Abstract
Background. Although current guidelines recommend the evaluation of mineral and bone metabolism in patients with all stages of chronic kidney disease (CKD), the prevalence of altered mineral ion homeostasis in the pediatric posttransplant population is unknown. Moreover, the contribution of abnormal mineral ion metabolism to graft outcomes in this population has not been evaluated.
Methods. Serum calcium, phosphorus, 25(OH)vitamin D, 1,25(OH)2vitamin D, parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF-23) levels were evaluated 4.9 ± 0.5 years after transplantation in 68 stable pediatric renal allograft recipients. Patients were subsequently followed for 2 years.
Results. At baseline, mean estimated glomerular filtration rate (GFR) was 60 ± 2 mL/min/1.73m2. Serum calcium and phosphorus values were within the reference interval. PTH values were elevated but did not differ by CKD stage. 25(OH)vitamin D levels were low in nearly half of all subjects. Tubular reabsorption of phosphate and 1,25(OH)2vitamin D values were lower, while FGF-23 and PTH values were higher in more advanced stages of CKD. Thirty percent of patients with FGF-23 values >110 RU/mL had a decrease in GFR of >50% (P < 0.05) and FGF-23 values predicted future episodes of rejection.
Conclusions. Despite normal serum calcium and phosphorus levels in the majority of prevalent pediatric renal transplant recipients, abnormalities in PTH, 25(OH)vitamin D and FGF-23 are common. FGF-23 levels may be associated with increased risk for deterioration of kidney function and episodes of rejection.
Keywords: acute rejection, FGF-23, indices of mineral metabolism, pediatric renal transplantation, progression of renal failure
Introduction
Successful renal transplantation corrects many of the abnormalities in mineral ion homeostasis and bone metabolism that develop during the course of chronic kidney disease (CKD). However, abnormalities in phosphate, parathyroid hormone (PTH) and vitamin D metabolism persist and may contribute to defects in skeletal mineralization, poor growth and bone fragility that prevail in children postrenal transplantation [1–3]. Current recommendations suggest routine assessment and treatment of hyperparathyroidism and hypovitaminosis D in CKD patients. Furthermore, levels of a new phosphaturic hormone, fibroblast growth factor 23 (FGF-23), are increased in patients with kidney transplants, perhaps as a result of corticosteroid therapy [4], and have recently been implicated in the development of secondary hyperparathyroidism in adult and pediatric patients with CKD [5]. However, the prevalence of mineral abnormalities in long-term pediatric renal transplant recipients has not been well characterized.
In addition to maintaining bone homeostasis, hormones that regulate mineral metabolism have been implicated in posttransplant graft function. Indeed, calcium, phosphorus and parathyroid hormone (PTH) levels have been linked to delayed graft function [6], while 25(OH)vitamin D and 1,25(OH)2vitamin D levels play a role in immune regulation [7]. FGF-23 regulates phosphate, 1,25(OH)2 vitamin D and potentially PTH levels [5,8] and has been linked to the rate of declining renal function in predialysis CKD patients [9]. However, the role of each of these hormones in long-term pediatric renal allograft survival is unknown. Thus, the current study was undertaken to characterize mineral metabolism across the spectrum of prevalent pediatric renal transplant recipients and to evaluate the effects of indices of mineral metabolism on long-term allograft function.
Materials and methods
In order to characterize the prevalence of mineral abnormalities in stable prevalent pediatric renal transplant recipients, a cross-sectional study was performed in subjects between the ages of 2 and 20 years and who had received their transplants at least 1 year previously. Stable allograft function was defined by no change in serum creatinine >0.2 mg/dL within the 6 months prior to the study. Exclusion criteria included the following: kidney transplantation within the past year, active infection, kidney transplant rejection at the time of enrollment, growth hormone therapy within the past 6 months, therapy with active vitamin D sterols within the past 6 months and a history of multiple organ transplantation. At a routine transplant clinic visit, a brief history and physical exam were performed and a list of current medications, blood pressure, height and weight were collected. Blood and urine samples were obtained at that visit for the determination of serum levels of calcium, albumin, phosphorus, alkaline phosphatase, 1,25(OH)2vitamin D and 25(OH)vitamin D and ethylenediaminetetraacetic acid-plasma levels of parathyroid hormone (PTH) and FGF-23, along with urine values of calcium, creatinine and phosphorus. Serum determinations of creatinine, calcium, albumin, creatinine, phosphorous and alkaline phosphatase and urine values of calcium, creatinine and phosphorus were performed using an Olympus AU5400 (Olympus America Incorporated, Center Valley, PA). PTH levels were determined by a first PTH-IMA (NicholsR, San Clemente, CA) that detects PTH(1-84) as well as amino-terminally truncated fragments up to and including PTH(7-84), while FGF-23 was determined by the second generation C-terminal assay (ImmutopicsR), which detects C-terminal as well as intact FGF-23. The reference interval for this assay (1–48 RU/mL) was determined from 26 healthy pediatric (11 males/15 females, age 12 ± 1 year, 73% Hispanic, 23% White, 4% other) controls recruited at our center during the same time interval as the study. Serum calcium levels were corrected for serum albumin levels by the formula: corrected calcium = measured calcium (mg/dL) + (0.8 × [4 − serum albumin (g/dL)]). 1,25(OH)2vitamin D and 25(OH)vitamin D levels were determined by radioimmunoassay [10]. Glomerular filtration was calculated by the formula derived by Schwartz et al. [11]. The tubular reabsorption of phosphate was calculated according to urine and serum values of creatinine and phosphate [tubular reabsorption of phosphate (TRP) =(1 − Ucreat × Uphos/Screat × Sphos) × 100].
To assess the value of baseline circulating ion and mineral hormone levels in predicting subsequent rejection episodes and renal function, all subjects in the cross-sectional cohort were followed at 6-month intervals for 2 years or until return to dialysis. During the follow-up period, calcineurin inhibitor levels were obtained to assess medication compliance and kidney biopsies were performed as deemed necessary by the treating physician. Biopsy-proven rejection episodes, as defined by modified Banff 2007 criteria [12], were recorded. At the conclusion of the 2 years of follow-up, an estimated glomerular filtration rate (GFR) was obtained in all subjects [11].
The study was approved by the UCLA Human Subject Protection Committee and informed consent was obtained from all patients and/or parents.
Statistical methods
Mean and standard error were used to summarize normally distributed variables; the median and interquartile range were utilized to describe variables with a skewed distribution (i.e. PTH, FGF-23 and alkaline phosphatase). Given the small number of patients with Stages 1 and 4 CKD, for purposes of analysis, Stages 1 and 2 CKD were considered together, as were Stages 3 and 4 CKD. Differences in variables were assessed using the t-test, the Mann–Whitney test or chi-squared analysis. Multiple linear and logistic regression analyses were used to determine interaction between variables. Odds ratios, receiver operating curves (ROC) and log-rank Kaplan–Meier survival curves were calculated to estimate predictive values of biochemical parameters for 2-year outcomes. Statistical analysis was performed using SAS software and all tests were two-sided with significance level P <0.05.
Results
Study participants
Between October 2005 and February 2006, 68 prevalent pediatric renal transplant recipients—32 with living-related and 36 with deceased donor allografts—were qualified for and agreed to participate in the study. The demographic data for this cohort are displayed in Table 1. Briefly, the majority of subjects were Hispanic (62%) and male (56%) and had a normal body mass index. The majority of patients (87%) had Stages 2 and 3 CKD and the average estimated GFR for the entire cohort was 62 ± 3 mL/min/1.73m2 (1.04 ± 0.05 mL/s/1.73m2). The time since transplantation varied between 1.0 and 18.4 years with a median of 3.1 years. Patients with more advanced stages of CKD tended to be older and had a longer time since transplantation. At time of transplantation, all subjects had received induction therapy with either daclizumab or anti-thymocyte globulin. After transplantation, the majority of study subjects were receiving standard maintenance immunosuppressive therapy, including steroids, calcineurin inhibition (cyclosporin or tacrolimus) and an antimetabolite (mycophenolate mofetil or azathioprine); a minority (22%) of patients were maintained on a steroid-free regimen, identical to standard therapy minus prednisone. One patient with Stage 3 CKD ingested calcium carbonate on a daily basis for acid reflux; no other participants were on any phosphate binder medications, active vitamin D sterols or 25(OH)vitamin D supplementation.
Table 1.
Demographics
| CKD 1 and 2 (n = 33) | CKD 3 and 4 (n = 35) | |
| Age (years) | 13.3 ± 0.9 | 16.7 ± 0.6a |
| Gender (n) (male/female) | 16/17 | 22/13 |
| Time since transplant (years) | 3.1 ± 0.5 | 6.6 ± 0.8a |
| Race | 9 | 9 |
| Caucasian | 1 | 1 |
| Black | 21 | 24 |
| Hispanic | 0 | 1 |
| Asian | 2 | 0 |
| Other | ||
| Steroid-based immunosuppression (%) | 67 | 91a |
| Body mass index (kg/m2) | 21.2 ± 1.0 | 22.3 ± 0.9 |
P <0.05 between CKD Stages 1 and 2 (combined) and Stages 3 and 4 (combined).
Of the 68 patients in the initial cohort, 2-year follow-up data were available in 64 patients. Of the four lost to follow-up, average baseline GFR was 62 (range 43–82) mL/min/1.73m2 [1.04 (range 0.72–1.37 mL/s/1.73m2)]. Baseline calcium, phosphorus, PTH, 25(OH)vitamin D, 1,25(OH)2vitamin D and FGF-23 levels were no different in those lost to follow-up from those included in the 2-year analysis.
Cross-sectional analysis: biochemical parameters
Baseline biochemical values are displayed in Table 2. Serum calcium levels were in the reference interval throughout all stages of CKD and all values, when corrected for serum albumin, were <10.2 mg/dL (2.55 mmol/L). Serum phosphorus levels were also within the reference interval in all stages of CKD; three patients (one with Stage 1; one with Stage 2 and one with Stage 3 CKD) displayed values <3 mg/dL (0.97 mmol/L), while 12 patients (two with Stage 1; five with Stage 2; four with Stage 3 and one with Stage 4 CKD) had values ≥5 mg/dL (1.62 mmol/L). PTH levels were increased in all stages of CKD—57% of the patients had plasma PTH levels above currently recommended target ranges for respective stages of CKD [13, 14]. PTH values did not differ between stages of CKD.
Table 2.
Biochemical parameters according to CKD stage
| Biochemical parameter | CKD 1 and 2 (n = 33) | CKD 3 and 4 (n = 35) |
| Calcium, mg/dL (mmol/L) | 9.6 ± 0.1 (2.40 ± 0.03) | 9.6 ± 0.1a (2.40 ± 0.03) |
| Albumin, g/dL (g/L) | 4.1 ± 0.1 (41 ± 1) | 4.1 ± 0.1 (41 ± 1) |
| Phosphorus, mg/dL (mmol/L) | 4.4 ± 0.2 (1.42 ± 0.13) | 4.1 ± 0.1 (1.32 ± 0.06) |
| Alkaline phosphatase (IU/L)a | 184 (87, 232) | 114 (79, 189)b |
| 1st PTH-IMA (pg/mL)a | 77 (53, 130) | 72 (54, 118) |
| 25(OH)D, ng/mL (nmol/L) | 26 ± 2 (65 ± 5) | 35 ± 2b (87 ± 5) |
| 1,25(OH)2D (pg/mL) (pmol/L) | 54 ± 4 (140 ± 10) | 39 ± 3b (101 ± 8) |
| FGF-23 (RU/mL)a | 77 (34, 113) | 151 (78, 217)b |
| Urine Ca/Cr, mg/mg (mmol/mmol) | 0.08 ± 0.01 (0.26 ± 0.03) | 0.06 ± 0.01b (0.19 ± 0.03) |
| TRP (%) | 88 ± 1 | 82 ± 2b |
Expressed as the median (interquartile range).
P < 0.05 between CKD Stages 1 and 2 (combined) and Stages 3 and 4 (combined).
Serum 25(OH)vitamin D levels were <30 ng/mL (75 nmol/L) in 33 of the 68 study participants [5 had values between 5 and 14 ng/mL (12 and 37 nmol/L), while the other 28 patients maintained levels between 15 and 29 ng/mL (38 and 74 nmol/L)]. 25(OH)vitamin D values were not related to circulating values of 1,25(OH)2vitamin D. Indeed, consistent with previous studies, 1,25(OH)2vitamin D levels were lower in more advanced stages of CKD, while 25(OH)vitamin D levels were higher in patients with Stages 3 and 4 CKD [5, 15]. PTH and alkaline phosphatase levels were higher in individuals with lower 25(OH)vitamin D concentrations.
Plasma FGF-23 levels were above the reference interval in 80% of all subjects and values were increased in the majority of patients in each stage of CKD. FGF-23 levels were lower in patients with Stages 1 and 2 CKD than in CKD Stages 3 and 4 and the urinary TRP was lower in more advanced CKD stages. Interestingly, five patients had FGF-23 values >500 RU/mL. These five patients were between 2.5 and 16.3 years posttransplantation, tended to be older (17.8 ± 1.2 years of age) and had varying degrees of renal dysfunction [from 29 to 62 mL/min/1.73m2 (0.48–1.04 mL/s/1.73m2)]. Serum phosphorus values in these five individuals were 4.9 ± 0.3 mg/dL (1.58 ± 0.10 mmol/L), the average TRP was 82.7 ± 4.4% and 1,25(OH)2vitamin D levels were 27.5 ± 3.4 pg/mL (71.5 ± 8.8 pmol/L). Overall, FGF-23 values were inversely related to estimated GFR (r = −0.35, P < 0.01) but did not correlate with serum 1,25(OH)2vitamin D values, serum phosphorus levels or the tubular reabsorption of phosphate.
Multivariable analysis, considering calcium, phosphorus, PTH, FGF-23, 25(OH)vitamin D, 1,25(OH)2vitamin D, estimated GFR and time since transplantation, revealed that GFR alone predicted TRP, while time since transplantation and 25(OH)vitamin D values were the sole significant predictors of 1,25(OH)2vitamin D and PTH levels, respectively.
Rejection episodes and deterioration of renal function during 2 years of follow-up
Sixty-four of the 68 patients from the initial cohort were followed for 2 years after the cross-sectional analysis of mineral metabolism. At the end of 2 years, renal function (estimated GFR) and the number of acute rejection episodes since entry into the study were assessed. Six of the 64 patients followed for 2 years had a decrease in GFR of ≥50% during the follow-up period, while 13 of the 64 patients developed biopsy-proved acute rejection [13 with acute cellular rejection/tubular interstitial type and 5 with concomitant humoral (C4d+) rejection]. To assess the role of biochemical markers as prognostic tools for the progression of kidney disease and for number of rejection episodes, ROC analysis was performed. Baseline GFR obtained the highest area under the curve (AUC) for predicting a decline in GFR over 2 years [AUC: 0.85; 95% confidence interval (CI) 0.72–0.98; P < 0.01), followed by circulating baseline FGF-23 values (AUC: 0.70; 95% CI 0.51–0.91; P < 0.05). From the ROC analysis, a value of 110 RU/mL was established as the cutoff yielding the highest sensitivity and specificity for decline in GFR. When patients with FGF-23 values ≥110 RU/mL were compared to those with lower values, as have been previously evaluated in CKD patients [9] (Table 3), 30% of patients with higher FGF-23 values had a decrease in GFR of ≥50% at 2 years of follow-up, in contrast to only one patient with an FGF-23 value <110 RU/mL. When patients with FGF-23 values >110 RU/mL were compared to those with lower values, the odds ratio for a ≥50% decrease in GFR at 2 years of 8.3 (95% CI 1.2–54.8). Similarly, time to first rejection was significantly shorter in patients with plasma FGF-23 values >110 RU/mL than in those with lower values (P < 0.05) (Figure 1). Apart from baseline GFR and FGF-23, no other biochemical parameters were able to predict a decline in GFR over time or future episodes of rejection.
Table 3.
Demographic and biochemical characteristics of patients based on FGF-23 values
| FGF-23 > 110 (n = 32) | FGF-23 < 110 (n = 36) | P-value | |
| Patient age | 16.1 ± 0.8 | 15.1 ± 0.8 | NS |
| Time since transplantation (years) | 5.5 ± 0.9 | 4.4 ± 0.7 | NS |
| Gender (% male) | 60 | 56 | NS |
| % Living related transplant | 53 | 47 | NS |
| % On tacrolimus | 80 | 79 | NS |
| % On prednisone | 90 | 67 | <0.05 |
| Mean tacrolimus level | 5.8 ± 0.2 | 6.2 ± 0.3 | NS |
| % With first transplant | 100 | 94 | NS |
| HLA mismatch (n) | 2.9 ± 0.3 | 3.1 ± 0.3 | NS |
| Calcium, mg/dL (mmol/L) | 9.7 ± 0.1 (2.43 ± 0.03) | 9.5 ± 0.1 (2.38 ± 0.03) | <0.05 |
| Phosphorus, mg/dL (mmol/L) | 4.4 ± 0.2 (1.42 ± 0.06) | 4.1 ± 0.1 (1.32 ± 0.03) | NS |
| PTH (pg/mL) | 116 ± 14 | 88 ± 12 | NS |
| 1,25(OH)2vitamin D, pg/mL (pmol/L) | 42 ± 4 (110 ± 9) | 48 ± 4 (126 ± 11) | NS |
| 25(OH)vitamin D, ng/mL (nmol/L) | 32 ± 2 (81 ± 6) | 29 ± 2 (72 ± 6) | NS |
| GFR, mL/min/1.73m2 (mL/s/1.73m2) | 56 ± 19 (0.94 ± 0.32) | 67 ± 24 (1.12 ± 0.40) | <0.05 |
| FGF-23 (RU/mL) | 194 (148, 298) | 60 (22, 86) | <0.05 |
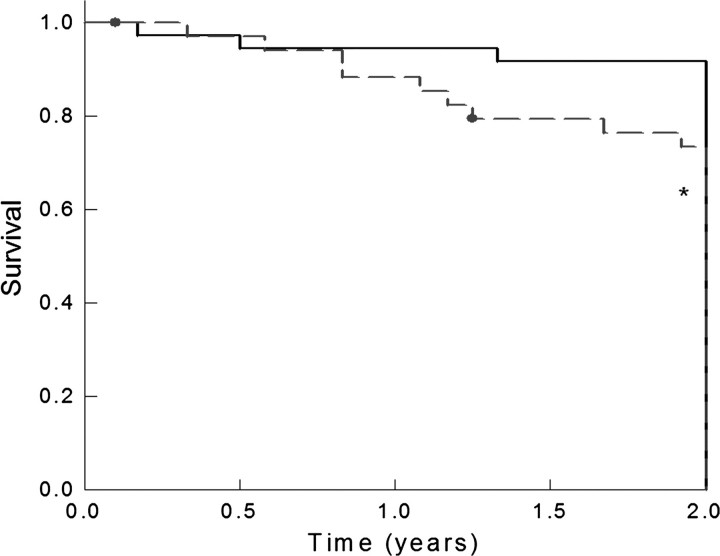
Fig. 1.
Kaplan–Meier survival curve comparing time to first episode of acute rejection in patients with baseline plasma FGF-23 greater than 110 RU/mL (dashed line) versus those with baseline plasma FGF-23 values <110 RU/mL (solid line). The asterisk signifies P <0.05 between groups.
Discussion
The current study demonstrates that alterations in mineral metabolism are common in long-term pediatric renal transplant recipients. Similar to predialysis CKD patients [5, 15], serum calcium and phosphorus levels were within the reference interval in the vast majority of patients. However, abnormal values of 25(OH)vitamin D, PTH and FGF-23 were present in ≥50% of the population. Although 25(OH)vitamin D and PTH have previously been implicated in immune regulation [7] and graft function [6], respectively, levels of these hormones had no relationship to 2-year graft function. Values of FGF-23, which have recently been demonstrated to be elevated in pediatric patients with solid organ transplantation [4], were elevated in all stages of post-transplant CKD and were inversely related to estimated GFR. Furthermore, estimated GFR and FGF-23 were associated with an increased risk of deterioration in renal function at 2 years follow-up and risk of rejection.
The high prevalence of abnormalities in vitamin D, PTH and FGF-23 in the present study highlights the need for the application of current KDIGO bone and mineral metabolism recommendations in the renal transplant population. Indeed, nearly half of all subjects, irrespective of renal function, had serum 25(OH)vitamin D levels below the currently recommended reference interval [16]. Although further studies are required to assess the longer-term consequences of 25(OH)vitamin D stores in this population, vitamin D metabolism has been implicated in immune function and bone strength and low levels in this immunosuppressed population may have long-term deleterious implications for the control of infection, malignancy and fractures [17]. Indeed, the higher alkaline phosphatase levels in patients with CKD Stages 1 and 2, occurring simultaneously with lower 25(OH)vitamin D values in these patients, as well as the inverse relationship between 25(OH)vitamin D and PTH values, suggest that 25(OH)vitamin D deficiency negatively impacts bone and mineral metabolism in stable transplant recipients.
As has been previously demonstrated [4], and similar to patients with predialysis CKD [5], FGF-23 values were increased in the majority (80%) of patients with all stages of CKD and were highest in those with the most advanced stages of kidney disease. FGF-23 was inversely correlated with GFR, as estimated by the Schwartz formula [11]; thus, it is possible that increased FGF-23 levels were attributable to poorer kidney function to some degree in these individuals. However, it is interesting to note that other factors must also contribute to increased FGF-23 levels in kidney transplant recipients since five subjects had high FGF-23 values (>500 RU/mL), despite a wide range in estimated GFR [ranging from 29 to 62 mL/min/1.73m2 (0.48–1.04 mL/s/1.73m2)], no exposure to vitamin D sterols for at least 6 months prior to entering the study and serum phosphate values in the reference interval. Pereira et al. [18] have demonstrated that skeletal expression of FGF-23 is increased in patients with CKD, suggesting that circulating levels of FGF-23 in patients with CKD may reflect both increased production and decreased clearance of the hormone. Moreover, consistent with the findings of Bacchetta et al. [4], steroid therapy was more common in patients with higher FGF-23 levels, suggesting a potential role for these agents in the modulation of skeletal FGF-23 synthesis.
Surprisingly, in the current study of 68 pediatric renal transplant recipients, FGF-23—by either bivariate or multivariable analysis—did not correlate with any other biochemical parameter. This is in contrast to data from the predialysis CKD population and from renal allograft recipients in the first year posttransplantation, in which higher FGF-23 values were correlated with increased phosphate excretion and decreased circulating 1,25(OH)2vitamin D levels [4, 5]. Although it is possible that a relationship between FGF-23 and other markers of mineral metabolism may have been evident in this population given a larger sample size, similarly sized cross-sectional studies in adult predialysis CKD patients have demonstrated relationships between these parameters [5], suggesting that the lack of relationship may, in fact, be due to something intrinsic to renal transplant recipients. Although Bacchetta et al. described the associations between FGF-23 and mineral metabolism in a large cohort of pediatric CKD patients, many of whom had undergone kidney transplantation, a separate analysis considering transplant recipients alone was not performed. Thus, the current study is the first to evaluate this relationship exclusively in pediatric patients with stable renal allografts. Interestingly, Bacchetta et al. described that CKD patients receiving glucocorticoid therapy had higher FGF-23 values than, other CKD patients, a finding which was confirmed in the current study, suggesting that glucocorticoid use may directly stimulate bone FGF-23 production and alter the relationship between FGF-23 values and other biochemical variables. Other immunosuppressive agents, many of which affect bone biology [20–25], may also affect skeletal FGF-23 expression, although studies are needed to evaluate this potential effect. Despite the lack of a direct relationship between FGF-23 and any biochemical parameter, elevated FGF-23 values may have contributed to some of the changes in mineral metabolism that were observed in later stages of CKD, including lower values of TRP and 1,25(OH)2vitamin D, and a larger sample size in which potential confounding factors, such as immunosuppressive regimens and age, could have been more optimally controlled may have revealed these relationships.
Consistent with data from the predialysis CKD population [9], elevated FGF-23 values were associated with an increased risk of ≥50% decline in GFR within the 2 years of follow-up. Interestingly, there was also an increased risk of subsequent biopsy-proven acute rejection and decline in renal function in those patients with higher baseline levels of FGF-23. In the current analysis, the cutoff level for FGF-23 as a predictor of future renal deterioration was determined by ROC analysis and it is interesting to note that the value of 110 RU/mL determined by the current analysis was very similar to the level of 104 RU/mL reported by Fliser et al. [9]. Moreover, the use of 104 RU/mL as a cutoff value does not change the findings of the current study. It is unclear whether elevated FGF-23 values predisposed to rejection, thus leading to a decline in renal function or whether increased FGF-23 levels may have led to a decline in renal function from some other etiology, such as interstitial fibrosis, which prompted the biopsy and led to the diagnosis of acute rejection. Since protocol biopsies were not performed in the current study, it is not possible to distinguish between these possibilities. However, FGF-23 binds to the FGFR1, the same receptor that is upregulated by anti-HLA class I antibodies and which may lead to vascular endothelial cell proliferation, atherosclerosis and renal fibrosis [26]. Alternatively, FGF-23 levels may be another marker of decreased GFR. Indeed, in ROC analysis, FGF-23 and estimated GFR had similar predictive value for both deterioration in renal function and subsequent acute rejection episodes. The correlation between estimated GFR and circulating FGF-23 values was imperfect, possibly reflecting the fact that the GFR was estimated by the Schwartz formula which has been shown to overestimate GFR in early stages of CKD [11, 27].
In conclusion, altered mineral ion homeostasis is common in the posttransplant period, even in patients with stable renal allograft function for >1 year, highlighting the need to apply KDIGO guidelines for bone metabolism and mineral ion homeostasis in the renal transplant population. Consistent with data from adult renal transplant recipients [28, 29], 25(OH)vitamin D deficiency and secondary hyperparathyroidism are highly prevalent in the pediatric renal transplant population, despite normal calcium and phosphorus levels. FGF-23 values are higher in more advance stages of post-transplant CKD, while serum 1,25(OH)2vitamin D levels and TRP values are lower. Elevated FGF-23 values may be associated with increased loss of kidney function and higher risk for rejection within 2 years of follow-up. Whether this represents a direct effect of FGF-23 on renal tissue or whether FGF-23 levels themselves are a marker of transplant renal dysfunction is unknown and further prospective studies are warranted to assess the role of FGF-23 in renal function postrenal transplantation.
Acknowledgments
This work was supported in part by USPHS grants DK-67563, DK-35423, DK-51081, DK-073039 and RR-00865; the National Kidney Foundation and funds from the Casey Lee Ball Foundation.
Conflict of interest statement. None declared.
References
- 1.Bartosh SM, Leverson G, Robillard D, et al. Long-term outcomes in pediatric renal transplant recipients who survive into adulthood. Transplantation. 2003;76:1195–1200. doi: 10.1097/01.TP.0000092524.75807.84. [DOI] [PubMed] [Google Scholar]
- 2.Groothoff JW, Cransberg K, Offringa M, et al. Long-term follow-up of renal transplantation in children: a Dutch cohort study. Transplantation. 2004;78:453–460. doi: 10.1097/01.tp.0000128616.02821.8b. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez CP, Salusky IB, Kuizon BD, et al. Bone disease in children and adolescents undergoing successful renal transplantation. Kidney Int. 1998;53:1358–1364. doi: 10.1046/j.1523-1755.1998.00866.x. [DOI] [PubMed] [Google Scholar]
- 4.Bacchetta J, Dubourg L, Harambat J, et al. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95:1741–1748. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadi F, li-Madadi A, Lessan-Pezeshki M, et al. Pre-transplant calcium-phosphate-parathormone homeostasis as a risk factor for early graft dysfunction. Saudi J Kidney Dis Transpl. 2008;19:54–58. [PubMed] [Google Scholar]
- 7.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 10.Hollis BW, Kamerud JQ, Selvaag SR, et al. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- 11.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. [PubMed] [Google Scholar]
- 14.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in children with chronic kidney disease. Am J Kidney Dis. 2005;46:S1–S121. [PubMed] [Google Scholar]
- 15.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 16.KDIGO Work Group. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int. 2009;76:s1–s130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 18.Pereira RC, Jüppner H, Azucena-Serrano CE, et al. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009;45:1161–1168. doi: 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wesseling-Perry K, Pereira RC, Wang H, et al. Relationship between plasma FGF-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab. 2009;94:511–517. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen DB, Goldberg BD. Stimulation of collagen synthesis and linear growth by growth hormone in glucocorticoid-treated children. Pediatrics. 1992;89:416–421. [PubMed] [Google Scholar]
- 21.Root AW, Bongiovanni AM, Eberlein WR. Studies of the secretion and metabolic effects of human growth hormone in children with glucocorticoid-induced growth retardation. J Pediatr. 1969;75:826–832. doi: 10.1016/s0022-3476(69)80306-9. [DOI] [PubMed] [Google Scholar]
- 22.Ortoft G, Oxlund H. Qualitative alterations of cortical bone in female rats after long-term administration of growth hormone and glucocorticoid. Bone. 1996;18:581–590. doi: 10.1016/8756-3282(96)00077-4. [DOI] [PubMed] [Google Scholar]
- 23.Wehrenberg WB, Bergman PJ, Stagg L, et al. Glucocorticoid inhibition of growth in rats: partial reversal with somatostatin antibodies. Endocrinology. 1990;127:2705–2708. doi: 10.1210/endo-127-6-2705. [DOI] [PubMed] [Google Scholar]
- 24.Aubia J, Serrano S, Marinoso L, et al. Osteodystrophy of diabetics in chronic dialysis: a histomorphometric study. Calcif Tissue Int. 1988;42:297–301. doi: 10.1007/BF02556363. [DOI] [PubMed] [Google Scholar]
- 25.Movsowitz C, Epstein S, Fallon M, et al. Cyclosporin-A in vivo produces severe osteopenia in the rat: effect of dose and duration of administration. Endocrinology. 1988;123:2571–2577. doi: 10.1210/endo-123-5-2571. [DOI] [PubMed] [Google Scholar]
- 26.Maciejewska I, Cowan C, Svoboda K, et al. The NH2-terminal and COOH-terminal fragments of dentin matrix protein 1 (DMP1) localize differently in the compartments of dentin and growth plate of bone. J Histochem Cytochem. 2009;57:155–166. doi: 10.1369/jhc.2008.952630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz GJ, Haycock GB, Edelmann CM, Jr, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 28.Sezer S, Yavuz D, Canoz MB, et al. Vitamin D status, bone mineral density, and inflammation in kidney transplantation patients. Transplant Proc. 2009;41:2823–2825. doi: 10.1016/j.transproceed.2009.06.141. [DOI] [PubMed] [Google Scholar]
- 29.Ewers B, Gasbjerg A, Moelgaard C, et al. Vitamin D status in kidney transplant patients: need for intensified routine supplementation. Am J Clin Nutr. 2008;87:431–437. doi: 10.1093/ajcn/87.2.431. [DOI] [PubMed] [Google Scholar]