Abstract
Tissue factor (TF), a membrane protein, is an initiator of blood coagulation in vivo. In this review we discuss how posttranslational modifications affect activity and other properties of TF. Glycosylation of the extracellular domain and the composition of carbohydrates at three glycosylation sites have an influence on TF activity in the extrinsic FXase by increasing the rate of FX proteolysis. No influence of TF glycosylation on the activity of the FVIIa/TF complex towards small synthetic substrates was observed, suggesting that glycosylation has no effect on TF interaction with FVIIa. There are no published data suggesting a direct influence of phosphorylation or palmitoylation in the cytoplasmic domain on TF procoagulant activity. There has been a debate in the recent literature related to the role and formation of the Cys186-Cys209 disulfide bond. Published opinions from various laboratories range from this bond being essential for the expression of cell TF activity to having no role in it. Overall, it is clear that some modifications of TF have an effect on TF procoagulant activity, signaling functions and trafficking. The influences of other modifications are debatable.
Keywords: Tissue Factor, Factor VIIa, Factor X activation, Mass-spectrometry, Carbohydrate composition, Review
2. INTRODUCTION
Tissue factor (TF) is a trans-membrane protein that is an essential component of the factor VIIa-TF enzymatic complex (extrinsic factor Xase). TF is expressed in a variety of cells and is found in the central nervous system, lungs, and placenta at relatively high concentrations(1–3). Some blood cells, such as monocytes and macrophages, can express detectable amounts of TF when they are stimulated in vitro by various, primarily by inflammation-related, agents(4–6). Additionally, TF has been identified in atherosclerotic plaques, which has suggested a role for TF in the progression of cardiovascular diseases(7, 8). In healthy individuals, however, cells in contact with blood do not contain physiologically active TF. Upon a damage of the vascular wall, subendothelial TF is expressed/exposed to the blood flow and binds factor VIIa, an enzymatic component of the extrinsic factor Xase, which circulates at a concentration of approximately 0.1 nM (9). This enzymatic complex activates the zymogens factor IX and factor X to their respective serine proteases, factor IXa and factor Xa, leading to thrombin generation and consequential initiation of the blood coagulation process.
Natural full-length TF is expressed as a 263 amino acid protein and is composed of three distinct domains: an NH2-terminal extracellular domain (residues 1–219), which is composed of two fibronectin type III domains and is responsible for factor VIIa binding and extrinsic factor Xase formation, the transmembrane domain (residues 220–242), which spans the membrane and anchors TF to it and a cytoplasmic carboxyterminal domain (residues 243–263), which is involved in signal transduction(10). Thus, two of the three domains of TF (extracellular and transmembrane) play distinct roles in the blood coagulation process and it has been generally accepted that TF lacking the cytoplasmic domain is functionally identical to the full-length protein in the initiation of blood coagulation.
The amino acid sequence data of TF indicate that there are four potential N-glycosylation sites; three of them are in the extracellular domain at Asn11, Asn124 and Asn137 and one in the cytoplasmic domain at Asn261. Although a partial identification of carbohydrates attached to the glycosylation sites of the extracellular domain was accomplished(11), a more detailed analysis of carbohydrate moieties and their role on TF affinity for factor VIIa and that of the complex enzyme (factor VIIa-TF) for its natural substrates factor IX and X are missing. Similarly, there are no convincing data published describing the influence of glycosylation on the TF-related related activity in processes leading to thrombin generation and clot formation. TF also contains two potential disulfide bonds (Cys49-Cys57 and Cys186-Cys209) located in the extracellular domain(12). The carboxy-terminal cytoplasmic domain contains a single Cys245 residue and two Ser residues (Ser253 and Ser258). The Cys245 residue is potentially linked to a palmitate or stearate fatty acyl chain(12) while one or both Ser residues can be phosphorylated by a protein kinase C-dependent mechanism(13).
A variety of human recombinant TF species have been produced, from those containing only the extracellular domain to the full-length protein. These recombinant proteins have been extensively used in research and clinical laboratories worldwide due to the limited availability of natural tissue factor. Alternatively, many laboratories have been using natural “thromboplastin” reagents as a source of TF in their experiments. These reagents are made by homogenizing natural tissues (most often non-human) that contain a relative abundance of TF such as brain, placenta and lung. Experimental results acquired using recombinant TF and thromboplastins in vitro are frequently used for understanding coagulation processes occurring in vivo. Unfortunately, the non-availability of isolated natural TF does not allow the confirmation (or rejection) of results obtained with recombinant TF or that present in homogenates of natural tissues. The goal of this review is to compare posttranslational modifications of natural TF purified from placenta with those of recombinant protein and to discuss the influence of these modifications on the functional activity of TF proteins.
3. POSTTRANSLATIONAL MODIFICATIONS AND ACTIVITY OF TISSUE FACTOR
3.1. Glycosylation
Posttranslational modifications are a common feature of proteins and usually have an effect on protein properties including their function, stability and localization. According to some estimates, between 140 and more than 200 types of posttranslational modifications of proteins have been described(14), ranging from quite common and widespread such as glycosylation, phosphorylation, acylation, methylation and ubiquitination to more rare such as sulfation, hydroxylation, etc.
Glycosylation, the attachment of carbohydrates to proteins and lipids, is the most common and complex form of posttranslational modifications and requires between 1–2% of human genes, which encode proteins responsible for this modification(15). The complexity of carbohydrates attached to the proteins makes their characterization difficult and challenging. Only advancements in methodology of the last decade (mass spectrometry; MS, high-pressure liquid chromatography; HPLC and nuclear magnetic resonance; NMR) allowed characterization of complex carbohydrate moieties of proteins more precisely and with lower labor intensity(16).
TF protein was purified and partially characterized for the first time 40 years ago(17). Shortly after, an evidence for the presence of carbohydrates on bovine TF was obtained by Pitlick(18). It was based upon the observation that concanavalin A, a plant lectin which binds preferentially to glucosyl and mannosyl residues(19), efficiently but reversibly inhibits TF procoagulant activity. Moreover, based on concanavalin binding, it was concluded later that glycosylation of TF from various species is different and there is certain heterogeneity in glycosylation of human TF extracted from various tissues(20). In 1985 Shands observed that tunicamysin, a mixture of homologous nucleoside antibiotics which prohibits posttranslational glycosylation(21), interfered with TF activity(22). Based on this observation, it was hypothesized that glycosylation of TF could be important for its function, although no direct evidence was provided. Two years later, the primary structure of the TF protein was established and potential glycosylation sites were suggested(10).
Although it has been known for almost 40 years that TF contains carbohydrate moieties, no attempts to precisely characterize their structure and abundance were taken. Moreover, the influence of glycosylation on functions of TF proteins, both natural and recombinant, is clearly underrepresented in the existing literature. In a side-by-side activity comparison for glycosylated and non-glycosylated full-length recombinant TF, it has been stated that glycosylation is not required for TF procoagulant activity(11). Since no quantitative data were provided, it is impossible to check the validity of that suggestion. Similarly, a statement suggesting that the activity of recombinant full-length TF is identical to that of natural protein from brain was not supported by any data(23). One of the rare studies addressing the issue of TF glycosylation and its influence on procoagulant activity of the protein was published by Stone and co-workers(24). The results of that study suggested that activity of a soluble form of TF, which is in fact the extracellular domain of the protein, is independent of the presence or absence of carbohydrate moieties on the protein. However, soluble forms of TF used by the authors of the study are physiologically irrelevant with respect to the initiation of blood coagulation due to their inability to bind to the membrane and in a complex with factor VIIa, efficiently activate factor X and factor IX(25).
Recently, the activity comparison of two forms of recombinant TF proteins (recombinant TF 1–243 produced in E. coli and recombinant TF 1–263 produced in insect Sf9 cells) and that of the natural human placental TF was accomplished in our laboratory(26). Deglycosylation, tryptic digestion, HPLC and MS techniques were used to accomplish this task. It was established that three of the four potential glycosylation sites have asparagine-linked (N-linked) oligosaccharides in their structure. All three of them were located in the extracellular domain of TF at Asn11, Asn124 and Asn137, i.e. at the sites predicted by the amino acid sequence of the protein(27). Similar to previous publications(10, 11), no carbohydrates were detected at Asn261. The N-linked glycosylation is typical for the majority of proteins that enter the secretory pathway in eukaryotic cells and occurs at the sites which contain Asn-X-Thr/Ser amino acid sequence(28, 29). It is catalyzed by the hetero-oligomeric oligosaccharyltransferase(30).
3.1.1. Carbohydrate composition
Based on the MS data, it was determined that, consistent with the expression system (bacteria), recombinant TF 1–243 had no carbohydrates attached to the backbone of the protein and that placental TF was modified more heavily than recombinant full-length protein rTF 1–263 (Table 1; 26). The extent of glycosylation and carbohydrate composition was different between the two proteins as well as between each glycosylated site within the protein. Additionally, a quite high heterogeneity of carbohydrates was observed at each glycosylation site. Only 20% of Asn11 in recombinant tissue factor 1–263 is glycosylated, predominantly with high mannose carbohydrates. In contrast, more than 75% of Asn11 is glycosylated in placental tissue factor. This site in placental tissue factor is heavily fucosylated and no mannose-containing carbohydrates are detected. Asn124 in both tissue factor proteins (natural and recombinant) is almost completely glycosylated, however the composition of carbohydrates is distinctly different. In recombinant tissue factor 1–263, more than 80% of this site is modified with high-mannose carbohydrates, whereas in placental tissue factor Asn124 is glycosylated with hybrid and sialylated carbohydrates. Similarly, pronounced differences in carbohydrate structure are observed for Asn137, although in both proteins this site is almost completely glycosylated. In recombinant tissue factor 1–263, the abundance of high-mannose carbohydrates is almost matched by that of fucosylated sugars, whereas in placental tissue factor Asn137 is modified exceptionally with fucosylated-(sialylated) carbohydrates. Differences observed in carbohydrate composition between human placental tissue factor and the recombinant protein produced in Sf9 cells are consistent with general patterns of protein glycosylation in mammalian and insect cells(31–33).
Table 1.
Molecular masses (Da) of TF proteins
| TF speciesa | Source | Calculatedb | MALDI-TOF | Differencec | SDS-PAGE |
|---|---|---|---|---|---|
| rTF1–243 | E.Coli | 27,423 | 27,800 | 377 | 31,000 |
| rTF1–243”D” | E.Coli | 27,423 | 27,800 | 377 | 31,000 |
| rTF1–263G | Sf9 | 29,592 | 33,196 | 3,604 | 37,000 |
| rTF1–263D | Sf9 | 29,592 | 30,202 | 610 | 33,000 |
| pTFG | Human placenta | 29,592 | 36,179 | 6,605 | 45,000 |
| pTFD | Human placenta | 29,592 | ND | ND | 34,000 |
p-placental, r-recombinant, G-glycosylated, D-deglycosylated
calculated from amino acid composition;
difference between calculated mass by amino acid composition and that obtained by MALDI-TOF.
From J Biol Chem 2010;285:3371–82 (26).
3.1.2. Activity
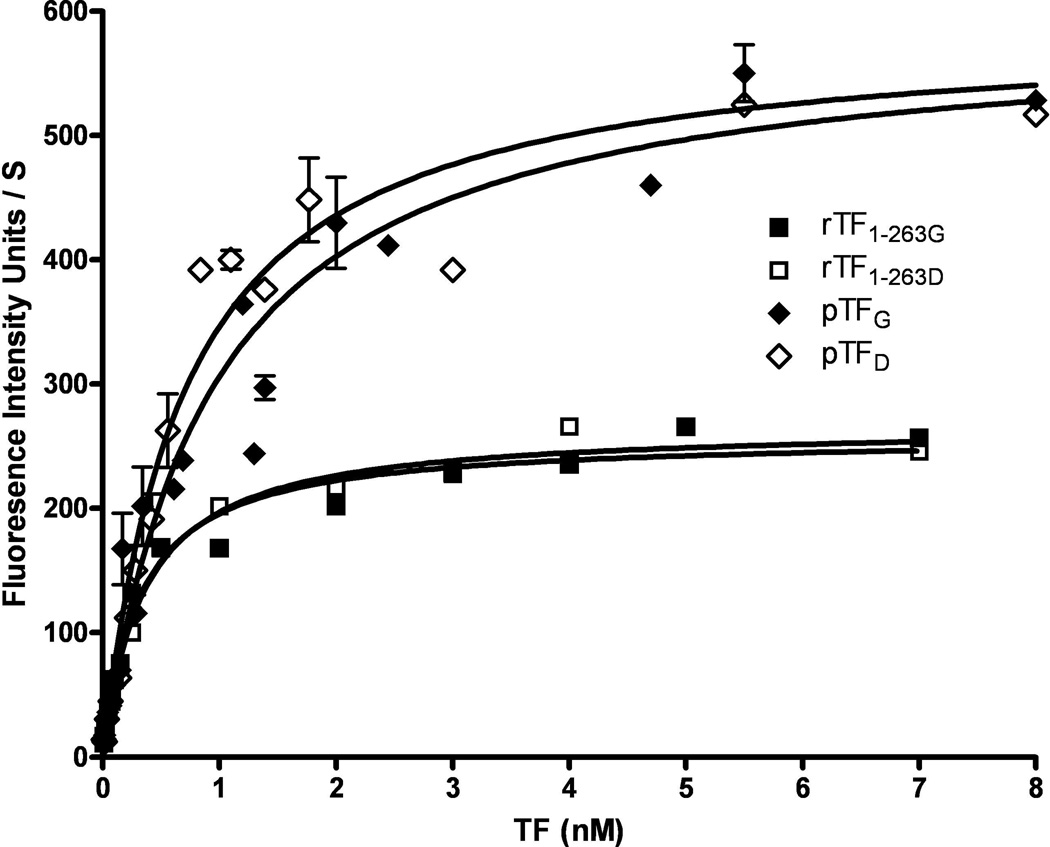
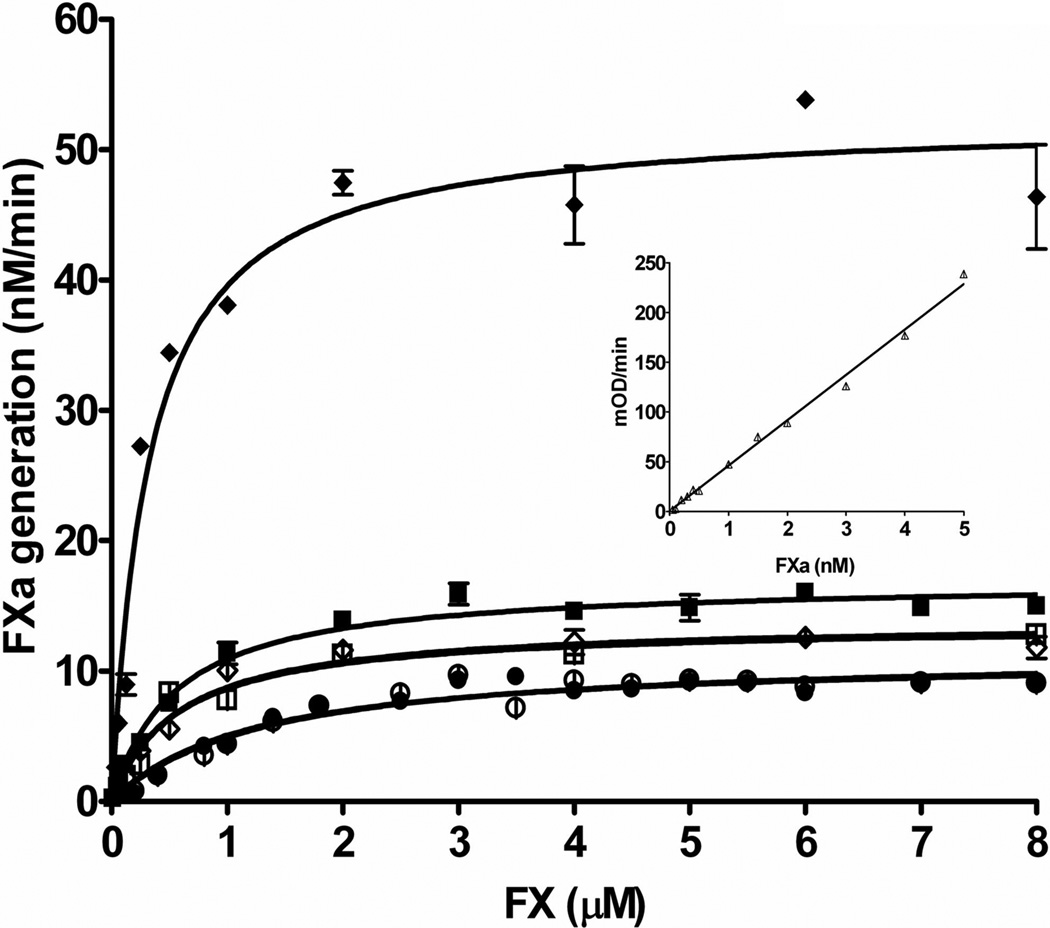
To evaluate the influence of glycosylation on TF activity, we compared glycosylated and fully deglycosylated forms of these three TF proteins. As expected, “deglycosylation” did not affect recombinant TF 1–243 activity and affinity for FVIIa and FX in the fluorogenic (membrane-independent) and the extrinsic factor Xase (membrane-dependent) assays (Table 2 and 3, respectively). Somewhat surprisingly, deglycosylation had no effect on the activity of recombinant and placental TF in the fluorogenic assay (Figure 1), suggesting that differences in activity in this assay observed for different forms of TF could be related to other posttranslational modifications. These results were consistent with observation of Stone et al. suggesting that the soluble form of TF, which does not interact with the membrane, is not influenced by deglycosylation(24). In contrast to the fluorogenic assay, deglycosylation of the full-length recombinant TF 1–263 slightly decreased the efficiency of this protein in the extrinsic factor Xase complex. However, the most pronounced decrease in the activity of this enzymatic complex was observed when natural placental TF was deglycosylated. It caused a decrease in affinity of factor X for the factor VIIa/TF complex, a decrease in the catalytic constant and, as a consequence of these changes, an almost 7-fold decrease in the second order rate constant (Table 3, Figure 2). Upon deglycosylation, all the parameters of the Michaelis-Menten kinetics of the extrinsic factor Xase were almost identical for both recombinant TF 1–263 and placental TF, suggesting that the differences in the activity observed for the glycosylated forms of these two TF proteins are related to the structure of carbohydrtates attached to the peptide chain and to the extent of glycosylation. A similar decrease in activity of the extrinsic factor Xase was observed by the Edgington’s laboratory when, upon mutation of two cysteines in the extracellular domain of TF, glycosylation of the mutant protein became impaired(34). In addition to the effect of glycosylation on TF procoagulant activity, it plays an important role in the transport of cell TF to the plasma membrane and in the microparticle generation(35).
Table 2.
Affinity and activity of the FVIIa/TF complex in a fluorogenic assay
| TF speciesa | Kdappb (nM) |
Vmaxc (pM/s) |
Stoichiometry (TF:FVIIa) |
|---|---|---|---|
| rTF1–243 | 0.41 ± 0.40 | 59.7 ± 1.2 | 1.1 : 1.0 |
| rTF1–243“D” | 0.47 ± 0.03 | 61.2 ± 1.0 | 0.9 : 1.0 |
| rTF1–263G | 0.31 ± 0.02 | 75.8 ± 1.6 | 0.9 : 1.0 |
| rTF1–263D | 0.35 ± 0.02 | 78.3 ± 1.4 | 1.0 : 1.0 |
| pTFG | 0.92 ± 0.15 | 173.1 ± 8.4 | 0.9 : 1.0 |
| pTFD | 0.69 ± 0.09 | 172.8 ± 10.0 | 1.0 : 1.0 |
p-placental, r-recombinant, G-glycosylated and D-deglycosylated
apparent dissociation constant
maximum rate of substrate hydrolysis at 0.5 nM FVIIa and saturating TF
From J Biol Chem 2010;285:3371–82 (26).
Table 3.
Activity of the TF/FVIIa complex in the extrinsic FXase assay
| TF speciesa | KMb (µM) |
kcatc (s−1) |
kcat/Km (µM−1•s−1) |
relative activityd |
Stoichiometry (TF:FVIIa) |
|---|---|---|---|---|---|
| rTF1–243 | 1.19 ± 0.22 | 1.8 | 1.5 | 1.0 | 1.0 : 1.0 |
| rTF1–243”D” | 1.31 ± 0.26 | 1.9 | 1.4 | 0.9 | 1.0 : 1.0 |
| rTF1–263G | 0.54 ± 0.05 | 2.8 | 5.2 | 3.5 | 1.0 : 1.0 |
| rTF1–263D | 0.57 ± 0.17 | 2.2 | 3.8 | 2.5 | 0.9 : 1.0 |
| pTFG | 0.32 ± 0.04 | 8.7 | 26.8 | 17.9 | 0.9 : 1.0 |
| pTFD | 0.57 ± 0.07 | 2.3 | 4.0 | 2.7 | 0.9 : 1.0 |
p-placental, r-recombinant, G-glycosylated and D-deglycosylated
Michaelis constant
catalytic constant
in comparison with rTF1–243
From J Biol Chem 2010;285:3371–82 (26).
Figure 1.
Amidolytic activity of FVIIa in complex with placental TF (pTF; diamonds) and recombinant full-length TF (rTF1–263; squares) both glycosylated (G; filled symbols) and deglycosylated (D; open symbols). Increasing concentrations of TF were incubated with 0.5 nM FVIIa followed by the addition of 50 µM fluorogenic substrate. The rate of substrate hydrolysis was recorded. From J Biol Chem 2010;285:3371–82 (26).
Figure 2.
Proteolytic activity of the extrinsic FXase formed by FVIIa and relipidated placental TF (◆ glycosylated and ◊ deglycosylated), full-length recombinant TF1–263 (■ glycosylated and □ deglycosylated) and truncated recombinant TF1–243 (● native and ○ “deglycosylated”). FVIIa (5 nM) was incubated with 0.1 nM relipidated TF and increasing concentrations of FX were added. FXa generation was monitored in a chromogenic assay. From J Biol Chem 2010;285:3371–82 (26).
3.2. Phosphorylation
Among numerous posttranslational modifications of proteins, phosphorylation is the most important because it plays a critical role in the regulation of many protein functions. Protein phosphorylation is carried out by protein kinases, which transfer phosphate to the hydroxyl groups of the side chains of three amino acids – serine, threonine and tyrosine(36). Hydroxyl groups of serine represent the major site of phosphorylation in proteins (90–95% of total phosphorylation sites) and 5–10% of total phosphorylation occurs at the threonine side chain, whereas tyrosine phosphate represents less than 1% of total protein phosphorylation in eukaryotic cells.
In 1992, Zioncheck et al. determined that TF protein contains two phosphorylation sites, both of them in the cytoplasmic domain(13). They suggested that phosphorylation is regulated by the protein kinase C-dependent mechanism, because it was induced by the PKC activator phorbol 12-myristate 13-acetate, whereas staurosporine, a potent PKC inhibitor, abolished phosphorylation of TF. From the alignment of cDNA sequences of several species (including human) it was concluded that phosphorylation sites contain a conserved amino acid sequence X-Ser*/Thr*-Pro-X with asterisk indicating the phosphorylation residue. In a later publication, Mody and Carson suggested that the cytoplasmic domain of TF can be phosphorylated in vitro at multiple sites, particularly at Ser253 and Ser258(37). The mutational data presented by Dorfleutner and Ruf suggested that initial phosphorylation at Ser253 enhances the subsequent phosphorylation at Ser258(38).
There are several publications describing the influence of protein kinase C activators and inhibitors on cell TF activity(39), primarily by altering expression of TF antigen on the cell surface(40). In addition to the regulation of TF expression, phosphorylation of TF influences cell signaling, migration and angiogenesis(41–43). Ryden and coworkers showed that TF phosphorylation related protease-activated receptor-2 signaling plays an important role in breast cancer recurrence(44).
3.3. Palmitoylation
One of the common posttranslational modifications of eukaryotic proteins is acylation with fatty acids, primarily with myristate and palmitate. Myristoylation usually takes place at the N-terminal glycine by the formation of an amide bond, whereas palmitoylation occurs at a cysteine residue via a thioester bond formation(45). S-palmitoylation corresponds to the binding of a saturated fatty acid containing 16 carbon atoms to an unpaired cysteine. Other fatty acids than palmitic of various length, both saturated and unsaturated can also participate in S-acylation of proteins(46). In addition to cysteine, ε-amino group of lysine can be modified with the palmitate residue(47). S-palmitoylation is almost an exclusive feature of membrane proteins, although there is no well-defined sequence for this modification other than the presence of a free cysteine. S-palmitoylation occurs in the vicinity of cell membranes and directs proteins to the membrane lipid rafts, presumably due to a high affinity of proteins modified with fatty acids for these subdomains of cell membranes(48).
It has been shown by Bach and coworkers that TF has one S-palmitoylation site in the intracellular domain at Cys245 (12). In accordance to general features of this modification, Cys245 is at the N-terminus of the intracellular domain and is located close to the membrane surface. The extent of palmitoylation at this site, however, is not clear because it has been shown in several publications that in purified TF proteins, Cys245 can also participate in dimer and higher multimer formation(12, 49–51). Based upon the data presented in those publications, it is hard to conclude whether those multimeric forms of TF are characteristic for the protein in its native environment or they occur during TF purification due to a relatively low stability of S-palmitoylation. On the other hand, the data suggesting the presence of TF dimers and multimers on the surface of a living cell(52–55) indicate that these forms of TF can exist in vivo. Although the existence of TF dimers and multimers has been known for more than a decade, the data related to the activity of these TF forms are scarce and somewhat contradicting. For example, Donate and coworkers showed that the dimerized form of TF has the same factor X-activating efficiency as the monomeric form(55), whereas two other groups of investigators suggested that TF dimers represent an “encrypted” form of protein, which has a relatively low activity(53, 54).
It has been suggested that, in accordance with the properties with S-palmitoylated membrane proteins, this modification should target TF to the cell membrane lipid rafts, which are enriched in sphingolipids and cholesterol(56). During the last decade, increasing experimental data suggest a role for these rafts in modification of TF expression(57, 58) and activity(59–62). It has been shown that one of the components of the lipid rafts, (cholesterol) might play an important role in TF activity(60, 61) although the effect observed by investigators was minimal and did not exceed 2 to 3-fold. Additionally, there was some contradiction related to the role of cholesterol for TF activity. Dietzen and coworkers suggested that cholesterol elimination from the cell membrane lipid rafts increases TF activity(61), whereas two other groups observed a decrease in TF activity caused by cholesterol removal(60) and changes in lipid raft environment(59). It is possible, however, that this controversy could be caused by the use of different cell types by the investigators. Another mechanism, by which S-palmitoylation can regulate TF activity, is its effect on the phosphorylation of the intracellular domain of the protein. It has been shown by Dorfleutner and Ruf that S-palmitoylation at Cys245 inhibits phosphorylation at Ser258 in the intracellular domain of TF(38). The authors suggested several hypotheses explaining this effect, with the leading one implying that palmitoylation favors the association of TF with caveolin-containing lipid rafts. Such association should efficiently inhibit protein kinase Cα, involved in TF phosphorylation, by the scaffolding domain of caveolin(63).
Thus S-palmitoylation of the intracellular domain of TF can indirectly alter its procoagulant activity by directing TF towards cell membrane lipid rafts and by influencing phosphorylation of that domain.
3.4. Disulfide bond(s) and “encryption-decryption” of TF
While there is common agreement about the leading role of TF in the initiation of blood coagulation in vivo, there are significant controversies related to the expression and regulation of TF activity on the cell surface. It has been suggested that the majority of TF molecules located on the cell surface have low activity (are “encrypted”) and that “decryption” is essential for the expression of TF function(64). Several mechanisms, often contradictory, have been hypothesized in attempts to explain “encryption-decryption” of TF activity.
One of the suggested methods for the “decryption” of TF on the cell surface consists of the treatment of TF-bearing cells with calcium ionophore(65–71). Ionophore increases TF activity by 2 to 10-fold, while some authors assign this increased TF activity to increased expression of TF protein(65), others suggest this arises from changes in the cell membrane environment, particularly in an increased expression of acidic phospholipids(66, 68, 71), sometimes related to cell death(67, 70). Several studies hypothesize a role for cholesterol in cell lipid rafts contributing to the “encryption-decryption” of TF activity(60, 61, 72), although there is little agreement between the suggested mechanisms of this process. An increase in TF activity has been reported when lipopolysaccharide (LPS)-stimulated monocytes are treated with platelets(73–75). This observed increase in activity was quite limited (2 to 3-fold) and could be (in part) assigned to an increase in TF antigen expression by monocytes(75).
It has been also suggested that cell surface TF “decryption” is related to the Cys186-Cys209 disulfide bond formation. The key data leading to this hypothesis were based on the mutational studies by Edgington’s group(34). They mutated either Cys49 and Cys57 (which potentially could form a disulfide bridge in the N-terminal part of the extracellular domain of TF) or Cys186 and Cys209 (which could form a disulfide bond in the vicinity of the membrane) substituting serine residues for cysteines. Mutations of Cys49 and Cys57 had no effect on TF activity in the extrinsic factor Xase, whereas mutations of Cys186 and Cys209 decreased the activity of this complex by approximately 3-fold due to an impaired binding of FVIIa to this TF mutant. Based on these data it was concluded that the N-terminal pair of cysteines plays no role in TF activity, whereas the disulfide bridge in the C-terminus of the extracellular domain is essential for the “decryption” of TF activity. However, the Ser186Ser209 mutant, in contrast to the wild-type protein and Ser49Ser57 mutant, lacked glycosylation. In a study from our laboratory we showed that deglycosylation of natural TF leads to a similar decrease in activity(26). Thus an impaired activity observed for the Ser186Ser209 mutant could be caused not by the lack of the disulfide bond but by the lack of glycosylation.
The role (or absence of it) of the Cys186-Cys209 disulfide in the regulation of TF and the mechanism by which it is formed on the cell surface has been the subject of debates for the last several years. Chen and coworkers observed an increase in cell TF activity upon the treatment of cells with an oxidizing agent mercuric chloride(76). They concluded that this reagent restores Cys186-Cys209 disulfide bond leading to the observed changes in TF activity. However, no data (experimental or theoretical) are provided supporting the re-formation of the hypothesized reduced disulfide bridge. Existing chemical records conclude that mercuric chloride will oxidize only a single thiol group(77, 78). Moreover, an increase in TF activity on cell surfaces similar to that caused by mercuric chloride can be achieved by treating TF-bearing cells with other metal compounds, such as silver nitrate and phenylmercuric acetate(79).
In several publications from Ruf’s laboratory, it has been suggested that protein disulfide isomerase (PDI) is responsible for the regulation of cell TF activity via its effect on the status of the Cys186-Cys209 disulfide bond(80–82). Similarly, studies from Engelmann’s laboratory proposed that TF activation by PDI occurs due to the isomerization of a mixed disulfide and an intramolecular Cys186-Cys209 bond formation(83, 84). However, publications from several other laboratories showed that the TF activity-enhancing effect of PDI is related to the presence of acidic phospholipid phosphatidylserine either present as a contaminant in the PDI preparations(85, 86) or due to its relocation to the TF-bearing cell surface upon treatment with PDI or mercuric chloride(87–90). Moreover, Kothari et al. suggested in a recent publication that Cys186-Cys209 disulfide bond is not essential for the “decryption” of cell TF activity(91).
Thus the question related to the role of this disulfide in the expression of cell TF activity remains open. Moreover, even the presence of this bond in a functional TF protein has to be verified.
4. PERSPECTIVE
Tissue factor has several types of posttranslational modifications, glycosylation being the most complex of them. Although the presence of carbohydrates on tissue factor was identified several decades ago, a thorough characterization of their structure and an evaluation of their influence on tissue factor activity were, with rare exceptions, somewhat neglected. This lack of interest could be explained by several statements in literature suggesting that posttranslational modifications are not important for the function of this protein. Unfortunately, no solid (if any) data were provided to support these statements. Additionally, most of the experiments were done using recombinant tissue factor proteins, which were different with respect to posttranslational modifications from the natural protein. These differences are translated into differences in physiologically-relevant activities of tissue factor. Lately, the interest in tissue factor structure-activity relationship has been rekindled, primarily by the controversies related to the role of disulfides of the extracellular domain and that of glycosylation. Additionally, there has been an increasing number of studies accomplished using natural human tissue factor or that present on the cell surface instead of recombinant proteins. This increased interest in the tissue factor protein leads us to believe that existing gap in the knowledge related to the structure-activity relationship will be filled with new research data.
ACKNOWLEDGEMENT
This study was supported by P01 HL46703 grant from the National Institutes of Health.
REFERENCES
- 1.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 2.Fleck RA, Rao LV, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;59:421–437. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 3.Eddleston M, de la Torre JC, Oldstone MB, Loskutoff DJ, Edgington TS, Mackman N. Astrocytes are the primary source of tissue factor in the murine central nervous system. A role for astrocytes in cerebral hemostasis. J Clin Invest. 1993;92:349–358. doi: 10.1172/JCI116573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kornberg A, Rahimi-Levene N, Yona R, Mor A, Rachmilewitz EA. Enhanced generation of monocyte tissue factor and increased plasma prothrombin fragment1 levels in patients with polycythemia vera: mechanism of activation of blood coagulation. Am J Hematol. 1997;56:5–11. doi: 10.1002/(sici)1096-8652(199709)56:1<5::aid-ajh2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Nijziel M, van Oerle R, van 't Veer C, van Pampus E, Lindhout T, Hamulyak K. Tissue factor activity in human monocytes is regulated by plasma: implications for the high and low responder phenomenon. Br J Haematol. 2001;112:98–104. doi: 10.1046/j.1365-2141.2001.02545.x. [DOI] [PubMed] [Google Scholar]
- 6.Broussas M, Cornillet-Lefebvre P, Potron G, Nguyen P. Adenosine inhibits tissue factor expression by LPS-stimulated human monocytes: involvement of the A3 adenosine receptor. Thromb Haemost. 2002;88:123–130. [PubMed] [Google Scholar]
- 7.Jude B, Zawadzki C, Susen S, Corseaux D. Relevance of tissue factor in cardiovascular disease. Arch Mal Coeur Vaiss. 2005;98:667–671. [PubMed] [Google Scholar]
- 8.Mumford AD, McVey JH. Tissue factor in the myocardium: evidence of roles in haemostasis and inflammation. Dis Markers. 2004;20:353–358. doi: 10.1155/2004/963402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrissey JH, Macik BG, Neuenschwander PF, Comp PC. Quantitation of activated factor VII levels in plasma using a tissue factor mutant selectively deficient in promoting factor VII activation. Blood. 1993;81:734–744. [PubMed] [Google Scholar]
- 10.Spicer EK, Horton R, Bloem L, Bach R, Williams KR, Guha A, Kraus J, Lin TC, Nemerson Y, Konigsberg WH. Isolation of cDNA clones coding for human tissue factor: primary structure of the protein and cDNA. Proc Natl Acad Sci U S A. 1987;84:5148–5152. doi: 10.1073/pnas.84.15.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paborsky LR, Harris RJ. Post-translational modifications of recombinant human tissue factor. Thromb Res. 1990;60:367–376. doi: 10.1016/0049-3848(90)90219-3. [DOI] [PubMed] [Google Scholar]
- 12.Bach R, Konigsberg WH, Nemerson Y. Human tissue factor contains thioester-linked palmitate and stearate on the cytoplasmic half-cystine. Biochemistry. 1988;27:4227–4231. doi: 10.1021/bi00412a004. [DOI] [PubMed] [Google Scholar]
- 13.Zioncheck TF, Roy S, Vehar GA. The cytoplasmic domain of tissue factor is phosphorylated by a protein kinase C-dependent mechanism. J Biol Chem. 1992;267:3561–3564. [PubMed] [Google Scholar]
- 14.Rattan SI, Derventzi A, Clark BF. Protein synthesis, posttranslational modifications, and aging. Ann N Y Acad Sci. 1992;663:48–62. doi: 10.1111/j.1749-6632.1992.tb38648.x. [DOI] [PubMed] [Google Scholar]
- 15.Lowe JB, Marth JD. A genetic approach to Mammalian glycan function. Annu Rev Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 16.Campbell CT, Yarema KJ. Large-scale approaches for glycobiology. Genome Biol. 2005;6:236. doi: 10.1186/gb-2005-6-11-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemerson Y, Pitlick FA. Purification and characterization of the protein component of tissue factor. Biochemistry. 1970;9:5100–5105. doi: 10.1021/bi00828a009. [DOI] [PubMed] [Google Scholar]
- 18.Pitlick FA. Concanavalin A inhibits tissue factor coagulant activity. J Clin Invest. 1975;55:175–179. doi: 10.1172/JCI107908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein IJ, Hollerman CE, Smith EE. Protein-Carbohydrate Interaction. Ii. Inhibition Studies on the Interaction of Concanavalin a with Polysaccharides. Biochemistry. 1965;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- 20.van den Besselaar AM, Bertina RM. Interaction of thromboplastin apoprotein of different tissues with concanavalin A--evidence for heterogeneous glycosylation of the human apoprotein. Thromb Haemost. 1984;52:192–195. [PubMed] [Google Scholar]
- 21.Mahoney WC, Duksin D. Biological activities of the two major components of tunicamycin. J Biol Chem. 1979;254:6572–6576. [PubMed] [Google Scholar]
- 22.Shands JW., Jr Macrophage factor X activator formation: metabolic requirements for synthesis of components. Blood. 1985;65:169–175. [PubMed] [Google Scholar]
- 23.Waxman E, Ross JB, Laue TM, Guha A, Thiruvikraman SV, Lin TC, Konigsberg WH, Nemerson Y. Tissue factor and its extracellular soluble domain: the relationship between intermolecular association with factor VIIa and enzymatic activity of the complex. Biochemistry. 1992;31:3998–4003. doi: 10.1021/bi00131a015. [DOI] [PubMed] [Google Scholar]
- 24.Stone MJ, Ruf W, Miles DJ, Edgington TS, Wright PE. Recombinant soluble human tissue factor secreted by Saccharomyces cerevisiae and refolded from Escherichia coli inclusion bodies: glycosylation of mutants, activity and physical characterization. Biochem J. 1995;310(Pt 2):605–614. doi: 10.1042/bj3100605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiore MM, Neuenschwander PF, Morrissey JH. The biochemical basis for the apparent defect of soluble mutant tissue factor in enhancing the proteolytic activities of factor VIIa. J Biol Chem. 1994;269:143–149. [PubMed] [Google Scholar]
- 26.Krudysz-Amblo J, Jennings ME, 2nd, Mann KG, Butenas S. Carbohydrates and activity of natural and recombinant tissue factor. J Biol Chem. 2010;285:3371–3382. doi: 10.1074/jbc.M109.055178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz-Canada C, Kelleher DJ, Gilmore R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell. 2009;136:272–283. doi: 10.1016/j.cell.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothman JE, Lodish HF. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977;269:775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Helenius J, Braakman I, Helenius A. Cotranslational folding and calnexin binding during glycoprotein synthesis. Proc Natl Acad Sci U S A. 1995;92:6229–6233. doi: 10.1073/pnas.92.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelleher DJ, Gilmore R. An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology. 2006;16:47R–62R. doi: 10.1093/glycob/cwj066. [DOI] [PubMed] [Google Scholar]
- 31.Tomiya N, Narang S, Lee YC, Betenbaugh MJ. Comparing N-glycan processing in mammalian cell lines to native and engineered lepidopteran insect cell lines. Glycoconj J. 2004;21:343–360. doi: 10.1023/B:GLYC.0000046275.28315.87. [DOI] [PubMed] [Google Scholar]
- 32.Altmann F, Kornfeld G, Dalik T, Staudacher E, Glossl J. Processing of asparagine-linked oligosaccharides in insect cells. N-acetylglucosaminyltransferase I and II activities in cultured lepidopteran cells. Glycobiology. 1993;3:619–625. doi: 10.1093/glycob/3.6.619. [DOI] [PubMed] [Google Scholar]
- 33.Geisler C, Aumiller JJ, Jarvis DL. A fused lobes gene encodes the processing beta-N-acetylglucosaminidase in Sf9 cells. J Biol Chem. 2008;283:11330–11339. doi: 10.1074/jbc.M710279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehemtulla A, Ruf W, Edgington TS. The integrity of the cysteine 186-cysteine 209 bond of the second disulfide loop of tissue factor is required for binding of factor VII. J Biol Chem. 1991;266:10294–10299. [PubMed] [Google Scholar]
- 35.Bona R, Lee E, Rickles F. Tissue factor apoprotein: intracellular transport and expression in shed membrane vesicles. Thromb Res. 1987;48:487–500. doi: 10.1016/0049-3848(87)90405-1. [DOI] [PubMed] [Google Scholar]
- 36.Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982;296:613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- 37.Mody RS, Carson SD. Tissue factor cytoplasmic domain peptide is multiply phosphorylated in vitro. Biochemistry. 1997;36:7869–7875. doi: 10.1021/bi9701235. [DOI] [PubMed] [Google Scholar]
- 38.Dorfleutner A, Ruf W. Regulation of tissue factor cytoplasmic domain phosphorylation by palmitoylation. Blood. 2003;102:3998–4005. doi: 10.1182/blood-2003-04-1149. [DOI] [PubMed] [Google Scholar]
- 39.Car BD, Slauson DO, Dore M, Suyemoto MM. Endotoxin-mediated bovine alveolar macrophage procoagulant induction is dependent on protein kinase C activation. Inflammation. 1990;14:681–689. doi: 10.1007/BF00916371. [DOI] [PubMed] [Google Scholar]
- 40.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 41.Versteeg HH, Hoedemaeker I, Diks SH, Stam JC, Spaargaren M, van Bergen En Henegouwen PM, van Deventer SJ, Peppelenbosch MP. Factor VIIa/tissue factor-induced signaling via activation of Src-like kinases, phosphatidylinositol 3-kinase, and Rac. J Biol Chem. 2000;275:28750–28756. doi: 10.1074/jbc.M907635199. [DOI] [PubMed] [Google Scholar]
- 42.Poulsen LK, Jacobsen N, Sorensen BB, Bergenhem NC, Kelly JD, Foster DC, Thastrup O, Ezban M, Petersen LC. Signal transduction via the mitogen-activated protein kinase pathway induced by binding of coagulation factor VIIa to tissue factor. J Biol Chem. 1998;273:6228–6232. doi: 10.1074/jbc.273.11.6228. [DOI] [PubMed] [Google Scholar]
- 43.Ahamed J, Ruf W. Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J Biol Chem. 2004;279:23038–23044. doi: 10.1074/jbc.M401376200. [DOI] [PubMed] [Google Scholar]
- 44.Ryden L, Grabau D, Schaffner F, Jonsson PE, Ruf W, Belting M. Evidence for tissue factor phosphorylation and its correlation with protease-activated receptor expression and the prognosis of primary breast cancer. Int J Cancer. 2010;126:2330–2340. doi: 10.1002/ijc.24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magee AI, Courtneidge SA. Two classes of fatty acid acylated proteins exist in eukaryotic cells. EMBO J. 1985;4:1137–1144. doi: 10.1002/j.1460-2075.1985.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 47.Stanley P, Koronakis V, Hughes C. Acylation of Escherichia coli hemolysin: a unique protein lipidation mechanism underlying toxin function. Microbiol Mol Biol Rev. 1998;62:309–333. doi: 10.1128/mmbr.62.2.309-333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci U S A. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broze GJ, Jr, Leykam JE, Schwartz BD, Miletich JP. Purification of human brain tissue factor. J Biol Chem. 1985;260:10917–10920. [PubMed] [Google Scholar]
- 50.Guha A, Bach R, Konigsberg W, Nemerson Y. Affinity purification of human tissue factor: interaction of factor VII and tissue factor in detergent micelles. Proc Natl Acad Sci U S A. 1986;83:299–302. doi: 10.1073/pnas.83.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carson SD, Ross SE, Gramzinski RA. Protein co-isolated with human tissue factor impairs recovery of activity. Blood. 1988;71:520–523. [PubMed] [Google Scholar]
- 52.Roy S, Paborsky LR, Vehar GA. Self-association of tissue factor as revealed by chemical crosslinking. J Biol Chem. 1991;266:4665–4668. [PubMed] [Google Scholar]
- 53.Bach RR, Moldow CF. Mechanism of tissue factor activation on HL-60 cells. Blood. 1997;89:3270–3276. [PubMed] [Google Scholar]
- 54.Wolberg AS, Kon RH, Monroe DM, Ezban M, Roberts HR, Hoffman M. Deencryption of cellular tissue factor is independent of its cytoplasmic domain. Biochem Biophys Res Commun. 2000;272:332–336. doi: 10.1006/bbrc.2000.2783. [DOI] [PubMed] [Google Scholar]
- 55.Donate F, Kelly CR, Ruf W, Edgington TS. Dimerization of tissue factor supports solution-phase autoactivation of factor VII without influencing proteolytic activation of factor X. Biochemistry. 2000;39:11467–11476. doi: 10.1021/bi000986p. [DOI] [PubMed] [Google Scholar]
- 56.Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 57.Fortin JP, Rivard GE, Adam A, Marceau F. Studies on rabbit natural and recombinant tissue factors: intracellular retention and regulation of surface expression in cultured cells. Am J Physiol Heart Circ Physiol. 2005;288:H2192–H2202. doi: 10.1152/ajpheart.01135.2004. [DOI] [PubMed] [Google Scholar]
- 58.Mandal SK, Pendurthi UR, Rao LV. Cellular localization and trafficking of tissue factor. Blood. 2006;107:4746–4753. doi: 10.1182/blood-2005-11-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sevinsky JR, Rao LV, Ruf W. Ligand-induced protease receptor translocation into caveolae: a mechanism for regulating cell surface proteolysis of the tissue factor-dependent coagulation pathway. J Cell Biol. 1996;133:293–304. doi: 10.1083/jcb.133.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mandal SK, Iakhiaev A, Pendurthi UR, Rao LV. Acute cholesterol depletion impairs functional expression of tissue factor in fibroblasts: modulation of tissue factor activity by membrane cholesterol. Blood. 2005;105:153–160. doi: 10.1182/blood-2004-03-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dietzen DJ, Page KL, Tetzloff TA. Lipid rafts are necessary for tonic inhibition of cellular tissue factor procoagulant activity. Blood. 2004;103:3038–3044. doi: 10.1182/blood-2003-07-2399. [DOI] [PubMed] [Google Scholar]
- 62.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 63.Oka N, Yamamoto M, Schwencke C, Kawabe J, Ebina T, Ohno S, Couet J, Lisanti MP, Ishikawa Y. Caveolin interaction with protein kinase C. Isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J Biol Chem. 1997;272:33416–33421. doi: 10.1074/jbc.272.52.33416. [DOI] [PubMed] [Google Scholar]
- 64.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26:456–461. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 65.Wakita K, Stearns-Kurosawa DJ, Marumoto Y. The effect of calcium ionophore A23187 on tissue factor activity and mRNA in endothelial cells. Thromb Res. 1994;74:95–103. doi: 10.1016/0049-3848(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 66.Bach R, Rifkin DB. Expression of tissue factor procoagulant activity: regulation by cytosolic calcium. Proc Natl Acad Sci U S A. 1990;87:6995–6999. doi: 10.1073/pnas.87.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henriksson CE, Klingenberg O, Hellum M, Landsverk KS, Joo GB, Westvik AB, Kierulf P. Calcium ionophore-induced de-encryption of tissue factor in monocytes is associated with extensive cell death. Thromb Res. 2007;119:621–630. doi: 10.1016/j.thromres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 68.Stampfuss JJ, Censarek P, Bein D, Schror K, Grandoch M, Naber C, Weber AA. Membrane environment rather than tissue factor expression determines thrombin formation triggered by monocytic cells undergoing apoptosis. J Leukoc Biol. 2008;83:1379–1381. doi: 10.1189/jlb.1207843. [DOI] [PubMed] [Google Scholar]
- 69.Carson SD, Perry GA, Pirruccello SJ. Fibroblast tissue factor: calcium and ionophore induce shape changes, release of membrane vesicles, and redistribution of tissue factor antigen in addition to increased procoagulant activity. Blood. 1994;84:526–534. [PubMed] [Google Scholar]
- 70.Henriksson CE, Hellum M, Landsverk KS, Klingenberg O, Joo GB, Kierulf P. Flow cytometry-sorted non-viable endotoxin-treated human monocytes are strongly procoagulant. Thromb Haemost. 2006;96:29–37. doi: 10.1160/TH06-01-0052. [DOI] [PubMed] [Google Scholar]
- 71.Rand ML, Wang H, Bang KW, Packham MA, Freedman J. Persistence of phosphatidylserine exposure on activated platelets in vivo in rabbits. Thromb Haemost. 2007;98:477–478. [PubMed] [Google Scholar]
- 72.Liu ML, Reilly MP, Casasanto P, McKenzie SE, Williams KJ. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arterioscler Thromb Vasc Biol. 2007;27:430–435. doi: 10.1161/01.ATV.0000254674.47693.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lorenzet R, Niemetz J, Marcus AJ, Broekman MJ. Enhancement of mononuclear procoagulant activity by platelet 12-hydroxyeicosatetraenoic acid. J Clin Invest. 1986;78:418–423. doi: 10.1172/JCI112592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Halvorsen H, Olsen JO, Osterud B. Granulocytes enhance LPS-induced tissue factor activity in monocytes via an interaction with platelets. J Leukoc Biol. 1993;54:275–282. doi: 10.1002/jlb.54.4.275. [DOI] [PubMed] [Google Scholar]
- 75.Osterud B, Rao LV, Olsen JO. Induction of tissue factor expression in whole blood: lack of evidence for the presence of tissue factor expression in granulocytes. Thromb Haemost. 2000;83:861–867. [PubMed] [Google Scholar]
- 76.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45:12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 77.Bednar RA, Fried WB, Lock YW, Pramanik B. Chemical modification of chalcone isomerase by mercurials and tetrathionate. Evidence for a single cysteine residue in the active site. J Biol Chem. 1989;264:14272–14276. [PubMed] [Google Scholar]
- 78.Weber GJ, Mehr AP, Sirota JC, Aller SG, Decker SE, Dawson DC, Forrest JN., Jr Mercury and zinc differentially inhibit shark and human CFTR orthologues: involvement of shark cysteine 102. Am J Physiol Cell Physiol. 2006;290:C793–C801. doi: 10.1152/ajpcell.00203.2005. [DOI] [PubMed] [Google Scholar]
- 79.Kaneko H, Kakkar VV, Scully MF. Mercury compounds induce a rapid increase in procoagulant activity of monocyte-like U937 cells. Br J Haematol. 1994;87:87–93. doi: 10.1111/j.1365-2141.1994.tb04875.x. [DOI] [PubMed] [Google Scholar]
- 80.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raturi A, Ruf W. Effect of protein disulfide isomerase chaperone activity inhibition on tissue factor activity. J Thromb Haemost. 2010;8:1863–1865. doi: 10.1111/j.1538-7836.2010.03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;282:25416–25424. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 83.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manukyan D, von Bruehl ML, Massberg S, Engelmann B. Protein disulfide isomerase as a trigger for tissue factor-dependent fibrin generation. Thromb Res. 2008;122 Suppl 1:S19–S22. doi: 10.1016/S0049-3848(08)70013-6. [DOI] [PubMed] [Google Scholar]
- 85.Kothari H, Sen P, Pendurthi UR, Rao LV. Bovine protein disulfide isomerase-enhanced tissue factor coagulant function: is phospholipid contaminant in it the real culprit? Blood. 2008;111:3295–3296. doi: 10.1182/blood-2007-12-129825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Persson E. Protein disulfide isomerase has no stimulatory chaperone effect on factor X activation by factor VIIa-soluble tissue factor. Thromb Res. 2008;123:171–176. doi: 10.1016/j.thromres.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 87.Pendurthi UR, Rao LV. Response: Tissue factor de-encryption: the cell model system. Blood. 2008;112:913. [Google Scholar]
- 88.Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood. 2010;116:993–1001. doi: 10.1182/blood-2009-10-249607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Popescu NI, Lupu C, Lupu F. Role of PDI in regulating tissue factor: FVIIa activity. Thromb Res. 2010;125 Suppl 1:S38–S41. doi: 10.1016/j.thromres.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pendurthi UR, Ghosh S, Mandal SK, Rao LV. Tissue factor activation: is disulfide bond switching a regulatory mechanism? Blood. 2008;112:912–913. doi: 10.1182/blood-2007-07-101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kothari H, Nayak RC, Rao LV, Pendurthi UR. Cystine 186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption. Blood. 2010;115:4273–4283. doi: 10.1182/blood-2009-09-241356. [DOI] [PMC free article] [PubMed] [Google Scholar]