Abstract
Background User fees for primary care tend to suppress utilization, and many countries are experimenting with fee removal. Studies show that additional inputs are needed after removing fees, although well-documented experiences are lacking. This study presents data on the effects of fee removal on facility quality and utilization in Afghanistan, based on a pilot experiment and subsequent nationwide ban on fees.
Methods Data on utilization and observed structural and perceived overall quality of health care were compared from before-and-after facility assessments, patient exit interviews and catchment area household surveys from eight facilities where fees were removed and 14 facilities where fee levels remained constant, as part of a larger health financing pilot study from 2005 to 2007. After a national user fee ban was instituted in 2008, health facility administrative data were analysed to assess subsequent changes in utilization and quality.
Results The pilot study analysis indicated that observed and perceived quality increased across facilities but did not differ by fee removal status. Difference-in-difference analysis showed that utilization at facilities previously charging both service and drug fees increased by 400% more after fee removal, prompting additional inputs from service providers, compared with facilities that previously only charged service fees or had no change in fees (P = 0.001). Following the national fee ban, visits for curative care increased significantly (P < 0.001), but institutional deliveries did not. Services typically free before the ban—immunization and antenatal care—had immediate increases in utilization but these were not sustained.
Conclusion Both pilot and nationwide data indicated that curative care utilization increased following fee removal, without differential changes in quality. Concerns raised by non-governmental organizations, health workers and community leaders over the effects of lost revenue and increased utilization require continued effort to raise revenues, monitor health worker and patient perceptions, and carefully manage health facility performance.
Keywords: User fees, health financing, utilization, quality, access, Afghanistan
KEY MESSAGES.
A health financing pilot study showed increased utilization, but little difference in quantitative measures of observed or perceived quality of care at primary care facilities where user fees were removed.
Following a national user fee ban for primary care services in April 2008, visits for curative care, but not necessarily for preventive care (which were primarily free before the ban), have increased significantly, without major adverse effects on drug stock-outs.
Continued donor support for primary care and monitoring of quality in Afghanistan have helped to ensure a smooth transition to free services, but additional research is warranted on mechanisms to provide discretionary income to facilities and to closely monitor health worker and patient perceptions of quality under free services.
Introduction
A consensus has emerged that user fees are likely to suppress utilization of care in low-income settings (Gilson 1997; Hutton 2004; Lagarde and Palmer 2008) and can contribute to indebtedness and poverty (Russell 2004). They have seldom produced their intended benefits in terms of quality, equity or efficiency (Gilson et al. 2000; van der Geest et al. 2000; Sepehri and Chernomas 2001), after becoming widespread in sub-Saharan Africa in the 1980s as a means to raise revenue, improve the quality of services locally and increase utilization (Akin et al. 1987; Hutton 2004). Several groups, including researchers, donors, non-governmental organizations (NGOs), and politicians, have in recent years called for the carefully planned abolition of user fees (Pearson 2004; Gilson and McIntyre 2005; Save the Children UK 2005; CHOGM 2009).
A handful of countries have abolished user fees for health services, and many more are actively experimenting with exemptions for specific services, such as deliveries (Witter 2009). Since 2007, Zambia, Burundi, Niger, Liberia, Kenya, Senegal, Lesotho, Sudan and Ghana have experimented with removal of fees for key primary health services (Yates 2009). Documentation of experiences from the few countries that have abolished all fees for public sector health services, including Uganda and South Africa, provides valuable lessons about the effects of fee removal. In Uganda, abolition of user fees in 2001 led to an increase in curative care and smaller increases in preventive and promotive care, and these increases were pro-poor (Burnham et al. 2004; Nabyonga et al. 2005). Removal of fees in Uganda was accompanied by increased funding (US$0.02 per capita for drugs and US$0.52 per capita overall) to compensate for the loss of user fee revenues and increased workloads (Nabyonga-Orem et al. 2008). Early evidence indicates that the availability of drugs did not decline in Uganda following fee removal, and users’ perceptions remained relatively favourable. Evidence on the effects of user fee removal and exemptions on quality of services is limited, although experiences in Ghana and Senegal with exemptions for deliveries indicated that their effect on quality was negligible, according to measured indicators of both inputs and processes, as well as perceptions among staff and community members (Witter 2009).
In South Africa, a study found significant increases in curative care utilization, but not preventive or promotive care, following removal of user fees for pregnant women and children and subsequent complete abolition of fees at primary health care clinics (Wilkinson et al. 2001). Critics contend that these reforms were implemented hastily without sufficient planning, leading to congestion in clinics and reduced consultation times (Wilkinson et al. 2001). Outpatient visits in Kenya, where fees were introduced in 1989, increased to pre-fee levels when fees were briefly suspended in 1990, having declined 27–46% under fees (Mbugua et al. 1995; Collins et al. 1996).
Although user fee removal has led to increases in curative, and in some cases, preventive care, health workers can be dissatisfied with fee removal, particularly if additional facility funds or health worker incentives are not made available (Burnham et al. 2004; Witter et al. 2007a; Witter et al. 2007b). Researchers have pointed to the need to maintain health worker motivation and appropriate staffing levels following fee removal (Campbell et al. 2009), and have advocated for additional funding to cope with increased utilization. User fee removal strategies need to address a number of related issues and unintended consequences, namely to: ensure adequate drugs are available at local levels; find mechanisms to allow discretionary funds at local levels; conduct widespread public information campaigns about fee removal; adequately communicate the strategy to health workers; and monitor key trends (e.g. utilization, stock-outs, health worker and patient perceptions) (Gilson and McIntyre 2005; Ridde and Morestin 2011). Still, carefully documented evidence about the effects of removing user fees is limited, particularly concerning experiences of fee removal on a large scale.
In 2005, Afghanistan implemented a health financing pilot study to examine the effects of various community financing approaches, including free services, on quality of services and financial access to care. In April 2008, the Ministry of Public Health (MoPH) of Afghanistan officially banned user fees at the primary care level, citing the results of the health financing pilot study. This paper draws from results of the pilot study, as well as trends following the nationwide user fee ban in Afghanistan, to synthesize lessons about the effects of user fee removal on quality—both observed facility structural quality and overall perceived quality of care—and utilization.
Background
Afghanistan is an extremely poor, post-conflict country that developed a basic package of primary care services following the fall of the Taliban regime in 2001. Access to primary care services was rapidly expanded to the population, using contracting-out of service delivery to NGOs in most provinces (covering about 65% of all health facilities), contracting-in mechanisms in three provinces (10% of facilities) and Ministry of Public Health provision without contracting in one-quarter of the health facilities (Arur et al. 2010). By 2006, 82% of the population lived in districts that had contracting mechanisms in place for delivering the primary care package (Waldman et al. 2006). Donors spend between US$2 and US$5 per capita (average ∼US$4 per capita) to fund the basic package of health services (BPHS), which remains almost entirely donor-financed (Palmer et al. 2006; Loevinsohn and Sayed 2008). When contracts began, there were no policies or guidelines on cost-sharing at local levels, and most facilities charged some type of fees to patients: in 2004, 70.4% of basic health centres (BHCs), comprehensive health centres (CHCs) and district hospital outpatient departments charged user fees, and this increased to 84.3% by 2007 (Ministry of Public Health 2008a). Most facilities had a fee for registration only, although some charged for both registration and drugs.
In addition to the BPHS, which comprises about one-third of the overall health budget, most of the Afghan health sector is heavily dependent on donor funding (Ministry of Public Health 2009). The Ministry of Finance estimates that donor funding comprised 56% of the 1385 budget (based on the solar calendar, roughly equivalent to March 2005 to March 2006) and 62% of the 1386 budget, accounting for well over half the total amount spent on health in Afghanistan (Ministry of Public Health 2007a). The Ministry of Public Health has been slow to develop a health financing policy, with the first draft approved only in 2007.
To assist the fledgling government in making key health financing policy decisions, the Ministry of Public Health commissioned a pilot study to compare various community financing mechanisms on their ability to: (1) raise revenues; (2) improve quality; (3) ensure financial access to care; and (4) enhance community ownership of health services. The pilot study was designed with technical assistance from a third-party research organization, implemented in summer–autumn 2005 and evaluated in spring 2007. Three interventions were piloted: (1) a standardized user fee scheme, with separate fees for registration and for drugs, and a fee waiver card scheme for very poor and female-headed households; (2) a community health fund (voluntary pre-payment scheme); and (3) free services, which were considered an intervention as the majority of facilities were charging fees at the time.
The pilots were implemented in 10 provinces. The managing service provider responsible for the province-wide BPHS contract (an NGO in seven provinces and the MoPH in three) nominated five facilities (four in the case of one province) to be eligible for participation in the pilot, based on having sound administrative systems and full staffing. Because of concerns about capacity to implement multiple schemes and to assure full participation of the provider organizations, the managing provider selected two of the three interventions to pilot. Two facilities were randomized to one intervention; two to the other; and the last facility served as the control. Control facilities simply continued whichever cost-sharing arrangement was in place at baseline, and baseline and follow-up data were collected from all sites. The community health fund was evaluated in autumn 2006 and subsequently discontinued due to low enrolment and is discussed elsewhere (Rao et al. 2009); after evaluation, the eight facilities involved were no longer part of the pilot study. In total, 41 clinics remained in the pilot study (21 randomized to user fees; 10 to free services; and 10 as controls). Table 1 provides an overview of the number of pilot facilities by province fee-status at baseline.
Table 1.
Number of health financing pilot clinics in selected provinces, and baseline fees
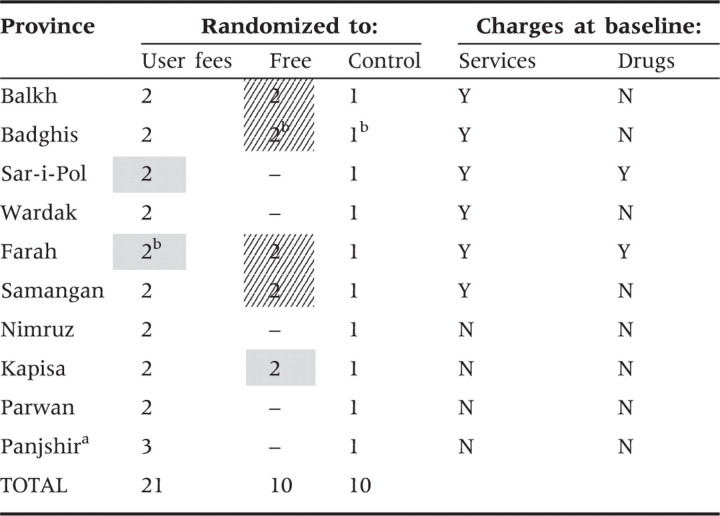 |
Note: Diagonally-shaded cells represent eight facilities where user fees were removed. Facilities randomized to ‘Control’ and cells shaded grey represent the comparison group for analysis in this study. The comparison group therefore includes facilities that experienced no change in user fee levels.
aPanjshir province implemented only user fees at three facilities.
bFacility not able to be surveyed at follow-up (one of two user fee facilities in Farah; both free facilities and the control facility in Badghis).
User fee ban
Citing the results from the health financing pilot study (which were presented to MoPH officials, donors, NGOs and other health sector stakeholders at several decision-making and information-sharing meetings), the MoPH decided to officially ban user fees at BPHS facilities in April 2008, issuing its first official policy on cost-sharing. MoPH officials used the policy-making process to hear additional opinions of donors, health system experts and consultants working in Afghanistan, as well as thoughts and concerns from various stakeholders. NGOs working in the health sector expressed concern about shortages of drugs and supplies, as well as decreases in their operating budgets, as a result of increased visits and loss of user fee revenues. Some of the NGOs at the time used fee revenues to supplement their central operating budgets, whereas others retained fees at facilities, where they were used locally as discretionary income. A major concern among NGOs providing BPHS services was a sudden increase in patient load due to healthier patients coming to take advantage of free services and medications, tying up the time of health workers. At the suggestion of NGOs, the MoPH held several meetings with donors around the time of the policy decision to see if they would be willing to increase funding for BPHS contracts under a user fee ban.
The top policy-making body of the MoPH banned user fees at the primary care level, effective on 17 April 2008. From that date, BPHS facilities were to cease charging any fees to patients. As a result of expressed concerns, the MoPH asked the Health Financing Task Force to conduct a survey of NGOs to assess their experiences with fee removal and gather suggestions for handling the user fee ban successfully. In addition, the HMIS and the Monitoring and Evaluation Departments were tasked with monitoring utilization and other trends following fee removal, to provide data to donors after 6 months to justify increased financial support and to assess any adverse effects, over a 3-year time period.
Methodology
This paper draws from data collected during the evaluation of the health financing pilot schemes in 2007, detailed below, as well as from the national routine administrative reporting database, to examine country-wide trends in new outpatient visits and stock-outs following the April 2008 user fee ban.
Health financing pilot data
Data for the health financing pilot evaluation come from baseline and follow-up facility assessments, patient exit interviews, catchment area household surveys and the health management information system (HMIS) database. Facility assessments were conducted to assess structural quality of care at pilot facilities, with data collected by trained surveyors about cleanliness/need for repairs, facility infrastructure, drug availability and equipment functionality, among other indicators, using visual verification techniques and interviews with health facility staff. Insecurity precluded the follow-up assessment from taking place at six facilities (three user fee, two free services and one control), leaving 35 facilities surveyed at both times, 20 of which are included in this analysis (six facilities with fee removal and 14 with no change in fee levels). At each surveyed facility, five exit interviews with caretakers of patients younger than 5 years and five interviews with patients (or their caretakers) 5 years and older were conducted using a systematic random sample of patients who were first observed during their encounter with the provider. Patients were asked to rate their level of agreement with eight statements about facility quality, and were asked additional information about health expenditures and travel to the facility. These assessments and exit interviews were part of a larger annual nationwide monitoring effort at BPHS facilities, and further details on sampling methodology are provided elsewhere (Peters et al. 2007).
At baseline, a household survey was conducted in two randomly selected villages with greater than 100 households in the catchment area of each pilot facility. In each village, a random start was selected and the nearest next-door method was used to select 25 women aged 18 years and older with a child aged 3 years and under for interview (Turner et al. 1996). The survey asked about illness in the past 30 days, care-seeking patterns, health expenditures and perceptions of the pilot facility by asking participants to rate their level of agreement (on a four-point scale) with eight statements about different aspects of facility quality. At baseline, only 68.9% of households had heard of the pilot health facility, and perceptions from only these households were included in the analysis. The survey was repeated at follow-up in the same villages whenever possible, and a third village was added to free services and control facilities to increase sample size, for a total of 75 interviews per facility. Insecurity precluded the follow-up household survey from taking place at six facilities (three user fee; one free services; and two control), only one of which overlapped with the facilities dropped from the facility assessment. Sixty-nine of the total 107 of villages surveyed (64.5% of villages, containing 77.4% of households surveyed) were common to both time periods. Further methodological details and results of the health financing pilot study are provided elsewhere (Ministry of Public Health 2008b), available at: http://www.moph.gov.af/en/reports/Health-Financing-Pilots-Report-2008-English.pdf.
Health financing pilot data analysis
Eight of the facilities randomized to free services previously charged user fees to patients, with two of these facilities charging both registration and drug fees (Table 1). Analysis of the facility assessment, patient exit interview and household survey data compared changes in quality and utilization from baseline to follow-up at these six facilities with complete before-and-after data (two clinics could not be surveyed due to insecurity at follow-up) to the group of facilities where the fee structure did not change over the course of the pilot (n = 14 with complete data, as two of these facilities could not be surveyed at follow-up). This latter group of facilities included nine control facilities (60% of which charged fees), two facilities randomized to free services where services were previously free, and three facilities randomized to user fees that previously charged for registration and for drugs. A sensitivity analysis was conducted omitting from the comparison group the four facilities randomized to user fees that previously charged both registration and medication fees. At these facilities, although the actual fee levels for curative care did not change, preventive/promotive services were made free under the pilot, a waiver card scheme was implemented, and revenues were retained at the facility and used to improve quality of care. A summary of the datasets used in the health financing pilot evaluation is provided in Table 2.
Table 2.
Sample sizes for health financing pilot evaluation data sources, by facility type
| Data source | No change |
Fees removed |
||
|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |
| Facility assessments | 14 | 14 | 6 | 6 |
| Patient exit interviews | 123 | 136 | 58 | 60 |
| Household survey | 479 | 796 | 198 | 573 |
| HMIS data on facility visits | 70a | 70 | 8 | 8 |
Note: Only 54.2% of households in facilities where fees were subsequently removed and 78.8% of facilities with no subsequent change in fee levels had heard of the pilot facility at baseline and were included in the household survey results. At follow-up, nearly all households had heard of the pilot facility, and an additional village was added to some facilities for data collection, significantly increasing the sample size at follow-up.
aIncludes 15 health financing pilot facilities and 55 other ‘control’ facilities in each province where fee levels did not change over the course of the pilot study.
From the facility assessments, a structural quality index was constructed by summing 31 binary items of observed facility quality across the four quality domains (possible range: 0 to 31). Perceived quality was measured by summing eight four-point Likert-scale items asking responding patients and household members to rate their level of agreement with statements such as, ‘The health unit is clean’ and ‘The health workers did a good job of explaining the illness’. The eight-item scale of key quality components, including items pertaining to convenience of facility location, cleanliness of the facility, respectfulness of facility staff, trust in the abilities and skills of the health workers, quality of health workers’ illness explanations, quality of health workers’ treatment explanations, ease of obtaining prescribed medications, and satisfaction with privacy during the visit, yielded a scale ranging from 8 to 32. Perceived quality among patients and households in this study therefore represents various aspects of quality of care, including some elements of structural quality (cleanliness, ease of obtaining medications) and some process quality measures—both technical (trust in abilities and skills of health workers) and interpersonal (respectfulness of facility staff, quality of health workers’ illness and treatment explanations, and privacy during visit).
Significant differences in changes over time between fee removal facilities and those where the fee structure did not change were tested by examining the β3 coefficient in the following difference-in-differences regression model, adjusting for clustering at the patient and household levels using the survey commands in Stata 10.0:1
In the difference-in-differences regression model above, β0 represents the average level of the outcome (e.g. perceived quality) at baseline in facilities where fees did not change during the pilot period; β1 represents the average change over time among facilities where fees did not change; β2 represents the average difference at baseline between facilities where fees did not change and those where fees were later removed; and β3 is the coefficient of interest, representing the difference in the change over time between facilities where fees were removed and those with no change in fees.
Data on new outpatient visits were extracted from the HMIS database for pilot facilities, for the 1-year period prior to pilot implementation and the 1-year period following implementation. Data for all facilities not participating in the pilot in a given province were added to the control facility arm, since their cost-sharing arrangements had not changed during the pilot period (see Table 2 for sample size). For each facility, the average monthly number of visits was calculated for the 1-year period prior to the pilot implementation and for the 1-year period after pilot implementation, resulting in two data points per facility. The difference-in-differences regression model specified above was used to test whether the changes in visit volumes over time differed significantly between the two groups. Due to large amounts of missing HMIS data prior to pilot implementation for other services, such as antenatal care, deliveries and DPT3 immunization, it was not possible to analyse changes in preventive/promotive services.
Routine administrative data analysis
Routine reporting data were also used to examine trends in utilization and stock-outs related to the nationwide user fee ban. HMIS data on facility utilization, including new outpatient visits, DPT3 doses given, all facility deliveries, antenatal care visits and drug stock-outs, were extracted for all BHCs, CHCs and district hospitals for the 3-year period prior to the user fee ban in April 2008, and for 14 months following the ban (total facilities = 1250). Facilities that were missing data for more than 3 months during this period (n = 549) were dropped from the analysis. Most of these dropped facilities (421, or 76.7%) were missing at least 1 year of data, as many facilities began their operations in the middle of this period, when Afghanistan was expanding its number of static facilities. Therefore, potential bias from dropping these facilities from analysis is minimal.
Service use data was seasonally de-trended by calculating the overall mean for each facility (across all years) for each month in the series. For each facility, the monthly mean was subtracted from the number of visits in that month in a given year. Therefore, the data represent monthly deviations from the series monthly mean for each facility. An interrupted time series regression model was used to test whether there was a significant increase in services once they were made free, by analysing both the increase in the month following the user fee ban, as well as the rate of increase in visits in the 14-month period following the ban compared with the rate in the 3-year period before. The following regression model was used:
where Yit is the visit level at facility i at time t. β1 is the slope coefficient for the time trend before the user fee ban at month 37 (with time points coded from 1 to 37, the intervention month, and 37 thereafter). Feeban is coded zero for months 1 to 37 and 1 for months 38 to 51; its coefficient β2 represents the immediate impact of the user fee ban. β3 represents the trend in visits after the user fee ban, and the difference between β3 and β2 represents a change in the visit trend following the ban. The Wooldridge test (Wooldridge 2002) detected serial correlation among the errors, due to repeated observations from each facility, and we therefore used a generalized estimating equations (GEE) model with a first-order autocorrelation modelling of the error term (Diggle et al. 2002).
Approval for the pilot study was obtained from the Johns Hopkins Bloomberg School of Public Health's Institutional Review Board, as well as from the Ethical Review Board of the Ministry of Public Health in Kabul, Afghanistan. Informed consent was obtained from all interviewed health workers, patients and households, primarily through verbal consent given the low literacy of Afghanistan's population.
Results
Facility assessments, exit interviews and household surveys
The structural quality index of observed facility quality improved from 18.5 to 28.0 (out of a possible total of 31 points) from 2004 to 2007, reflecting the continually improving quality at health facilities as they were being monitored. The gains were not significantly different among the facilities where fees were removed compared with those with no change in fees (10.1 vs 9.2 points). Perceived quality among patients was relatively high at baseline and increased by 1.6 points overall at follow-up, but this did not differ by facility fee status (Table 3). Similarly, the small change in households’ perceived quality of care from baseline to follow-up (0.4 points overall) did not differ by fee status of the facility.
Table 3.
Observed and perceived quality at pilot facilities, by time period and facility fee status (mean, se)
| No change |
Fees removed |
|||||
|---|---|---|---|---|---|---|
| Observed structural quality | Patient perceived quality | Household perceived quality | Observed structural quality | Patient perceived quality | Household perceived quality | |
| Baseline | n = 14 | n = 123 | n = 479 | n = 6 | n = 58 | n = 198 |
| 18.4 | 26.1 | 24.8 | 18.7 | 26.5 | 24.6 | |
| (1.7) | (0.7) | (0.5) | (3.0) | (1.6) | (1.4) | |
| Follow-up | n = 14 | n = 136 | n = 796 | n = 6 | n = 60 | n = 573 |
| 27.6 | 27.8 | 25.0 | 28.8 | 28.0 | 25.3 | |
| (1.0) | (0.4) | (0.3) | (0.7) | (1.0) | (0.3) | |
| Difference | 9.2 | 1.8 | 0.2 | 10.1 | 1.4 | 0.7 |
Note: Possible range of structural quality scale: 0 to 31; possible ranges of patient and household perceived quality: 8 to 32. Changes among the facilities where fees were removed not significantly different than those at facilities with no change in fees, for any of the indicators. Significance of changes assessed by β3 in the linear regression: Y = β0 + β1 Post + β2 Feesremoved + β3 Post*Feesremoved + ε. Patient- and household-level models use the Taylor Linearization series adjustments for survey data in Stata 10.0.
Sources: Facility assessments, patient exit interviews and catchment area household surveys.
Despite randomization of facilities to one of two selected interventions within each province, facilities randomized to free services had significantly lower awareness of their services among catchment area households at baseline, and sick household members were only half as likely to seek care there first when ill (P = 0.01). These facilities experienced a greater increase in the percentage of sick people in the catchment area seeking care there first from baseline to follow-up (24.2 percentage points compared with 12.1), but this difference was not statistically significant when accounting for clustering in the survey design (P = 0.20) (Table 4). Excluding the four user fee facilities from the ‘no change’ group did not significantly change the results for any of the outcomes. Changes in care-seeking were also not related to the baseline levels of poverty in the districts where each pilot facility was located (data not shown).
Table 4.
Percentage of sick household members seeking care first at pilot facility, by facility fee status (mean, se)
| No change | Fees removed | |
|---|---|---|
| Baseline | n = 683 | n = 388 |
| 58.4% | 34.3% | |
| (4.5%) | (7.1%) | |
| Follow-up | n = 1090 | n = 672 |
| 70.6% | 58.5% | |
| (3.7%) | (4.1%) | |
| Difference | 12.1 | 24.2 |
Note: Facilities in the fees removed group significantly less likely to seek care at pilot facility at baseline (P = 0.01). Change in the fee removal group not significantly greater than the change in the ‘no change’ group [Odds Ratio (OR) = 1.58, P = 0.20]. Change assessed by β3 coefficient in logistic regression model using survey commands in Stata 10.0: Log(careseek/1-careseek) = β0 + β1 Post + β2 Feesremoved + B3 Post*Feesremoved.
Source: Catchment area household surveys.
HMIS data for pilot facilities
Routine reporting data from the HMIS also reflected the greater increase in care seeking at pilot facilities where fees were removed. Visits at most facilities in Afghanistan were increasing during the study period, as communities were becoming more accustomed to using services and their quality was improving. The average number of monthly visits increased by more than 1000 patients in facilities where fees were removed (110% increase), compared with an increase of 317 visits (37% increase) at facilities with no change in their fee levels, P = 0.004 (Table 5). Facilities that previously charged both service and medication fees showed a significantly greater increase in visits compared with facilities that previously only charged for services (P < 0.001) and compared with facilities with no change in fee levels (P = 0.001). The two facilities randomized to free services where both medication and service fees were previously charged were in Farah province in western Afghanistan. Monthly visits at these two facilities increased by an average of 2588 visits in the year following user fee removal compared with the year before—about five times the rate at which visits increased in other facilities where fees were removed (Table 5).
Table 5.
Average monthly outpatient visits, 1 year pre-pilot study compared with 1 year post-pilot, by facility fee status (mean, sd)
| No change | Fees removed | |||
|---|---|---|---|---|
| All | Service fee only | Service & drug fees | ||
| Pre-pilot | n = 70 | n = 8 | n = 6 | n = 2 |
| 855.9 | 916.5 | 1071.8 | 450.3 | |
| (41.3) | (199.4) | (233.6) | (66.7) | |
| Post-pilot | n = 70 | n = 8 | n = 6 | n = 2 |
| 1172.9 | 1922.4 | 1550.3 | 3038.7 | |
| (53.5) | (348.4) | (318.7) | (463.6) | |
| Difference | 317.0 | 1006.0 | 478.5 | 2588.4 |
Note: Only the change in the fee removal facilities that previously charged both service and medications fees is significantly greater than the change in the group that had ‘no change’ in fees (P = 0.001), as well as compared with the fee removal group that previously only had service fees (P < 0.001).
Source: Health management information system (HMIS) data.
Trends in utilization and stock-outs following user fee ban
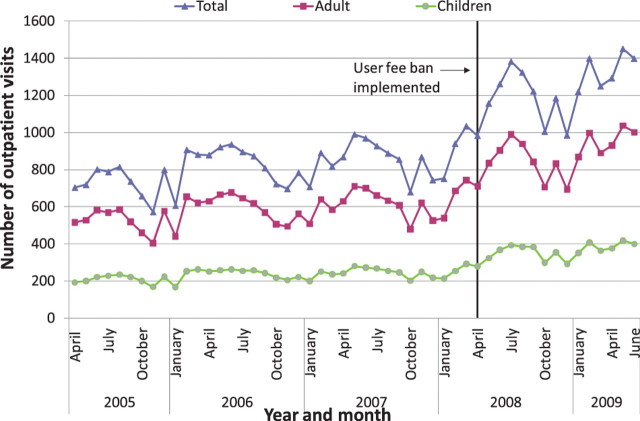
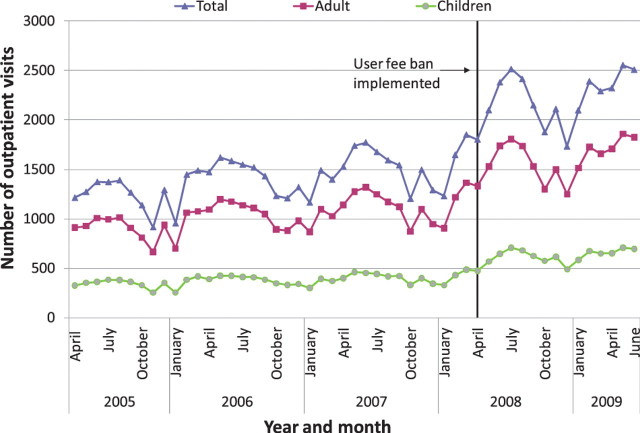
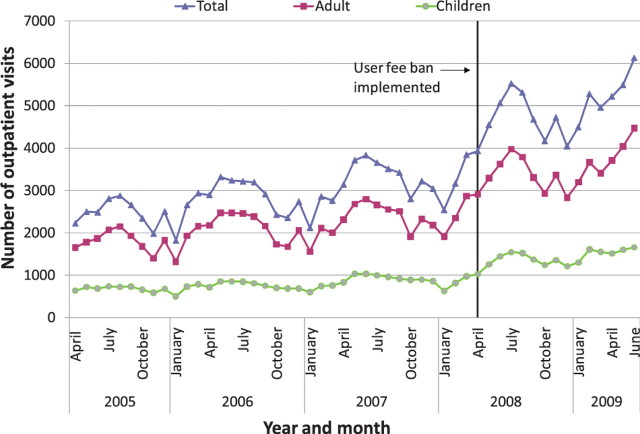
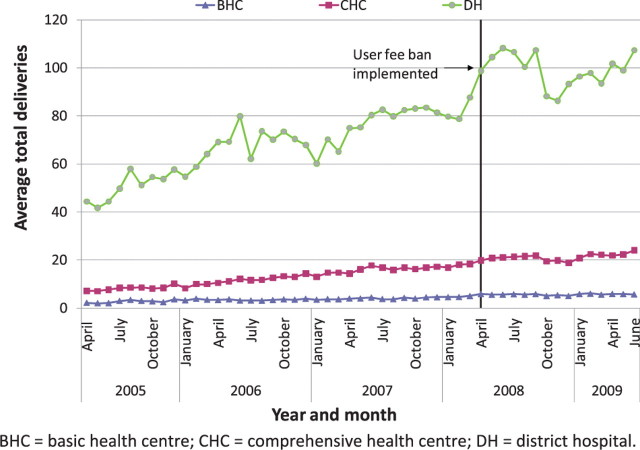
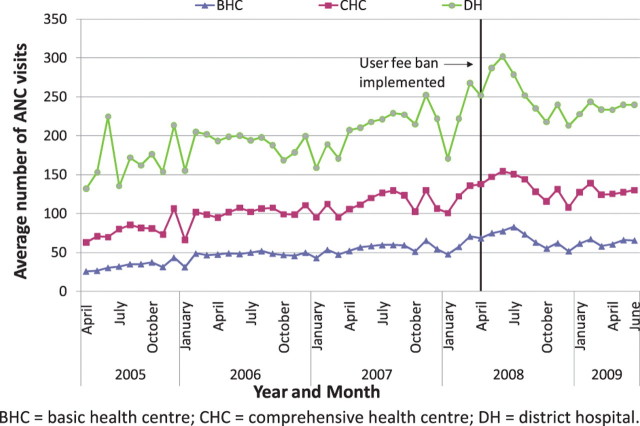
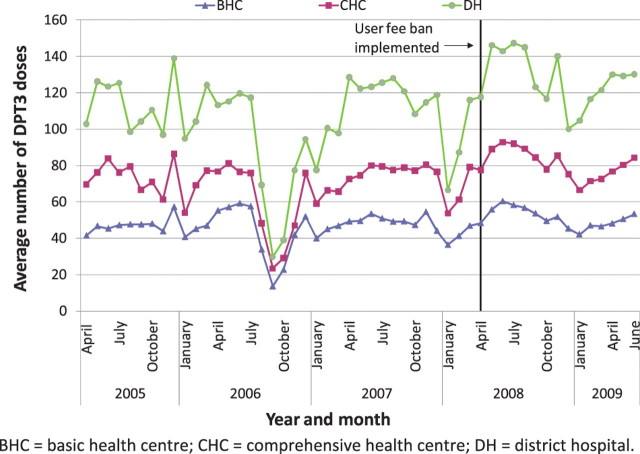
Nationally, it appears that new outpatient visits, which were rising overall before the user fee ban, increased immediately after the user fee ban, and continued to rise (Figures 1–3). The trend in antenatal care visits and institutional deliveries, where user fees had not been applied at most facilities previously, other than for drug costs for deliveries at some facilities, had an upward trend before the ban at district hospitals, but was flatter at CHCs and BHCs. These appeared to increase following the ban at district hospitals, but not noticeably at lower-level facilities (Figures 4 and 5). DPT3 doses given, which fluctuated significantly in the 14-month period before the ban, seemed to rise after the ban, but the gains were not sustained (Figure 6).
Figure 1.
Average outpatient visits at basic health centres (BHCs)
Figure 2.
Average outpatient visits at comprehensive health centres (CHCs)
Figure 3.
Average outpatient visits at district hospitals
Figure 4.
Average total deliveries, by facility type
Figure 5.
Average number of antenatal care visits, by facility type
Figure 6.
Average doses of DPT3, by facility type
As shown in Table 6, interrupted time series regression models confirmed that monthly outpatient visits increased significantly following the ban (by 149.1 visits on average at BHCs and by 560.7 at district hospitals, P < 0.001). Although increasing on average before the ban, monthly outpatient visits rose even faster after the ban at BHCs and CHCs, on average by 9.2 and 13.4 more visits per month (P < 0.001), and at district hospitals by 26.2 more visits per month, although this increase was not statistically significant. Regression models revealed no significant increase in deliveries following the user fee ban, and little change in delivery trends, which had been slowly rising. However, models indicated that deliveries increased slightly more slowly at CHCs and district hospitals following the user fee ban (P < 0.05 for both). Antenatal care visits increased significantly after the user fee ban, by 5.0, 7.2 and 15.5 visits on average, respectively, at BHCs, CHCs and district hospitals (P < 0.001, P < 0.01 and P < 0.05, respectively). However, these gains were not sustained and antenatal care visits decreased significantly following the initial increase, reversing the upward trend present before the ban (Table 6). The regression models for DPT3 doses compared only the 14-month period prior to the user fee ban with the 14-month period afterwards, given a huge increase in drug stock-outs 2 years prior to the ban resulting in dramatically reduced DPT3 doses given. DPT3 doses rose significantly in the month following the ban, from 7.5 at BHCs (P < 0.001) to 26.0 at district hospitals (P < 0.01), but this increase did not appear to be sustained, as DPT3 doses trended downward slightly at all facilities after the ban, and decreased significantly compared with the 14-month pre-ban period at CHCs (P < 0.01).
Table 6.
Interrupted time series regression coefficients for outpatient department visits, deliveries and DPT3 doses
| N | Fee removal coefficient (se) | Pre-slope coefficient (se) | Post-slope coefficient (se) | Post-pre coefficient (se) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Outpatient visits | |||||||||
| BHC | 329 | 149.14*** | (13.69) | 6.13*** | (0.42) | 15.33*** | (1.52) | 9.21*** | (1.65) |
| CHC | 226 | 256.72*** | (26.41) | 13.12*** | (0.86) | 26.48*** | (3.03) | 13.36*** | (3.32) |
| DH | 33 | 560.72*** | (134.33) | 36.14*** | (3.96) | 62.31*** | (14.67) | 26.17 | (15.78) |
| Deliveries | |||||||||
| BHC | 296 | 0.41 | (0.23) | 0.07*** | (0.01) | 0.03 | (0.02) | −0.04 | (0.03) |
| CHC | 266 | 0.38 | (0.47) | 0.35*** | (0.02) | 0.18** | (0.06) | −0.17* | (0.07) |
| DH | 39 | 7.32 | (4.25) | 1.27*** | (0.16) | −0.03 | (0.53) | −1.31* | (0.59) |
| Antenatal visits | |||||||||
| BHC | 320 | 5.02*** | (1.12) | 0.489*** | (0.03) | −0.66*** | (0.12) | −1.15*** | (0.13) |
| CHC | 226 | 7.26** | (2.15) | 0.706*** | (0.06) | −0.91*** | (0.23) | −1.62*** | (0.24) |
| DH | 33 | 15.45* | (7.37) | 1.71*** | (0.21) | −2.79*** | (0.80) | −4.50*** | (0.86) |
| DPT3 | |||||||||
| BHC | 377 | 7.50*** | (1.42) | −0.06 | (0.13) | −0.49* | (0.20) | −0.43 | (0.31) |
| CHC | 257 | 10.48*** | (2.32) | 0.64** | (0.21) | −1.70*** | (0.32) | −2.34*** | (0.50) |
| DH | 38 | 23.53** | (8.06) | 0.35 | (0.78) | −1.91 | (1.18) | −2.26 | (1.84) |
Note: Coefficients from a GEE regression with autoregressive model of first order [AR(1)] error correlation modelled on the seasonally de-trended data. Model for DPT3 only considers 1 year and 1 month prior to intervention and 14 months post-intervention, due to drug stock-outs 2 years prior to the intervention that severely reduced DPT3 doses given.
*P < 0.05; **P < 0.01; ***P < 0.001.
N represents the number of facilities included across the 51 time points (27 for DPT3) in the model.
BHC = basic health centre; CHC = comprehensive health centre; DH = district hospital.
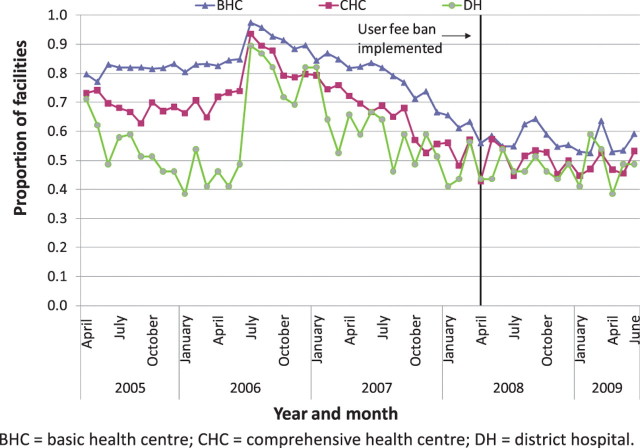
Similar to DPT3, the regression models for drug stock-outs considered only the 14-month period before the user fee ban as opposed to the full 3 years, as huge increases in stock-outs 2 years before the ban would make the pre-ban trend difficult to tease out (Figure 7). Stock-outs were steadily declining in the 14-month period before the user fee ban, and the decline appeared to level off following the ban.
Figure 7.
Proportion of facilities with any drug stock-out, by facility type
A Prais-Wintsen interrupted time series regression model on the combined proportion of facilities with stock-outs at each time period was run separately for BHCs, CHCs and district hospitals, for the 14-month period prior to the user fee ban compared with the 14-month period afterwards. Although the models indicated that stock-outs did not increase immediately following the ban, they provided evidence that the declining trend in stock-outs in the 14 months before the user fee ban was flattened following the ban, with stock-out rates holding relatively steady rather than continuing to decline (P < 0.001 for the difference between the postslope and preslope coefficients for all facility types, data not shown).
Discussion
Experiences from countries where user fees have been removed are increasingly relevant as more low- and middle-income countries are considering this approach to increasing access to health services. Evidence from the health financing pilot study in Afghanistan indicates that there were no significant differences in changes over time in observed or perceived quality of care between facilities where fees were removed and those where fee structures remained constant during the pilot study. Analysis of facility utilization data for both the health financing pilot study and the nationwide user fee ban indicated that utilization of curative care increased when fees are removed, a positive finding in a country with minimal access to health services during much of the previous two decades. Outpatient visits represented only 0.97 contacts per inhabitant per year in 2007, one year before the ban (Loevinsohn and Sayed 2008), and overutilization of health services is not currently an overriding concern in Afghanistan, given the large unmet health needs of its population (Ministry of Public Health 2008a).
Similar to what has been found in other settings, there was a more pronounced effect on curative care use following fee removal, in comparison with preventive and promotive service use. One reason for the lack of increases in preventive and promotive care utilization might be that these services were largely free before the ban. National facility assessment data indicated that of BPHS facilities charging fees in 2007, 99.2% reported that Expanded Programme on Immunization (EPI) services were free, 92.6% that antenatal care was free, and 83.6% that deliveries were free of charge (although at some facilities patients still had to pay for drugs needed during delivery) (authors' calculations based on Ministry of Public Health 2007b). Even so, increased use of facilities for curative care did not translate into increased use of preventive and promotive services, at least in the immediate period following fee removal, similar to findings in South Africa following fee removal (Wilkinson et al. 2001).
Although curative care utilization during the pilot study increased significantly more at facilities that previously charged both service and medication fees, as opposed to those charging only service fees, it is not possible to draw generalizable conclusions about this effect. The NGO managing BPHS services in Farah province responded swiftly to the initial increases in visits at the two facilities randomized to free services, following local radio campaigns and information dissemination by community shuras (committees) about the newly free services. The NGO added a second doctor to each clinic, implemented a triage system to prioritize sicker patients, expanded working hours and increased supplies of medicine accordingly. In addition, they also made other improvements, including a generator, landscaping and painting of the clinics. The concerted efforts made to increase staffing, drugs and other resources at the two facilities previously charging medication and service fees may have had stronger effects on utilization than the fee removal itself. Nonetheless, the experience of fee removal in Farah province suggests that even extremely large increases in utilization can be handled effectively with responsive monitoring and increased resource allocation, as needed.
Data from both the pilot study and the user fee ban indicated that measures of structural quality of care were not significantly affected by user fee removal. In addition, data from the pilot study revealed that patients’ and households’ perceptions of quality of care, including both structural and process quality of care, were not negatively impacted by removal of fees. One possible reason for this might be the relatively low amounts contributed by user fees (on average US$103.7 per month at BHCs and US$211.6 per month at CHCs) to the overall facility operating budget and the limited impact these funds could therefore potentially have on structural quality improvements (Ministry of Public Health 2008b). The differences these funds could make might have been palpable to health workers and community leaders who were directly involved in spending them, but may not have made a big enough difference on the infrastructural quality or health workers’ behaviour to have influenced patients and households’ perceptions of quality.
Despite the lack of evidence from patient and household surveys that removal of fees negatively impacted perceived quality, additional data from qualitative interviews and focus groups conducted during the pilot evaluation indicated a pervasive sentiment among health workers and community leaders that free services lead to facility overcrowding, as patients come when they are not seriously ill and waste staff time and medicines (Ministry of Public Health 2008b). Additional analyses of data from facility assessments and patient–provider interactions conducted for the follow-up survey indicated no significant differences in waiting times or consultation times between facilities where fees were removed and those with no change in fee levels (Ministry of Public Health 2008b).
Another source of frustration noted in interviews of health workers, compounding their feelings of an increased workload, was the loss of discretionary revenues, which could no longer be used for real-time expenses such as repairs, drug purchases to prevent stock-outs and other quality improvement activities (Ministry of Public Health 2008b). Similar perceptions were documented among health workers following the abolition of fees in Uganda, where staff reported that free services increased access to health services but decreased their own morale (Burnham et al. 2004; Nabyonga-Orem et al. 2008), and in Niger (Ridde and Diarra 2009). It is critically important to try to find ways to maintain discretionary income at facilities even when services are free, as such funds can give health facilities some degree of autonomy and can be very important for ensuring timely upkeep and maintenance of facility quality. Data from Farah province indicate that it is possible to maintain very positive facility staff and community leader perceptions following fee removal when the appropriate staffing, drugs, equipment and other resources are added to cope with significantly increased demand for services (Ministry of Public Health 2008b).
Lessons learned from the fee removal
Five of 40 NGOs contacted by the MoPH responded to questions about their experiences after the user fee ban, reporting a range of experiences. NGOs reported that strategies they found useful for successfully handling the user fee ban included: enhanced health education and awareness raising in communities about rational drug use; stricter prescription practices and training for pharmacists; and closer supervision of facilities and monitoring of patient demand to ensure drug supplies, equipment and staffing were adequate. Some NGOs indicated no effect from the lack of revenues following the user fee ban. Others reported using saved user fee revenues or additional budget from other sources to compensate for increased demand and loss of fee revenues. One NGO noted it had to cut back on activities such as paying community health workers for priority referrals and paying the ambulance to transport patients. A few NGOs noted that loss of discretionary income at facilities from user fees resulted in a lack of budget for small-scale local activities, including purchase of additional supplies and drugs, undertaking small repairs and maintenance, and other rehabilitation and construction activities and running costs as needed.
Broader factors that likely contributed to the successful removal of fees at the primary care level include consideration of stakeholder concerns and mechanisms to monitor these, attentiveness of the managing service providers to changes in utilization patterns, and continued external funding for health. Two of the three major donors funding the BPHS continued their support at roughly the same per capita levels, and one of the donors slightly increased its funding levels for the subsequent 3 years. It is clear that Afghanistan will depend on donor assistance to fund the health sector in the short to medium term, and perhaps longer as well. Recent research indicates that funding for reconstruction of health systems and services of post-conflict countries needs to be secured with longer-term time horizons in mind, up to between 15 and 27 years for even the best-case scenarios (Chand and Coffman 2008). Even stable countries are increasingly recognizing the role that donor funding can play in supporting user fee removal (CHOGM 2009), and donors have expressed willingness to do this (International Health Partnership 2009). The experience of the NGO in Farah in very successfully coping with large increases in demand following fee removal, by adding staff, drugs and equipment directly, speaks to the importance of being ready with additional preparations and resources when fees are removed. It is important to think about potential additional investments that may be necessary, aside from money for drugs, equipment and other items. Investment in human resource capacity, for example through training additional health workers to staff facilities where visits increase substantially, is an important consideration for fee removal and one that requires a longer-term planning horizon and close co-ordination with human resources departments (Campbell et al. 2009).
Limitations of data sources
Data used in the analyses presented in this paper have several notable limitations. First, the number of facilities included in the health financing pilot study was small, limited primarily due to practical considerations but further reduced by insecurity at follow-up. This, combined, with the higher-than-anticipated design effect of some of the indicators measured meant that the power to detect differences between facility groups was relatively low. Although observed and perceived quality showed no discernable trends, the large increase in care seeking at facilities randomized to free services was not statistically greater than the increase among facilities with no fee change. Second, the routine HMIS data used to assess changes in utilization during the pilot study and following the user fee ban were incomplete, and analysis was limited to facilities missing no more than 3 months of data. Routine reporting systems in many developing countries, such as Afghanistan, suffer from questionable data accuracy, although they can still be useful for detecting trends over time.
Conclusions and policy implications
The Afghanistan health financing pilot study and subsequent nationwide user fee ban at the primary care level represent the successful application of a pilot study to make an informed national policy decision. The user fee ban was implemented swiftly after presentation of the pilot study results, but following deliberations and further information gathering about best practices in user fee removal and discussion of health sector stakeholder concerns. Early results indicate that visits for curative care, but not necessarily for preventive and promotive care, have increased following the ban, without major adverse effects on drug stock-outs.
There is a need for continued monitoring following the user fee ban, a best practice recommended by user fee experts (Gilson and McIntyre 2005; Ridde and Morestin 2011). For example, some concerns about quality did not emerge in Uganda until 2 to 3 years after user fees were abolished (Nabyonga-Orem et al. 2008). Afghanistan has taken the positive decision to use a 3-year time-frame to monitor the effects of the user fee ban. During this period, in addition to continued analysis of routine reporting data, further research should be conducted on mechanisms to provide discretionary income to facilities, and on health worker and patient perceptions, in order to better understand the longer-term effects of increased service utilization on health worker morale and consumer perceptions of free government services.
Funding
This study was funded by a contract with the Afghanistan Ministry of Public Health and the Johns Hopkins University Bloomberg School of Public Health, in collaboration with the Indian Institute of Health Management Research. Financial support (Grant # H050474) was also provided by the UK Department for International Development (DFID) for the Future Health Systems research programme consortium.
Conflict of interest
None declared.
Acknowledgements
The authors would like to thank colleagues at the Afghanistan Ministry of Public Health, Johns Hopkins University Bloomberg School of Public Health, Indian Institute of Health Management Research and the data collectors and participants in the health financing pilot study. The authors also express their appreciation for the financial support (Grant # H050474) provided by the UK Department for International Development (DFID) for the Future Health Systems research programme consortium. This document is an output partly funded from a project financed by DFID for the benefit of developing countries. The views expressed are not necessarily those of DFID.
Endnote
1 The binary outcome of seeking care first at the pilot facility was tested using the logistic regression model: Log[P(careseek)/(1-P(careseek))] = β0 + β1 Post + β2 Feesremoved + β3 Post*Feesremoved.
References
- Akin JS, Birdsall N, de Ferranti D. Financing Health Services in Developing Countries: An Agenda for Reform. A World Bank Policy Study. Washington, DC: The World Bank; 1987. [Google Scholar]
- Arur A, Peters D, Hansen P, et al. Contracting for health and curative care use in Afghanistan between 2004 and 2005. Health Policy and Planning. 2010;25:135–44. doi: 10.1093/heapol/czp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham GM, Pariyo G, Galiwango E, Wabire-Mangen F. Discontinuation of cost sharing in Uganda. Bulletin of the World Health Organization. 2004;82:187–95. [PMC free article] [PubMed] [Google Scholar]
- Campbell J, Oulton J, McPake B, Buchan J. Removing user fees? Engage the health workforce. The Lancet. 2009;374:1966. doi: 10.1016/S0140-6736(09)62118-8. [DOI] [PubMed] [Google Scholar]
- Chand S, Coffman R. 2008. How soon can donors exit from post-conflict states? Working Paper No. 141. Washington, DC: Center for Global Development. [Google Scholar]
- CHOGM. 2009. Commonwealth Heads of Government Meeting Communique. CHOGM, 27–29 November 2009, Republic of Trinidad & Tobago. Online at: http://www.thecommonwealth.org/files/216904/FileName/TrinidadandTobagoCHOGMCommunique.pdf, accessed 14 September 2011.
- Collins D, Quick JD, Musau SN, Kraushaar K, Hussein IM. The fall and rise of cost sharing in Kenya: the impact of phased implementation. Health Policy and Planning. 1996;11:52–63. doi: 10.1093/heapol/11.1.52. [DOI] [PubMed] [Google Scholar]
- Diggle P, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. Oxford: Oxford University Press; 2002. [Google Scholar]
- Gilson L. The lessons of user fee experience in Africa. Health Policy and Planning. 1997;12:273–85. doi: 10.1093/oxfordjournals.heapol.a018882. [DOI] [PubMed] [Google Scholar]
- Gilson L, McIntyre D. Removing user fees for primary care in Africa: the need for careful action. British Medical Journal. 2005;331:762–5. doi: 10.1136/bmj.331.7519.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson L, Kalyalya D, Kuchler F, et al. The equity impacts of community financing activities in three African countries. International Journal of Health Planning and Management. 2000;15:291–317. doi: 10.1002/hpm.599. [DOI] [PubMed] [Google Scholar]
- Hutton G. London: DFID; 2004. Charting the path to the World Bank's “No blanket policy on user fees”: a look over the past 25 years at the shifting support for user fees in health and education, and reflections on the future. DFID Health Resource Centre. [Google Scholar]
- International Health Partnership. Outcome document of the meeting of the Taskforce on Innovative International Financing for Health Systems, 23 September, New York. 2009 Online at: http://www.internationalhealthpartnership.net//CMS_files/documents/un_general_assembly_meeting_outcome_document_EN.pdf, accessed 14 September 2011. [Google Scholar]
- Ministry of Public Health. Kabul: Islamic Republic of Afghanistan, Ministry of Public Health; 2007a. National Policy on Health Financing and Sustainability. [Google Scholar]
- Ministry of Public Health. Kabul: Islamic Republic of Afghanistan, Ministry of Public Health; 2007b. Balanced Scorecard for the Basic Package of Health Services 2007. [Google Scholar]
- Ministry of Public Health. Kabul: Johns Hopkins University/Indian Institute of Health Management Research and Islamic Republic of Afghanistan, Ministry of Public Health; 2008a. Afghanistan Health Survey 2006: Estimates of Priority Indicators for Rural Afghanistan. [Google Scholar]
- Ministry of Public Health. Kabul: Johns Hopkins University/Indian Institute of Health Management Research and Islamic Republic of Afghanistan, Ministry of Public Health; 2008b. Final Evaluation on Health Financing Pilots: Effects of User Fees versus Free Services on Primary Care in Afghanistan. [Google Scholar]
- Ministry of Public Health. Kabul: Islamic Republic of Afghanistan, Ministry of Public Health; 2009. National strategy on health care financing and sustainability 2009-2013. [Google Scholar]
- Lagarde M, Palmer N. The impact of user fees on health service utilization in low- and middle-income countries: how strong is the evidence? Bulletin of the World Health Organization. 2008;86:839–48. doi: 10.2471/BLT.07.049197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loevinsohn B, Sayed GD. Lessons from the health sector in Afghanistan: how progress can be made in challenging circumstances. Journal of the American Medical Association. 2008;300:724–6. doi: 10.1001/jama.300.6.724. [DOI] [PubMed] [Google Scholar]
- Mbugua JK, Bloom GH, Segall MM. Impact of user charges on vulnerable groups: the case of Kibwezi in rural Kenya. Social Science & Medicine. 1995;41:829–35. doi: 10.1016/0277-9536(94)00400-n. [DOI] [PubMed] [Google Scholar]
- Nabyonga J, Desmet M, Karamagi H, et al. Abolition of cost-sharing is pro-poor: evidence from Uganda. Health Policy and Planning. 2005;20:100–8. doi: 10.1093/heapol/czi012. [DOI] [PubMed] [Google Scholar]
- Nabyonga-Orem J, Karamagi H, Atuyambe L, et al. Maintaining quality of health services after abolition of user fees: a Uganda case study. BMC Health Services Research. 2008;8:102. doi: 10.1186/1472-6963-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer N, Strong L, Wali A, Sondorp E. Contracting out health services in fragile states. British Medical Journal. 2006;332:718–21. doi: 10.1136/bmj.332.7543.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Gauld R, Norris P, Rades T. “This body does not want free medicines”: South African consumer perceptions of drug quality. Health Policy and Planning. 2010;25:61–9. doi: 10.1093/heapol/czp039. [DOI] [PubMed] [Google Scholar]
- Pearson M. London: DFID Health Systems Resource Centre; 2004. Issues Paper: The case for abolition of user fees for primary health services. [Google Scholar]
- Peters DH, Noor AA, Singh LP, et al. A balanced scorecard for health services in Afghanistan. Bulletin of the World Health Organization. 2007;85:146–51. doi: 10.2471/BLT.06.033746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KD, Waters H, Steinhardt L, et al. An experiment with community health funds in Afghanistan. Health Policy and Planning. 2009;24:301–11. doi: 10.1093/heapol/czp018. [DOI] [PubMed] [Google Scholar]
- Ridde V, Diarra A. A process evaluation of user fees abolition for pregnant women and children under five years in two districts in Niger (West Africa) BMC Health Services Research. 2009;9:89. doi: 10.1186/1472-6963-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridde V, Morestin F. A scoping review of the literature on the abolition of user fees in health care services in Africa. Health Policy and Planning. 2011;26:1–11. doi: 10.1093/heapol/czq021. [DOI] [PubMed] [Google Scholar]
- Russell S. The economic burden of illness for households in developing countries: a review of studies focusing on malaria, tuberculosis, and human immunodeficiency virus/acquired immunodeficiency syndrome. American Journal of Tropical Medicine & Hygiene. 2004;71(2 Suppl):147–55. [PubMed] [Google Scholar]
- Save the Children UK. An unnecessary evil? User fees for health care in low-income countries. London: Save the Children Fund; 2005. [Google Scholar]
- Sepehri A, Chernomas R. Are user charges efficiency- and equity-enhancing? A critical review of economic literature with particular reference to experience from developing countries. Journal of International Development. 2001;13:183–209. [Google Scholar]
- Turner AG, Magnani RJ, Shuaib M. A not quite as quick but much cleaner alternative to the Expanded Programme on Immunization (EPI) Cluster Survey design. International Journal of Epidemiology. 1996;25:198–203. doi: 10.1093/ije/25.1.198. [DOI] [PubMed] [Google Scholar]
- van der Geest S, Macwan'gi M, Kamwanga J. User fees and drugs: what did the health reforms in Zambia achieve? Health Policy and Planning. 2000;15:59–65. doi: 10.1093/heapol/15.1.59. [DOI] [PubMed] [Google Scholar]
- Waldman R, Strong L, Wali A. Kabul: Afghanistan Research and Evaluation Unit; 2006. Afghanistan's health system since 2001: condition improved, prognosis cautiously optimistic. [Google Scholar]
- Wilkinson D, Gouws E, Sach M, Karim SS. Effect of removing user fees on attendance for curative and preventive primary health care services in rural South Africa. Bulletin of the World Health Organization. 2001;79:665–71. [PMC free article] [PubMed] [Google Scholar]
- Witter S. Service- and population-based exemptions: are these the way forward for equity and efficiency in health financing in low-income countries? Advances in Health Economics and Health Services Research. 2009;21:251–88. [PubMed] [Google Scholar]
- Witter S, Arhinful DK, Kusi A, Zakariah-Akoto S. The experience of Ghana in implementing a user fee exemption policy to provide free delivery care. Reproductive Health Matters. 2007a;15:61–71. doi: 10.1016/S0968-8080(07)30325-X. [DOI] [PubMed] [Google Scholar]
- Witter S, Kusi A, Aikins M. Working practices and incomes of health workers: evidence from an evaluation of a delivery fee exemption scheme in Ghana. Human Resources for Health. 2007b;5:2. doi: 10.1186/1478-4491-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge J. Econometric Analysis of Cross Section and Panel Data. Cambridge, MA: The MIT Press; 2002. [Google Scholar]
- Yates R. Universal health care and the removal of user fees. The Lancet. 2009;373:2078–81. doi: 10.1016/S0140-6736(09)60258-0. [DOI] [PubMed] [Google Scholar]