Abstract
Background. As research has identified a wide array of biological functions of vitamin D, the consequences of vitamin D deficiency in persons with chronic kidney disease has attracted increased attention. The objective of this study was to determine the extent of 25-hydroxyvitamin D (25-OH vitamin D) deficiency and its associations with self-reported physical activity and health-related quality of life (HRQoL) among participants of the Comprehensive Dialysis Study (CDS).
Methods. The nutrition substudy of the CDS enrolled patients new to dialysis from 68 dialysis units throughout the USA. Baseline 25-OH vitamin D concentration was measured using the Direct Enzyme Immunoassay (Immunodiagnostic Systems Inc.). Physical activity was measured with the Human Activity Profile (HAP); the Medical Outcomes Study Short Form-12 (SF-12) was employed to measure HRQoL.
Results. Mean age of the participants (n = 192) was 62 years. There were 124 participants (65%) with 25-OH vitamin D concentrations < 15 ng/mL, indicating deficiency, and 64 (33%) with 25-OH vitamin D≥ 15 to <30 ng/mL, indicating insufficiency. After adjusting for age, sex, race/ethnicity, diabetes, season and center, lower 25-OH vitamin D concentrations were independently associated with lower scores on the HAP and on the Mental Component Summary of the SF-12 (P < 0.05 for both), but not with the Physical Component Summary of the SF-12.
Conclusion. In a well-characterized cohort of incident dialysis patients, lower 25-OH vitamin D concentrations were associated with lower self-reported physical activity and poorer self-reported mental health.
Keywords: dialysis, epidemiology and outcomes, mineral metabolism, United States Renal Data System, vitamin D
Introduction
Over the past decade, low 25-hydroxyvitamin D (25-OH vitamin D) concentrations have been associated with a range of adverse outcomes beyond those strictly associated with bone and mineral metabolism. In the general population, 25-OH vitamin D concentrations < 20 ng/mL are associated with higher risk of death [1] and poorer physical performance [2]; concentrations < 15 ng/mL are associated with musculoskeletal pain [3].
The Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines recommend annual testing for 25-OH vitamin D deficiency in individuals with chronic kidney disease (CKD). Supplementation with 25-OH vitamin D is recommended for patients with a concentration < 30 ng/mL, primarily to prevent or attenuate secondary hyperparathyroidism and osteomalacia [4]. The role of regular testing for and treatment of 25-OH vitamin D deficiency in patients on dialysis is undefined, as patients on dialysis are presumed to lack the ability to convert 25-OH vitamin D to the active 1,25-(OH)2 vitamin D form of the molecule [4, 5]. However, preliminary studies report a higher risk of death among hemodialysis patients with 25-OH vitamin D deficiency [6, 7], similar to the general population.
One potential mechanism for the deleterious effect of 25-OH vitamin D deficiency in the dialysis population is reduction or absence of its autocrine activity, important in the regulation of cellular proliferation and apoptosis [8]. In particular, 25-OH vitamin D promotes skeletal muscle growth and differentiation. Deficiency is associated with muscle atrophy, musculoskeletal pain and poorer physical function in the general population [2, 9, 10]. Several randomized clinical trials and meta-analyses have demonstrated that supplementation of 25-OH vitamin D decreases the risk for falls and fractures in the elderly [11–14]; one trial also demonstrated improved muscle strength among persons receiving 25-OH vitamin D supplementation [13].
Using data on individuals new to dialysis from the Comprehensive Dialysis Study (CDS), we evaluated the prevalence of 25-OH vitamin D deficiency and the associations among 25-OH vitamin D concentrations, self-reported physical activity and health-related quality of life (HRQoL). We hypothesized that a large fraction of the CDS population would be 25-OH vitamin D deficient and that 25-OH vitamin D concentrations would be inversely correlated with self-reported physical activity and HRQoL, independent of age and other confounders.
Materials and methods
The primary aim of the CDS was to determine the relations among nutritional status, inflammatory response, physical activity, rehabilitation and HRQoL in patients new to dialysis. The study was carried out through the United States Renal Data System Center and the Special Studies Centers in Nutrition and Rehabilitation/Quality of Life at 296 dialysis units throughout the USA. Institutional review boards at University of California San Francisco and Davis, Emory University and University of Minnesota approved the study. All participants provided informed consent.
The study design and methods have been previously described in detail [15]. Patients starting dialysis between June 2005 and January 2007 at the selected units were invited to participate. A participant was excluded if he or she had a speech, hearing or cognitive impairment, prior or imminent transplantation or plans to move out of the area. In addition, since participants had to complete the patient questionnaire via a telephone interview, individuals who did not have phones or did not speak English or Spanish were not eligible to participate. The average length of time that CDS participants had been on dialysis at their baseline interview was ∼4 months (median 122 days; range 78–357 days).
As part of a nutrition substudy of CDS, 266 patients recruited from 68 dialysis units also provided serum samples. One individual had a 25-OH vitamin D concentration greater than the assay limit and was excluded from further analysis. Of the remaining participants, 192 (72%) completed the physical activity and HRQoL questionnaires within 120 days of baseline serum sample collection. These participants comprise the analytic cohort.
Self-reported physical activity and HRQoL
Baseline physical activity was assessed using the Human Activity Profile (HAP) questionnaire [16], in which participants report whether they are still doing, have stopped doing or have never done 94 activities of increasing intensity. The activities range from 1 to 10 Metabolic Equivalent of Task, from transferring out of bed (Ranked 1) to running 3 miles in ≤30 min (Ranked 94). Other examples of the kinds of everyday activities captured in the HAP include: dressing and undressing (Ranked 18), carrying a light load of groceries (Ranked 36) and walking 1 mile (Ranked 63).
Two scores are available from the HAP: a maximum activity score (MAS), reflective of the most taxing activity a respondent is still performing and an adjusted activity score (AAS), reflective of the respondent’s daily activity level. The MAS is the number of the highest ranked activity the respondent is still performing. The AAS is calculated by subtracting from the MAS the number of activities that the respondent has stopped performing that rank below his or her most metabolically intense activity. The HAP has been validated in a range of healthy and diseased groups of patients [17]. In the dialysis population, it has been shown to have a high degree of reproducibility [18]; it also correlated closely with physical accelerometry and tests of physical function, such as gait speed and chair rising time [19]
The 12-item Medical Outcomes Study Short Form survey (SF-12) was used to assess HRQoL [20]. The SF-12 evaluates eight domains: physical functioning, physical role limitation, bodily pain, general health, vitality, social functioning, emotional role limitation and mental health. Two summary scores, Physical Component Summary (PCS) and Mental Component Summary (MCS), are then created. A higher score indicates better quality of life in each of the domains.
25-OH vitamin D and other covariates
Baseline serum samples were collected from participants in the nutrition substudy at the time of their routine laboratory studies and shipped overnight to University of California Davis. Specimens were received as serum. They were shipped on ice via overnight delivery the day they were drawn following centrifugation at the dialysis unit. They were aliquoted and stored over liquid nitrogen until assay. Concentrations of 25-OH vitamin D were measured via the Direct Enzyme Immunoassay (Immunodiagnostic Systems Inc.). The assay has a mean intra-assay coefficient of variation of 5.5% and a mean inter-assay coefficient of variation of 6.6%. The range of the assay is 2.4–144 ng/mL. All measurements were made in duplicate and the mean employed in the analyses. According to K/DOQI guidelines, 25-OH vitamin D deficiency was defined as concentration < 15 ng/mL and insufficiency was defined as concentration 15 to <30 ng/mL.
Information about participants’ demographic characteristics, comorbidities and dialysis treatment characteristics were obtained from a patient questionnaire administered by DataBanque Research Services (Pittsburgh, PA) and from the Centers for Medicare and Medicaid Services Medical Evidence Report (form 2728). Comorbidities utilized in the analysis included: diabetes, congestive heart failure and atherosclerotic disease (defined as atherosclerotic heart disease, cerebrovascular disease, peripheral vascular disease or amputation). Based on a previous National Health and Nutrition Examination Survey analysis, participants’ state of residence was categorized as a state of low sunlight exposure versus medium or high sunlight exposure [21]. Low sunshine state was defined as residence in Maine, Vermont, New Hampshire, Massachusetts, Connecticut, Rhode Island, New York, Pennsylvania, Ohio, Michigan, Minnesota and Washington. Concentrations of 25-OH vitamin D concentrations drawn between May and August were classified as summer and compared to the rest of the year.
Statistical analysis
Continuous data are presented using means (± SD) and compared using Student’s t-test. Categorical data are presented as proportions and compared using chi-square. Since 25-OH vitamin D concentrations were skewed, we log transformed them prior to inference testing.
We used linear regression to determine the unadjusted associations among 25-OH vitamin D concentrations and patient characteristics. The multivariable analyses adjusted for age, sex, race, diabetes, season (summer versus other season) and characteristics significantly associated with 25-OH vitamin D concentrations in unadjusted analyses (at a P-value <0.1). A random effects model was utilized in the multivariable analyses, which included sample weights to account for the probability of dialysis facility inclusion in the nutrition substudy as well as clustering of participants within dialysis facilities.
A similar procedure was utilized to determine the unadjusted association of 25-OH vitamin D concentrations with scores on the HAP and the SF-12 scales. For the multivariable analyses, we constructed a parsimonious model that adjusted for age, sex, race, diabetes, season and center using a random effects model. We then substituted the dichotomous variable 25-OH vitamin D deficiency, defined as a 25-OH vitamin D concentration < 15 ng/mL, to assess the consistency of our results.
All analyses were performed using SAS v9.1.3 (SAS Institute Inc., Cary, NC).
Results
Clinical characteristics of the analytic cohort are presented in Table 1. Compared to CDS participants missing 25-OH vitamin D measurements or complete questionnaire data, participants in the analytic cohort had generally similar demographic characteristics and modestly higher serum albumin concentrations. The mean age of participants in the analytic cohort was 62 ± 14 years; approximately half were male, and approximately half were college educated.
Table 1.
Characteristics of participants in the CDSa
| Characteristics | Participants (N = 1432) | Analytic cohort (n = 192) | P-value |
| Age (years) | 60.3 ± 14.2 | 61.6 ± 13.9 | 0.21 |
| Female | 640 (44.7%) | 93 (48.4%) | 0.32 |
| Race, non-white | 457 (31.9%) | 51 (26.6%) | 0.13 |
| Low sunshine state | 368 (25.7%) | 30 (15.6%) | <0.01 |
| College education or more | 629 (43.9%) | 94 (49.0%) | 0.20 |
| Body mass index (kg/m2) | 29.7 ± 8.1 | 30.1 ± 7.8 | 0.52 |
| Current smoker | 222 (15.5%) | 26 (13.5%) | 0.48 |
| Peritoneal dialysis | 148 (10.3%) | 20 (10.4%) | 0.97 |
| Hemodialysis access via catheterb | 722 (56.6%) | 85 (50.0%) | 0.25 |
| Diabetes | 823 (57.5%) | 116 (60.4%) | 0.44 |
| Atherosclerotic disease | 485 (33.9%) | 69 (35.9%) | 0.57 |
| Congestive heart failure | 430 (30.0%) | 67 (34.9%) | 0.17 |
| Creatinine (mg/dL) | 6.9 ± 3.5 | 6.8 ± 3.53 | 0.78 |
| Albumin (g/dL)c | 3.2 ± 0.7 | 3.4 ± 0.5 | <0.01 |
Data are expressed as mean ± SD or as N (%) where appropriate.
Proportions presented in this row are for patients on hemodialysis only.
Albumin values for the analytic cohort (n = 192) were measured in the CDS, and values for the excluded cohort (n = 1083) were taken from the Centers for Medicare and Medicaid Services Medical Evidence Report (form 2728).
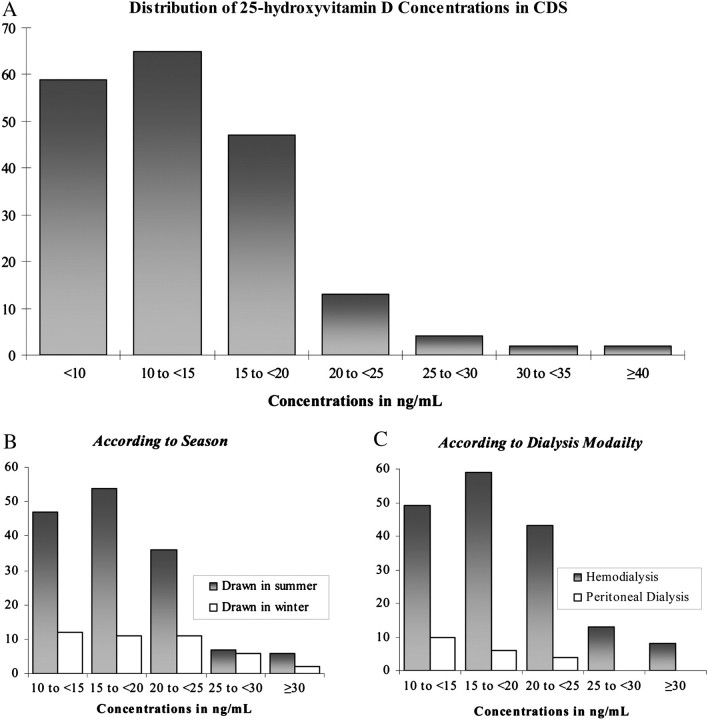
Median 25-OH vitamin D concentration was 12.6 (10th percentile 7.3, 90th percentile 20.7) ng/mL (Figure 1, panel A). The majority of patients [124 (65%)] had frank 25-OH vitamin D deficiency and the vast majority of participants [188 (98%)] had 25-OH vitamin D insufficiency or deficiency. Forty-two (22%) of our samples were collected in the summer season (Figure 1, panel B). Most participants [172 (90%)] were on hemodialysis (Figure 1, panel C). Of the patients on hemodialysis, a majority [170 (99%)] were receiving hemodialysis three times a week for a median of 3.5 h (range 2.3–4.5 h).
Fig. 1.
Distribution of 25-OH vitamin D concentrations in (A) the CDS cohort. (B) Distribution according to season of serum draw. Summer was defined as May–August. (C) Distribution according to dialysis modality.
Correlates of 25-OH vitamin D concentration
In unadjusted analyses, female sex, current smoking, higher body mass index and peritoneal dialysis (versus hemodialysis) were associated with lower 25-OH vitamin D concentrations (Table 2). Older age, summer season and higher serum creatinine and serum albumin concentrations were associated with ‘higher’ 25-OH vitamin D concentrations (Table 2). With the exception of smoking (P = 0.08) and serum creatinine (P = 0.08) these associations remained significant in multivariable analysis.
Table 2.
Demographic and clinical correlates of 25-OH vitamin D concentrationsa
| Characteristics | Unadjusted percent change in 25-OH vitamin D concentration | Adjusted percent change in 25-OH vitamin D concentration | ||
| βb ± SE | P-value | βb ± SE | P-value | |
| Age (per 10 years) | 5.6 ± 2.2 | 0.01 | 5.3 ± 1.8 | 0.003 |
| Female sex | −16.2 ± 6.1 | 0.003 | −11.5 ± 6.1 | 0.04 |
| Race, non-white | −7.5 ± 7.1 | 0.26 | 6.3 ± 6.3 | 0.30 |
| Low sunshine state | −1.8 ± 8.7 | 0.83 | NA | NA |
| Summer (versus other season) | 33.3 ± 7.3 | <0.0001 | 35.3 ± 5.7 | <0.0001 |
| Current smoker | −16.1 ± 9.2 | 0.05 | −12.3 ± 7.8 | 0.08 |
| College education or more | −2.4 ± 6.2 | 0.69 | NA | NA |
| Body mass index | −1.1 ± 0.4 | 0.003 | −1.2 ± 0.3 | 0.0007 |
| Peritoneal dialysis | −15.6 ± 10.3 | 0.09 | −19.6 ± 7.2 | 0.002 |
| Diabetes | −8.4 ± 6.4 | 0.16 | −4.2 ± 4.8 | 0.36 |
| Atherosclerotic disease | −1.4 ± 6.1 | 0.82 | NA | NA |
| Congestive Heart Failure | −8.2 ± 6.5 | 0.18 | NA | NA |
| Creatinine | 1.8 ± 0.9 | 0.04 | 0.8 ± 0.5 | 0.08 |
| Albumin | 29.7 ± 6.6 | <0.0001 | 24.9 ± 6.2 | 0.0003 |
NA, Not applicable.
β coefficient can be interpreted as the percent change expected in 25-OH vitamin D for each unit change in the independent variable.
25-OH vitamin D concentration, self-reported physical activity and HRQoL
In analyses adjusted for age, sex, race, diabetes, season and center, a 25% higher 25-OH vitamin D concentration was associated with a 1.7 ± 0.7 point higher score on the AAS (P = 0.03) and a 1.3 ± 0.6 point higher score on the MAS (P = 0.03) (Table 3). In addition, a 25% higher 25-OH vitamin D concentration was associated with a 1.0 ± 0.4 point higher score on the MCS (P = 0.03). There was no significant association between 25-OH vitamin D concentrations and PCS scores.
Table 3.
Association of 25-OH vitamin D concentrations with physical activity and HRQoL
| Model | Unadjusted | Adjusted for age, sex, race, diabetes status, season and center | ||
| βa ± SE | P-value | βa ± SE | P-value | |
| AAS | 1.34 ± 0.66 | 0.04 | 1.68 ± 0.74 | 0.03 |
| MAS | 1.00 ± 0.53 | 0.06 | 1.29 ± 0.60 | 0.03 |
| SF-12 PCS | 0.22 ± 0.41 | 0.60 | 0.60 ± 0.41 | 0.15 |
| SF-12 MCS | 0.81 ± 0.43 | 0.06 | 0.95 ± 0.44 | 0.03 |
β coefficient and SE values can be interpreted as: for a 25% change in 25-OH vitamin D concentration, the predicted variable (adjusted or maximum activity score, SF-12 scores) would change by β ± SE.
In sensitivity analyses, 25-OH vitamin D deficiency, defined as 25-OH vitamin D concentration < 15 ng/mL, was independently associated with lower scores on the AAS (β coefficient ± SE: −5.5 ± 2.5, P = 0.03), MAS (β coefficient ± SE: −4.4 ± 2.1, P = 0.04) and PCS (β coefficient ± SE: −4.2 ± 1.7 P = 0.01), but not with scores on the MCS (β coefficient ± SE: −2.5 ± 1.8, P = 0.18).
Discussion
In this diverse sample of incident dialysis patients, two-thirds were 25-OH vitamin D deficient and >95% were 25-OH vitamin D insufficient or deficient based on National Kidney Foundation K/DOQI guidelines. Furthermore, lower 25-OH vitamin D concentrations were significantly, albeit modestly, associated with lower levels of self-reported physical activity and poorer HRQoL.
The prevalence of 25-OH vitamin D deficiency seen in our study was more pronounced than in prior reports. In a study of 908 incident hemodialysis patients from the USA, Bhan et al. [22] found that ∼37% of participants were 25-OH vitamin D deficient. Another study of 84 hemodialysis patients from Argentina reported that 23% of participants were 25-OH vitamin D deficient [23]. The difference in prevalence among the three studies may be explained, in part, by differences in latitude, the season 25-OH vitamin D was assayed and/or patient characteristics. For example, in contrast to the earlier studies involving only hemodialysis patients, 10% of our study sample was receiving peritoneal dialysis, and these patients had significantly lower 25-OH vitamin D concentrations. A majority (78%) of our samples were collected in the winter season, another significant correlate of lower 25-OH vitamin D concentration.
The serum concentration of 25-OH vitamin D that can be considered ‘sufficient’ remains a matter of debate. K/DOQI guidelines, which recommend 25-OH vitamin D concentrations > 30 ng/mL are largely opinion based and apply only to patients with CKD Stages III and IV [4]. The Institute of Medicine has recently recommended that concentrations > 20 ng/mL are sufficient for maintaining bone health in the general population [24]. Using this cut off, ∼171 (89%) patients in our study would be considered 25-OH vitamin D deficient. However, whether this threshold is appropriate for attenuation of other adverse outcomes associated with vitamin D deficiency such as falls, cancer and cardiovascular mortality is uncertain.
The clinical correlates of 25-OH vitamin D concentration noted in our analysis, such as sex, season and serum albumin concentration, are consistent with the findings of Bhan et al. [22]. We also identified body mass index and peritoneal dialysis as significant correlates of 25-OH vitamin D concentration. An inverse correlation between body mass index and 25-OH vitamin D concentrations has been previously reported in the general population [25–28]. Potential reasons include reduced nutritional intake of 25-OH vitamin D [28], reduced sunlight exposure [25] or an increased volume of distribution of 25-OH vitamin D in obese patients [26, 27]. Peritoneal dialysis was associated with a 20% lower 25-OH vitamin D concentration in comparison to hemodialysis in our study. Shah et al. [29] have also reported strikingly low 25-OH vitamin D concentrations in patients on peritoneal dialysis in their center; all 29 participants in their study were 25-OH vitamin D insufficient (concentration < 30 ng/mL) and ∼80% had undetectable concentrations. Older participants had significantly higher 25-OH vitamin D concentrations in our study. Potential explanations for this finding include an increased intake of 25-OH vitamin D rich foods and supplements among older adults [28] or the possibility that over time younger individuals may be spending relatively more time indoors [30].
Our study expands on current understanding of the importance of 25-OH vitamin D to examine its relation to physical activity and quality of life. We found that each 25% decrease in 25-OH vitamin D concentration was associated with about a 1.5-point lower score on the MAS and AAS of the HAP profile. The magnitude of this difference is approximately equivalent to the difference in physical activity between climbing 12 steps versus 9 steps. The clinical significance of this difference is not clear, though previous studies have indicated that larger differences in physical activity are associated with higher mortality risk among incident hemodialysis patients [31].
Our findings appear similar to reports in the general elderly population. For example, the CHIANTI study of 976 elderly Italians reported significantly worse physical performance in participants with 25-OH vitamin D < 10 ng/mL [9]. A recent study of 4100 ambulatory elderly participants in the National Health and Nutrition Examination Survey also found a positive association between 25-OH vitamin D concentrations and performance on the sit-to-stand test as well as the eight-foot walk test [32].
Skeletal muscle fibers carry vitamin D receptors; activation of these receptors is proposed to cause muscle growth and proliferation [5, 8, 14, 33, 34]. Biopsy and imaging findings consistent with muscular atrophy have been associated with 25-OH vitamin D deficiency [34, 35]. Furthermore, supplementation with calcium and vitamin D improves the number and size of muscle fibers [13] as well as lower extremity function [36]. Alternatively, 25-OH vitamin D deficiency may be a marker of poor health and nutritional status, but not a direct cause for lower physical activity. It is also possible that lower physical activity led to poor nutritional intake and reduced sun exposure, so that the association could be bidirectional.
There are limited data regarding the association between 25-OH vitamin D concentrations and HRQoL in the general population [37, 38], and none to our knowledge in the dialysis population. We found that each 25% decrease in 25-OH vitamin D concentration was associated with a 1-point lower score on the SF-12 MCS, but not the PCS. In patients on maintenance dialysis, there is a 1.2% reduction in the risk of death and 0.6% reduction in the risk of first hospitalization per 1-point increase in the MCS [39].
The study has several strengths. First, we used a diverse sample of incident dialysis patients drawn from multiple dialysis facilities, including a substantial number of patients receiving peritoneal dialysis, a group underrepresented in previous studies. The sample size of the analytic cohort was relatively large and characteristics of participants were similar to those of the overall CDS cohort. Second, we used validated instruments to assess physical activity and HRQoL.
Several limitations of this study should also be noted. Since 25-OH vitamin D levels are variable within individuals depending on season, nutrition and time spent outdoors, a mean value based on repeated measures at different time points for each participant would have served as a better indicator of his or her time-averaged 25-OH vitamin D serum concentration. We also did not collect data on whether our participants were taking 25-OH vitamin D supplements. While the HAP has been validated in the general population and in patients on dialysis, the study findings may have been strengthened with direct measurement of physical function and/or physical performance, especially measures of muscle strength, which are not captured by the HAP. Furthermore, because of the cross-sectional nature of our study, we cannot determine causality. Finally, other factors, such as baseline hemoglobin concentrations, residual renal function or dialysis dose could impact serum 25-OH vitamin D concentrations and/or physical activity and HRQoL, but these data were not collected in CDS.
In summary, in a well-characterized cohort of patients new to dialysis, 25-OH vitamin D deficiency was present in almost two-thirds of patients, while 25-OH vitamin D insufficiency was nearly universal. Lower 25-OH vitamin D concentrations were associated with lower self-reported physical activity and poorer self-reported mental health, suggesting that this common finding may have far reaching and important consequences. Further research is required to determine whether hypovitaminosis D is causally linked to reduced activity, and whether supplementation can improve physical activity and HRQoL in the dialysis population.
Supplementary data
Supplementary data is available online at http://ndt.oxfordjournals.org.
Acknowledgments
This work was funded by contract N01-DK-7-0005 from the NIDDK. Dr S.A. was supported by F32 DK 084697. Dr G.M.C. was supported by K24 DK 080645. Dr M.K.T. is supported by K23 AG028952 and an ASN-ASP-Junior Development award in Geriatric Nephrology, funded through Atlantic Philanthropies, the American Society of Nephrology, and the John A. Hartford Foundation. Drs G.M.C, K.L.J., B.G. and G.K. were also supported by N01-DK012450.
Conflict of interest statement. None declared.
An abstract describing these findings was presented at the meeting of the American Society of Nephrology 2010. They have not been published elsewhere. The data reported here have been supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the USA government.
References
- 1.Melamed ML, Michos ED, Post W, et al. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wicherts IS, van Schoor NM, Boek AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 3.McBeth J, Pye SR, O'Neill TW, et al. Musculoskeletal pain is associated with very low levels of vitamin D in men: results from the European Male Ageing Study. Ann Rheum Dis. 2010;69:1448–1452. doi: 10.1136/ard.2009.116053. [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. http://www.kidney.org/professionals/KDOQI/guidelines_bone/index.htm. New York, NY: National kidney foundation; 2003. [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Pecovnik-Balon B, Jakopin E, Bevc S, et al. Vitamin D as a novel nontraditional risk factor for mortality in hemodialysis patients. Ther Apher Dial. 2009;13:268–272. doi: 10.1111/j.1744-9987.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- 7.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 8.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 9.Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62:440–446. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon J, Suzuki T, Yoshida H, et al. Concomitant lower serum albumin and vitamin D levels are associated with decreased objective physical performance among Japanese community-dwelling elderly. Gerontology. 2007;53:322–328. doi: 10.1159/000103257. [DOI] [PubMed] [Google Scholar]
- 11.Flicker L, MacInnis RJ, Stein MS, et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc. 2005;53:1881–1888. doi: 10.1111/j.1532-5415.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 12.Broe KE, Chen TC, Weinberg J, et al. A higher dose of vitamin d reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc. 2007;55:234–239. doi: 10.1111/j.1532-5415.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 13.Sato Y, Iwamoto J, Kanoko T, et al. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20:187–192. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of Vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 15.Kutner NG, Johansen KL, Kaysen GA, et al. The Comprehensive Dialysis Study (CDS): a USRDS Special Study. Clin J Am Soc Nephrol. 2009;4:645–650. doi: 10.2215/CJN.05721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fix A, Daughton D. Human Activity Profile Professional Manual. Odessa, FL: Psychological Assessment Resources Inc; 1988. [Google Scholar]
- 17.Davidson M, de Morton N. A systematic review of the Human Activity Profile. Clin Rehabil. 2007;21:151–162. doi: 10.1177/0269215506069475. [DOI] [PubMed] [Google Scholar]
- 18.Wellard S. Validation of physical activity measurement for people on dialysis treatment. EDTNA ERCA J. 2003;29:140–142. doi: 10.1111/j.1755-6686.2003.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 19.Johansen KL, Painter P, Kent-Braun JA, et al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int. 2001;59:1121–1127. doi: 10.1046/j.1523-1755.2001.0590031121.x. [DOI] [PubMed] [Google Scholar]
- 20.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 21.John EM, Schwartz GG, Dreon DM, et al. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971–1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999;8:399–406. [PubMed] [Google Scholar]
- 22.Bhan I, Burnett-Bowie SA, Ye J, et al. Clinical measures identify vitamin d deficiency in dialysis. Clin J Am Soc Nephrol. 2010;5:460–467. doi: 10.2215/CJN.06440909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Valle E, Negri AL, Aguirre C, et al. Prevalence of 25(OH) vitamin D insufficiency and deficiency in chronic kidney disease stage 5 patients on hemodialysis. Hemodial Int. 2007;11:315–321. doi: 10.1111/j.1542-4758.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 24.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konradsen S, Ag H, Lindberg F, et al. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur J Nutr. 2008;47:87–91. doi: 10.1007/s00394-008-0700-4. [DOI] [PubMed] [Google Scholar]
- 26.Ramel A, Jonsson PV, Bjornsson S, et al. Vitamin D deficiency and nutritional status in elderly hospitalized subjects in Iceland. Public Health Nutr. 2009;12:1001–1005. doi: 10.1017/S1368980008004527. [DOI] [PubMed] [Google Scholar]
- 27.Arunabh S, Pollack S, Yeh J, et al. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88:157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 28.Kamycheva E, Joakimsen RM, Jorde R. Intakes of calcium and vitamin d predict body mass index in the population of Northern Norway. J Nutr. 2003;133:102–106. doi: 10.1093/jn/133.1.102. [DOI] [PubMed] [Google Scholar]
- 29.Shah N, Bernardini J, Piraino B. Prevalence and correction of 25(OH) vitamin D deficiency in peritoneal dialysis patients. Perit Dial Int. 2005;25:362–366. [PubMed] [Google Scholar]
- 30.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Hare AM, Tawney K, Bacchetti P, et al. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. 2003;41:447–454. doi: 10.1053/ajkd.2003.50055. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or = 60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 33.Boonen S, Bischoff-Ferrari HA, et al. Addressing the musculoskeletal components of fracture risk with calcium and vitamin D: a review of the evidence. Calcif Tissue Int. 2006;78:257–270. doi: 10.1007/s00223-005-0009-8. [DOI] [PubMed] [Google Scholar]
- 34.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13:187–194. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- 35.Tagliafico AS, Ameri P, Bovio M, et al. Relationship between fatty degeneration of thigh muscles and vitamin D status in the elderly: a preliminary MRI study. AJR Am J Roentgenol. 2010;194:728–734. doi: 10.2214/AJR.09.3130. [DOI] [PubMed] [Google Scholar]
- 36.Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 37.Porthouse J, Cockayne S, King C, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330:1003. doi: 10.1136/bmj.330.7498.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witham MD, Crighton LJ, Gillespie ND, et al. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail. 2010;3:195–201. doi: 10.1161/CIRCHEARTFAILURE.109.907899. [DOI] [PubMed] [Google Scholar]
- 39.Lacson E, Jr., Xu J, Lin SF, et al. A comparison of SF-36 and SF-12 composite scores and subsequent hospitalization and mortality risks in long-term dialysis patients. Clin J Am Soc Nephrol. 2010;5:252–260. doi: 10.2215/CJN.07231009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.