Abstract
Background. Despite growing literature of the dialysis patients’ high burden of illness and a compromised quality of life, little is known about their daily life experiences.
Methods. A cross-sectional study using the day reconstruction method, an experience sampling method, was used. Seventy-one dialysis patients recruited from three dialysis centers systematically reconstructed their activities and experiences of the preceding day. Time spent on their activities, settings and associated emotions were assessed to compute U-Index scores (the percentage of time a person spent in an unpleasant or undesirable state). Patients also completed the Illness Effects Questionnaire-Self-Report (IEQ-S) and the Short-Form Health Survey-36 v2 (SF-36v2).
Results. Patients spent ∼6 h of their day (excluding sleep hours) in an unpleasant or undesirable state (U-Index = 34.45 ± 29.26). U-Index scores did not differ by race, age, sex or years on dialysis and were moderately associated with IEQ-S scores (r = 0.43, P ≤ 0.001) and weakly associated with SF-36v2 physical component scores (r = −0.34, P = 0.003). U-Index scores differed significantly between dialysis days and non-dialysis days for hemodialysis patients (P = 0.012). Those who had depression or used antidepressants and reported income not meeting basic needs showed significantly higher U-Index scores than their counterparts (P < 0.05).
Conclusions. The findings may assist clinicians to better understand the daily activities and burdens experienced by dialysis patients and suggest areas for future research and clinical considerations to improve the quality of their lives.
Keywords: daily life experience, dialysis, quality of life
Introduction
Patients with end-stage renal disease (ESRD) are living longer and enduring more complications from their illness than before. Those on maintenance dialysis often live lives of compromised quality due to the burden of illness, which usually includes managing multiple concurrent symptoms, comorbidities and the demands of dialysis [1–3]. Although it varies, 25–30% of peritoneal dialysis (PD) and hemodialysis (HD) patients have mild-to-moderate physical and cognitive impairment due to comorbidity [4]. Poor sleep quality is a significant problem in PD patients, associated with depression and quality of life [5]. HD patients report higher symptom distress and lower quality of life over time, whereas PD patients report stable symptom distress and quality of life over time but significant social dysfunction [6].
Researchers and clinicians typically rely on global reports of health-related quality of life and well-being to describe the impact of illness. Research has shown that health-related quality of life is associated with increased mortality and morbidity [7–9]. However, this assessment using standardized measures (e.g., SF-36 Health Survey or Kidney Disease-Quality of Life Questionnaire) does not provide detailed information about the level of illness burden that patients experience on a daily basis and emotional well-being and activities in their own setting or situations. Furthermore, global standardized measures result in reports that are highly context dependent because people’s responses are likely influenced by memory bias and social desirability [10].
The purpose of this study was to obtain detailed information about daily activities and events that dialysis patients engage in on a daily basis, the emotional experiences associated with performing those activities and to quantify the level of well-being experienced in the dialysis patients’ home settings. We attained such data using the day reconstruction method (DRM) [11], an innovative approach designed to characterize an individual’s daily life activities by quantifying information about time use and subjective emotional experiences associated with performing those activities. We examined the following research questions: (i) what is the level of daily illness burden or well-being experienced by dialysis patients on a typical day and what are its relationships with global standardized measures of health-related quality of life; (ii) what daily activities or events are associated with the level of burden and (iii) does daily illness burden or emotional well-being differ by dialysis modality, years on dialysis and sociodemographic characteristics. Secondarily, we explored whether racial differences and their interaction effects with other variables existed in daily illness burden or emotional well-being.
Materials and methods
Study design, setting and participants
This study was a cross-sectional descriptive survey. The sample comprised 71 patients on maintenance dialysis recruited from three dialysis centers in North Carolina, USA. To be eligible, patients had to meet the following inclusion criteria: (i) receiving either in-center HD or PD for at least 6 months prior to enrollment (to help ensure that patients had acclimated to dialysis and medical regimens); (ii) normal cognitive functioning as evidenced by less than three errors on the Short Portable Mental Status Questionnaire (SPMSQ) [12]; (iii) ≥18 years and (iv) ability to speak English (for telephone interview). Patients who were too sick to participate in an hour-long telephone interview or who required special care and assistance (e.g. living in a nursing home) were excluded. The study was approved by the University of North Carolina Institutional Review Board.
The social worker of each clinic screened patients for eligibility and assessed their willingness to be contacted. After a patient granted permission, a research assistant called the patient to explain the study and conducted the cognitive screening using SPMSQ. If the number of errors on the tool was less than three, the patient was formally invited to participate in the study. Data collection was done over the telephone at the time of the patient’s consent or was scheduled for a separate day if the patient preferred. Participants were mailed a $20 money order as compensation for their time.
Of 124 patients, who were deemed eligible and were willing to be contacted, 3 failed the SPMSQ, 2 were too sick to participate and 2 lived in a nursing home. Additionally, the phone numbers provided by 23 patients were no longer in service at the time of contact. Of the remaining 94 patients, 23 patients refused to participate (24.5%).
Variables and measurement
The DRM [11] was used to obtain detailed descriptions of the daily activities and experiences of dialysis patients. The DRM is a survey designed to collect data regarding an individual’s experiences on a given day, through a systematic mental reconstruction about the previous day. Patients first recreated the previous day into working memory by producing a short diary consisting of a sequence of episodes. Then, patients were asked to answer a series of questions to describe key features of each episode, such as when the episode began and ended, what they were doing, where they were, with whom they were interacting and what emotional affect they had during that time. Patients were provided with a list of emotions that included four positive affects (happy, enjoying myself, competent and peaceful) and eight negative affects (frustrated, depressed, tired, anxious/worried, angry, in pain, hassled and impatient for it to end) or expressed their own adjectives if their desired affect was not included in the list. Durations of time spent in an unpleasant or undesirable state (negative affects) were summed and divided by the total waking time (= 24—sleep hours) to compute U-Index for each patient. Higher U-Index scores indicate lower well-being states (possible range, 0–100%). This survey took 30–45 min for participants to complete.
The Illness Effects Questionnaire-Self-Report (IEQ-S; Multi-Health Systems Inc.), a 20-item 7-point Likert scale was administered to assess an individual’s perception of how the illness interfered with or modified personal and social behavior. Questions include items about perceived family and personal disruptions, physical problems and fears about the consequences of illness. Good internal consistency reliability and test–retest reliability have been reported (0.93 and 0.99, respectively) [13]. This instrument has been used in patients with ESRD [14]. Higher total scores indicate greater levels of perceived life disruption from illness (possible range, 7–140).
The Short-Form Health Survey v2 (SF-36™) [15] was used to assess generic health-related quality of life, including physical, emotional, social and role functioning and general health perceptions. Scores were summed after being multiplied by their respective physical or mental factor score coefficients to compute two standardized summary scores, the physical and mental component summaries. Validity, psychometric testing and comparison data for healthy and chronically ill populations, including ESRD patients, are well established [15]. Higher scores indicate greater physical and mental functioning.
Finally, patients answered sociodemographic questions and questions about their satisfaction with life as a whole and satisfaction with current health. Medical records were reviewed to obtain information about dialysis modality, years on dialysis and the diagnosis of depression or use of antidepressants.
Statistical analysis
Descriptive statistics (e.g. frequency, mean, SD, 95% confidence interval) of the sample characteristics were computed. To assess racial differences in sociodemographic, clinical and the preceding day characteristics, t-test and χ2 test were used as appropriate. The P-values <0.05 were considered significant when statistical tests were applied. Logistic regression models were used to determine the significance of racial difference in depression or use of antidepressants when accounting for other significantly differing characteristics between blacks and whites. To examine factors associated with U-Index, t-test, analysis of variance, Pearson correlation and linear regression analysis were employed. The backward selection criteria were applied when determining significant factors in the multivariate analysis. Model diagnostics based on residuals were implemented to ensure that our approach satisfies the statistical assumptions of general linear models.
Results
Characteristics of the participants and the previous day
Roughly 30% of the participants (n = 21) were ≥61 years, 42.2% were male, 69% were non-Hispanic black and 83.1% completed at least a high school education. The majority of the participants (n = 56) received in-center HD. Time on dialysis was 4.42 years (SD = 3.35) on average. Medical records of 17 patients (23.9%) indicated depression or current use of antidepressants. The mean SF-36v2 physical and mental component scores were 35.69 (SD = 8.42) and 39.97 (SD = 8.72), respectively. The mean IEQ-S was 67.61 (SD = 32.34). Table 1 presents the sociodemographic and clinical characteristics of the participants by race. In this sample, non-Hispanic whites were older (59.95 versus 48.78, P = 0.005), had more years of education (14.14 versus 12.31, P = 0.007), fewer years on dialysis (2.71 versus 5.19, P = 0.003) and were more likely to be diagnosed with depression or use antidepressants (40.9% versus 16.3%, P = 0.025). When adjusted for age, years of education and years on dialysis, the racial difference in depression or use of antidepressants became statistically insignificant (the odds ratio for blacks to be diagnosed with depression/use of antidepressants = 0.4, P = 0.21).
Table 1.
Sample characteristics
| Black (n = 49) | White (n = 22) | ||
| Sociodemographic | |||
| Age (mean ± SD) | 48.78 ± 15.03 | 59.95 ± 15.10 | t = 2.89, P = 0.005 |
| Male, n (%) | 18 (36.7) | 12 (54.5) | n.s. |
| Married | 13 (26.5) | 11 (50.0) | n.s. |
| Number of adults living together, median | 2 | 2 | n.s. |
| Years of education | 12.31 ± 1.88 | 14.14 ± 3.63 | t = 2.80, P = 0.007 |
| Full-time or part-time employment | 5 (10.2) | 3 (13.6) | n.s. |
| Disabled/unable to work | 27 (55.1) | 11 (50.0) | n.s. |
| Annual household income <$20 000 | 32 (65.3) | 10 (45.5) | n.s. |
| Yes, income meets basic needs | 28 (57.1) | 16 (72.7) | n.s. |
| No religious preference | 8 (16.3) | 7 (31.8) | n.s. |
| Extent of following religious customs and practices | n.s. | ||
| Never or sometimes | 9 (22.0) | 6 (40.0) | |
| Frequently or always | 32 (78.0) | 9 (60.0) | |
| Importance of spirituality in life | n.s. | ||
| Not at all-somewhat important | 9 (18.4) | 8 (36.4) | |
| Very-extremely important | 40 (81.6) | 14 (63.6) | |
| Insurancea | |||
| Medicare | 40 (81.6) | 17 (77.3) | |
| Medicaid | 24 (49.0) | 6 (27.3) | |
| Private | 8 (16.3) | 4 (18.2) | |
| Clinical | |||
| In-center HD | 40 (81.6) | 16 (72.7) | n.s. |
| PD (all CCPDb) | 9 (18.4) | 6 (27.3) | |
| Years on dialysis (mean ± SD) | 5.19 ± 3.64 | 2.71 ± 1.60 | t = 3.98, P = 0.003 |
| Depression or use of antidepressants | 8 (16.3) | 9 (40.9) | χ2 = 5.04, P = 0.025 |
| Health-related quality of life, illness effects and satisfaction | |||
| SF-36 physical component score | 35.47 ± 9.06 | 36.19 ± 6.97 | n.s. |
| Mental component score | 39.95 ± 9.16 | 40.01 ± 7.84 | n.s. |
| Illness effects questionnaire score | 66.31 ± 33.88 | 70.51 ± 29.15 | n.s. |
| Satisfaction with life | n.s. | ||
| Not at all satisfied or not satisfied | 8 (16.3) | 5 (22.7) | |
| Satisfied or very satisfied | 41 (83.7) | 17 (77.3) | |
| Satisfaction with health | n.s. | ||
| Not at all satisfied or not satisfied | 21 (42.9) | 12 (54.5) | |
| Satisfied or very satisfied | 28 (57.1) | 10 (45.5) | |
| Yesterday | |||
| Day of the week | n.s. | ||
| Weekday (Monday to Friday) | 34 (69.4) | 16 (72.7) | |
| Weekend (Saturday, Sunday) | 15 (30.6) | 6 (27.3) | |
| Hours of actual sleep | 6.70 ± 2.61 | 7.80 ± 3.05 | n.s. |
| A total number of events/activities | 8.80 ± 2.74 | 10.05 ± 2.97 | n.s. |
| Compared to a typical day, yesterday was … | n.s. | ||
| Much worse | 3 (6.1) | 1 (4.5) | |
| Somewhat worse | 5 (10.2) | 3 (13.6) | |
| Pretty typical | 28 (57.1) | 15 (68.2) | |
| Somewhat better | 11 (22.4) | 3 (13.6) | |
| Much better | 2 (4.1) | 0 |
n.s., not significant
Multiple response.
CCPD, continuous cycling peritoneal dialysis.
Yesterday was rated ‘a pretty typical day’ for 60.6% of the participants (n = 43). Of the 56 HD patients, the preceding day was a dialysis day for 33 patients and a non-dialysis day for 23 patients. Eleven of the 71 previous days of the total participants were Sundays. On average, the participants reported 7 h of sleep the previous night (SD = 2.78), and 69% reported that their sleep quality had been ‘fairly good’ or ‘very good’ during the past month. There was no significant difference in hours of sleep between dialysis days and non-dialysis days for HD patients and between HD patients and PD patients.
Participants’ activities and experiences of the previous day
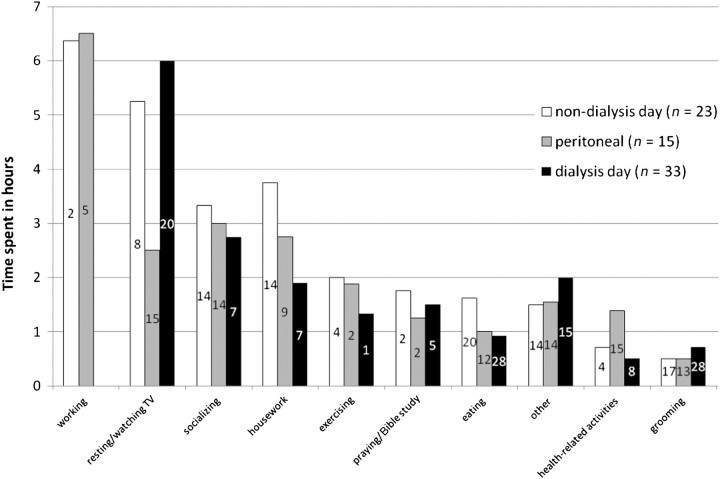
Figure 1 presents the activities performed by the participants during the previous day and includes duration of each activity, excluding sleep hours. As expected, the majority of the waking time on dialysis days (64.7%) was consumed by dialysis treatment and some combination of resting or watching TV afterward. Only seven participants (9.9%) reported exercise activities (e.g. going for a walk, walking the dog, practicing Yoga, lifting weights) performed the previous day. All PD patients reported health-related activities (e.g. taking vitals, monitoring blood glucose and taking medications), excluding setting up or disconnecting PD, whereas only 12 HD patients (21.4%) reported performing any health-related activity. Although PD patients reported a slightly higher number of activities performed the previous day (= 10.67) compared to those HD patients reported on dialysis days (= 8.27) and HD patients reported on non-dialysis days (= 9.52), no statistically significant difference was found.
Fig. 1.
Activities performed ‘yesterday’. Note. The number in each bar indicates the number of patients who performed the activity. Grooming (e.g. showering and dressing), health-related activities (e.g. medication taking, monitoring blood glucose and taking vitals), socializing (e.g. visits to/from family members, friends or neighbors and going out, going to church) and other (e.g. phoning, computer/emailing, paying bills).
Table 2 lists the aspects or activities of ‘yesterday’ that were associated with positive and negative emotions. Noticeably, the positive or negative emotions of the previous day were marked by dialysis. For instance, the first emotion reported on dialysis days was trepidation (‘dreading dialysis’) for the 19 of the HD patients. Eighteen patients (25.4%) reported moderate-to-severe pain the previous day that lasted from 30 min to 10 h (3.06 h ± 2.75). Of these patients, two were on PD, seven were HD patients reporting non-dialysis days, and nine were HD patients reporting dialysis days.
Table 2.
Aspects or activities of ‘yesterday’ associated with positive and negative affectsa
| Associated with positive affects | n (%) | Associated with negative affects | n (%) | |
| 1. | Spending time with family or friends | 21 (29.6) | Dialysis | 19 (26.8) |
| 2. | No dialysis | 15 (21.1) | Being tired/sick/in pain, can’t do anything | 8 (11.3) |
| 3. | Getting out of dialysis | 6 (8.5) | Housework and chores | 8 (11.3) |
| 4. | Church activities, reading/studying Bible | 5 (7.0) | Family conflict | 4 (5.6) |
| 5. | Having meals | 4 (5.6) | Financial and legal issues | 2 (2.8) |
| 6. | Watching TV | 3 (4.2) | Busy at work | 1 (1.4) |
| 7. | Going out (bowling/shopping) | 3 (4.2) | Child caring | 1 (1.4) |
| 8. | Cooking/cleaning | 2 (2.8) | No appetite | 1 (1.4) |
Positive affects (e.g. happy, enjoying myself); negative affects (e.g. frustrated, anxious, tired, depressed).
U-Index and associated factors
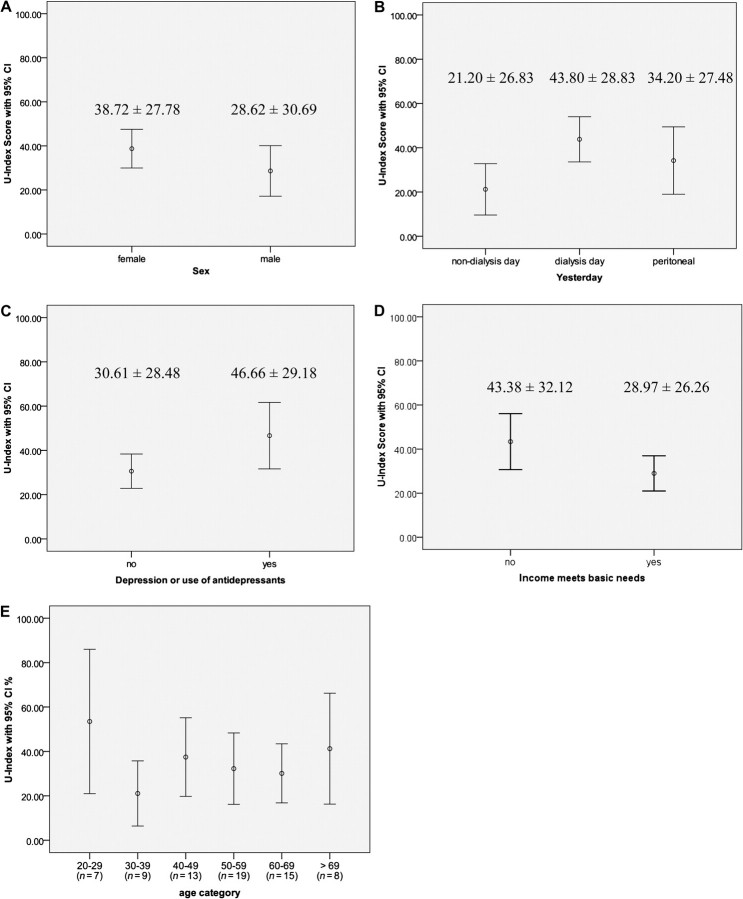
The U-Index or the percentage of time a person spent in an unpleasant or undesirable state was 34.45 on average (SD = 29.26). This percentage represents ∼6 h of yesterday, excluding sleep hours (= 7–8 h). Of the sociodemographic and clinical characteristics of the participants, only two factors were significantly associated with U-Index: whether or not household income meets basic needs (t = 2.06, P = 0.043) and depression or use of antidepressants (t = 2.02, P = 0.048) (see Figure 2). The mean U-Index of females (38.72 ± 27.78) was higher than that of males (28.62 ± 30.69), but this difference was not statistically significant (t = 1.45, P =0.15). No significant difference in U-Index was observed between blacks (34.15 ± 30.80) and whites (35.13 ± 26.18; t = 0.13, P = 0.89). Broken down by age category, the youngest (20–29 years) and the oldest (>69 years) groups appeared to have higher U-Index scores than those of the remainder, but with wide confidence intervals due to the small sample size. As expected, U-Index scores differed significantly among dialysis days (HD patients), non-dialysis days (HD patents) and previous days of PD patients (F2,68 = 4.44, P = 0.015); however, only the difference between dialysis days and non-dialysis days was significant (P = 0.012 < 0.05/3 comparisons = 0.016).
Fig. 2.
U-Index by sex, dialysis day, depression, income meets basic needs and age.
U-Index was significantly correlated with IEQ-S scores (r = 0.43, P ≤ 0.001) and SF-36v2 physical component scores (r = −0.34, P = 0.003) but not mental component scores (r = −0.20, P = 0.09). IEQ-S scores and SF-36 scores did not differ by dialysis day. The ratings of satisfaction with life and satisfaction with health were not associated with U-Index (rs < 0.20, P > 0.05).
A multivariate analysis was performed to confirm the association between U-Index and dialysis days when accounting for other significant factors. Initially, the model included depression/use of antidepressants, income meeting basic needs, age, race and dialysis day. Due to the lack of association, the last three variables were eliminated from the model. When adjusted for depression/use of antidepressants and income meeting basic needs, the association between U-Index and dialysis days remained unchanged (F = 5.17, P = 0.001, R2 = 0.24).
Subgroup analysis of patients who rated the previous day as ‘a pretty typical day’
U-Index scores significantly differed by whether the previous day was ‘a pretty typical day (n = 43)’, ‘somewhat worse or much worse (n = 12)’ or ‘somewhat better or much better (n = 16)’: 30.67, 58.45 and 26.62, respectively (F = 5.62, P = 0.006). Within the group of patients who rated the previous day as a pretty typical day, U-Index scores significantly differed by dialysis day: 42.31 ± 22.81, 19.24 ± 27.86 and 26.87 ± 24.68 for dialysis days (HD patients), non-dialysis days (HD patents) and previous days of PD patients, respectively (F = 3.61, P = 0.036). However, the difference between dialysis days and non-dialysis days was not statistically significant (P = 0.035 > 0.05/3 comparisons = 0.016).
U-Index was significantly correlated with IEQ-S scores (r = 0.51, P ≤ 0.001) and SF-36v2 physical component scores (r = −0.31, P = 0.043) but not mental component scores (r = −0.28, P = 0.07). U-Index was significantly associated with the rating of satisfaction with life (r = −0.36, P = 0.02) but not with the rating of satisfaction with health (r = −0.07, P = 0.65).
Discussion
In recent years, there has been a growing literature describing dialysis patients’ a compromised health-related quality of life and its association with survival and mortality [6, 8, 16–18]. Although periodic assessment of health-related quality of life for dialysis patients is now mandated in the USA, how such information is actually used to improve their quality of life is unknown. Dialysis treatment demands significant life-style changes for patients with renal failure, yet little is known about their daily life experiences.
We used the DRM to demonstrate how dialysis patients spend their days, jointly with their subjective experiences in their everyday context. Notably, a high proportion of ‘yesterday’s’ activities for HD patients were spent resting (5–6 h on average) regardless of whether it was a dialysis day or a non-dialysis day. In contrast, PD patients spent <3 h on resting.
Less than 10% of the total sample engaged in any type of exercise activities (not labor activities) that lasted >15 min. Even though we specifically inquired about health-related activities and provided examples, only 12 of 56 HD patients reported one of those activities, whereas all PD patients did. Nearly a half of the sample engaged in socializing activities, but those activities were largely visits from friends or other family members.
Although the contrast between dialysis days and non-dialysis days for HD patients’ subjective well-being is not surprising, our study is the first to quantify the difference. The greater subjective well-being of HD patients on non-dialysis days seemed to be driven by the sense of liberation from dialysis for the day. Contrary to our expectation that a greater sense of well-being of HD patients on non-dialysis days might in turn help patients engage in more activities or different types of activities was not supported. There were no notable differences between dialysis days and non-dialysis days in types and the total number of activities performed. We speculate that this might be because of profound fatigue commonly experienced by HD patients as evidence by the durations of resting on both dialysis days and non-dialysis days.
As anticipated, the associations of U-Index scores with other global assessment of health-related quality of life and satisfaction with life or health were weak to none. The DRM is a measure of the satisfaction people derive from their activities [11]. The DRM assesses specific day-to-day activities and associated affects, whereas SF-36v2 measures overall effects of physical and mental health. As such, the dimensions measured by the DRM and those global reports may differ. It is also possible that the DRM might be less susceptible to retrospective reporting biases or social desirability than typical global reports [10, 19]. Although U-Index scores were significantly associated with SF-36v2 physical component scores but not with mental component scores, neither correlation was strong enough to yield meaningful interpretations. The majority of the sample reported that they ‘frequently’ or ‘always’ engaged in religious activities, yet only <6% of them actually did the previous day. It is likely that dialysis and fatigue associated with the treatment and comorbid conditions prevent them from maintaining a religious or spiritual life that the patients desire. But also, it is possible that the participants chose such responses to the global religious question because those options would be considered more desirable or expected.
The finding that whether or not family income meets basic needs, not the level of household income, was significantly associated with U-Index also reflects the source of dialysis patients’ everyday burden. It would be important for clinicians to recognize and be sensitive to these financial difficulties.
The effect size of racial difference in depression or use of antidepressants is interesting, even though we did not achieve statistical significance after accounting for other group differences due to the small sample size. Despite the significant association between depression or use of antidepressants and U-Index and nearly the same U-Index scores between blacks and whites, a higher proportion of whites were diagnosed with depression or used antidepressants. Future studies are warranted to examine the relationships among race, depression and U-Index in dialysis patients given the inconsistency in previous empirical data of racial differences in depression, health-related quality of life and psychological well-being [20–23]. Such future research may consider study designs with a matched sample to better manage potential confounding factors.
Our study has several limitations. This study was a cross-sectional survey in which each patient was interviewed only ∼1 day, and thus, possible variations across several days of the week and over time could not be captured. The study sample recruited from three dialysis centers and its small sample size limit generalizability of the study findings. Our primary analysis for this study was based on combining information across centers. Due to small sample sizes, there was insufficient information to reliably conduct analyses within the centers. The sample did not include patients on home HD (daily dialysis and nocturnal HD) or continuous ambulatory PD, who are likely to have different daily life experiences. Yet our findings suggest areas for future research and considerations in clinical practice. The use of joint assessments of activities and subjective well-being allows for a better understanding of lives of patients. The information about time use, the frequency and intensity of stress and affective states associated with daily activities can be useful for assessing the burden of illness or difficulties in adhering to prescribed medical regimens in the patients’ home settings. The assessment of daily life experiences could be used to explore the level of coping and adjustment in dialysis patients overtime including those who are starting dialysis or switching to a different modality and regimens and contribute to the development of individualized interventions and strategies that are likely to be integrated into the patient’s daily life.
Acknowledgments
The authors sincerely thank the social workers (Mr. Bradley Menton, Ms. Sandra Cameron, Ms. Yolanda Davis and Ms. Judy Baldwin) in the participating dialysis units for their assistance in potential patient identification and recruitment. This research was supported by National Center for Research Resources, the North Carolina Translational and Clinical Sciences Institute, a pilot project under UL1RR025747.
References
- 1.Buemi M, Lacquaniti A, Bolignano D, et al. Dialysis and the elderly: an underestimated problem. Kidney Blood Press Res. 2008;31:330–336. doi: 10.1159/000164277. [DOI] [PubMed] [Google Scholar]
- 2.Kimmel PL, Cohen SD, Weisbord SD. Quality of life in patients with end-stage renal disease treated with hemodialysis: survival is not enough! J Nephrol. 2008;21(Suppl 13):S54–S58. [PubMed] [Google Scholar]
- 3.Weisbord SD, Bossola M, Fried LF, et al. Cultural comparison of symptoms in patients on maintenance hemodialysis. Hemodial Int. 2008;12:434–440. doi: 10.1111/j.1542-4758.2008.00307.x. [DOI] [PubMed] [Google Scholar]
- 4.Athienites NV, Miskulin DC, Fernandez G, et al. Comorbidity assessment in hemodialysis and peritoneal dialysis using the index of coexistent disease. Semin Dial. 2000;13:320–326. doi: 10.1046/j.1525-139x.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- 5.Guney I, Biyik M, Yeksan M, et al. Sleep quality and depression in peritoneal dialysis patients. Ren Fail. 2008;30:1017–1022. doi: 10.1080/08860220802406419. [DOI] [PubMed] [Google Scholar]
- 6.Ginieri-Coccossis M, Theofilou P, Synodinou C, et al. Quality of life, mental health and health beliefs in haemodialysis and peritoneal dialysis patients: investigating differences in early and later years of current treatment. BMC Nephrol. 2008;9:14. doi: 10.1186/1471-2369-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelstein FO, Story K, Firanek C, et al. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:33–38. doi: 10.2215/CJN.00630208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelstein FO, Wuerth D, Finkelstein SH. Health related quality of life and the CKD patient: challenges for the nephrology community. Kidney Int. 2009;76:946–952. doi: 10.1038/ki.2009.307. [DOI] [PubMed] [Google Scholar]
- 9.Mujais SK, Story K, Brouillette J, et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4:1293–1301. doi: 10.2215/CJN.05541008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz N, Oyserman D. Asking questions about behavior: cognition, communication and questionnaire construction. Am J Eval. 2001;22:127–160. [Google Scholar]
- 11.Kahneman D, Krueger AB, Schkade DA, et al. A survey method for characterizing daily life experience: the day reconstruction method. Science. 2004;306:1776–1780. doi: 10.1126/science.1103572. [DOI] [PubMed] [Google Scholar]
- 12.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 13.Wise TN, Mann LS, Jani N, et al. Convergent validation of the Illness Effects Questionnaire. Psychol Rep. 1994;75:248–250. doi: 10.2466/pr0.1994.75.1.248. [DOI] [PubMed] [Google Scholar]
- 14.Kimmel PL, Peterson RA, Weihs KL, et al. Psychosocial factors, behavioral compliance and survival in urban hemodialysis patients. Kidney Int. 1998;54:245–254. doi: 10.1046/j.1523-1755.1998.00989.x. [DOI] [PubMed] [Google Scholar]
- 15.Ware J, Jr., Kosinski M, Turner-Bowker DM, et al. How to score version 2 of the SF-12 Health Survey. Lincoln, Rhode Island: QualityMetric Inc.; 2002. [Google Scholar]
- 16.Kimmel PL, Emont SL, Newmann JM, et al. ESRD patient quality of life: symptoms, spiritual beliefs, psychosocial factors, and ethnicity. Am J Kidney Dis. 2003;42:713–721. doi: 10.1016/s0272-6386(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 17.Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64:339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 18.Patel SS, Shah VS, Peterson RA, et al. Psychosocial variables, quality of life, and religious beliefs in ESRD patients treated with hemodialysis. Am J Kidney Dis. 2002;40:1013–1022. doi: 10.1053/ajkd.2002.36336. [DOI] [PubMed] [Google Scholar]
- 19.Krueger AB, Stone AA. Assessment of pain: a community-based diary survey in the USA. Lancet. 2008;371:1519–1525. doi: 10.1016/S0140-6736(08)60656-X. [DOI] [PubMed] [Google Scholar]
- 20.Kutner NG, Zhang R, Brogan D. Race, gender, and incident dialysis patients' reported health status and quality of life. J Am Soc Nephrol. 2005;16:1440–1448. doi: 10.1681/ASN.2004080639. [DOI] [PubMed] [Google Scholar]
- 21.Lopes AA, Bragg-Gresham JL, Satayathum S, et al. Health-related quality of life and associated outcomes among hemodialysis patients of different ethnicities in the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2003;41:605–615. doi: 10.1053/ajkd.2003.50122. [DOI] [PubMed] [Google Scholar]
- 22.Unruh M, Miskulin D, Yan G, et al. Racial differences in health-related quality of life among hemodialysis patients. Kidney Int. 2004;65:1482–1491. doi: 10.1111/j.1523-1755.2004.00529.x. [DOI] [PubMed] [Google Scholar]
- 23.Weisbord SD, Fried LF, Unruh ML, et al. Associations of race with depression and symptoms in patients on maintenance haemodialysis. Nephrol Dial Transplant. 2007;22:203–208. doi: 10.1093/ndt/gfl521. [DOI] [PubMed] [Google Scholar]