Background: Antimicrobial peptide LL-37 activates leukocytes, mast cells, and endothelial cells.
Results: LL-37 causes chemotaxis, degranulation, and chemokine production in human mast cells expressing MrgX2, but it does not induce receptor desensitization.
Conclusion: MrgX2 is a novel receptor for LL-37 in human mast cells, and this receptor is resistant to regulation.
Significance: This study has important implications for innate immunity and inflammation.
Keywords: Antimicrobial Peptides, Calcium Intracellular Release, Cellular Immune Response, G Protein-coupled Receptors (GPCR), G Proteins, Inflammation, Mast Cell, Receptor Regulation, Signal Transduction
Abstract
Human LL-37 is a multifunctional antimicrobial peptide that promotes inflammation, angiogenesis, wound healing, and tumor metastasis. Most effects of LL-37 are mediated via the activation of the cell surface G protein-coupled receptor FPR2 on leukocytes and endothelial cells. Although LL-37 induces chemotaxis, degranulation, and chemokine production in mast cells, the receptor involved and the mechanism of its regulation remain unknown. MrgX2 is a member of Mas-related genes that is primarily expressed in human dorsal root ganglia and mast cells. We found that a human mast cell line LAD2 and CD34+ cell-derived primary mast cells, which natively express MrgX2, responded to LL-37 for sustained Ca2+ mobilization and substantial degranulation. However, an immature human mast cell line, HMC-1, that lacks functional MrgX2 did not respond to LL-37. shRNA-mediated knockdown of MrgX2 in LAD2 mast cell line and primary CD34+ cell-derived mast cells caused a substantial reduction in LL-37-induced degranulation. Furthermore, mast cell lines stably expressing MrgX2 responded to LL-37 for chemotaxis, degranulation, and CCL4 production. Surprisingly, MrgX2 was resistant to LL-37-induced phosphorylation, desensitization, and internalization. In addition, shRNA-mediated knockdown of the G protein-coupled receptor kinases (GRK2 and GRK3) had no effect on LL-37-induced mast cell degranulation. This study identified MrgX2 as a novel G protein-coupled receptor for the antibacterial peptide LL-37 and demonstrated that unlike most G protein-coupled receptors it is resistant to agonist-induced receptor phosphorylation, desensitization, and internalization.
Introduction
Antimicrobial peptides such as defensins and cathelicidins are secreted by activated epithelial cells as well as by invading leukocytes and play an important role in host defense (1). Cathelicidins consist of a putative N-terminal signal peptide, a highly conserved cathelin-like domain, and a C-terminal antimicrobial domain corresponding to the mature antibacterial peptide. About 30 cathelicidin members have been identified in mammals. However, only one cathelicidin, hCAP18 (human cationic antibacterial protein of 18 kDa), has been found in humans thus far, and its C-terminal mature antibacterial peptide (LL-37), comprising 37 amino acid residues, has direct antibacterial effects against Gram-positive and Gram-negative bacteria (2). In addition, LL-37 displays immunomodulatory properties via the recruitment of monocytes and T cells (3, 4). LL-37 stimulates angiogenesis to promote wound healing and tumor invasiveness (5–8). Most effects of LL-37 appear to be mediated via the activation of G protein-coupled formyl peptide receptor 2 (FPR2; earlier known as FPRL1) on monocytes, T cells, and endothelial cells (3, 8–11). LL-37 also activates chemokine receptor CXCR2 in human neutrophils (12), purinergic receptor P2X7 in fibroblasts (13), and insulin growth factor receptors in epithelial cells (14).
Mast cells are known to play a critical role in innate immunity, and this function requires mast cell degranulation and subsequent neutrophil recruitment (15, 16). LL-37 induces Ca2+ mobilization, chemotaxis, and degranulation in rat peritoneal mast cells (17, 18). It also causes increased vascular permeability in wild-type but not in mast cell-deficient rats (19). Thus, LL-37-induced mast cell activation could contribute to the innate immune function of mast cells. LL-37 is also thought to be involved in chronic inflammatory diseases. The level of LL-37 in human skin increases dramatically from 1 μm in normal individuals to ∼304 μm in psoriatic patients (20, 21). LL-37 induces degranulation and pruritogenic cytokine generation in human mast cells (22, 23). Although LL-37 activates mast cells via pertussis toxin-sensitive G protein and phospholipase C-mediated signaling pathway (18), the GPCRs2 it utilizes have not been determined.
A large family of GPCRs called Mas-related genes (Mrgs, also known as sensory neuron-specific receptors) has recently been identified in rodents (24, 25). These receptors are selectively expressed in small diameter sensory neurons of dorsal root ganglia and are thought to be involved in the sensation and modulation of pain. Interestingly, a subgroup of these receptors (MrgX1–MrgX4) are expressed in human but not in murine neurons (24, 26). Recent studies have shown that MrgX2 is also expressed in human mast cells and activated by basics peptides (27, 28). Given that LL-37 also displays basic properties, we hypothesized that it activates human mast cells via MrgX2. Using shRNA-mediated knockdown of MrgX2 in human mast cells, we provide the novel finding that LL-37 causes sustained Ca2+ mobilization and degranulation via MrgX2. Moreover, using transfected mast cell lines that do not natively express MrgX2 (27), we demonstrate that LL-37 promotes chemotaxis and induces chemokine, CCL4 via MrgX2.
Agonist-occupied GPCRs are phosphorylated by a family of protein kinases, collectively known as G protein-coupled receptor kinases (GRKs) (29). Of the seven known GRKs, four (GRK2, GRK3, GRK5, and GRK6) are expressed ubiquitously. It is well established that GPCR phosphorylation by GRKs leads to the recruitment of β-arrestin, which results in receptor desensitization and internalization (29). A goal of this study was to determine the role of agonist-induced receptor phosphorylation on the regulation of MrgX2. Our studies clearly demonstrate that unlike most other known GPCRs, MrgX2 is resistant to LL-37-induced receptor phosphorylation, desensitization, and internalization. It is noteworthy that LL-37-induced mast cell degranulation leads to the release of tryptase, which degrades LL-37, rendering it inactive (23). Thus, in the absence of MrgX2 receptor desensitization, enhanced mast cell degranulation likely provides a novel feedback mechanism to regulate receptor function by limiting ligand availability for the receptor.
EXPERIMENTAL PROCEDURES
Materials
Frozen human G-CSF-mobilized peripheral blood CD34+ progenitors were obtained from The Fred Hutchinson Cancer Center (Seattle, WA). All cell culture reagents and pertussis toxin were purchased from Invitrogen. Amaxa cell transfection kits and reagents were purchased from Lonza (Gaithersburg, MD). Anti-HA antibody (12CA5) and anti-HA (HA-7)-agarose beads were purchased from Roche Applied Science and Sigma, respectively. All recombinant human cytokines were purchased from PeproTech (Rocky Hill, NJ). Phorbol 12-myristate 13-acetate (PMA) was purchased from Calbiochem. Anti-human C3a receptor antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phycoerythrin (PE)-labeled donkey anti-mouse IgG was purchased from eBioscience (San Diego). Bovine adrenal medulla docosapeptide (BAM-22P) and cortistatin-14 (CST) were obtained from American Peptide (Vista, CA). Native complement C3a was from Complement Technology (Tyler, TX). LL-37 (Leu-Leu-Gly-Asp-Phe-Phe-Arg-Lys-Ser-Lys-Glu-Lys-Ile-Gly-Lys-Glu-Phe-Lys-Arg-Ile-Val-Gln-Arg-Ile-Lys-Asp-Phe-Leu-Arg-Asn-Leu-Val-Pro-Arg-Thr-Glu-Ser) was from Anaspec (Freemont, CA).
Differentiation of Human Mast Cells from CD34+ Progenitors and Culture of Human Mast Cell Lines
To obtain primary mast cells, human CD34+ progenitors were cultured in StemPro-34 medium supplemented with l-glutamine (2 mm), penicillin (100 IU/ml), streptomycin (100 μg/ml), rhSCF (100 ng/ml), rhIL-6 (100 ng/ml), and rhIL-3 (30 ng/ml) (1st week only). Hemidepletions were performed weekly with media containing rhSCF (100 ng/ml) and rhIL-6 (100 ng/ml) (30). Cells were used for experiments after 7–10 weeks in culture. HMC-1 cells were cultured in Iscove's modified Dulbecco's medium supplemented with 10% FCS, glutamine (2 mm), penicillin (100 IU/ml), and streptomycin (100 μg/ml) (31). LAD2 cells were maintained in complete StemPro-34 medium supplemented with 100 ng/ml rhSCF (32). RBL-2H3 and HEK-293T cells were maintained as monolayer cultures in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, l-glutamine (2 mm), penicillin (100 IU/ml), and streptomycin (100 μg/ml) (33).
Lentivirus-mediated Knockdown of MrgX2, GRK2, and GKR3 in Human Mast Cells
MrgX2-targeted Mission shRNA lentiviral plasmids were purchased from Sigma. The clone that gave the highest knockdown efficiency (TRCN0000009174) was used. A scrambled control nontarget vector (SHC002), which does not bind to any known human mRNAs, was also purchased from Sigma. Lentivirus generation was performed according to the manufacturer's manual. Cell transduction was conducted by mixing 1.5 ml of viral supernatant with 3.5 ml of LAD2 (5 × 106 cells) or CD34+ mast cells (3 × 106 cells). Eight hours post-infection, medium was changed to virus-free complete medium, and antibiotic (puromycin, 2 μg/ml, Sigma) selection was initiated 16 h later. Knockdown of GRK2 and GRK3 in LAD2 cells was performed as described previously (34). Cells were analyzed for MrgX2, GRK2, or GRK3 knockdown and used for subsequent assays 4 days following initiation of puromycin selection.
Quantitative PCR
Total RNA from mast cells was extracted using TRIzol (Invitrogen), treated with DNase I, and reverse-transcribed to cDNA using first strand cDNA synthesis kit (GE Healthcare). Gene expression was analyzed using real time PCR with TaqMan® fast universal PCR master mix on a TaqMan 7500 fast real time PCR system (Applied Biosystems, Foster City, CA). TaqMan probes for hGAPDH, hMrgX2, and hGRK3 were used for real time PCR to analyze the knockdown efficiency. The amplification conditions were as follows: initial denaturation at 95 °C for 20 s, followed by 40 cycles of amplification: 95 °C for 3 s and 60 °C for 30 s. Analysis was performed according to ΔΔCt method. The results are expressed as a ratio of MrgX2 or GRK3 to GAPDH (34, 35).
Western Blotting
Protein extracts of control and GRK2 knockdown LAD2 cells following lysis with RIPA buffer were separated by SDS-PAGE and transferred to Hybond ECL nitrocellulose membranes (GE Healthcare). Blots were incubated with anti-human GRK2 antibody (Santa Cruz Biotechnology) in blocking buffer (PBS, 0.5% Tween 20, 5% skim milk) followed by HRP-labeled goat anti-rabbit IgG (Thermo Scientific, 1:5000 in blocking buffer) secondary antibody. Bound antibody was detected using the SuperSignal® West Femto maximum sensitivity substrate kit (Thermo Scientific) according to the manufacturer's protocol.
Stable Transfection of RBL-2H3 and HMC-1 Cells
RBL-2H3 cells stably expressing MrgX1 and MrgX2 were generated as described previously (27, 36). For HMC-1 cells, 2 × 106 cells were transfected with plasmids encoding HA-tagged MrgX2 using the Amaxa nucleofector device and Amaxa kit V according to the manufacturer's protocol. Following nucleofection, cells were cultured in the presence of G418 (1 mg/ml), and cells expressing equivalent receptors were sorted using an anti-HA-specific antibody 12CA5/FITC-conjugated anti-mouse IgG and used for studies on Ca2+ mobilization, chemotaxis, receptor internalization, and CCL4 chemokine generation.
Calcium Mobilization
Ca2+ mobilization was determined as described previously (37, 38). Briefly, cells (human mast cells, 0.2 × 106) and (RBL-2H3 or HMC-1 cells, 1.0 × 106) were loaded with 1 μm indo-1 AM for 30 min at room temperature. Cells were washed and resuspended in 1.5 ml of HEPES-buffered saline. Ca2+ mobilization was measured in a Hitachi F-2500 spectrophotometer with an excitation wavelength of 355 nm and an emission wavelength of 410 nm (38).
Degranulation
Human mast cells (5 × 103) and RBL-2H3 cells (5 × 104) were seeded into 96-well plates in a total volume of 50 μl of HEPES buffer containing 0.1% BSA and exposed to different concentrations of peptides. In some assays, cells were pretreated with pertussis toxin (100 ng/ml; 16 h) or La3+ (lanthanum chloride, 1 μm; 5 min). For total β-hexosaminidase release, unstimulated cells were lysed in 50 μl of 0.1% Triton X-100. Aliquots (20 μl) of supernatants or cell lysates were incubated with 20 μl of 1 mm p-nitrophenyl-N-acetyl-β-d-glucosamine for 1.5 h at 37 °C. Reaction was stopped by adding 250 μl of a 0.1 m Na2CO3, 0.1 m NaHCO3 buffer, and absorbance was measured at 405 nm (33).
Chemotaxis Assay
LL-37 or buffer (30 μl) was added to the lower wells of a 96-well chemotaxis chamber (8-μm pore size; NeuroProbe, Gaithersburg, MD). Mock-transfected HMC-1 cells or cells expressing MrgX2 (0.5 × 106 in 50 μl of buffer) were placed on the upper chamber. After 3 h of incubation at 37°C, the migrated cells were collected from the lower chambers. Triplicate wells were pooled, and the cells were resuspended in 30 μl of complete Iscove's modified Dulbecco's medium. The cells were counted with a hemocytometer, and the results are expressed as absolute number of cells that had migrated.
Receptor Internalization
Cells (0.25 × 106) were exposed to buffer or different agonists for indicated time intervals at 37 °C. Cells were washed twice with ice-cold FACS buffer (PBS containing 2% FBS) and labeled with anti-HA antibody (12CA5) or isotype control and incubated on ice for 30 min. After washing twice with cold FACS buffer, cells were stained with PE-labeled anti-mouse IgG antibody on ice for 30 min. Cells were washed twice and fixed in 300 μl of 2% formaldehyde solution. The samples were acquired and analyzed using LSR II flow cytometer (BD Biosciences).
Chemokine CCL4 Generation
Chemokine release assay was performed as described previously (38). Briefly, mock- or MrgX2-transfected HMC-1 cells (0.2 × 106) were stimulated with indicated concentrations of LL-37 for 6 h. CCL4 chemokine levels in the supernatants were quantified by DuoSet ELISA kit from R&D Systems (Minneapolis, MN) according to the manufacturer's protocol.
Receptor Expression and Phosphorylation
Transient transfections were performed on 80% confluent HEK-293T monolayers in 60-mm dishes in 4 ml of OptiMEM® medium (Invitrogen) containing 1 μg of plasmid DNA (encoding hemagglutinin (HA)-tagged human MrgX2 and C3aR) and 7 μl of Lipofectamine reagent (Invitrogen). To detect receptor expression, cells (0.5 × 106) were incubated with anti-HA (12CA5) antibody followed by PE-conjugated anti-mouse IgG as the secondary antibody at 4 °C for 30 min and analyzed by flow cytometry.
Receptor phosphorylation experiments were performed via modification of procedures described previously (33, 39). Briefly, HEK-293T cells expressing HA-tagged human MrgX2 and C3aR were labeled with 0.15 mCi/ml [32P]orthophosphate for 90 min and stimulated with indicated agonists at 37 °C for 5 min. The reaction was stopped by adding 3 volumes of ice-cold PBS, and cells were lysed in immunoprecipitation buffer (50 mm Tris, pH 8.0, 150 mm NaCl, 1.0% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 5 mm EDTA, and protease and phosphatase inhibitors). The pre-cleared cell lysate was incubated with 15 μl of anti-HA-agarose beads for 2 h. Samples were washed three times with lysis buffer and resolved by 10% SDS-PAGE. The gels were dried, and 32P-incorporated protein bands were imaged with Kodak image film.
RESULTS
LL-37 Induces Degranulation in Human Mast Cells via Pertussis Toxin-sensitive G Protein-dependent and -independent Pathways
Previous studies demonstrated that LL-37 causes substantial degranulation in primary human lung mast cells in a dose-dependent manner within a concentration range of 1–10 μm (23, 40). As shown in Fig. 1A, we also found that LL-37 stimulates degranulation in LAD2 mast cells within the same concentration range. In rat peritoneal mast cells, LL-37 induces a sustained Ca2+ mobilization and degranulation, and both of these responses are inhibited by pertussis toxin (PTx), indicating the involvement of a Gi family of G proteins (18). However, the signaling pathway by which LL-37 induces degranulation in human mast cells is unknown. We therefore tested the effects of PTx on LL-37-induced Ca2+ mobilization and degranulation in LAD2 mast cells. We found that LL-37 caused sustained Ca2+ mobilization in LAD2 cells, but PTx had no effect on this response (Fig. 1B). By contrast, PTx caused substantial inhibition of LL-37-induced degranulation (Fig. 1C). La3+ has been shown to inhibit both Ca2+ influx and mast degranulation (41, 42). As shown in Fig. 1B, unlike PTx, La3+ (1 μm) almost completely inhibited LL-37-induced Ca2+ response, and this was associated with a substantial inhibition of LL-37-induced mast cell degranulation (Fig. 1D). These findings suggest that in contrast to the situation in rat peritoneal mast cells, LL-37 causes degranulation in human mast cells via the synergistic interaction of a Gαi-independent Ca2+ influx and another Gαi-mediated pathway (33).
FIGURE 1.
LL-37 stimulates degranulation and Ca2+ mobilization in LAD2 mast cells. A, LAD2 mast cells were stimulated with different concentrations of LL-37 (0.1–10 μm), and percent degranulation (β-hexosaminidase release) was determined. B, cells were treated with or without PTx (100 ng/ml, 16 h), loaded with Indo-1AM, and Ca2+ mobilization in response to LL-37 (1 μm) was determined. Indo-1-loaded cells were also exposed to La3+ (1 μm), and LL-37-induced Ca2+ mobilization was determined. C and D, LAD2 cells were pretreated with vehicle or PTx (100 ng/ml) (C) or La3+ (1 μm; 5 min) (D) and stimulated with different concentrations of LL-37 (1–10 μm), and percent degranulation was determined. Data are mean ± S.E. of three experiments. Statistical significance was determined by one-way ANOVA (A) or two-way ANOVA with Bonferroni's post test (C and D). ** indicates p < 0.001.
LL-37 Activates Human Mast Cells via MrgX2
LL-37 activates neutrophils, monocytes, eosinophils, and T cells via FPR2 (3, 9). Although mast cells express transcript for FPR2, the effects of LL-37 do not appear to be mediated via this receptor (17, 40). It is noteworthy that mast cells are the only known cells outside the dorsal ganglia that express MrgX2 (28). Furthermore, this receptor is activated by basic peptides (27, 28) Given that LL-37 displays basic properties, we hypothesized that it could activate mast cells via MrgX2. Although LAD2 cells endogenously express MrgX2, an immature human mast cell line, HMC-1 cells do not express functional receptors (27). By contrast both cell lines express GPCR for C3a (C3aR) (43). We therefore used these cell lines to determine whether there was a correlation between MrgX2 expression and responsiveness to LL-37. As shown in Fig. 2 (A and D), C3a caused transient Ca2+ mobilization in both LAD2 and HMC-1 cells. However, the known MrgX2 neuropeptide ligand CST and LL-37 caused sustained Ca2+ responses in LAD2 cells (Fig. 2, B and C) but not in HMC-1 cells (Fig. 2, E and F). These findings suggest that MrgX2 could serve as a receptor for LL-37 in LAD2 mast cells.
FIGURE 2.
Cortistatin and LL-37 induce Ca2+ mobilization in LAD2 mast cells but not in HMC-1 cells. LAD2 (A–C) and HMC-1 (D–F) cells were loaded with Indo-1AM and stimulated with C3a (10 nm; A and D), CST (1 μm) (B and E), or 10 μm LL-37 (C and F), and intracellular Ca2+ mobilization was determined. E and F, cells were also stimulated at 500 s with 10 nm C3a following initial stimulation. Data shown are representative of three similar experiments.
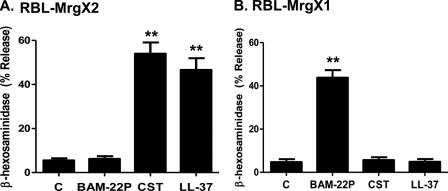
To confirm the role of MrgX2 on LL-37-induced mast cell responses in human mast cells, we generated stable transfectants expressing this receptor in HMC-1 cells. FACS analysis demonstrated cell surface expression of HA-tagged MrgX2 in receptor-transfected but not in mock-transfected cells (Fig. 3A). As shown in Fig. 3, B–D, LL-37 induced sustained Ca2+ mobilization, chemotaxis, and chemokine CCL4 in cells expressing MrgX2 but not in mock-transfected cells. Our next goal was to test the role of MrgX2 on LL-37-induced mast cell degranulation. Because HMC-1 cells do not have the capacity to undergo degranulation, we used rat basophilic leukemia cells, RBL-2H3, stably expressing human MrgX2 (27). In this system, both CST and LL-37 induced substantial mast cell degranulation (Fig. 4A). In addition to MrgX2, human mast cells express MrgX1 (27, 36). To determine the specificity of LL-37 for MrgX2, we also utilized RBL-2H3 cells stably expressing MrgX1 (36). As shown in Fig. 4B, cells expressing MrgX1 responded to its known ligand BAM-22P for degranulation, but they were resistant to LL-37. Moreover, cells expressing MrgX2 did not respond to BAM-22P. These findings support the notion that LL-37 utilizes human MrgX2 to activate mast cells.
FIGURE 3.
LL-37 induces Ca2+ mobilization, chemotaxis, and chemokine CCL4 generation in HMC-1 cells stably expressing MrgX2. A, representative histogram of expression level of HA-MrgX2 (MrgX2) in receptor-transfected (thick bold line) or mock-transfected control cells (dotted line) as analyzed by flow cytometry is shown. B, mock-transfected HMC-1 cells or cells expressing MrgX2 were loaded with Indo-1, and intracellular Ca2+ mobilization in response to LL-37 (3 μm) was determined. C and D, cells (as in B) were exposed to different concentrations of LL-37, and chemotaxis as well as chemokine CCL4 generation were determined. Data are means ± S.E. of three experiments. Statistical significance was determined by two-way ANOVA with Bonferroni's post test. * indicates p < 0.01, and ** indicates p < 0.001.
FIGURE 4.
LL-37 induces degranulation in RBL-2H3 cells stably expressing MrgX2 but not MrgX1. RBL-2H3 cells stably expressing MrgX2 (A) or MrgX1 (B) were stimulated with buffer (bar C), BAM-22P, cortistatin (CST) (both at 1 μm) or LL-37 (3 μm) for 30 min, and β-hexosaminidase release was measured. Data are mean ± S.E. of three experiments. Statistical significance was determined by one-way ANOVA with Bonferroni's post test. ** indicates p < 0.001.
To further confirm the role of MrgX2 on LL-37-induced mast cell degranulation, we used lentiviral Mission shRNA to knock down the expression of MrgX2 in LAD2 cells. Cells were transduced with five different shRNA constructs targeting different regions of MrgX2. For control, we used a scrambled shRNA construct. After transduction and selection with puromycin, quantitative PCR was performed to determine the extent of MrgX2 knockdown. We found that clone 4 (TRCN0000009174) was the most effective in knocking down MrgX2 expression. We therefore used this clone for subsequent studies. As shown in Fig. 5A, we were able to knock down the mRNA expression for MrgX2 by ∼60%. We could not determine MrgX2 protein expression due to the absence of a suitable antibody for Western blotting studies. When compared with shRNA control cells, knockdown of MrgX2 resulted in almost complete inhibition of LL-37-induced Ca2+ mobilization. This effect was specific for MrgX2 as silencing the expression of this receptor had little or no effect on C3a-induced Ca2+ response (Fig. 5, B and C). At a functional level, silencing MrgX2 expression caused substantial inhibition of LL-37 and CST but not C3a-induced mast cell degranulation (Fig. 5D).
FIGURE 5.
Knockdown of MrgX2 inhibits LL-37-induced Ca2+ mobilization and degranulation in LAD2 mast cells. LAD2 mast cells were stably transduced with scrambled shRNA control lentivirus or shRNA lentivirus targeted against MrgX2. A, quantitative PCR was performed to determine mRNA expression in control and MrgX2 knockdown (KD) cells. Results are expressed as a ratio of MrgX2 to GAPDH mRNA levels. B, shRNA control or C, MrgX2 KD cells were loaded with Indo-1 and stimulated with LL-37 (100 nm) at 100 s followed by a second stimulation of C3a (10 nm) at 300 s, and intracellular Ca2+ mobilization was determined. D, shRNA control and MrgX2 KD cells were stimulated with different concentrations of LL-37 (1–10 μm), CST (10 and 100 nm), or C3a (1 nm), and percent degranulation (β-hexosaminidase release) was determined. Data are mean ± S.E. of three experiments. Statistical significance was determined by t test (A) or two-way ANOVA with Bonferroni's post test (D). * indicates p < 0.01, and ** indicates p < 0.001.
MrgX2 Is Resistant to Agonist-induced Receptor Phosphorylation, Desensitization, and Internalization
C3aR is highly susceptible to agonist-induced GRK-mediated phosphorylation (39, 44). Although GRKs have no clear consensus sequence in their receptor substrates for phosphorylation, some prefer acidic residues, and others require basic residues in close proximity to serine/threonine residues (29). As shown in Fig. 6A, the C terminus of C3aR possesses 10 serine/threonine residues, whereas MrgX2 possesses only 5 residues. Furthermore, the MrgX2 C terminus contains fewer acidic and basic residues than C3aR. To compare agonist-induced phosphorylation of MrgX2 with C3aR, we generated transfectants expressing HA-tagged receptors in HEK-293T cells. Flow cytometry analysis using an antibody specific to the HA tag indicated that MrgX2 is expressed at a higher level than C3aR (Fig. 6, B and C). 32P-Labeled cells were stimulated with the appropriate ligand; C3a and CST or LL-37 and receptor phosphorylations were determined by immunoprecipitation followed by autoradiography. As expected, C3a caused robust phosphorylation of its receptor (Fig. 6D). Despite the fact that MrgX2 was expressed at a higher level than C3aR, MrgX2 was relatively resistant to CST and LL-37 for phosphorylation (Fig. 6D).
FIGURE 6.
MrgX2 is resistant to agonist-induced phosphorylation. A, sequence comparisons of the C termini of C3aR and MrgX2 is shown. B and C, HEK-293T cells transiently expressing HA-C3aR or HA-MrgX2 were incubated with anti-HA antibody followed by PE-conjugated anti-mouse IgG. Representative histograms of expression levels of C3aR (B) and MrgX2 (C) (thick bold lines) in receptor-transfected and mock-transfected cells (dotted lines) are shown. D, HEK-293T cells expressing C3aR were labeled with 32P and stimulated with C3a (100 nm), and C3aR phosphorylation was determined. Similarly, cells expressing MrgX2 were stimulated with CST (100 nm) or LL-37 (10 μm) for 5 min, and receptor phosphorylation was determined. An autoradiograph from a 3-h exposure is shown. Data shown are representative of three independent experiments.
Using intracellular Ca2+ mobilization as an assay, we have previously shown that C3aR and platelet-activating factor receptors are highly susceptible to desensitization (33, 45, 46). Thus, although the wild-type receptors expressed in RBL-2H3 cells respond to ligand for transient Ca2+ mobilization, cells expressing phosphorylation-deficient receptors respond to ligand for sustained Ca2+ response. We have recently shown that RBL-2H3 cells expressing wild-type MrgX2 respond to its ligands for sustained Ca2+ mobilization (27). Furthermore, unlike the situation for C3a, both LL-37 and CST induce sustained Ca2+ responses in LAD2 mast cells (Fig. 2, A–C). These findings suggest that, consistent with the relative resistance of MrgX2 to undergo phosphorylation (Fig. 6D), it does not undergo desensitization.
We performed additional sets of experiments to assess desensitization of MrgX2. In each case, we used C3aR for control. In the absence of added extracellular Ca2+, C3a and LL-37 induced transient Ca2+ responses that returned to basal levels within ∼1 min (Fig. 7, A and B). Adding back Ca2+ (1 mm) at 5 min after the initial stimulation with C3a resulted only in a marginal increase in intracellular Ca2+ (Fig. 7A). However, for LL-37, subsequent addition of Ca2+ resulted in substantial increase in Ca2+ mobilization (Fig. 7B).
FIGURE 7.
MrgX2 is resistant to desensitization and internalization. A and B, LAD2 cells were incubated with Indo-1AM, washed in a Ca2+-free buffer, and stimulated with C3a (10 nm) (A) and LL-37 (3 μm) (B), and Ca2+ mobilization in the absence of extracellular Ca2+ was determined. After 5 min, cells were exposed to Ca2+ at a final concentration of 1 mm, and intracellular Ca2+ mobilization was again determined. Traces represent results from three similar experiments. C, MrgX2-expressing HMC-1 cells were exposed to C3a (100 nm) for different time periods, and cell surface C3aR expression was determined by flow cytometry. MrgX2-expressing HMC-1 cells were exposed to LL-37 (1 μm) (D) or PMA (50 ng/ml) (E) for different time periods, and MrgX2 expression levels were determined by flow cytometry using 12CA5 antibody. Data are represented as percent receptor surface expression levels of control. Data are mean ± S.E. of three experiments. Statistical significance was determined by one-way ANOVA with Bonferroni's post test. ** indicates p < 0.001.
Agonist-induced phosphorylation of most GPCRs is associated with their internalization (29). To determine whether MrgX2 is internalized following LL-37 exposure, we utilized HMC-1 cells stably expressing HA-tagged MrgX2. Because these cells endogenously express C3aR, which is very sensitive to internalization (34), we used C3a as control. Consistent with phosphorylation, C3aR was internalized by ∼75% upon stimulation with C3a for 1–5 min (Fig. 7C). By contrast, LL-37 did not induce internalization of MrgX2 even after cells were exposed to the ligand for up to 30 min (Fig. 7D). To assess if MrgX2 receptor was internalized by heterologous mechanisms, we stimulated the cells with the protein kinase C activator PMA. As for LL-37, PMA did not promote MrgX2 internalization (Fig. 7E).
For most GPCRs, agonist-induced receptor phosphorylation and desensitization are mediated by GRKs (29). We have recently shown that LAD2 mast cells express GRK2 and GRK3 and that these protein kinases are involved in C3aR desensitization (34). We therefore generated LAD2 mast cells with stable knockdown of GRK2 and GRK3. As shown in Fig. 8A, lentiviral shRNA almost completely silenced GRK2 protein expression. Because we were unable to utilize any of the commercially available antibodies to assess GRK3 knockdown, we performed quantitative PCR. As shown in Fig. 8B, we were able to achieve ∼75% knockdown of GRK3 mRNA expression. Consistent with our previous findings (34), GRK2 and GRK3 knockdown enhanced C3a-induced mast cell degranulation (Fig. 8C). However, silencing the expression of these GRKs had no effect on CST or LL-37-induced degranulation (Fig. 8D). These findings clearly demonstrate that MrgX2 is resistant to agonist-induced receptor phosphorylation and desensitization.
FIGURE 8.
Knockdown of GRK2 or GRK3 has no effect on cortistatin and LL-37-induced degranulation in LAD2 mast cells. LAD2 mast cells were stably transduced with scrambled shRNA control lentivirus or shRNA lentivirus targeted against GRK2 or GRK3. A, representative immunoblot of LAD2 cells with GRK2 knockdown is shown. B, quantitative PCR was performed to assess GRK3 mRNA levels in shRNA control and GRK3 knockdown (KD) cells. Results are expressed as a ratio of GRK3 to GAPDH mRNA levels. C and D, control shRNA, GRK2, and GRK3 KD cells were stimulated with different concentrations of C3a (0.1 and 1 nm) (C) or LL-37 (1 and 3 μm) and CST (100 nm) (D), and percent degranulation was determined. Data are mean ± S.E. of three experiments. Statistical significance was determined by t test (B) and two-way ANOVA with Bonferroni's post test. * indicates p < 0.01 and ** indicates p < 0.001.
LL-37 Induces Signaling and Degranulation in Human CD34+ Cell-derived Mast Cells via MrgX2
All of the signaling and functional studies described above were performed with a human mast cell line that endogenously expresses MrgX2 as well as transfected HMC-1, RBL-2H3, and HEK-293T cells. To confirm the biological relevance of these studies, we repeated selected experiments in CD34+-derived primary human mast cells. We found that, as for LAD2 cells (Fig. 1A), LL-37 induced degranulation in CD34+-derived mast cells in the concentration range of 1–10 μm. However, the maximum response observed in CD34+-derived mast cells was ∼50% that found in LAD2 cells (compare Fig. 1A with 9A). Similar to the situation in LAD2 cells, LL-37 induced a transient Ca2+ response in primary mast cells in the absence of extracellular Ca2+, but restoration of extracellular Ca2+ resulted in a sustained intracellular Ca2+ response (compare Fig. 7B with 9B.). Most importantly, we were able to knock down the expression of MrgX2 in primary mast cells (Fig. 9C). Furthermore, silencing MrgX2 expression resulted in a substantial inhibition of LL-37-induced degranulation (Fig. 9D).
FIGURE 9.
LL-37 activates CD34+ cell-derived primary mast cells via MrgX2. A, CD34+ primary mast cells were stimulated with different concentrations of LL-37 (1–10 μm), and percent degranulation was determined. B, cells were incubated with Indo-1AM in Ca2+-free buffer and stimulated with 3 μm LL-37 at 100–200 s, and intracellular Ca2+ mobilization was determined. After 5 min, cells were exposed to 1 mm Ca2+, and intracellular Ca2+ mobilization was determined. C, mast cells were stably transduced with scrambled shRNA control lentivirus or shRNA lentivirus targeted against MrgX2. Quantitative PCR was performed to assess MrgX2 mRNA levels in shRNA control and MrgX2 knockdown (KD) cells. Results are expressed as a ratio of MrgX2 to GAPDH mRNA levels. D, control shRNA or MrgX2 KD cells were stimulated with different concentrations of LL-37 (3 and 10 μm), and percent degranulation was determined. Data are mean ± S.E. of three experiments. Statistical significance was determined by one-way ANOVA (A), t test (C), or two-way ANOVA with Bonferroni's post test (D). ** indicates p < 0.001.
DISCUSSION
Human LL-37 is a multifunctional antimicrobial peptide that promotes innate immunity, inflammation, angiogenesis, wound healing, and tumor metastasis. Although LL-37 mediates its responses in most immune cells and endothelial cells via FPR2, it activates CXCR2 in neutrophils, insulin growth factor receptor in gingival fibroblasts, and P2X7 in transfected HEK293 cells (8, 11–14, 47). LL-37 induces chemotaxis, degranulation and chemokine production in mast cells (17–19, 22, 23). Although much effort has been directed toward delineating the signaling pathways by which LL-37 activates mast cells, little progress has been made due to the lack of information regarding the receptor type involved (17–19, 22, 48). In this study, we have identified MrgX2 as a novel GPCR for LL-37 in human mast cells. We also demonstrated that unlike most GPCR, MrgX2 is resistant to agonist-induced phosphorylation, desensitization, and internalization. We propose a novel mechanism for the regulation of mast cell activation by LL-37.
Mrg receptors belong to the GPCR family, and they are also known as the sensory neuron-specific GPCRs. In humans, four MrgX genes, MrgX1–X4 are known (24, 26). Although originally thought to be specifically expressed in dorsal root ganglia, it now appears that human skin mast cells, cord blood-derived mast cells, CD34+ cell-derived mast cells, and a human mast cell line, LAD2, express MrgX2 (27, 28, 49). Most interestingly, this receptor is not present in human lymph nodes, spleen, or peripheral blood leukocytes (28). In fact, of the 42 human cell types tested, only mast cells express MrgX2 (28). LL-37 is an amphipathic peptide, and given the recent demonstrations that MrgX2 serves as a receptor for a variety of cationic peptides (27, 28), we hypothesized that it could serve as a receptor for LL-37 in human mast cells. Indeed, three lines of evidence clearly support this contention. First, LAD2 and CD34+ cell-derived human mast cells that endogenously express MrgX2 responded to LL-37 for Ca2+ mobilization and degranulation. Second, mast cell lines stably expressing MrgX2 responded to LL-37 for chemotaxis, degranulation, and chemokine production. Third, shRNA-mediated knockdown of MrgX2 in LAD2 cells and CD34+ cell-derived primary mast cells resulted in substantial decreases in LL-37-induced Ca2+ mobilization and degranulation.
Consistent with previous reports in rat peritoneal mast cells (18), we found that LL-37-induced degranulation in human mast cells is inhibited by PTx. However, an important difference was that although LL-37-induced Ca2+ influx in rat peritoneal mast cells was blocked by PTx (18), it had no effect on the Ca2+ response in human mast cells (Fig. 1). It is noteworthy that MrgX2 couples to the Gαq family of G proteins for Ca2+ mobilization in transfected HEK-293 cells (50). This raises the interesting possibility that, unlike the situation in rat mast cells, MrgX2 couples to Gαq to promote Ca2+ influx (inhibited by La3+) and that this response synergizes with PTx-sensitive signals, likely protein kinase C, to promote degranulation in human mast cells. The reason for the difference in specificity of LL-37 for G protein coupling between rat and human mast cells is unknown, but it could reflect the utilization of different GPCRs. It is noteworthy that unlike most GPCRs, Mrg receptors display substantial species-specific differences. Interestingly, human Mrg receptors share only 45–65% amino acid sequence identity with rat receptors. In addition, although there are only four Mrg genes known in humans, the rat genome possesses one each of the MrgA, MrgC, and MrgD genes and 10 MrgB genes (51). Rat peritoneal mast cells express a number of Mrg receptors, including MrgB1, MrgB2, MrgB3, MrgB6, MrgB8, and MrgB9 (28). Thus, although LL-37 couples to MrgX2 in human mast cells, it likely activates one or more of the Mrg receptors expressed in rat peritoneal mast cells to induce degranulation.
An important property of most GPCRs is that upon ligand stimulation they undergo GRK-mediated receptor phosphorylation, desensitization, and receptor internalization (52). Accordingly, the uridine nucleotide-activated P2Y6 receptor, which does not possess a consensus phosphorylation site for GRKs, is resistant to agonist-induced desensitization and internalization (53). Furthermore, PKC activation by PMA also has no effect on the regulation of P2Y6. In this study, we have demonstrated that MrgX2 is also resistant to agonist-induced receptor phosphorylation, desensitization, and internalization. We also showed that silencing of GRK2 and GRK3 had no effect on LL-37-induced mast cell degranulation. Furthermore, similar to the situation for the P2Y6 receptor (53), MrgX2 was resistant to internalization in response to PKC activation by PMA. These findings suggest that MrgX2 and P2Y6 are among only a few GPCRs, which are resistant to regulation by receptor phosphorylation. It is noteworthy that both MrgX2 and P2Y6 are expressed in human mast cells (27, 54). Furthermore, P2Y6 contributes to leukotriene-mediated mast cell activation and survival (54). Thus, the lack of desensitization of these receptors likely has important consequences for their biological functions in mast cells.
It is important to note that outside the dorsal root ganglia mast cells are the only cell type that expresses MrgX2 (28). Human peripheral blood leukocytes, thyroid, bone marrow cells, monocytes, T cells, fibroblasts, and epithelial cells have all been shown not to express MrgX2 (28). Many of these cells respond to LL-37 for chemotaxis and cytokine generation via the activation of GPCRs and growth factor receptors. Mast cells are unique among LL-37-responsive cells for two important reasons. First, they respond to LL-37 via MrgX2; second the resulting release of mast cell protease degrades and inactivates LL-37 (23). This raises the interesting possibility that protease released by LL-37-activated mast cells not only provides a negative feedback loop to control further mast cell activation but cross-regulates other effects of LL-37 such as angiogenesis, wound healing, tumor metastasis, and inflammation.
Acknowledgments
We are grateful to Dr. Joseph Butterfield (Mayo Clinic, Rochester, MN) for supplying us with HMC-1 cells. We also thank Drs. Arnold Kirshenbaum and Dean Metcalfe (NIAID, National Institutes of Health) for providing LAD2 mast cells and the FACS core facilities of the Schools of Medicine and Dental Medicine, University of Pennsylvania for acquisition, analysis, and cell sorting.
This work was supported, in whole or in part, by National Institutes of Health Grant HL085774.
- GPCR
- G protein-coupled receptor
- CST
- cortistatin-14
- Mrg
- Mas-related gene
- PTx
- pertussis toxin
- ANOVA
- analysis of variance
- rh
- recombinant human
- PMA
- phorbol 12-myristate 13-acetate
- PE
- phycoerythrin
- GRK
- G protein-coupled receptor kinase.
REFERENCES
- 1. Yang D., Chertov O., Oppenheim J. J. (2001) J. Leukocyte Biol. 69, 691–697 [PubMed] [Google Scholar]
- 2. Zanetti M. (2004) J. Leukocyte Biol. 75, 39–48 [DOI] [PubMed] [Google Scholar]
- 3. De Yang, Chen Q., Schmidt A. P., Anderson G. M., Wang J. M., Wooters J., Oppenheim J. J., Chertov O. (2000) J. Exp. Med. 192, 1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bowdish D. M., Davidson D. J., Hancock R. E. (2006) Curr. Top. Microbiol. Immunol. 306, 27–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koczulla R., von Degenfeld G., Kupatt C., Krötz F., Zahler S., Gloe T., Issbrücker K., Unterberger P., Zaiou M., Lebherz C., Karl A., Raake P., Pfosser A., Boekstegers P., Welsch U., Hiemstra P. S., Vogelmeier C., Gallo R. L., Clauss M., Bals R. (2003) J. Clin. Invest. 111, 1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDermott A. M. (2009) Ophthalmic Res. 41, 60–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coffelt S. B., Marini F. C., Watson K., Zwezdaryk K. J., Dembinski J. L., LaMarca H. L., Tomchuck S. L., Honer zu Bentrup K., Danka E. S., Henkle S. L., Scandurro A. B. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 3806–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coffelt S. B., Tomchuck S. L., Zwezdaryk K. J., Danka E. S., Scandurro A. B. (2009) Mol. Cancer Res. 7, 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tjabringa G. S., Ninaber D. K., Drijfhout J. W., Rabe K. F., Hiemstra P. S. (2006) Int. Arch. Allergy Immunol. 140, 103–112 [DOI] [PubMed] [Google Scholar]
- 10. Lee S. Y., Lee M. S., Lee H. Y., Kim S. D., Shim J. W., Jo S. H., Lee J. W., Kim J. Y., Choi Y. W., Baek S. H., Ryu S. H., Bae Y. S. (2008) FEBS Lett. 582, 273–278 [DOI] [PubMed] [Google Scholar]
- 11. Li Y., Cai L., Wang H., Wu P., Gu W., Chen Y., Hao H., Tang K., Yi P., Liu M., Miao S., Ye D. (2011) Oncogene 30, 3887–3899 [DOI] [PubMed] [Google Scholar]
- 12. Zhang Z., Cherryholmes G., Chang F., Rose D. M., Schraufstatter I., Shively J. E. (2009) Eur. J. Immunol. 39, 3181–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Montreekachon P., Chotjumlong P., Bolscher J. G., Nazmi K., Reutrakul V., Krisanaprakornkit S. (2011) J. Periodontal Res. 46, 327–337 [DOI] [PubMed] [Google Scholar]
- 14. Girnita A., Zheng H., Gronberg A., Girnita L., Stahle M. (2011) Oncogene, inn press [Google Scholar]
- 15. Stevens R. L., Adachi R. (2007) Immunol. Rev. 217, 155–167 [DOI] [PubMed] [Google Scholar]
- 16. Thakurdas S. M., Melicoff E., Sansores-Garcia L., Moreira D. C., Petrova Y., Stevens R. L., Adachi R. (2007) J. Biol. Chem. 282, 20809–20815 [DOI] [PubMed] [Google Scholar]
- 17. Niyonsaba F., Iwabuchi K., Someya A., Hirata M., Matsuda H., Ogawa H., Nagaoka I. (2002) Immunology 106, 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niyonsaba F., Someya A., Hirata M., Ogawa H., Nagaoka I. (2001) Eur. J. Immunol. 31, 1066–1075 [DOI] [PubMed] [Google Scholar]
- 19. Chen X., Niyonsaba F., Ushio H., Nagaoka I., Ikeda S., Okumura K., Ogawa H. (2006) J. Dermatol. Sci. 43, 63–66 [DOI] [PubMed] [Google Scholar]
- 20. Ong P. Y., Ohtake T., Brandt C., Strickland I., Boguniewicz M., Ganz T., Gallo R. L., Leung D. Y. (2002) N. Engl. J. Med. 347, 1151–1160 [DOI] [PubMed] [Google Scholar]
- 21. Murakami M., Ohtake T., Dorschner R. A., Schittek B., Garbe C., Gallo R. L. (2002) J. Invest. Dermatol. 119, 1090–1095 [DOI] [PubMed] [Google Scholar]
- 22. Niyonsaba F., Ushio H., Hara M., Yokoi H., Tominaga M., Takamori K., Kajiwara N., Saito H., Nagaoka I., Ogawa H., Okumura K. (2010) J. Immunol. 184, 3526–3534 [DOI] [PubMed] [Google Scholar]
- 23. Schiemann F., Brandt E., Gross R., Lindner B., Mittelstädt J., Sommerhoff C. P., Schulmistrat J., Petersen F. (2009) J. Immunol. 183, 2223–2231 [DOI] [PubMed] [Google Scholar]
- 24. Dong X., Han S., Zylka M. J., Simon M. I., Anderson D. J. (2001) Cell 106, 619–632 [DOI] [PubMed] [Google Scholar]
- 25. Lembo P. M., Grazzini E., Groblewski T., O'Donnell D., Roy M. O., Zhang J., Hoffert C., Cao J., Schmidt R., Pelletier M., Labarre M., Gosselin M., Fortin Y., Banville D., Shen S. H., Ström P., Payza K., Dray A., Walker P., Ahmad S. (2002) Nat. Neurosci. 5, 201–209 [DOI] [PubMed] [Google Scholar]
- 26. Burstein E. S., Ott T. R., Feddock M., Ma J. N., Fuhs S., Wong S., Schiffer H. H., Brann M. R., Nash N. R. (2006) Br. J. Pharmacol. 147, 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Subramanian H., Kashem S. W., Collington S. J., Qu H., Lambris J. D., Ali H. (2011) Mol. Pharmacol. 79, 1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tatemoto K., Nozaki Y., Tsuda R., Konno S., Tomura K., Furuno M., Ogasawara H., Edamura K., Takagi H., Iwamura H., Noguchi M., Naito T. (2006) Biochem. Biophys. Res. Commun. 349, 1322–1328 [DOI] [PubMed] [Google Scholar]
- 29. Pitcher J. A., Freedman N. J., Lefkowitz R. J. (1998) Annu. Rev. Biochem. 67, 653–692 [DOI] [PubMed] [Google Scholar]
- 30. Radinger M., Jensen B. M., Kuehn H. S., Kirshenbaum A., Gilfillan A. M. (2011) Curr. Protoc. Immunol. Chapter 7, Unit 7.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Butterfield J. H., Weiler D. A. (1989) Int. Arch. Allergy Appl. Immunol. 89, 297–300 [DOI] [PubMed] [Google Scholar]
- 32. Kirshenbaum A. S., Akin C., Wu Y., Rottem M., Goff J. P., Beaven M. A., Rao V. K., Metcalfe D. D. (2003) Leuk. Res. 27, 677–682 [DOI] [PubMed] [Google Scholar]
- 33. Ali H., Richardson R. M., Tomhave E. D., DuBose R. A., Haribabu B., Snyderman R. (1994) J. Biol. Chem. 269, 24557–24563 [PubMed] [Google Scholar]
- 34. Guo Q., Subramanian H., Gupta K., Ali H. (2011) PLoS One 6, e22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vibhuti A., Gupta K., Subramanian H., Guo Q., Ali H. (2011) PLoS One 6, e19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kashem S. W., Subramanian H., Collington S. J., Magotti P., Lambris J. D., Ali H. (2011) Eur. J. Pharmacol. 668, 299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ali H., Richardson R. M., Tomhave E. D., Didsbury J. R., Snyderman R. (1993) J. Biol. Chem. 268, 24247–24254 [PubMed] [Google Scholar]
- 38. Ali H., Ahamed J., Hernandez-Munain C., Baron J. L., Krangel M. S., Patel D. D. (2000) J. Immunol. 165, 7215–7223 [DOI] [PubMed] [Google Scholar]
- 39. Ahamed J., Haribabu B., Ali H. (2001) J. Immunol. 167, 3559–3563 [DOI] [PubMed] [Google Scholar]
- 40. Yoshioka M., Fukuishi N., Kubo Y., Yamanobe H., Ohsaki K., Kawasoe Y., Murata M., Ishizumi A., Nishii Y., Matsui N., Akagi M. (2008) Biol. Pharm. Bull. 31, 212–216 [DOI] [PubMed] [Google Scholar]
- 41. Chang W. C., Di Capite J., Singaravelu K., Nelson C., Halse V., Parekh A. B. (2008) J. Biol. Chem. 283, 4622–4631 [DOI] [PubMed] [Google Scholar]
- 42. Hide M., Beaven M. A. (1991) J. Biol. Chem. 266, 15221–15229 [PubMed] [Google Scholar]
- 43. Venkatesha R. T., Berla Thangam E., Zaidi A. K., Ali H. (2005) Mol. Immunol. 42, 581–587 [DOI] [PubMed] [Google Scholar]
- 44. Langkabel P., Zwirner J., Oppermann M. (1999) Eur. J. Immunol. 29, 3035–3046 [DOI] [PubMed] [Google Scholar]
- 45. Ahamed J., Ali H. (2002) J. Biol. Chem. 277, 22685–22691 [DOI] [PubMed] [Google Scholar]
- 46. Venkatesha R. T., Ahamed J., Nuesch C., Zaidi A. K., Ali H. (2004) J. Biol. Chem. 279, 44606–44612 [DOI] [PubMed] [Google Scholar]
- 47. Tomasinsig L., Pizzirani C., Skerlavaj B., Pellegatti P., Gulinelli S., Tossi A., Di Virgilio F., Zanetti M. (2008) J. Biol. Chem. 283, 30471–30481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Niyonsaba F., Hirata M., Ogawa H., Nagaoka I. (2003) Curr. Drug Targets Inflamm. Allergy 2, 224–231 [DOI] [PubMed] [Google Scholar]
- 49. Kajiwara N., Sasaki T., Bradding P., Cruse G., Sagara H., Ohmori K., Saito H., Ra C., Okayama Y. (2010) J. Allergy Clin. Immunol. 125, 1137–1145.e6 [DOI] [PubMed] [Google Scholar]
- 50. Robas N., Mead E., Fidock M. (2003) J. Biol. Chem. 278, 44400–44404 [DOI] [PubMed] [Google Scholar]
- 51. Zylka M. J., Dong X., Southwell A. L., Anderson D. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10043–10048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Krupnick J. G., Benovic J. L. (1998) Annu. Rev. Pharmacol. Toxicol. 38, 289–319 [DOI] [PubMed] [Google Scholar]
- 53. Brinson A. E., Harden T. K. (2001) J. Biol. Chem. 276, 11939–11948 [DOI] [PubMed] [Google Scholar]
- 54. Jiang Y., Borrelli L., Bacskai B. J., Kanaoka Y., Boyce J. A. (2009) J. Immunol. 182, 1129–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]