Abstract
We hypothesized that the formation and differentialtion of osteoclasts are accelerated and the potential of bone resorption is increased in the hemiplegic bone marrow in the early stage of stroke. We randomly divided white female Sprague-Dawley (SD) rats (n = 30) into two groups, stroke (n = 15) and sham group (n = 15). On the 7th day after stroke, after cutting away the epiphyses of the femurs and tibias, diaphyseal channels were flushed using α-minimum essential medium (α-MEM) and bone marrow cells were collected. Bone marrow stem cells, which were extracted from the femur and tibia, were cultured on the 7th day after middle cerebral artery occlusion. We then estimated the ratio of non-adherent cells to total bone marrow cells that included osteoclast precursor cells. After culturing these cells separately, cells that tested positive on the tartrate resistant acid phosphatase (TRAP) were counted and bone resorption was evaluated by using the OAAS™ plate. In comparison to the control group, the stroke group showed a higher increase of non-adherent cells in the hemiplegic side bone marrow. In addition, after the primary culture, the stroke group showed an increased number of TRAP positive cells and a higher degree of bone resorption estimated by OAAS™ plate. As a result, osteoclastogenesis and osteoclast differentiation are accelerated and the potential of bone resorption is increased in the hemiplegic bone marrow and these changes are detected as early as within the first week after middle cerebral artery occlusion in SD rats.
Keywords: Bone Resorption, Osteoclasts, Osteoporosis, Stroke
INTRODUCTION
Osteoporosis is the most common disease among metabolic bone diseases and can be defined clinically as existence of a fracture, histomorphologically as reduction in bone matrix per unit volume, and epidemiologically as increased risk of fracture (1). Osteoporotic fracture increases with age and the frequency of it increases according to aging of society. In the United States, about 1.5 million osteoporotic fracture patients occur every year, resulting in an extremely high social medical expense of about 18 billion dollars (2).
According to recent reports, the bone mineral density of the hemiplegic limb is significantly lower than that of non-hemiplegic side, and the incidence of hip fracture increases by about 2-4 times after stroke, among which more than 79% occur on the hemiplegic side (3-5). Sato et al. (6, 7) suggested two causes of osteoporosis by stroke. First, bone resorption increases by immobilization from paralysis. Second, lack of nutrition and reduced exposure to sunlight bring deficiency of vitamin D, which induces osteoporosis. In this study, Sato et al. (6) demonstrated increase in bone resorption by increased serum carboxy-terminal telopeptide of type I collagen (ICTP; a marker for bone resorption) in patients one week after stroke.
Many studies using serum bone markers have reported on the induction of osteoporosis on the hemiplegic side since the initial stages of stroke, but there are no studies yet on the effect of stroke on osteoclast differentiation in bone marrow cells. We hypothesized that the formation and differentiation of osteoclasts are accelerated and the potential of bone resorption is increased in the hemiplegic bone marrow in the early stage of stroke. To confirm this, we conducted a study with SD rats by extracting and culturing bone marrow cells in the femur and tibia after one week of stroke.
MATERIALS AND METHODS
Animals and treatments
A group of 30 (12 weeks, 220-260 mg) white female Sprague-Dawley rats were bred at a clean animal breeding room of the College of Medicine at the Catholic University of Korea. All experimental procedures were approved by the Animal Experimental Committee of the Catholic University of Korea. The feed (Samyang Oil & Feed Co., Ltd., Seoul, Korea) containing 1.15% calcium and 0.75% phosphorous and water were supplied without limits.
Subjects were randomly divided into the control and experimental groups, and each group consisted of 15 rats. The experimental group received ischemic stroke surgery and the control group received a sham operation. Both groups were maintained at the same sleep-wake cycle with 12 hr light-dark cycle (light-out 18:00-06:00).
The experimental protocol was approved by the institutional animal care and use committee at the College of Medicine, the Catholic University of Korea.
Preparation of ischemic stroke rats
Ketamine (30 mg/kg) (Yuhan Co., Seoul, Korea) and xylazine hydrochloride (4 mg/kg) (Bayer Korea, Seoul, Korea) were used to anesthetize rats. While maintaining body temperature of 37℃ ± 1℃ during the surgery, cerebral ischemia was induced using the method of Longa et al. (8). For the method of surgery, the central neck of rats was cut in supine position and the left common carotid artery was dissected. Following the common carotid artery, external carotid artery and internal carotid artery were separated for ligation of the common carotid artery and external carotid artery. The tip of monofilament nylon (4.0) was pushed into the common carotid artery bifurcation to block the middle cerebral artery. The same procedure was performed on the control group until the dissection of the carotid artery, but the rat was sutured without ligation of the artery. After ligation of the middle cerebral artery, permanent local ischemic stroke was confirmed using the following two methods. Three days after operation, neurologic examination was performed on the ischemic stroke and control rats, following the protocols used by Garcia et al. (9). Seven days after ischemic stroke, the femur and tibia were extracted, and the brain was taken out and placed for 30 min in 2,3,5-Triphenyl-2H-tetrazolium chloride dye (Sigma Diagnostic, St. Louis, MO, USA). After 20 min of culturing at 37℃, it was fixed in 2% paraformaldehyde solution (Sigma Diagnostic) to verify ischemic stroke (10).
Culturing of bone marrow
Seven days after ischemic stroke, when all rats in the experimental and control groups were sacrificed, right femurs and tibias of the control group and right femurs and tibias of the experimental group were extracted. The epiphyses of the femurs and tibias were cut away, and bone marrow was collected by flusing the diaphyseal channel with α-minimum essential medium (α-MEM). After centrifugation and counting the number of bone marrow cells, they were resuspended on α-MEM containing 15% fetal calf serum. Cells were incubated for three hours under 37℃ vapor saturated atmosphere with 5% carbon dioxide in a tissue culture flasks (106 cells/cm2). Then, the adherent cells and the non-adherent cells were separated.
Measurement of Tartrate-Resistant Acid Phosphatase (TRAP) positive cells
The ratio of the number of the non-adherent cells to bone marrow cells was measured and the non-adherent cells were diluted to a concentration of 1 × 106 cells/mL before moving to a culture dish. The cells were cultured on a 24-well plate (1 mL/well) in order to examine the number of TRAP positive cells. After 8 days of culturing, cells were placed in citrate/aceton solution for 30 sec and dyed for one hour in a dark room of 37℃ using Sigma Acid Phosphatase Leukocyte Kit (Sigma Diagnostic) to find TRAP positive cells.
Measurement of bone resorption
Among the non-adherent cells, 3 × 105 cells were cultured on an OAAS™ plate (Osteogenic Core Technology, Cheonan, Korea) (1 mL/well) for evaluation of bone resorption. OAAS™ plate is a product covered on the surface by calcium phosphate as a thin layer to allow measurement of bone resorption while culturing the osteoclast. 3 × 105 cells per well were cultured for 13 days. After cell culture, OAAS™ plate was washed using distilled water, dried and observed the percentage of resorbed area. The image was stored using a digital camera under optical microscope and the area ratio of bone resorption to total area was evaluated using an image analysis program called Scion Image.
Statistical analysis
Ratio of the non-adherent cells, the number of TRAP positive cells and the percentage of resorbed area on OAAS™ plate were analyzed. Comparison between the control group that received sham operation and the hemiplegic side of the stroke group was analyzed using Mann-Whitney U test. Comparison between the hemiplegic side and the non-hemiplegic side of the experimental group was analyzed using Wilcoxon sign rank test.
RESULTS
Throughout the experimental period, four rats died both in the experimental and control groups. The number of subjects was reduced to 22 rats for analysis of results, each group containing 11 rats.
Body weight
Body weights before sham operation or ischemic stroke surgery and seven days after operations were 230.7 ± 9.5 g and 237.6 ± 4.3 g in the control group and 231.9 ± 9.5 g and 235.4 ± 10.9 g in the experimental group, which showed no significant difference (P = 0.551).
Verification of ischemic stroke
Three days after ischemic stroke surgery, neurologic examination as proposed by Garcia et al. (9) was conducted. Scores of the control group and experimental group were 13.68 ± 0.5 and 17.8 ± 0.4 points. A significant difference (P < 0.001) was shown between the two groups.
Measurement of osteoclastic precursor cell ratio
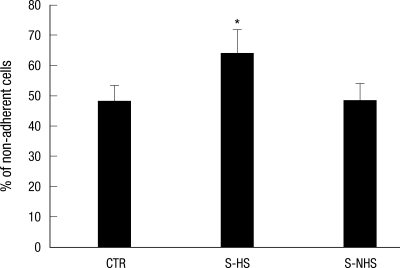
The ratio of the non-adherent cells containing osteoclastic precursor cells was 47.8% ± 12.5% for the control group, 64.2% ± 13.8% on the hemiplegic side of the experimental group and 48.2% ± 12.8% on the non-hemiplegic side of the experimental group. In comparison to the control group, significantly higher ratio was observed on the hemiplegic side of the experimental group (P = 0.013). There also was a significant difference between the hemiplegic side and the non-hemiplegic side of the experimental group (P = 0.001) (Fig. 1).
Fig. 1.
Percentage of non-adherent cells from bone marrow. CTR, controls; S-HS, hemiplegic side of the stroke group; S-NHS, non-hemiplegic side of the stroke group. Data are the mean ± SEM. *P < 0.05 control.
Measurement of TRAP positive cells
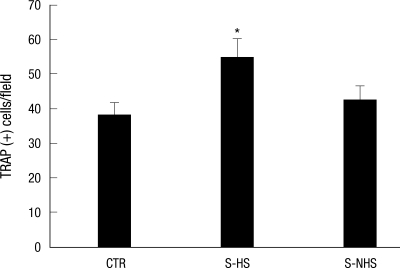
The number of TRAP positive cells was 38.1 ± 8.2 for the control group, 54.9 ± 11.1 for the hemiplegic side of the experimental group, and 42.6 ± 11.3 for the non-hemiplegic side of the experimental group. Compared to the control group, the number was significantly higher (P = 0.001) on the hemiplegic side of the experimental group. In comparison of the hemiplegic and the non-hemiplegic side of the experimental group, significant difference was observed (P = 0.29) (Fig. 2, 3).
Fig. 2.
Tartrate resistant acid phosphatase (TRAP) positive cells from bone marrow. CTR, controls; S-HS, hemiplegic side of the stroke group; S-NHS, non-hemiplegic side of the stroke group. Data are the mean ± SEM. *P < 0.05 control.
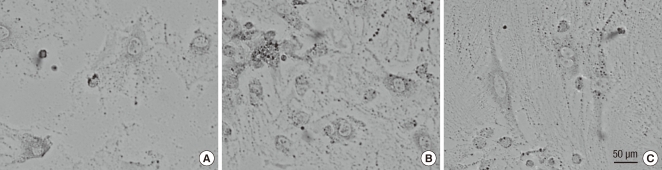
Fig. 3.
Photomicrographs of osteoclasts in primary culture obtained from bone marrow of rats, TRAP stain, magnification ×100. (A) Control. (B) Hemiplegic side of the stroke rats. (C) Non-hemiplegic side of the stroke rats.
Measurement of bone resorption area
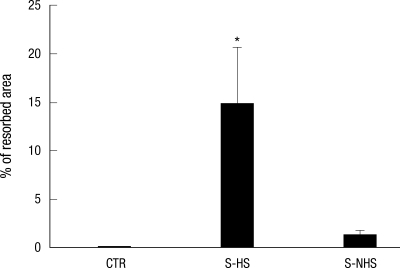
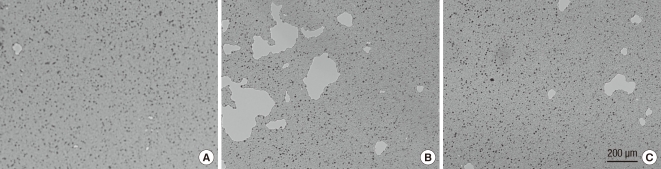
The percentage of resorbed area on an OAAS™ plate was measured to be 0.08% ± 0.17% in the control group, 14.8% ± 6.5% on the hemiplegic side of the experimental group and 1.3% ± 1.4% on the non-hemiplegic side of the experimental group. Compared to the control group, a significant difference was observed on the hemiplegic side of the experimental group (P < 0.001). Significant difference was also shown between hemiplegic and non-hemiplegic side of the experimental group (P = 0.003) (Fig. 4, 5).
Fig. 4.
The percentage of mineral surface resorbed by osteoclasts on OAAS™ plate. CTR, controls; S-HS, hemiplegic side of the stroke group; S-NHS, non-hemiplegic side of the stroke group. Data are the mean ± SEM. *P < 0.05 control.
Fig. 5.
Photomicrographs of pits formed by osteoclasts from rats, magnification ×25. (A) Control. (B) Hemiplegic side of the stroke group. (C) Non-hemiplegic side of the stroke group.
DISCUSSION
Monocytes and macrophage precursor cells in the bone marrow differentiate to preosteoclast. Then, TRAP gene is gradually expressed, and several cells fuse together to form a polykaryocyte. The final form is an activated osteoclast (11).
In this study, the ratio of the non-adherent cells to total bone marrow cells was significantly increased on the hemiplegic side of the experimental group compared to the control group and the non-hemiplegic side. Although the total number of bone marrow cells did not differ by large, the ratio of the non-adherent cells to total bone marrow cells increased. Since the non-adherent cells containing osteoclastic precursors also contained monocytes and macrophage precursor cells, the above results suggest that compositional changes occur with a propensity of increased ratio of osteoclastic precursor cells in bone marrow from the early stages of stroke. The number of TRAP positive cells after osteoclastic precursors culture showed a 40% increase on the hemiplegic side of the MCA occlusion group compared to the control group. We cultured the same number of the non-adherent cells, but a larger number of TRAP positive cells was measured for the hemiplegic side of the MCA occlusion group compared to the control group. This indicates that the formation and differentiation of osteoclasts in bone marrow are accelerated from the early stage of stroke. Bone resorption area measured after culturing the osteoclastic precursors on OAAS™ plate was increased by a factor of over 100 in the hemiplegic side of ischemic stroke compared to the control group. The number of TRAP positive cells was about 40% higher in the experimental group than the control group, but a difference of over 100 times for bone resorption area measured on OAAS™ plate showed an increased potential of osteoclastic activity in the MCA occlusion group. As such, the formation and differentiation of osteoclasts in the hemiplegic bone marrow are accelerated from the early stages of stroke, thereby increasing the potential of bone resorption and accelerating the development of osteoporosis on the hemiplegic side.
According to recent studies, excessive bone loss is present in infectious disease. Activated T cells increase the expression of receptor activator of nuclear factor κB ligand (RANKL), and thereby promote osteoclastic activity (12, 13). Acute post-stroke period is vulnerable to infection for humans as well as experimental animals. Therefore, the possible post-stroke infection may be another cause of promoting osteoclastic activity.
A recent study reported that 16.4%-38.5% of hip fracture patients have a past history of stroke (3). In stroke patients with unilateral stroke and persisting paresis at the time of fracture, 62.5% have their fracture on the paretic side. Hip fracture is more common in patients with less severe stroke than in patients with more severe stroke because mobility is better in those with less severe stoke and they have more chance to fall down. This study suggests that reduced bone density on the lower limb on the hemiplegic side as an important cause (3).
Grano et al. (14) reported on the increased bone resorption and formation of osteoclasts in the bone marrow within five days of removal of weight bearing on rat hindlimb. Sato et al. (6) reported on the increased biochemical marker which shows bone resorption one week after stroke. In accordance to the results from previous studies, we hypothesize that the increase in osteoclastogenesis in the bone marrow cells and bone resorption that were detected in this study, were also caused by reduced weight bearing from immobilization of the hemiplegic side. Looking at this, active weight bearing on the hemiplegic side from the initial stages of stroke should be an important therapeutic point in order to reduce the effect of immobilization.
This study has some limitations. Firstly, we demonstrated only the change of osteoclast differentiation and the potential of bone resorption in the early stage of stroke, because we speculated that the rate of bone loss in the early stage of stroke was probably accelerated with a disproportionate increase in bone resorption (15). However, osteoporosis is influenced not only by the changes of osteoclast differentiation and bone resorption, but also by the changes of osteoblast differentiation and bone formation. Therefore, additional studies about the changes of osteoblast differentiation and bone formation might be needed. Secondly, the percentage of resorbed area on OAAS™ plate was measured to be 0.08% in the control group, but this result was so small that it may be doubted whether the 3 × 105 cells cultured on the plate were not enough to measure bone resortion area or an OAAS™ plate was not adequate for measuring a tiny resorption area. Lastly, this study has another limitation from small sample size.
Since osteoclastogenesis and osteoclast differentiation accelerate and bone resorption increases at the early stage of stroke, bone resorption inhibitors such as bisphosphonates might have the prevention effect against the osteoporosis on the hemiplegic limb (16). Poole et al. (17) reported that zoledronate therapy is associated with a reduction in osteoclastsic cell numbers. Another study showed that treatment with etidronate increases bone mineral density in chronically hospitalized patients with stroke, and may prevent hip fracture (18). Further studies are necessary to measure the effectiveness of prevention of the osteoporotic fracture on the hemiplegic side by the use of bisphosphonates in the early stage of stroke. Furthermore, additional studies are needed to estimate the amount of weight bearing time required in order to exert its preventive effect on osteoporosis in the early stage of stroke.
In summary, this study demonstrates that osteoclastogenesis and osteoclast differentiation are accelerated and the potential of bone resorption is increased in the hemiplegic bone marrow and these changes are detected as early as within the first week after MCA occlusion in SD rats. After stroke, changes that lead to osteoporosis occur from the early periods of stroke; since hemiplegic patients have an increased propensity to have an unstable gait or movement pattern, falling down on the hemiplegic side can easily result in hip fractures. The results of this study emphasize the importance of active prevention and management of osteoporosis from the early stages of stroke.
References
- 1.Kanis JA, Glüer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000;11:192–202. doi: 10.1007/s001980050281. [DOI] [PubMed] [Google Scholar]
- 2.Gabriel SE, Tosteson AN, Leibson CL, Crowson CS, Pond GR, Hammond CS, Melton LJ., 3rd Direct medical costs attributable to osteoporotic fractures. Osteoporos Int. 2002;13:323–330. doi: 10.1007/s001980200033. [DOI] [PubMed] [Google Scholar]
- 3.Ramnemark A, Nilsson M, Borssén B, Gustafson Y. Stroke, a major and increasing risk factor for femoral neck fracture. Stroke. 2000;31:1572–1577. doi: 10.1161/01.str.31.7.1572. [DOI] [PubMed] [Google Scholar]
- 4.Ramnemark A, Nyberg L, Lorentzon R, Englund U, Gustafson Y. Progressive hemiosteoporosis on the paretic side and increased bone mineral density in the nonparetic arm the first year after severe stroke. Osteoporos Int. 1999;9:269–275. doi: 10.1007/s001980050147. [DOI] [PubMed] [Google Scholar]
- 5.Choi EK. The effects of decreased physical activity to bone mineral density in hemiparetic stroke patients. Korean J Med. 2005;69:387–394. [Google Scholar]
- 6.Sato Y, Kuno H, Kaji M, Etoh K, Oizumi K. Influence of immobilization upon calcium metabolism in the week following hemiplegic stroke. J Neurol Sci. 2000;175:135–139. doi: 10.1016/s0022-510x(00)00298-7. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y, Maruoka H, Oizumi K, Kikuyama M. Vitamin D deficiency and osteopenia in the hemiplegic limbs of stroke patients. Stroke. 1996;27:2183–2187. doi: 10.1161/01.str.27.12.2183. [DOI] [PubMed] [Google Scholar]
- 8.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 9.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 10.Mehta SH, Dhandapani KM, De Sevilla LM, Webb RC, Mahesh VB, Brann DW. Tamoxifen, a selective estrogen receptor modulator, reduces ischemic damage caused by middle cerebral artery occlusion in the ovariectomized female rat. Neuroendocrinology. 2003;77:44–50. doi: 10.1159/000068332. [DOI] [PubMed] [Google Scholar]
- 11.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 12.Rauner M, Sipos W, Pietschmann P. Osteoimmunology. Int Arch Allergy Immunol. 2007;143:31–48. doi: 10.1159/000098223. [DOI] [PubMed] [Google Scholar]
- 13.Jones D, Glimcher LH, Aliprantis AO. Osteoimmunology at the nexus of arthritis, osteoporosis, cancer, and infection. J Clin Invest. 2011;121:2534–2542. doi: 10.1172/JCI46262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grano M, Mori G, Minielli V, Barou O, Colucci S, Giannelli G, Alexander C, Zallone AZ, Vico L. Rat hindlimb unloading by tail suspension reduces osteoblast differentiation, induces IL-6 secretion, and increases bone resorption in ex vivo cultures. Calcif Tissue Int. 2002;70:176–185. doi: 10.1007/s00223-001-2034-6. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Fujimatsu Y, Kikuyama M, Kaji M, Oizumic K. Influence of immobilization on bone mass and bone metabolism in hemiplegic elderly patients with a long-standing stroke. J Neurol Sci. 1998;156:205–210. doi: 10.1016/s0022-510x(98)00041-0. [DOI] [PubMed] [Google Scholar]
- 16.Carano A, Teitelbaum SL, Konsek JD, Schlesinger PH, Blair HC. Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J Clin Invest. 1990;85:456–461. doi: 10.1172/JCI114459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole KE, Vedi S, Debiram I, Rose C, Power J, Loveridge N, Warburton EA, Reeve J, Compston J. Bone structure and remodeling in stroke patients: early effects of zoledronate. Bone. 2009;44:629–633. doi: 10.1016/j.bone.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato Y, Iwamoto J, Honda Y. Beneficial effect of etidronate therapy in chronically hospitalized, disabled patients with stroke. J Stroke Cerebrovasc Dis. 2010;19:198–203. doi: 10.1016/j.jstrokecerebrovasdis.2009.04.006. [DOI] [PubMed] [Google Scholar]