Abstract
(See the editorial commentary by Tanton et al, on pages 1822–6.)
Background. Standard-dose HSV-2 suppressive therapy (acyclovir 400 mg twice daily) reduces plasma HIV-1 levels by 0.25–0.50 log10 copies/mL. It is not known if higher doses might further suppress HIV-1 levels.
Methods. We enrolled 32 HIV-1/HSV-2 dually infected Kenyan individuals who were not on antiretroviral therapy (ART) into a randomized, crossover trial of 2 dosing regimens of HSV-2 suppression: valacyclovir 1.5 g vs acyclovir 400 mg, both twice daily for 12 weeks, then a 2-week washout, and then the alternative for 12 weeks. Weekly plasma HIV-1 RNA quantity was measured (ClinicalTrials.gov number NCT01026454).
Results. Mean plasma HIV-1 levels were significantly lower on valacyclovir compared with acyclovir: 2.94 vs 3.56 log10 copies/mL, an average difference of 0.62 log10 copies/mL (95% confidence interval [CI]: −0.68, −0.55; P < .001), a 76% decrease. Valacyclovir resulted in a 1.23 log10 copies/mL decrease compared with baseline HIV-1 levels without HSV-2 suppression. Adherence was similar (99.4% of dispensed study tablets taken), and high-dose valacyclovir was well tolerated.
Conclusions. High-dose valacyclovir reduced plasma HIV-1 viral levels by 0.62 log10 copies/mL compared with standard-dose acyclovir. The potential for higher-dose HSV-2 suppressive therapy to slow HIV-1 disease progression and reduce HIV-1 infectiousness among HIV-1/HSV-2 coinfected persons not yet eligible for ART warrants further evaluation.
Herpes simplex virus type 2 (HSV-2) infection is common among persons infected with HIV-1, with prevalences exceeding 90% in some settings [1]. Among HIV-1/HSV-2 coinfected persons, HSV-2 reactivation, including asymptomatic genital shedding, has been associated with increased plasma and genital HIV-1 concentrations [2–4], suggesting that HSV-2 replication enhances HIV-1 infectiousness and may accelerate HIV-1 disease progression.
The antiviral nucleoside analogue acyclovir and its prodrug, valacyclovir, are safe and effective treatments for HSV-2 infection. They are routinely used as episodic treatment for symptomatic genital herpes and as daily suppressive therapy to decrease the frequency of both symptomatic reactivation and asymptomatic genital HSV-2 shedding [5]. Six randomized trials [6–11] among HIV-1/HSV-2 dually infected persons not taking antiretroviral therapy (ART) found that standard doses of daily HSV-2 suppressive therapy (400–800 mg of acyclovir or 500 mg of valacyclovir orally twice daily) reduced plasma HIV-1 levels by 0.25–0.50 log10 copies/mL. In the largest trial among 3408 HIV-1/HSV-2 dually infected persons, the Partners in Prevention HSV/HIV Transmission Study, acyclovir 400 mg twice daily reduced plasma HIV-1 levels by an average of 0.25 log10 copies/mL, an effect that was sustained over 24 months of follow-up [12]. In that study, HSV-2 suppression modestly slowed HIV-1 disease progression. Compared with participants randomized to placebo, those in the standard-dose acyclovir arm had a 16% reduced risk of progression to a composite endpoint of CD4 <200 cells/μL, ART initiation, or death and a 19% reduced risk of CD4 decline to <350 cells/μL among those who had an initial CD4 count of ≥350 cells/μL [11].
The results of the Partners in Prevention HSV/HIV Transmission Study [12] indicate that HSV-2 suppression could delay HIV-1 disease progression before initiation of ART. We conducted a randomized, open-label, crossover trial to evaluate whether a higher dose of HSV-2 suppressive therapy would have a greater effect on reducing plasma HIV-1 RNA levels than a standard HSV-2 suppressive dose.
METHODS
Study Design and Population
Between March and November 2010, we conducted a randomized, open-label, crossover trial of 2 doses of HSV-2 suppressive therapy among HSV-2/HIV-1 dually infected persons from Thika, Kenya. Eligible participants were ≥18 years of age, HIV-1 and HSV-2 seropositive, and had CD4 counts >250 cells/μL (the Kenyan CD4 threshold for ART initiation at the time of the study) and HIV-1 plasma levels of >400 copies/mL. Exclusion criteria included current participation in another HIV-1 therapeutic trial, pregnancy, current use of ART or anti-HSV medications, a history of seizure or adverse reaction to any anti-HSV medication, or abnormal laboratory studies (serum creatinine >2.0 mg/dL, serum transaminases >3× the upper limit of normal, hematocrit <30%, absolute neutrophil count <1000 cells/μL, or platelet count <75 000/μL). The institutional review boards of the University of Washington and the Kenyatta National Hospital approved the study protocol. Participants provided written informed consent.
Study Drug and Randomization
Participants were randomly assigned to receive either valacyclovir 1.5 g orally twice daily or acyclovir 400 mg twice daily. After 12 weeks, participants crossed over to the alternative treatment for an additional 12 weeks. Treatment periods were separated by a 2-week washout. A simple randomization was generated, with each participant having equal probability of starting on valacyclovir or acyclovir. Study medication was dispensed every 2 weeks, and adherence was assessed by pill counts of returned bottles. Acyclovir was formulated as 400-mg tablets (thus, 1 tablet taken twice daily); valacyclovir was formulated as 500-mg tablets (thus, 3 tablets taken twice daily). The trial was conducted as an open-label study because the primary outcome measure (plasma HIV-1 levels) and adverse event monitoring were unlikely to be influenced by knowledge of treatment assignment. Laboratory staff performing plasma HIV-1 assays were not aware of treatment assignment. Study medication was donated by GlaxoSmithKline.
Specimen Collection and Laboratory Procedures
Blood specimens for plasma HIV-1 RNA quantification were collected at enrollment and then weekly thereafter. To explore the kinetics of viral suppression with initiation and rebound after withdrawal of HSV-2 suppression, additional samples were taken at 1, 2, and 3 days after initiation and at 3, 7, and 14 days after discontinuing each treatment arm. All study participants were known to be HIV-1 seropositive; HIV-1 serostatus was confirmed by ELISA (Vironostika® HIV Uni-Form II Ag/Ab, bioMérieux bv). HSV-2 serostatus was determined by ELISA (HerpeSelect-2, Focus Technologies), with an index value cutoff of 3.5 used to improve specificity [13]. CD4 counts were determined by standard flow cytometric methods at enrollment and at the end of each treatment period. HIV-1 RNA was quantified in plasma using the M2000SP Abbott real-time HIV-1 assay; the lower limit of HIV-1 quantification was 40 (1.60 log10) HIV-1 RNA copies/mL. Chemistry and hematological profiles were obtained at study screening and at the end of each treatment period. Urine pregnancy testing (QuickVue®, Quidel Corporation) was done at screening and at the participant’s request during follow-up, based on missed menses. Women who became pregnant were exited from the study at that time.
Sample Size
A sample size of 32 was estimated to provide >80% power to detect at least 0.25 log10 copies/mL difference in plasma HIV-1 levels (the primary study endpoint) between the treatment arms, allowing for 10% drop-out. The 0.25 log10 difference in plasma HIV-1 RNA was selected based on a difference in plasma HIV-1 RNA thought to be potentially clinically significant, plasma HIV-1 RNA variance measures seen in prior longitudinal studies [6–8]. The crossover design was chosen for its efficiency in sample size because within-person HIV-1 RNA variability is generally less than between-person variability. Previous studies of the effect of HSV-2 suppression on HIV-1 levels used a similar crossover design, with similar sample sizes [6, 8].
Data Analysis
Plasma HIV-1 levels were log10 transformed to stabilize the variances, and undetectable HIV-1 RNA levels were imputed at the midpoint between zero and the lower limit of quantification (40 copies/mL). The primary analysis was comparison of weekly mean plasma HIV-1 levels in the valacyclovir vs acyclovir treatment periods; the analysis was intent-to-treat. Linear random mixed-effects models were used to assess the effect of HSV-2 suppressive treatment on plasma HIV-1 quantity. Negative binomial regression using generalized estimating equations was used to assess the frequency of HIV-1 RNA detection in plasma. Coefficients (β) from the linear mixed-effects models were exponentiated and compared with 1 to determine the percent change in HIV-1 RNA quantity. Sequence effects were evaluated to assess whether the effect of valacyclovir differed depending on whether an individual was randomized to receive valacyclovir in the first or second study arm. Multivariate models adjusting for CD4 count were fit with linear mixed-effects modeling; there was no substantive change in the valacyclovir effect after adjustment for CD4 count. Consequently, results from the multivariate analyses are not presented. Analyses were conducted using SAS for Windows, version 9.2.
RESULTS
Study Participants
Thirty-two HIV-1/HSV-2 coinfected individuals were enrolled. An additional 34 were screened and excluded for the following reasons: plasma HIV-1 levels <400 copies/mL (n = 10), HSV-2 seronegative (n = 11), absolute neutrophil count <1000 cells/μL (n = 5), not being able to attend regular visits (n = 4), CD4 count <250 cells/μL (n = 1), platelet count <75 000/μL (n = 1), hematocrit <30% (n = 1), and declined study participation (n = 1). Of the 32 persons enrolled, 17 (53%) were women. At enrollment, the median age was 37 years (range 23–60) and the median CD4 count was 441 cells/μL (range 283–977). One woman had previously taken zidovudine monotherapy for prophylaxis against mother-to-child HIV-1 transmission, with the last dose 12 months before enrollment. Fourteen (43.8%) participants had previously participated in the Partners in Prevention HSV/HIV Transmission Study [12], which had ended participant follow-up by November 2008. Only 1 participant reported a history of genital ulcers. The mean plasma HIV-1 RNA level at enrollment was 4.10 log10 copies/mL (SD 0.75).
Follow-up
Of 384 expected weekly visits during each treatment arm, 364 (94.8%) during the valacyclovir arm and 376 (97.9%) during the acyclovir arm were completed. Twenty-nine participants provided all weekly plasma samples. Three women became pregnant during the study and were discontinued from follow-up at that time: 1 at the end of the first treatment period (acyclovir; she did not cross over to the valacyclovir arm) and the other 2 at week 4 of the second treatment period (1 in each study arm). There was no reported use of other antiretroviral or anti-HSV medications during follow-up.
Study Drug Adherence
Adherence, as measured by pill count of returned study product, was high. Participants took a median of 99.4% of tablets dispensed (range 83.1–100% for the valacyclovir arm and 73.8–100% for the acyclovir arm). Drug adherence was similar in both treatment periods (P = .8).
Effect of HSV-2 Suppression Dosing Regimen on Plasma HIV-1 Concentrations
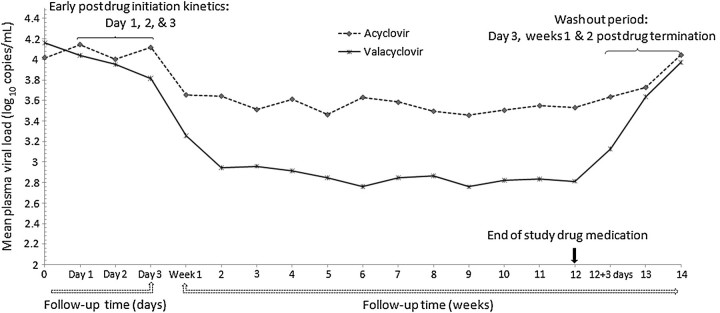
Mean plasma HIV-1 levels were significantly lower during the valacyclovir arm (2.94 log10 copies/mL, 95% confidence interval [CI], 2.65, 3.24) compared with the acyclovir arm (3.56 log10 copies/mL, 95% CI, 3.26, 3.85), for an average difference between the study arms of 0.62 log10 copies/mL (95% CI, −.68, −.55; P < .001) lower in the valacyclovir arm, a 76% decrease (Figure 1). Compared with baseline plasma HIV-1 levels, the valacyclovir effect was a decrease of 1.23 log10 copies/mL (95% CI, −1.38, −1.07; P < .001). Mean plasma HIV-1 RNA levels at the start of the second period were similar (ie, 4.08 log10 copies/mL) to enrollment HIV-1 levels. No statistically significant sequence effect was observed. The change in plasma HIV-1 levels appeared to follow a quadratic curve (P = .02), with an accelerated reduction in the first 2 weeks, followed by a slower rate of change thereafter, tending to plateau. The shape of this curve was similar for the 2 treatment arms (P = .2). The difference in HIV-1 levels by treatment arm was achieved within the first week of study drug initiation, and the effect was sustained through the 12-week treatment period. Plasma HIV-1 levels were below the limit of detection (<40 copies/mL) in 13.5% of 364 weekly specimens during the valacyclovir arm and 3.2% of 376 weekly specimens during the acyclovir arm (risk ratio 4.2, 95% CI, 1.2, 14.2; P = .02).
Figure 1.
Mean plasma HIV-1 RNA concentrations, by treatment arm (acyclovir dashed line, valacyclovir solid). Plasma viral load measurements were obtained weekly except for the day 1, 2, and 3 measurements at the beginning of each arm in order to assess early viral kinetics after drug initiation and day 3 in the washout period to assess viral rebound after drug discontinuation.
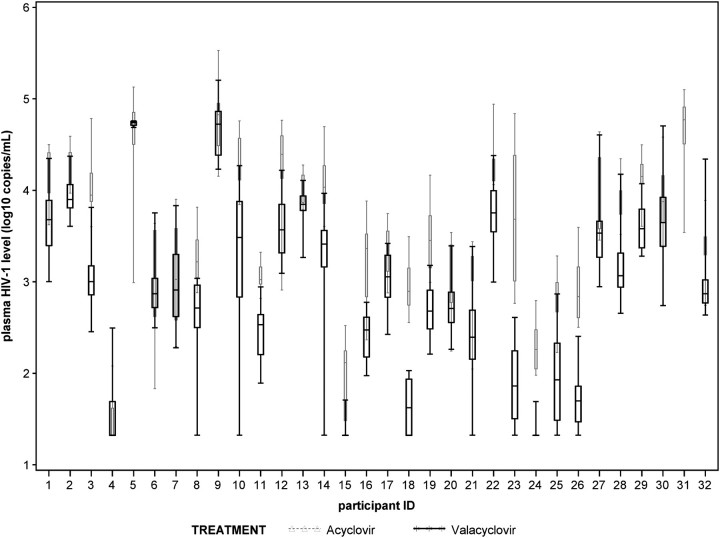
The magnitude of reduction in plasma HIV-1 levels varied across individual participants (Figure 2). Mean plasma HIV-1 levels were lower among 27 participants, similar in 3 (ie, within 0.1 log10 copies/mL), and higher in 1 participant during the valacyclovir vs the acyclovir period. Among the 27 participants with lower HIV-1 levels on valacyclovir, HIV-1 levels were ≤2 log10 copies/mL lower for 6 and ≥2 log10 copies/mL lower for 21. One participant did not cross over to the valacyclovir arm due to pregnancy. There was no statistically significant difference in valacyclovir effect for individuals with higher vs lower baseline plasma HIV-1 RNA levels (data not shown).
Figure 2.
Plasma HIV-1 concentrations for each study participant by treatment arm (acyclovir grey, valacyclovir black). The line represents the median and whiskers indicate the range. Undetectable levels were assigned values between 0 and the lower limit of quantification.
Daily sampling for the first 3 days in each arm demonstrated a significant difference by treatment group by day 3: a 0.26 log10 copies/mL (95% CI, 0.09, 0.43) mean difference in plasma HIV-1 RNA (Figure 1). In the postdrug termination period, plasma HIV-1 concentrations rebounded to baseline levels (within 0.1 log10 copies/mL) within 2 weeks for both arms.
Safety
There was high drug tolerability and safety observed during the study follow-up period. No hepatic or renal impairment was observed based on laboratory testing during the study. Absolute neutrophil counts decreased to <1000 cells/μL in 2 participants, 1 in the acyclovir arm and the other in the valacyclovir arm, both at the end of the second treatment period. No cases of thrombocytopenia were observed. A total of 79 symptomatic illnesses were reported during the course of study follow-up; the type and frequency were consistent with those expected for the study population (eg, malaria, respiratory tract infections). The most commonly reported symptoms (>3 episodes) were abdominal pain, headache, body itching, cough and flu-like symptoms, and back and joint pains; no symptom was more common in the high-dose valacyclovir arm compared with the acyclovir arm. CD4 cell counts increased by a median of 86 cells/μL (Mann–Whitney U test: P = .3 vs baseline) and 69 cells/μL (Mann–Whitney U test: P = .7) during the 12 weeks of HSV-2 suppressive treatment with valacyclovir and acyclovir, respectively.
DISCUSSION
In this randomized, crossover trial, with multiple observations per participant, we found that high-dose HSV-2 suppressive therapy significantly decreased plasma HIV-1 levels compared with standard dosing. Specifically, we found a 0.62 log10 copies/mL reduction for high-dose valacyclovir compared with standard-dose acyclovir. The net effect of high-dose valacyclovir was a >1 log10 copies/mL decrease in plasma HIV-1 concentrations compared with baseline HIV-1 levels without HSV-2 suppression.
HSV-2 is common in persons infected with HIV-1. In sub-Saharan Africa, the region hardest hit by the HIV-1 epidemic, HSV-2 seroprevalences of 50% to >90% have been reported among HIV-1−infected individuals [1, 7, 14]. In the Americas and Europe, HSV-2 seroprevalence is >50% among HIV-1−infected men who have sex with men [15, 16]. Worldwide, countries are struggling with demands to provide ART, even in countries where national guidelines recommend initiation at CD4 counts lower than the World Health Organization−recommended threshold of 350 cells/μL [17]. Thus, HSV-2 suppressive therapy could provide a targeted strategy to delay the time to initiation of ART in such settings and for individuals reluctant to initiate ART at high CD4 counts, with few side effects and low risk for accumulated toxicity and antiretroviral resistance. In a prior study, standard-dose HSV-2 suppression reduced plasma HIV-1 and the risk of HIV-1 disease progression [11]. The quantity of HIV-1 in plasma is a strong predictor of the risk of HIV-1 disease progression [18, 19] and HIV-1 transmission [20, 21]. Thus, it is plausible the >1 log10 reduction in plasma HIV-1 levels with high-dose HSV-2 suppression would result in substantially greater delayed HIV-1 progression and reduced HIV-1 transmission than observed with acyclovir 400 mg bid [11, 12]. Modeling of the relationship of plasma HIV-1 level and HIV-1 transmission risk from the Partners in Prevention HSV/HIV Transmission Study indicates a 67% reduction in HIV-1 transmission probability, with a 1.2 log10 reduction in plasma HIV-1 levels [20]. Mathematical modeling is needed to assess whether high-dose HSV-2 suppression could have meaningful effects on HIV-1 disease progression at a population level. Importantly, cost-effectiveness analysis is needed to determine the price point that would favor generic valacyclovir as an ART-sparing regimen in HIV-1−infected persons not eligible for ART.
We observed a reduction in plasma HIV-1 concentrations within 1 week after treatment initiation, and this effect was sustained through 12 weeks of follow-up. Plasma HIV-1 levels decreased with additional time on HSV-2 suppressive therapy, similar to findings from an earlier study of standard-dose HSV-2 suppression from Burkina Faso [7].There were some differences in mean plasma HIV-1 levels by treatment arm across individuals. Whether additional reductions in HIV-1 levels could be achieved with longer duration of HSV-2 suppression should be explored. Plasma HIV-1 concentrations rebounded to prerandomization levels within 2 weeks of termination of HSV-2 suppressive therapy, consistent with the short half-life and lack of intracellular accumulation of the study medications.
The mechanism by which HSV-2 suppressive therapy reduces HIV-1 concentrations has been thought to be mediated through decreasing HSV-2 replication and related immune activation, particularly in the genital tract [22], and direct effects of HSV proteins on HIV-1 that result in increased HIV-1 transcription [23]. Recent in vitro studies have suggested that acyclovir may also inhibit HIV-1 replication, a direct antiretroviral effect [24–26]. In those in vitro studies, acyclovir exposure selected for a mutation in HIV-1 reverse transcriptase, resulting in an amino acid substitution at codon 75 (V75I) and concomitant loss of an anti-HIV-1 effect of acyclovir. However, we previously reported no evidence of V75I resistance among 168 HIV-1/HSV-2 coinfected persons treated with standard-dose HSV-2 suppression for up to 24 months [27]. In the present study, we saw no waning of effect after 12 weeks of high-dose valacyclovir. However, it is not known whether longer-term high-dose HSV-2 suppression might select for HIV-1 resistance. We did not collect genital samples to measure HSV-2 reactivation. Studies of the effect of high-dose valacyclovir therapy on genital HSV-2 shedding might contribute to understanding whether plasma HIV-1 RNA effects are due to a direct effect on HIV-1 replication or an indirect effect through HSV suppression.
Acyclovir and valacyclovir are available as generic preparations and have been in clinical use for more than 20 years. Long-term suppressive therapy with standard doses of these medications has been shown to be safe for persons with HIV-1 [5]. The dose of valacyclovir used in this study is equivalent to that used for treatment of herpes zoster in HIV-1−infected individuals. Studies of single- and multiple-dose high-dose valacyclovirindicate rapid conversion of valacyclovir to acyclovir and 3- to 5-fold greater bioavailability of valacyclovir compared with acyclovir 800 mg [28, 29]. Our results provide encouraging data about the clinical and laboratory safety profile of high-dose valacyclovir suppressive therapy. In our population, adherence to and tolerance of the study medications was very high, as was compliance with weekly sample collection.
In conclusion, high-dose valacyclovir HSV-2 suppressive therapy reduced plasma HIV-1 viral levels by 0.62 log10 copies/mL compared with standard-dose suppressive therapy and by a net >1 log10 copies/mL from baseline HIV-1 levels prior to HSV-2 suppression. This suggests that high-dose HSV-2 suppressive therapy could substantially slow HIV-1 disease progression and reduce HIV-1 transmission among HIV-1/HSV-2 coinfected persons not yet eligible for ART. Given the constraints on ART programs and preference of some HIV-1−infected individuals to delay ART initiation, the potential for high-dose HSV-2 suppression to delay ART initiation should be evaluated. Long-term efficacy and safety of high-dose HSV-2 suppression warrants evaluation in larger studies with CD4 decline and clinical disease progression as outcomes.
Notes
Acknowledgments.
We gratefully acknowledge the study participants for their time and dedication. Additionally, we thank Dr Ruth Wamae and the staff at the Thika Partners Clinic for their dedication and contribution to this work, the Immunology Laboratory at the University of Nairobi for HIV-1 PCR testing, the Clinical Trials Laboratory at the University of Nairobi for additional laboratory testing, and Dr Meredith Potochnic for assistance with study drug packaging. We are grateful to Drs Lawrence Corey and Anna Wald for their advice on the study design.
Authorship.
All authors contributed to the design and execution of the study. K. M., with J. M. B., wrote the first draft of the manuscript. All authors contributed to the analysis and interpretation of the data and to the final draft of the article.
Financial support.
This work was supported by the Bill and Melinda Gates Foundation (grant 26469) and the US National Institutes of Health (grant R01 AI-083034). Study drug was donated by GlaxoSmithKline. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gwanzura L, McFarland W, Alexander D, Burke RL, Katzenstein D. Association between human immunodeficiency virus and herpes simplex virus type 2 seropositivity among male factory workers in Zimbabwe. J Infect Dis. 1998;177:481–4. doi: 10.1086/517381. [DOI] [PubMed] [Google Scholar]
- 2.Celum CL, Robinson NJ, Cohen MS. Potential effect of HIV type 1 antiretroviral and herpes simplex virus type 2 antiviral therapy on transmission and acquisition of HIV type 1 infection. J Infect Dis. 2005;191(Suppl 1):S107–14. doi: 10.1086/425272. [DOI] [PubMed] [Google Scholar]
- 3.McClelland RS, Wang CC, Overbaugh J, et al. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002;16:2425–30. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 4.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186:1718–25. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 5.Conant MA, Schacker TW, Murphy RL, Gold J, Crutchfield LT, Crooks RJ. Valaciclovir versus aciclovir for herpes simplex virus infection in HIV-infected individuals: two randomized trials. Int J STD AIDS. 2002;13:12–21. doi: 10.1258/0956462021924550. [DOI] [PubMed] [Google Scholar]
- 6.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–8. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–9. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–8. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 9.Delany S, Mlaba N, Clayton T, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: a randomized placebo-controlled trial in South Africa. AIDS. 2009;23:461–9. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunne EF, Whitehead S, Sternberg M, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr. 2008;49:77–83. doi: 10.1097/QAI.0b013e3181831832. [DOI] [PubMed] [Google Scholar]
- 11.Lingappa JR, Baeten JM, Wald A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375:824–33. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laeyendecker O, Henson C, Gray RH, et al. Performance of a commercial, type-specific enzyme-linked immunosorbent assay for detection of herpes simplex virus type 2-specific antibodies in Ugandans. J Clin Microbiol. 2004;42:1794–6. doi: 10.1128/JCM.42.4.1794-1796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baeten AL, Natzke MK, Hayney MS. Vaccinations during pregnancy and breast-feeding. J Am Pharm Assoc (2003) 2008;48:554–6. doi: 10.1331/JAPhA.2008.08050. [DOI] [PubMed] [Google Scholar]
- 15.Brown EL, Wald A, Hughes JP, et al. High risk of human immunodeficiency virus in men who have sex with men with herpes simplex virus type 2 in the EXPLORE study. Am J Epidemiol. 2006;164:733–41. doi: 10.1093/aje/kwj270. [DOI] [PubMed] [Google Scholar]
- 16.Konda KA, Klausner JD, Lescano AG, et al. The epidemiology of herpes simplex virus type 2 infection in low-income urban populations in coastal Peru. Sex Transm Dis. 2005;32:534–41. doi: 10.1097/01.olq.0000175413.89733.ae. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach (2010 revision) Geneva: WHO; 2010. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. Accessed 10 March 2011. [PubMed] [Google Scholar]
- 18.Lavreys L, Baeten JM, Chohan V, et al. Higher set point plasma viral load and more-severe acute HIV type 1 (HIV-1) illness predict mortality among high-risk HIV-1-infected African women. Clin Infect Dis. 2006;42:1333–9. doi: 10.1086/503258. [DOI] [PubMed] [Google Scholar]
- 19.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 20.Lingappa JR, Hughes JP, Wang RS, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One. 2010;5:e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 22.Rebbapragada A, Wachihi C, Pettengell C, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589–98. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 23.Mosca JD, Bednarik DP, Raj NB, et al. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987;325:67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- 24.Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4:260–70. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMahon MA, Siliciano JD, Lai J, et al. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem. 2008;283:31289–93. doi: 10.1074/jbc.C800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMahon MA, Parsons TL, Shen L, Siliciano JD, Siliciano RF. Consistent inhibition of HIV-1 replication by acyclovir in CD4+ T cells without detection of human herpes viruses. J Virol. 2011;85:4618–22. doi: 10.1128/JVI.02423-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baeten JM, Lingappa J, Beck I, et al. Herpes simplex virus type 2 suppressive therapy with acyclovir or valacyclovir does not select for specific HIV-1 resistance in HIV-1/HSV-2 dually infected persons. J Infect Dis. 2011;203:117–21. doi: 10.1093/infdis/jiq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vezina HE, Balfour HH, Jr, Weller DR, Anderson BJ, Brundage RC. Valacyclovir pharmacokinetics and exploratory pharmacodynamics in young adults with Epstein-Barr virus infectious mononucleosis. J Clin Pharmacol. 2010;50:734–42. doi: 10.1177/0091270009351884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weller S, Blum MR, Doucette M, et al. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin Pharmacol Ther. 1993;54:595–605. doi: 10.1038/clpt.1993.196. [DOI] [PubMed] [Google Scholar]