Background: Albumin interacts with megalin and triggers cellular responses in proximal tubule cells.
Results: Albumin modulates the PI3K/protein kinase B, protein kinase C, and protein kinase A pathways promoting the regulation of the (Na+ + K+)-ATPase expression.
Conclusion: Variation in the albumin concentration in the proximal tubule affects sodium reabsorption.
Significance: These results open new avenues to understanding the role of albumin in proximal tubule cells.
Keywords: Epithelial Cell; Kidney; Na,K-ATPase; Protein Kinases; Sodium Transport; Albuminuria; Proximal Tubule; Renal Disease; Signaling Pathways; Sodium Pump
Abstract
In recent decades, evidence has confirmed the crucial role of albumin in the progression of renal disease. However, the possible role of signaling pathways triggered by physiologic concentrations of albumin in the modulation of proximal tubule (PT) sodium reabsorption has not been considered. In the present work, we have shown that a physiologic concentration of albumin increases the expression of the α1 subunit of (Na+ + K+)-ATPase in LLC-PK1 cells leading to an increase in enzyme activity. This process involves the sequential activation of PI3K/protein kinase B and protein kinase C pathways promoting inhibition of protein kinase A. This integrative network is inhibited when albumin concentration is increased, similar to renal disease, leading to a decrease in the α1 subunit of (Na+ + K+)-ATPase expression. Together, the results indicate that variation in albumin concentration in PT cells has an important effect on PT sodium reabsorption and, consequently, on renal sodium excretion.
Introduction
Albumin filtrated in the renal glomerulus is reabsorbed by proximal tubule (PT)2 cells via receptor-mediated endocytosis, which involves a complex of three proteins: megalin, cubilin, and amnionless (1). Injury to the glomerular filtration membrane, observed in many renal diseases, leads to an increase in filtration of albumin and, consequently, albumin overload in PT and albuminuria (1). In the past 3 decades, several studies have shown that albumin has an active role in the progression of renal disease mediated by its proinflammatory effects (2). However, the possible role of albumin-triggered signaling pathways in the modulation of PT sodium reabsorption has not been investigated.
Most sodium filtrated in the glomerulus is reabsorbed in PT cells mainly through an active transcellular pathway (3). This process depends on basolateral (Na+ + K+)-ATPase responsible for creating an electrochemical gradient used by cotransporters located in the luminal membrane. Consequently, any change in the activity of this enzyme leads to changes in PT sodium reabsorption, renal sodium excretion, and, consequently, in salt homeostasis (4). To keep renal sodium excretion within narrow limits, this enzyme is finely regulated by hormones and autacoids. This process involves both short and long term regulation (4). Short term regulation could involve enzyme phosphorylation, which depends on the balance between the activities of protein kinases and protein phosphatases (5). Long term regulation usually involves protein synthesis, which also depends on the previous activation of different protein kinases (4).
Today, it is well known that albumin interacts with megalin and triggers cellular responses (2, 6). Megalin contains specific motifs in its short carboxyl-terminal cytoplasmic tail that are involved in protein-protein interactions, such as Src homology 3 and PDZ domains, and protein kinase-phosphorylated sites (1, 7). The functionality of these motifs has been shown by identification of proteins associated to megalin such as Dab-2 (disabled-2) and protein kinase B (PKB) (1, 8). Furthermore, Yuseff et al. (7) showed that megalin could be phosphorylated in vitro by protein kinase C (PKC), protein kinase A (PKA), glycogen synthase kinase-3, and casein kinase. This complex integrated network of signaling pathways has been associated in most studies with the proinflammatory effects of albumin in pathological conditions (2, 6). However, little is known about its possible role in PT cell function in physiological conditions.
PT cells work in an integrative way, they reabsorb sodium, and are exposed to different albumin concentrations. Based on exposure to albumin, it is plausible to postulate that the binding of albumin to megalin in PT cells triggers a cellular response that could modulate sodium reabsorption through regulation of (Na+ + K+)-ATPase activity. If this hypothesis is true, another question arises: is the modulation of the sodium pump dependent on the changes in albumin concentration in PT observed in renal disease? The aim of this work is to investigate the modulation of PT (Na+ + K+)-ATPase activity by albumin in physiological and pathological concentration and identify the molecular pathways involved. We used LLC-PK1 cells, a well characterized porcine PT cell line. We found that lower albumin concentrations increased (Na+ + K+)-ATPase expression and activity. This effect was mediated by PI3K/PKB-dependent PKC activation and inhibition of PKA. In addition, we observed that this effect was abolished when the albumin concentration was increased in LLC-PK1 cells. These results suggest a new role for albumin in PT and open new possibilities in understanding the physiological and pathophysiologic actions of albumin in sodium homeostasis.
EXPERIMENTAL PROCEDURES
Materials and Reagents
Bovine serum albumin (BSA), ATP (sodium salt), ouabain, sodium chloride, potassium chloride, magnesium chloride, EDTA, EGTA, Hepes, Tris, histone type II-S, PKA inhibitor peptide, PMSF, forskolin, Bt2cAMP, sodium deoxycholate, sodium orthovanadate, and mouse monoclonal α1 subunit (Na+ + K+)-ATPase antibody (clone M7-PB-E9) were purchased from Sigma-Aldrich. Sodium pyrophosphate was purchased from Reagen S.A. (Rio de Janeiro, Brazil). SDS was purchased from USB Corporation. Sucrose was from Merck. Calphostin C and wortmannin were purchased from Calbiochem. Polyclonal phospho-PKB (Ser-473), polyclonal PKB, and polyclonal β-actin antibodies were from Cell Signaling Technology. Mouse monoclonal α1 subunit (Na+ + K+)-ATPase antibody (clone C464.6) was purchased from Millipore. 32Pi was obtained from the Brazilian Institute of Energetic and Nuclear Research (São Paulo, Brazil). LLC-PK1 cells were obtained from American Type Culture Collection (Rockville, MD). [γ-32P]ATP was synthesized according to the procedures described by Maia et al. (9). All other reagents were of the highest purity available.
Cell Culture and Biochemical Assay
LLC-PK1 cells, a well characterized porcine PT cell line, were maintained in low glucose Dulbecco's modified Eagle's medium with 10% fetal bovine serum/1% penicillin/streptomycin (37 °C and 5% CO2). The cells were used 1 day after 95–98% confluence was reached, typically 3 days after seeding. Previously, the cells were preincubated overnight with different compounds indicated in the figure legends in serum-depleted medium. Cell fractionation, immunofluorescence, (Na+ + K+)-ATPase activity, protein kinases activities, immunoblotting, and immunoprecipitation were performed as described (8, 10, 11).
Preparation of Renal Cortex Homogenate
The renal cortex homogenate was obtained as described previously (10). Briefly, the kidneys were removed and homogenized in cold solution (250 mm sucrose, 10 mm Hepes (pH 7.6), 2 mm EDTA, 1 mm PMSF). The homogenate was cleared by centrifugation at 4 °C for 10 min at 7,000 × g and stored at −80 °C.
Cell Fractionation
Cells were washed with PBS2+ and harvested in buffer containing 20 mm Hepes (pH 7.4), 2 mm EGTA, 400 μm PMSF, 50 mm NaF, 2× Complete protease inhibitor (Roche Diagnostics) and lysed by 30 strokes in a Dounce homogenizer. Nuclei were removed by centrifugation at 4 °C for 10 min at 1,000 × g. The membrane fractions were obtained by centrifugation at 4 °C for 60 min at 100,000 × g. The pellet was resuspended in buffer as described above.
Assessment of (Na+ + K+)-ATPase α1 Subunit on Cell Surface
The proteins expressed on the surface of the basolateral membrane were detected by biotinylation assay (12). LLC-PK1 cells were grown in 6-well plates containing Transwell inserts. Cells were incubated overnight with 0.01 or 20.0 mg/ml albumin and washed three times with PBS2+. The proteins expressed at the basolateral membrane were biotinylated by incubation with 1.5 mg/ml EZ-Link® Sulfo-NHS-SS-biotin purchased from Pierce Biotechnology for 30 min at 4 °C. Biotinylated proteins were quenched with PBS-glycine buffer and rinsed with PBS2+. Cells were lysed in radioimmunoprecipitation assay buffer and clarified by centrifugation at 4 °C for 10 min at 7,000 × g. The biotinylated proteins were precipitated with NeutraAvidin® UltraLink® resin beads (Pierce Biotechnology) overnight at 4 °C. The precipitate was then subjected to SDS-PAGE and immunoblotting for detection of (Na+ + K+)-ATPase α1 subunits.
(Na+ + K+)-ATPase Activity Assay
The ATPase activity was measured according to the method described by Grubmeyer and Penefsky (13). Treated cells were washed in PBS2+, harvested, and cleared by centrifugation at 4 °C for 5 min at 600 × g, and incubated for 30 min in lysis buffer (1 mm EGTA, 0.1% sodium deoxycholate, 20 mm Hepes (pH 7.4), 400 μm PMSF, 2× Complete protease inhibitor, 250 mm sucrose). The composition of the standard assay medium was 10 mm MgCl2, 5 mm ATP (specific activity 0.27 μCi/nmol [γ-32P]ATP), 20 mm Hepes (pH 7.0), 120 mm NaCl, and 30 mm KCl. The reaction was started by the addition of 0.3 mg/ml protein from cell or renal cortex homogenate. After 10 min, the reaction was stopped with charcoal activated by 0.1 n HCl. The 32Pi released was measured by liquid scintillation counter (Packard Tri-Carb 2100 TR). Spontaneous hydrolysis of [γ-32P]ATP was <1% of the total ATPase activity. The (Na+ + K+)-ATPase activity was determined by sensitivity to 1 mm ouabain.
Protein Kinase Activity Assay
The protein kinase C, A, and G activities were measured by phosphorylation of histone type II-S using ATP/[γ-32P]ATP as substrate. The composition of the reaction medium was 4 mm MgCl2, 10 μm ATP (specific activity 7 × 103 μCi/nmol [γ-32P]ATP), 20 mm Hepes (pH 7.0), 1.5 mg/ml histone, and 0.7 mg/ml protein from the homogenate fraction. After 20 min, the reaction was stopped with 20% trichloroacetic acid (TCA), and the sample was immediately placed on ice. An aliquot (0.2 ml) was filtered through a Millipore filter (0.45-μm pore size) and washed with ice-cold 20% TCA solution and 0.1 m phosphate buffer (pH 7.0). The radioactivity was quantified using a liquid scintillation counter (Packard Tri-Carb 2100 TR). The PKC, PKA, and PKG activities were determined by sensitivity to 10−8 m calphostin C, 10−8 m PKA inhibitor peptide, or 10−6 m KT5823, respectively.
Immunoprecipitation and Immunoblotting
After treatment, the cells were washed with PBS2+, harvested, and incubated for 40 min in lysis buffer (25 mm Tris (pH 7.5), 50 mm NaCl,1 mm EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 10 mm SDS, 10 mm NaF, 5 mm sodium vanadate, 5 mm sodium pyrophosphate, protease inhibitor mixture (Roche Diagnostics)) and cleared by centrifugation at 4 °C for 10 min at 15,000 × g. The supernatant was retained, and the protein concentrations were determined by the Folin phenol method (14), using BSA as standard. When indicated, PKC was immunoprecipitated according to the manufacturer's instructions. Proteins were resolved on SDS-polyacrylamide gels and transferred to polyvinylidene fluoride or nitrocellulose membranes (Millipore), according to the manufacturer's instructions. After antibody labeling, detection was performed with ECL-plus (Amersham Biosciences).
Statistical Analysis
The results are expressed as means ± S.E. Statistical significance was assessed by two-way analysis of variance (ANOVA), considering the treatments as factors. The significance of the differences was verified by the Bonferroni t test. Statistical analysis was performed using absolute values. Significance was determined as p < 0.05.
RESULTS
Modulation of (Na+ + K+)-ATPase by Albumin
LLC-PK1 cells were preincubated overnight with different compounds in medium without serum. The cells were then washed out with PBS2+ and used in different experimental protocols. Cell viability in the presence of low and high albumin concentrations was determined by lactate dehydrogenase measurements in the supernatant and propidium iodide staining analyzed by fluorescence-activated cell sorting. Cell viability >was 95% in all conditions tested.
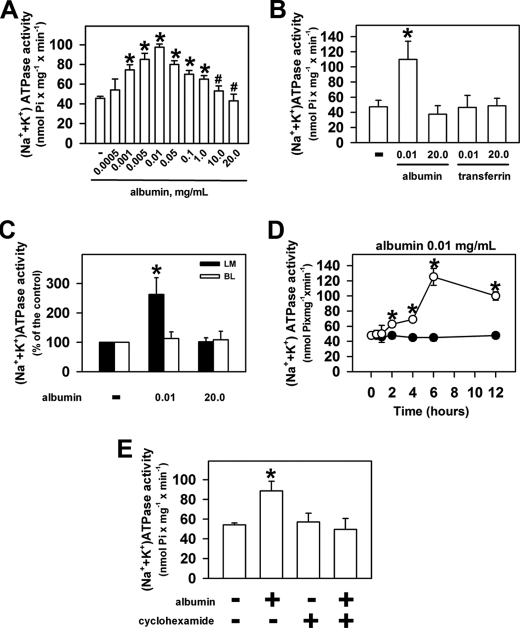
Initially, we tested whether albumin could modulate PT (Na+ + K+)-ATPase activity. Fig. 1A shows that an increase in albumin concentration from 0.0005 up to 0.01 mg/ml increased enzyme activity from 45.7 ± 1.9 (in the absence of albumin) to 97.5 ± 3.1 nmol Pi/mg per min. Further increase in albumin concentration decreased the enzyme activity to the control level. Higher albumin concentrations (10 and 20 mg/ml) did not change the enzyme activity compared with the control in the absence of albumin. In addition, we investigated whether the activation of the (Na+ + K+)-ATPase activity was specific to albumin. It was observed that transferrin at 0.01 and 20 mg/ml did not change the enzyme activity, showing that the effect is specific for albumin (Fig. 1B).
FIGURE 1.
Albumin modulates (Na+ + K+)-ATPase activity. LLC-PK1 cells were grown on 6-well plates (A, B, D, and E) or on Transwell cell culture inserts (C), kept overnight (except in D) in medium depleted of serum in the absence or in the presence of compounds indicated in the figure. After treatment, the cells were washed with PBS2+, and (Na+ + K+)-ATPase activity was measured. A, dose response of albumin on (Na+ + K+)-ATPase activity (n = 7). B, effect of transferrin (n = 4). C, effect of albumin added on the luminal (LM) or basolateral (BL) side of Transwell cell culture inserts (n = 5). D, time course of the effect of albumin (n = 8). E, modulation of the effect of 0.01 mg/ml albumin on the enzyme activity by cyclohexamide (n = 6). Error bars, S.E. *, statistically significant in relation to the control (in the absence of albumin) or to #, 0.01 mg/ml albumin (p < 0.05).
In the next step, we tested the effect of albumin on the (Na+ + K+)-ATPase activity in LLC-PK1 cells grown on permeable supports (Transwell plates, Fig. 1C). Under these conditions, albumin was added at the luminal or at basolateral side and kept overnight in a medium depleted of serum. After treatment, the (Na+ + K+)-ATPase was evaluated. When 0.01 mg/ml albumin was added at luminal side, the enzyme activity was increased by 162%. In contrast, when albumin, at same concentration, was added at basolateral side the enzyme activity was not changed. The addition of 20 mg/ml albumin did not change the enzyme activity when added at both sides. These results are similar to those obtained in LLC-PK1 cells grown on nonpermeable supports, showing that the effect of albumin is mediated by the luminal receptor.
(Na+ + K+)-ATPase activity can be modulated by rapid mechanisms (minutes) that do not involve protein synthesis or by mechanisms that involve protein synthesis that takes hours. In the next experimental group, the LLC-PK1 cells were preincubated with different concentrations of albumin (0.01–20 mg/ml) for 30 min. Under these conditions (Na+ + K+)-ATPase activity was not changed by any albumin concentration. The time course of the effect of albumin on the enzyme activity revealed that the stimulatory effect of 0.01 mg/ml albumin was only observed after 2 h of preincubation, and the maximal stimulatory effect was observed for 6 h of preincubation and maintained for up to 12 h (Fig. 1D). Furthermore, the stimulatory effect of albumin on enzyme activity was completely abolished by the addition of 25 μm cyclohexamide, a protein synthesis inhibitor (Fig. 1E).
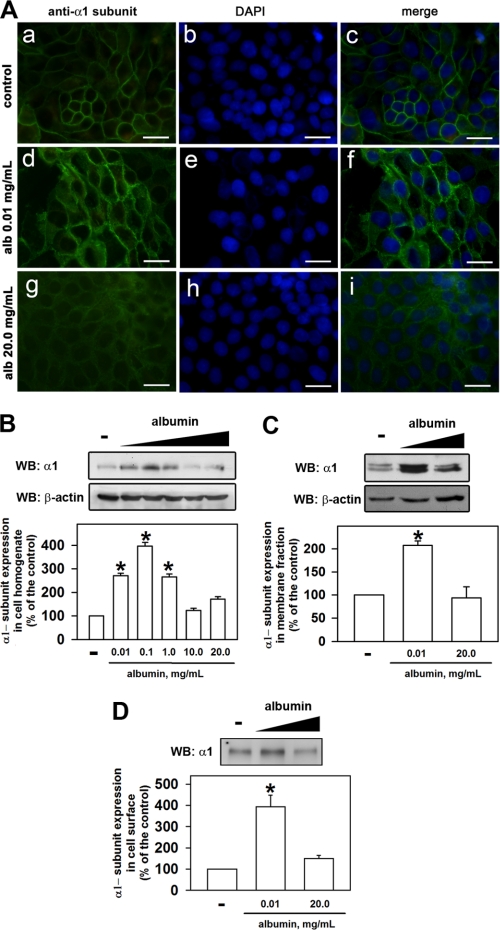
Immunofluorescence was performed to assess the modulation of expression of the α1 subunit of (Na+ + K+)-ATPase in LLC-PK1 cells (Fig. 2A). 4′,6-Diamidino-2-phenylindole (DAPI) (blue) was used to mark the nucleus. Expression of the α1 subunit of (Na+ + K+)-ATPase (green) increased when the cells were preincubated with 0.01 mg/ml albumin but did not change with 20 mg/ml albumin (compare Fig. 2A, c, f, i). Immunoblotting was performed to confirm the increase in α1 subunits. Overnight preincubation of the cells at lower albumin concentrations (0.01–1.0 mg/ml) increased α1 subunits in the homogenate (Fig. 2B), the membrane fraction (Fig. 2C), and on the cell surface (Fig. 2D), but higher albumin concentrations (10–20 mg/ml) did not (Fig. 2B). These results show that the α1 subunit expression induced by albumin takes place in the plasma membrane.
FIGURE 2.
Albumin induces the expression of (Na+ + K+)-ATPase α1 subunits. A, LLC-PK1 cells were grown on coverslips, kept in medium depleted of serum in the absence (a–c) or in the presence of 0.01 mg/ml (d–f) or 20.0 mg/ml (g–i) albumin. After treatment, the cells were washed with PBS2+, and immunofluorescence was performed. The (Na+ + K+)-ATPase α1 subunits are shown in green. Blue (DAPI) represents the nucleus. Scale bar, 10 μm (n = 3). B–D, LLC-PK1 cells were kept overnight in medium depleted of serum in the absence or presence of 0.01 mg/ml albumin. Immunoblotting was performed for the (Na+ + K+)-ATPase α1 subunit in cell homogenate (B, n = 5), in the membrane fraction (C, n = 6), or on the cell surface of basolateral membrane (D, n = 4). Error bars, S.E. *, statistically significant in relation to the control (in the absence of albumin).
Albumin-triggered Cellular Response Involves Modulation of Protein Kinase Activities
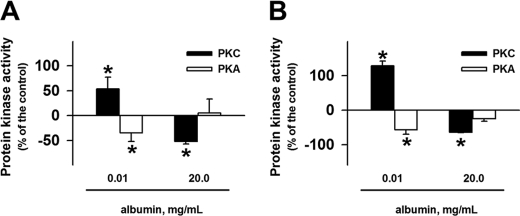
The observation that PT (Na+ + K+)-ATPase activity is modulated by serine/threonine protein kinases could be a clue to determine the molecular mechanism involved in the albumin effect. We decided to verify whether albumin modulates serine/threonine protein kinases such as PKA, PKC, and PKG activities. The cells were preincubated with albumin (0.01 and 20 mg/ml) for 30 min (acute treatment) or overnight (chronic treatment). An inverse correlation for the albumin effect in PKC and PKA activities was found. Albumin at a lower concentration (0.01 mg/ml) increased PKC activity and decreased PKA activity in both acute (Fig. 3A) and chronic treatment (Fig. 3B). On the other hand, a higher albumin concentration (20 mg/ml) decreased PKC activity and did not change PKA activity for both treatments (Fig. 3). Albumin did not change the PKG activity for any of the conditions tested.
FIGURE 3.
Effect of albumin on PKA and PKC activity. LLC-PK1 cells were grown in 6-well plates, incubated for 30 min (A) or overnight (B) in medium depleted of serum in the absence or in the presence of 0.01 or 20 mg/ml albumin. After treatment, the cells were washed with PBS2+, and the protein kinase activities were measured using histone II-S as substrate (n = 5). Error bars, S.E. *, statistically significant in relation to the control (in the absence of albumin).
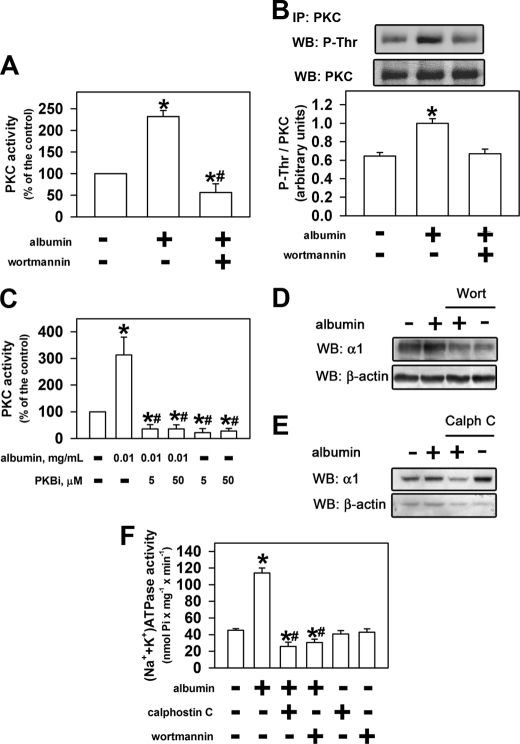
In a previous work, it was observed that in LLC-PK1 cells, a lower concentration of albumin stimulates PKB activity and a higher concentration inhibits it (8). These results suggest that there could be a correlation between the mechanisms of modulation of PKB and PKC activity by albumin. We decided to investigate whether activation of PKC depends on previous activation of PKB. Using 10−7 m wortmannin, an inhibitor of the PI3K/PKB pathway, the PKC activation induced by albumin did not occur (Fig. 4A).
FIGURE 4.
PKC and PKB mediated the effect of albumin on the sodium pump. LLC-PK1 cells were kept overnight (D–F) or for 30 min (A–C) in medium depleted of serum in the absence or in the presence of 0.01 mg/ml albumin. After treatment, the cells were washed with PBS2+. A, effect of albumin on PKC activity in the presence or in the absence of 0.1 μm wortmannin (n = 5). B, effect of albumin on PKC phosphorylation in the presence or in the absence of 0.1 μm wortmannin. When indicated, PKC was immunoprecipitated (IP) followed by immunoblotting (WB) for phosphothreonine residue (P-Thr) or PKC (n = 3). C, modulation of the effect of albumin on PKC activity by PKB inhibitor (PKBi; n = 6). D and E, effect of albumin on the α1 subunit of (Na+ + K+)-ATPase expression in the presence or in the absence of 0.1 μm wortmannin or 0.1 μm calphostin C. The α1 subunit bands were quantified and normalized by β-actin expression (n = 4). F, modulation of the effect of albumin on (Na+ + K+)-ATPase activity by 0.1 μm wortmannin or 0.1 μm calphostin C. *, statistically significant in relation to the control (in the absence of albumin) or #, to 0.01 mg/ml albumin (p < 0.05).
Complete activation of all PKC isoforms involves phosphorylation of the threonine (Thr) residue in the activation segment promoted by phosphoinositide-dependent kinase 1 (15). To address this issue, the cells were treated with 0.01 mg/ml albumin for 30 min, and PKC was immunoprecipitated and detected by immunoblotting against phosphorylated threonine residues (Thr(P)) (Fig. 4B). Albumin increased the phosphorylation of threonine residues, and this effect was abolished by 10−7 m wortmannin. Furthermore, we observed that the stimulatory effect of 0.01 mg/ml albumin on PKC activity was abolished by preincubation of the LLC-PK1 cells with PKB inhibitor (Fig. 4C).
The increase in expression of the α1 subunit of the (Na+ + K+)-ATPase induced by albumin was completely abolished when wortmannin (Fig. 4D) or calphostin C (Fig. 4E) was added. The same effect was observed in relation to (Na+ + K+)-ATPase activity (Fig. 4F). Our data indicate that PI3K-dependent activation of PKB by lower albumin concentration leads to activation of PKC and, consequently, an increase in (Na+ + K+)-ATPase expression. The addition of wortmannin and calphostin C in the presence of albumin decreased both the enzyme activity and expression below that of the control (Fig. 4, D–F). This observation could indicate that there are two different components involved in modulation of the expression of the α1 subunit of (Na+ + K+)-ATPase: (i) one stimulatory, PI3K/PKB/PKC pathway, which is activated by lower albumin concentration; (ii) the other inhibitory, which is blocked by lower albumin concentrations. One possible candidate to inhibitory component could be PKA, as shown in Fig. 3.
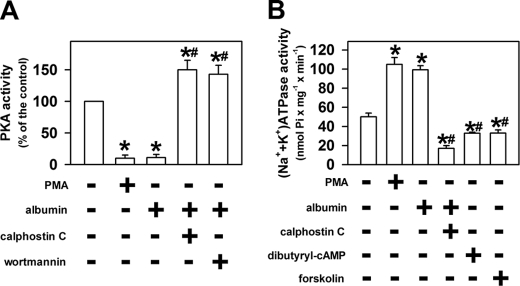
Inhibition of PKA Activity Plays Important Role in Activation of (Na+ + K+)-ATPase by Albumin
In this step we investigated whether there is some correlation among activation of the PI3K/PKB/PKC pathway, PKA inhibition, and the (Na+ + K+)-ATPase α1 subunit expression. We observed that 10−12 m phorbol 12-myristate 13-acetate (PMA), an activator of PKC, inhibited PKA activity in a similar way to 0.01 mg/ml albumin (Fig. 5A). In addition, the inhibitory effect of albumin on PKA activity was reversed by 10−8 m calphostin C or 10−7 m wortmannin. In the presence of these inhibitors, PKA activity was increased by 0.01 mg/ml albumin. Fig. 5B shows the correlation between the PI3K/PKB/PKC and PKA pathways on the modulation of (Na+ + K+)-ATPase. PMA (10−12 m) had the same stimulatory effect as 0.01 mg/ml albumin on (Na+ + K+)-ATPase activity, but in the presence of PKC inhibitor, 0.01 mg/ml albumin inhibited (Na+ + K+)-ATPase activity in a way similar to 10−6 m Bt2cAMP or 10−6 m forskolin, an activator of adenylyl cyclase. These data show that the PI3K/PKB/PKC pathway mediates the inhibitory effect of albumin on PKA activity, which contributes to the increase in (Na+ + K+)-ATPase activity.
FIGURE 5.
Inhibition of PKA activity is involved in the effect of albumin on the sodium pump. LLC-PK1 cells were grown in 6-well plates and incubated for 30 min (A) or overnight (B) in medium depleted of serum in the absence or in the presence of 0.01 mg/ml albumin. After treatment, the cells were washed with PBS2+, and PKA or (Na+ + K+)-ATPase activities were measured. A, modulation of the effect of albumin on the PKA activity by 10 pm PMA, 0.1 μm wortmannin, or 0.1 μm calphostin C (n = 7). B, role of PKA in modulation of the sodium pump by albumin. 10 pm PMA, 0.1 μm wortmannin, 0.1 μm calphostin C, 1 μm Bt2cAMP, or 1 μm forskolin was added when indicated (n = 5). *, statistically significant in relation to the control (in the absence of albumin) or to #, 0.01 mg/ml albumin (p < 0.05).
DISCUSSION
In general, the focus of research on the effects of albumin has turned to the action of the higher albumin concentration found in renal diseases (2, 6, 17, 18). However, our results reveal an important role of albumin in PT sodium reabsorption. At physiological concentrations, albumin increases (Na+ + K+)-ATPase activity by increasing α1 subunits. This effect of lower albumin concentration on PT (Na+ + K+)-ATPase activity agrees with the high level of sodium reabsorption in this segment. Albumin could be responsible for maintaining a high level of expression and activity of PT (Na+ + K+)-ATPase, contributing to a high level of PT sodium reabsorption in physiological conditions (3). These results indicate that the action of albumin in physiological conditions is more important for PT function than has been supposed and opens new avenues to understanding the role of albumin in PT cell function in both physiological and pathological conditions.
One question that arises from our results is what the sensor is that is involved in the effect of albumin on the PT (Na+ + K+)-ATPase activity. One possible candidate is megalin, an albumin receptor, located on the luminal side of PT cells and LLC-PK1 cells (1). This idea is supported by the observation that albumin on the basolateral side did not change the PT (Na+ + K+)-ATPase activity. In addition, a correlation between albumin concentration and megalin expression has been shown; lower albumin concentrations increase megalin expression, and higher albumin concentrations decrease it (8). These results indicate that there is a correlation between megalin expression and PT (Na+ + K+)-ATPase activity.
Because megalin is a scavenger receptor belonging to the low density lipoprotein receptor family (1), it could be expected that other proteins that bind to megalin could trigger similar effects. However, we observed that transferrin, a constituent of nephrotic urine, did not change the (Na+ + K+)-ATPase activity, indicating that this effect is specific to albumin. Similar specificity to albumin was observed by Tang et al. (19) by measuring IL-8 secretion in PT endothelial cells. They showed that transferrin had only a small effect compared with albumin and IgG had no effect. This specificity indicates that albumin binds to specific domains of the megalin, triggering a specific cellular response.
Recently, a debate about the amount of albumin filtered has emerged. Russo et al. (20), using two-photon microscopy, showed in male normal Munich-Wistar rats that the glomerular sieving coefficient (GSC) for albumin is 0.02–0.04, which is about 50 times higher than has been proposed from micropuncture studies (GSC = 0.0006) (21). In this model, it was proposed that albumin filtration only involves size selectivity. Most of the albumin filtered is reabsorbed by the retrieval pathway that returns this albumin to the peritubular blood supply. Assuming that the GSC for albumin is higher, the concentration of albumin in the lumen of the PT could range from 0.8 to 2.0 mg/ml. If this observation is true, why does the high albumin filtration not cause toxic effects in PT cells? In general, it has been shown that only albumin concentrations higher than 10 mg/ml have a toxic effect on PT cells. In a previous work, we showed that only albumin concentrations of 10–20 mg/ml induce apoptosis in LLC-PK1 cells (8). Then, even though the GSC for albumin is higher than has been proposed, the albumin concentration in the lumen of the PT does not reach the concentration that causes toxic effects on these cells (17, 21–23). In the present work, we showed that albumin concentrations ranging from 0.001 up to 1.0 mg/ml increased (Na+ + K+)-ATPase activity. These data indicate that the significance of our results does not change even though the level of albumin filtration is higher than has been proposed. Regardless of the amount of albumin filtered, the involvement of megalin in albumin reabsorption in PT cells is well documented and accepted (1). Thus, even in the presence of a large amount of filtered albumin, the role of the albumin-megalin interaction in the function of PT cells or in tubule-interstitial injury should not be ruled out. In agreement with this hypothesis, several studies have correlated albuminuria with tubule-interstitial injury and, consequently, progression of renal disease (16, 17).
Several lines of evidence suggest that the albumin-megalin interaction has signaling functions beyond its role in albumin endocytosis (2, 6, 17). It was shown that cellular responses such as activation of PKB, JAK/STAT, NF-κB, and MAPK pathways are triggered by interaction between albumin and megalin (8, 17, 22–24). In the present work, we showed that PI3K/PKB activation is involved in the effect of lower albumin concentrations on PT (Na+ + K+)-ATPase activity. This result agrees with that observed by Brunskill et al. (25) in OK cells and by our group in LLC-PK1 cells (8). In a previous paper, we observed that the activation of this pathway is involved in the effect of albumin on the cell survival (8). In this context, it is plausible to postulate that the final effect of albumin depends on the PI3K/PKB downstream pathway.
Our data indicate clearly that the activation of the PI3K/PKB pathway leads to PKC activation and PKA inhibition. The PI3K-dependent PKC activation was also observed in muscle of rats fed a high fat diet (26). The impairment of PI3K was associated with a malfunction in an atypical PKC type (26). Similar correlation between PI3K/PKB and PKC pathways was observed in B cell chronic lymphocytic leukemia cells (27). However, the molecular mechanism involved in the activation of PKC by the PI3K/PKB pathway has not yet been determined. One possibility could be the involvement of phosphoinositide-dependent kinase 1, which anchors to PI3K-formed in D3-phosphorylated phospholipids and phosphorylates threonine residues in the activation segment of both PKC and PKB (15). On the other hand, the observation that PKB inhibitor and wortmannin abolished albumin-induced PKC activation suggests that PKB could promote direct phosphorylation of PKC. This hypothesis agrees with the observation that PKB coprecipitated with PKC in BT-549 breast cancer (28, 29) and CHO cells (27, 28).
Lee and Han (24) showed that a higher albumin concentration (3–20 mg/ml) activates Ca2+-dependent PKC, which phosphorylates the epidermal growth factor receptor in primary rabbit PT cells leading to cell growth. However, Li et al. (30) showed that albumin (10–40 mg/ml) activates PKCδ, promoting tubular cell injury and death during albuminuria in a rat proximal tubular cell line. Here, we observed that a higher albumin concentration (10–20 mg/ml) did not change PKC activity compared with controls in the absence of albumin. These apparent contradictory effects of albumin could be explained by activation of specific protein PKC types. In support of this hypothesis, it was observed that PKCϵ acts as a negative modulator of growth factor-induced activation of PKB in CHO cells (31), and a positive cooperation between PKCθ and PKB in the NF-κB transactivation cascade was observed in Jurkat T cells (32).
Little is known about the interaction between PKB and PKC to modulate PT function. PKC activator (PMA) increases ouabain-sensitive rubidium uptake in well oxygenated rat PT (4). Carranza et al. (33) have shown that insulin activates PKCζ and PKB downstream of PI3K and that these pathways contribute to the increase in l-dopa uptake into PT cells. Similarly, it was shown that C peptide increases PKB, PKCϵ, and PKCδ, which could mediate the activation of PT (Na+ + K+)-ATPase activity (34). However, no interdependent activation between PKB and PKC was observed. Our data suggest a sequential activation of PKB and PKC pathways and a PKB-dependent PKC effect on (Na+ + K+)-ATPase expression. The observation that PKC and PI3K inhibitors did not change (Na+ + K+)-ATPase activity in the absence of albumin indicates that these pathways did not modify the basal expression of the enzyme and they are specifically mediating the albumin effect on the expression of the sodium pump.
The albumin-induced activation of the PI3K/PKB/PKC pathway inhibits PKA activity. The significance of this inhibition in the activation of (Na+ + K+)-ATPase activity is not completely clear. We observed that addition of cell-permeant BT2cAMP or forskolin inhibited the enzyme activity. Similarly, Zhang and Yuan (35) showed that D1-mediated dopamine inhibition of PT (Na+ + K+)-ATPase activity involves an increase in intracellular cAMP level. Based on these observations, we postulate that blocking the inhibitory effect of the cAMP/PKA pathway by activation of the PI3K/PKB and PKC pathways is crucial to enhance (Na+ + K+)-ATPase activity. This hypothesis is supported by the observation that (Na+ + K+)-ATPase activity is decreased and PKA activity is increased in LLC-PK1 cells in the simultaneous presence of PKC inhibitor and lower albumin concentration. The inhibition of the cAMP/PKA pathway probably occurs one step before the activation of PKA by cAMP because the addition of cell-permeant Bt2cAMP abolished the stimulatory effect of albumin on (Na+ + K+)-ATPase activity. In agreement with this idea, it was observed that PKC inhibits adenylyl cyclase through its phosphorylation (36).
In summary, we postulate that albumin is a messenger molecule modulating PT sodium reabsorption. The key element between albumin concentration and PT sodium reabsorption could be megalin that senses albumin concentration triggering intracellular signaling. The final effect depends on the intracellular balance between the PI3K/PKB/PKC and cAMP/PKA pathways. In a previous work we showed that PKB anchors to megalin at the luminal membrane in a PI3K-independent manner (8). However, albumin-induced PKB activation depends on previous PI3K activation (8). Albumin at lower concentrations induces the increase in PI3K activity, PKB phosphorylation, activation, and its dissociation from megalin. The increase in PI3K/PKB leads to activation of PKC and a decrease in PKA activity. The consequence of this process is to increase α1 subunit expression and (Na+ + K+)-ATPase activity. On the other hand, a decrease in megalin expression leads to shut down of the PI3K/PKB/PKC pathway and activation of PKA, promoting the decrease in (Na+ + K+)-ATPase expression and activity similar to that found in patients with chronic kidney disease (37, 38).
Acknowledgments
We thank Shanserley Leite do Espírito Santo and Mario Luiz da Silva Bandeira (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro technical training fellowship) for technical support.
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Instituto Nacional de Ciência e Tecnologia para Pesquisa Translacional em Saúde e Ambiente na Região Amazônica (INPeTAm/CNPq), and Instituto Nacional de Ciência e Tecnologia em Biologia Estrutural e Bioimagem (INBEB/CNPq).
- PT
- proximal tubule
- Bt2cAMP
- dibutyryl cyclic AMP
- GSC
- glomerular sieving coefficient
- PMA
- phorbol 12-myristate 13-acetate.
REFERENCES
- 1. Christensen E. I., Verroust P. J., Nielsen R. (2009) Pflugers Arch. 458, 1039–1048 [DOI] [PubMed] [Google Scholar]
- 2. Baines R. J., Brunskill N. J. (2008) Nephron Exp. Nephrol. 110, 67–71 [DOI] [PubMed] [Google Scholar]
- 3. O'Shaughnessy K. M., Karet F. E. (2006) Annu. Rev. Nutr. 26, 343–365 [DOI] [PubMed] [Google Scholar]
- 4. Féraille E., Doucet A. (2001) Physiol. Rev. 81, 345–418 [DOI] [PubMed] [Google Scholar]
- 5. Poulsen H., Morth P., Egebjerg J., Nissen P. (2010) FEBS Lett. 584, 2589–2595 [DOI] [PubMed] [Google Scholar]
- 6. Theilig F. (2010) Ann. Anat. 192, 125–132 [DOI] [PubMed] [Google Scholar]
- 7. Yuseff M. I., Farfan P., Bu G., Marzolo M. P. (2007) Traffic 8, 1215–1230 [DOI] [PubMed] [Google Scholar]
- 8. Caruso-Neves C., Pinheiro A. A., Cai H., Souza-Menezes J., Guggino W. B. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 18810–18815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maia J. C., Gomes S. L., Juliani M. H. (1993) Genes of Antigens of Parasites: A Laboratory Manual, pp. 144–157, Fundação Oswaldo Cruz, Rio de Janeiro [Google Scholar]
- 10. Landgraf S. S., Wengert M., Silva J. S., Zapata-Sudo G., Sudo R. T., Takiya C. M., Pinheiro A. A., Caruso-Neves C. (2011) Am. J. Physiol. Renal Physiol. 300, F499–510 [DOI] [PubMed] [Google Scholar]
- 11. Caruso-Neves C., Kwon S. H., Guggino W. B. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 17513–17518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pierre S. V., Belliard A., Sottejeau Y. (2011) Am. J. Physiol. Cell Physiol. 300, C42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grubmeyer C., Penefsky H. S. (1981) J. Biol. Chem. 256, 3718–3727 [PubMed] [Google Scholar]
- 14. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 15. Pearce L. R., Komander D., Alessi D. R. (2010) Nat. Rev. Mol. Cell Biol. 11, 9–22 [DOI] [PubMed] [Google Scholar]
- 16. Eddy A. A. (1989) Am. J. Pathol 135, 719–733 [PMC free article] [PubMed] [Google Scholar]
- 17. Abbate M., Zoja C., Remuzzi G. (2006) J. Am. Soc. Nephrol. 17, 2974–2984 [DOI] [PubMed] [Google Scholar]
- 18. National Kidney Foundation (2002) Am. J. Kidney Dis. 39, S1–266 [PubMed] [Google Scholar]
- 19. Tang S., Leung J. C., Abe K., Chan K. W., Chan L. Y., Chan T. M., Lai K. N. (2003) J. Clin. Invest. 111, 515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Russo L. M., Sandoval R. M., McKee M., Osicka T. M., Collins A. B., Brown D., Molitoris B. A., Comper W. D. (2007) Kidney Int. 71, 504–513 [DOI] [PubMed] [Google Scholar]
- 21. Gekle M. (2007) Kidney Int. 71, 419–481 [DOI] [PubMed] [Google Scholar]
- 22. Nakajima H., Takenaka M., Kaimori J. Y., Hamano T., Iwatani H., Sugaya T., Ito T., Hori M., Imai E. (2004) J. Am. Soc. Nephrol. 15, 276–285 [DOI] [PubMed] [Google Scholar]
- 23. Morigi M., Macconi D., Zoja C., Donadelli R., Buelli S., Zanchi C., Ghilardi M., Remuzzi G. (2002) J. Am. Soc. Nephrol. 13, 1179–1189 [PubMed] [Google Scholar]
- 24. Lee Y. J., Han H. J. (2008) Am. J. Physiol. Renal Physiol. 294, F534–541 [DOI] [PubMed] [Google Scholar]
- 25. Brunskill N. J., Stuart J., Tobin A. B., Walls J., Nahorski S. (1998) J. Clin. Invest. 101, 2140–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tremblay F., Lavigne C., Jacques H., Marette A. (2001) Diabetes 50, 1901–1910 [DOI] [PubMed] [Google Scholar]
- 27. Barragán M., de Frias M., Iglesias-Serret D., Campàs C., Castaño E., Santidrián A. F., Coll-Mulet L., Cosialls A. M., Domingo A., Pons G., Gil J. (2006) J. Leukoc. Biol. 80, 1473–1479 [DOI] [PubMed] [Google Scholar]
- 28. Doornbos R. P., Theelen M., van der Hoeven P. C., van Blitterswijk W. J., Verkleij A. J., van Bergen en Henegouwen P. M. (1999) J. Biol. Chem. 274, 8589–8596 [DOI] [PubMed] [Google Scholar]
- 29. Mao M., Fang X., Lu Y., Lapushin R., Bast R. C., Jr., Mills G. B. (2000) Biochem. J. 352, 475–482 [PMC free article] [PubMed] [Google Scholar]
- 30. Li X., Pabla N., Wei Q., Dong G., Messing R. O., Wang C. Y., Dong Z. (2010) J. Am. Soc. Nephrol. 21, 1115–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsumoto M., Ogawa W., Hino Y., Furukawa K., Ono Y., Takahashi M., Ohba M., Kuroki T., Kasuga M. (2001) J. Biol. Chem. 276, 14400–14406 [DOI] [PubMed] [Google Scholar]
- 32. Bauer B., Krumböck N., Fresser F., Hochholdinger F., Spitaler M., Simm A., Uberall F., Schraven B., Baier G. (2001) J. Biol. Chem. 276, 31627–31634 [DOI] [PubMed] [Google Scholar]
- 33. Carranza A., Musolino P. L., Villar M., Nowicki S. (2008) Am. J. Physiol. Cell Physiol. 295, C1602–1609 [DOI] [PubMed] [Google Scholar]
- 34. Zhong Z., Davidescu A., Ehrén I., Ekberg K., Jörnvall H., Wahren J., Chibalin A. V. (2005) Diabetologia 48, 187–197 [DOI] [PubMed] [Google Scholar]
- 35. Zhang Y. R., Yuan Z. Y. (2010) Clin. Exp. Pharmacol. Physiol. 37, 613–618 [DOI] [PubMed] [Google Scholar]
- 36. Beazely M. A., Watts V. J. (2006) Eur. J. Pharmacol. 535, 1–12 [DOI] [PubMed] [Google Scholar]
- 37. Vlachojannis J., Tsakas S., Petropoulou C., Kurz P. (1997) Nephrol. Dial. Transplant. 12, 470–473 [DOI] [PubMed] [Google Scholar]
- 38. Cianciaruso B., Bellizzi V., Minutolo R., Colucci G., Bisesti V., Russo D., Conte G., De Nicola L. (1996) J. Am. Soc. Nephrol. 7, 306–313 [DOI] [PubMed] [Google Scholar]