death, public-use with state, Total U.S. (1969-2007), Underlying mortality data provided by National Center for Health Statistics. 2007. [Google Scholar]
- 19.Korea National Statistics Office. The Statistics Korea: annual report on the cause of death statistics (based on vital registration) Daejeon: Korea National Statistics Office; 2007. [Google Scholar]
- 20.Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010;25:1113–1121. doi: 10.3346/jkms.2010.25.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antenor JA, Han M, Roehl KA, Nadler RB, Catalona WJ. Relationship between initial prostate specific antigen level and subsequent prostate cancer detection in a longitudinal screening study. J Urol. 2004;172:90–93. doi: 10.1097/01.ju.0000132133.10470.bb. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Ito K, Ohi M, Kubota Y, Suzuki K, Fukabori Y, Kurokawa K, Yamanaka H. Diagnostic significance of digital rectal examination and transrectal ultrasonography in men with prostate-specific antigen levels of 4 NG/ML or less. Urology. 2001;58:994–998. doi: 10.1016/s0090-4295(01)01409-1. [DOI] [PubMed] [Google Scholar]
- 23.Carvalhal GF, Smith DS, Mager DE, Ramos C, Catalona WJ. Digital rectal examination for detecting prostate cancer at prostate specific antigen levels of 4 ng/ml or less. J Urol. 1999;161:835–839. [PubMed] [Google Scholar]
- 24.Vis AN, Hoedemaeker RF, Roobol M, van der Kwast TH, Schröder FH. Tumor characteristics in screening for prostate cancer with and without rectal examination as an initial screening test at low PSA (0.0-3.9 ng/mL) Prostate. 2001;47:252–261. doi: 10.1002/pros.1069. [DOI] [PubMed] [Google Scholar]
- 25.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA., Jr Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]