Background: PPARδ is a ligand-activated transcriptional factor that has been implicated in the vascular homeostasis.
Results: Activation of PPARδ significantly attenuated Ang II-induced senescence of VSMCs by up-regulation of PTEN and ensuing modulation of the PI3K/Akt signaling.
Conclusion: PPARδ inhibits Ang II-induced senescence of VSMCs via PTEN-mediated inhibition of ROS generation.
Significance: PPARδ provides a novel insight into the treatment of atherosclerotic vascular disease.
Keywords: Akt PKB, Gene Expression, Peroxisome Proliferator-activated Receptor (PPAR), Reactive Oxygen Species (ROS), Transcription Factors, Angiotensin II, Cellular Senescence, PTEN
Abstract
Cellular senescence-associated changes in blood vessels have been implicated in aging and age-related cardiovascular disorders. Here, we demonstrate that peroxisome proliferator-activated receptor (PPAR) δ coordinates angiotensin (Ang) II-induced senescence of human vascular smooth muscle cells (VSMCs). Activation of PPARδ by GW501516, a specific ligand for PPARδ, significantly attenuated Ang II-induced generation of superoxides and suppressed senescence of VSMCs. A marked increase in the levels of p53 and p21 induced by Ang II was blunted by the treatment with GW501516. Ligand-activated PPARδ up-regulated expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and suppressed the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Knockdown of PTEN with siRNA abrogated the effects of PPARδ on cellular senescence, on PI3K/Akt signaling, and on generation of ROS in VSMCs treated with Ang II. Finally, administration of GW501516 to apoE-deficient mice treated with Ang II significantly reduced the number of senescent cells in the aorta, where up-regulation of PTEN with reduced levels of phosphorylated Akt and ROS was demonstrated. Thus, ligand-activated PPARδ confers resistance to Ang II-induced senescence by up-regulation of PTEN and ensuing modulation of the PI3K/Akt signaling to reduce ROS generation in vascular cells.
Introduction
Peroxisome proliferator-activated receptor (PPARδ),4 a member of the PPAR nuclear receptor family, is a ligand-activated transcriptional factor with multiple biological functions (1, 2). This receptor regulates gene expression by forming heterodimers with the retinoid X receptor via PPAR response elements (PPRE), which are located in the target genes (1, 2). PPARδ is ubiquitously expressed in a variety of cell lineages, including vascular smooth muscle cells (VSMCs). It has been postulated that ligand-activated PPARδ exerts antiatherosclerotic effects by anti-inflammatory mechanisms (2–4). In fact, activation of PPARδ suppressed the inflammation and proliferation of VSMCs through up-regulation of transforming growth factor (TGF)-β1 (5), an anti-inflammatory and profibrotic mediator. Furthermore, we have recently demonstrated that PPARδ-induced TGF-β1 increased deposition of extracellular matrix in vascular cells to regulate the stability of atherosclerotic plaque (6). Based on its beneficial properties including regulation of vascular inflammation and fibrosis, modulation of lipoprotein metabolism, and improvement of metabolic profiles (3–8), PPARδ is a promising new target for the treatment of vascular diseases associated with metabolic disorders. However, the therapeutic efficacy of PPARδ ligands in atherosclerosis remains controversial (9, 10).
Cellular senescence is a phenomenon of the irreversible and permanent growth arrest in cells cultured in vitro, where they show characteristic phenotypic changes in gene expression and morphology (11). Primary cultured cells undergo replicative senescence characterized by shortened telomere length, eventually leading to incomplete chromosomal replication (12). Unlike replicative senescence, stress-induced premature senescence (SIPS) is triggered by a variety of chemical agents (13, 14) and stresses including oxidative stress and activation of oncogenes (15, 16).
Reactive oxygen species (ROS) break strands and modify bases of DNA to elicit both replicative senescence and SIPS (17, 18). Increased levels of ROS are detected in atherosclerotic plaques (19), suggesting a role for ROS in cellular senescence. Recently, angiotensin II (Ang II) has been demonstrated to promote senescence of vascular cells, leading to vascular remodeling and atherosclerosis (20, 21). Because NADPH oxidase has been implicated in Ang II-dependent ROS generation, vascular NADPH oxidase may participate in cellular senescence.
We and others (3–6) have shown that PPARδ regulates the stability of atherosclerotic plaques through modulation of inflammation and extracellular matrix homeostasis. A senescent cell phenotype has been identified in human and experimental animal arteries at sites prone to atherosclerosis (22–24). Accordingly, a question was raised as to whether PPARδ induces anti-senescent responses in vascular cells. Here we demonstrate that activation of PPARδ prevents premature senescence and reduces generation of ROS in VSMCs. Interestingly, up-regulation of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) was involved in this process.
EXPERIMENTAL PROCEDURES
Cell Culture
Human aortic vascular smooth muscle cells (VSMCs) were obtained from ScienCell Research Laboratories (Carlsbad, CA). The cells were cultured in Smooth Muscle Cell Medium containing smooth muscle cell growth supplement at 37 °C in an atmosphere of 95% air and 5% CO2., based on the manufacturer's recommendations.
SA-β-Gal Staining
Senescent cells were detected using an SA-β-galactosidase staining kit according to the manufacturer's instructions (Sigma-Aldrich). Briefly, VSMCs pretreated with or without indicated reagents for 30 min were incubated with GW501516 for 24 h, followed by treatment with Ang II for 3 days in 6-well plates. After washing twice with ice-cold phosphate-buffered saline (PBS), the cells were incubated with staining-solution (4.2 mm citric acid, 12.5 mm sodium phosphate, 158 mm sodium chloride, 0.21 mm magnesium chloride, 2.21 mg/ml potassium ferrocyanide, 1.68 mg/ml potassium ferricyanide, 1 mg/ml X-Gal, pH 6.0) for 6 h at 37 °C. Senescent cells were visualized using an Olympus JP/1X71 fluorescence microscope (Tokyo, Japan). For detection of senescent cells in the aortas of apoE-deficient mice, serial cryosections (5 μm) mounted on gelatin-coated slides were incubated in the staining solution, and SA-β-galactosidase was visualized as described above.
Gene Silencing with siRNA
Cells were seeded into 100-mm culture dishes 18–24 h prior to transfection. Cells were transfected with 80 nm control siRNA (Ambion, Austin, TX), human PPARδ siRNA (Ambion), human PPARδ siRNA_2 (Dharmacon Research, Chicago, IL), or human PTEN siRNA (Cell Signaling, Beverly, MA) in serum-free medium using Welfect-Q (WelGENE, Daegu, Korea). Following incubation for 6 h, the transfection medium was replaced with fresh medium, and the cells were incubated for an additional 48 h, at which point they were treated with the indicated reagents for the indicated time.
Confocal Immunofluorescence Microscopy
Cells seeded on 35-mm cover glass bottom dishes (SPL Life Sciences) were transfected with or without the indicated siRNA for 48 h. The cells were treated with GW501516 or vehicle (DMSO) for 24 h, and then treated with Ang II. After incubation for 24 h, the cells were fixed using neutral buffered solution (4% formaldehyde). After permeabilization with PBS containing 0.1% Tween-20 for 3 min, the fixed cells were stained overnight at 4 °C using anti-γ-H2A.X antibody (Cell Signaling, Beverly, MA) at a dilution of 1:200. Secondary goat anti-rabbit IgG conjugated to Cy-3 (Invitrogen, Carlsbad, CA) was applied for 2 h at room temperature. Confocal imaging analysis was performed using an Olympus FV-1000 confocal laser fluorescence microscope (Tokyo, Japan).
Western Blot Analysis
Cells treated with the indicated reagents were washed in ice-cold PBS and lysed in PRO-PREP Protein Extraction Solution (iNtRON Biotechnology, Seoul, Korea). An aliquot of the cell lysate was subjected to SDS-polyacrylamide gel electrophoresis and transferred onto a Hybond-P+ polyvinylidene difluoride membrane (Amersham Biosciences UK Ltd.). Membranes blocked with 5% nonfat milk in Tris-buffered saline (TBS) containing 0.1% Tween-20 overnight at 4 °C were reacted with the indicated specific antibodies in TBS containing 1% BSA and 0.05% Tween-20 overnight at 4 °C and then incubated with peroxidase-conjugated goat antibody diluted to 1:3000 for 2 h at room temperature. After extensive washing in TBS containing 0.1% BSA and 0.1% Tween-20, immunoreactive bands were detected using West-ZOL Plus (iNtRON Biotechnology, Seoul, Korea). For detection of protein expression in the aorta of apoE-deficient mice, aortic tissue was homogenized using the FastPrep-24 instrument with ceramic spheres (MP Biomedicals). After centrifugation (13,000 rpm for 10 min), an aliquot of the protein was subjected Western blot analysis as described above. Polyclonal antibodies specific for p53, p21, pAkt, and Akt were obtained from Cell Signaling. A rabbit anti-α-SMA antibody was purchased from Abcam plc. (Cambridge, UK). Polyclonal antibody specific for PPARδ, and a monoclonal anti-PTEN antibody, as well as horseradish peroxidase (HRP)-conjugated IgG, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Northern Blot Analysis
Aliquots of 5 μg of total RNA were heat-denatured at 65 °C for 15 min in gel-running buffer (40 mm MOPS, 10 mm sodium acetate, and 1 mm EDTA, pH 7.0) containing 50% formamide and then subjected to electrophoresis on a 1% agarose gel containing 2.2 m formaldehyde. Size-fractionated RNA was transferred onto a Hybond-N+ nylon membrane (Amersham Biosciences UK Ltd.) overnight by capillary action and then hybridized with the indicated 32P-labeled probes at 68 °C in QuikHyb solution (Stratagene, La Jolla, CA). The membrane was washed, and the radioactivity on the membrane was detected by a Fuji BAS-2500 Bioimaging Analyzer (Tokyo, Japan). The blots were stripped and rehybridized with a 32P-labeled GAPDH cDNA probe. The cDNA probe was generated by PCR using primers that were specific for nucleotides 1083–1518 of human PTEN.
Assay for Intracellular Superoxide
Intracellular superoxide production was measured by lucigenin-amplified chemiluminescence or a fluorescent indicator, dihydroethidium (DHE), as described previously (25). To determine the level of superoxide anion by lucigenin, VSMCs treated with each reagent for the indicated time were trypsinized and collected by centrifugation at 1,000 rpm for 5 min. After washing twice with ice-cold PBS, the cells were resuspended in HBSS buffer (5.4 mm KCl, 0.3 mm Na2HPO4, 0.4 mm KH2PO4, 4.2 mm NaHCO3, 1.3 mm CaCl2, 0.5 mm MgCl2, 0.6 mm MgSO4, 137 mm NaCl, 5.6 mm glucose, pH 7.4) containing 5 μm of lucigenin. The lucigenin-derived chemiluminescence was determined every 50 s for a total of 5 min, by a LB96V luminometer (EG&G Berthold, Bad Wildbad, Germany). For analysis of intracellular superoxide production, cells treated as described above were washed with ice-cold PBS and incubated for 30 min at 37 °C with 10 μm of DHE in PBS. Following incubation in a humidified chamber protected from light, the red fluorescence was detected through a 580 nm long-pass filter using a fluorescence microscope (Olympus, Tokyo, Japan). For detection of intracellular superoxide production in the aorta of apoE-deficient mice, serial cryosections (5 μm) mounted on gelatin-coated slides were reacted with DHE and the red fluorescence was detected as described above.
Assessment of PIP3 Levels
Phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) activity was quantified using a PIP3 mass strip assay kit according to the manufacturer's protocol (Echelon Biosciences, Salt Lake City, UT). Briefly, human VSMCs plated in 100 mm culture dishes were transfected with 100 nm human PTEN siRNA for 48 h and then pretreated with GW501516. After incubation for 24 h, cells were stimulated with Ang II for 3 h. Cells were collected using cold 0.5 m trichloroacetic acid (TCA) and washed with 5% TCA solution containing 1 mm EDTA. After extraction of neutral lipids with MeOH:CHCl3 (2:1), acidic lipids were extracted with CHCl3:MeOH:12 n HCl (40:80:1) and vacuum-dried. Samples were dissolved in CHCl3 and spotted onto nitrocellulose membrane. After blocking in PBS containing 3% fatty acid-free BSA, chemiluminescent signals were detected.
Animal Study
All animal studies were approved by the Institutional Animal Care Committee of Gyeongsang National University. ApoE-deficient mice were obtained from Japan SLC, Inc. (Shizuoka, Japan). Animals were housed under controlled environmental conditions with a 12 h light/dark cycle and received food and tap water ad libitum. 3–5-month-old male apoE-deficient mice were anesthetized and osmotic mini-pumps (model 2004, Alzet, Palo Alto, CA) containing vehicle (DMSO), GW501516 (3.3 mg/kg/day in DMSO), and/or Ang II (1.44 mg/kg/day in DMSO) were implanted to deliver indicated reagent(s) subcutaneously for 4 weeks. Mice were sacrificed, and the aortas were removed for histological examination and analysis for protein expression.
Immunohistochemical Analysis
Following fixation with 4% paraformaldehyde and cryoprotection in 20% sucrose, tissues were embedded in OCT (Sakura Finetech Co., Tokyo, Japan) and snap-frozen in iso-pentene, prechilled in liquid nitrogen. Serial cryosections (5 μm) were mounted on gelatin-coated slides and boiled in 10 mm sodium citrate for 3 min. Sections were blocked in 0.1 m TBS containing 1% BSA for 2 h at room temperature, followed by incubation with antibody at 4 °C overnight, and then washed three times. Staining was performed using the manufacturer's protocol (ABC Kit, Vector Labs) and color development was achieved using 0.05 m Tris-HCl (pH 7.4), 0.2 mg/ml diaminobenzidine (DAB), and 0.003% H2O2, for 30 s and observed using fluorescence microscope (Olympus, Tokyo, Japan). Nuclei and cytoplasm were identified using H & E counterstaining.
Statistical Analysis
Data are expressed as the means ± S.E. Statistical significance was determined by Student's t test or ANOVA with post hoc Bonferroni test. A value of p < 0.05 was considered statistically significant.
RESULTS
Ligand-activated PPARδ Inhibits Ang II-induced Senescence in VSMCs
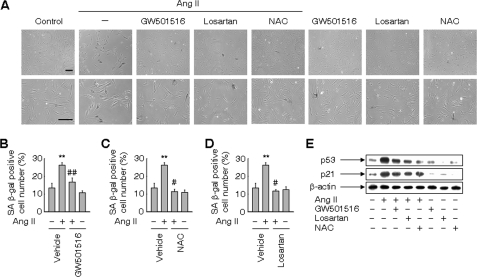
We examined whether ligand-activated PPARδ affects the premature senescence of VSMCs induced by Ang II (20). When VSMCs were treated with Ang II, the senescence-associated β-galactosidase (SA β-gal) activity, a biomarker for cellular senescence, was significantly increased in Ang II-treated VSMCs (Fig. 1, A–D). This increase was significantly suppressed in the presence of GW501516, suggesting the involvement of PPARδ in the inhibition of Ang II-induced premature senescence (Fig. 1, A and B). Furthermore, a significant reduction in SA β-gal activity was observed by pretreatment with losartan, an Ang II type 1 (AT1) receptor-specific blocker, or N-acetyl-cysteine (NAC), a thiol antioxidant. These findings suggest that AT1 receptor and ROS mediate Ang II-induced senescence in VSMCs (Figs. 1, A, C, and D). Consistent with SA β-gal activity, the Ang II-induced high frequency of phosphorylated H2A histone family member X (γ-H2A.X) foci, another biomarker for cellular senescence (26), was also significantly attenuated in the presence of GW501516 (supplemental Fig. S1), corroborating the effects of PPARδ in the inhibition of Ang II-induced senescence.
FIGURE 1.
Ligand-activated PPARδ inhibits Ang II-induced senescence in VSMCs. A, SA β-gal staining of VSMCs. Cells pretreated with 30 μm NAC or 1 μm losartan for 30 min were incubated for 24 h in the presence or absence of 50 nm GW501516, a specific ligand for PPARδ. Cells were subsequently incubated with 100 nm Ang II for 3 days. Bars indicate 200 μm. B—D, number of SA β-gal-positive cells. E, expression of p53 and p21. Whole-cell lysates of VSMCs pretreated as above and incubated with Ang II for 24 h were subjected to Western blot analyses. Representative images from four independent determinations are shown. The results are expressed as the means ± S.E. (n = 4). **, p < 0.05 versus untreated group; #, p < 0.01; ##, p < 0.05 versus Ang II-treated vehicle group.
To characterize cellular senescence blocked by GW501516, we examined levels of p53 and p21, key proteins in the senescence pathway (27). Up-regulation of these proteins was demonstrated in Ang II-treated VSMCs as well as in a mouse model of atherosclerosis (20). As shown in Fig. 1E, a marked increase in the levels of p53 and p21 was observed in VSMCs treated with Ang II, whereas treatment with GW501516 clearly blunted the effect of Ang II on the levels of these proteins. We next examined the effects of NAC on the levels of p53 and p21. The Ang II-induced increase in both proteins was attenuated in the presence of NAC (Fig. 1E), suggesting the involvement of ROS in the premature senescence of VSMCs induced by Ang II. Losartan also suppressed the Ang II-induced increase in both proteins, which indicated the involvement of AT1 receptor in this senescence pathway.
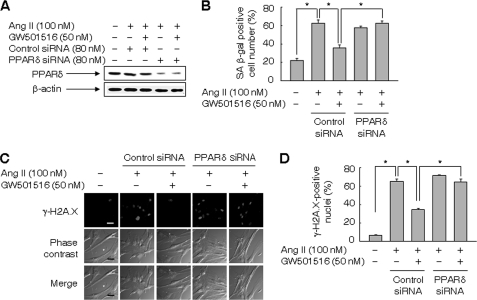
To verify the role of PPARδ in blocking Ang II-induced senescence of VSMCs, we examined the effect of GW501516 in cells treated with a small interfering (si)RNA against PPARδ. The level of PPARδ in VSMCs was markedly reduced upon transfection with PPARδ siRNA in the presence of Ang II and/or GW501516, whereas control siRNA, consisting of a pool of nonspecific sequences, had no effect on PPARδ levels (Fig. 2A). As expected, the siRNA-mediated down-regulation of PPARδ significantly suppressed the GW501516-mediated inhibition of premature senescence of VSMCs induced by Ang II (Fig. 2, B--D). Similar results were obtained when the cells were analyzed with another PPARδ siRNA_2 (supplemental Fig. S2)
FIGURE 2.
A small interfering RNA against PPARδ abolished the effect of GW501516 on Ang II-induced cellular senescence. A, expression of PPARδ in VSMCs transfected with siRNA against PPARδ or control siRNA. Transfected cells pretreated with GW501516 for 24 h were incubated with Ang II for another 24 h. B, number of SA β-gal-positive cells. VSMCs transfected with siRNA against PPARδ or control were pretreated with GW501516 for 24 h, and then incubated with Ang II for 3 days. C and D, assessment of γ-H2A.X foci. VSMCs transfected with indicated siRNA were pretreated with GW501516 for 24 h, and then treated with Ang II for 24 h. Representative images from four independent experiments are shown (panel C). Bars indicate 50 μm. Cells were chosen from at least four randomly selected fields and scored for γ-H2A.X-positive nuclei (panel D). The results are expressed as the means ± S.E. (n = 4). *, p < 0.01.
Ligand-activated PPARδ Attenuates Generation of ROS Induced by Ang II
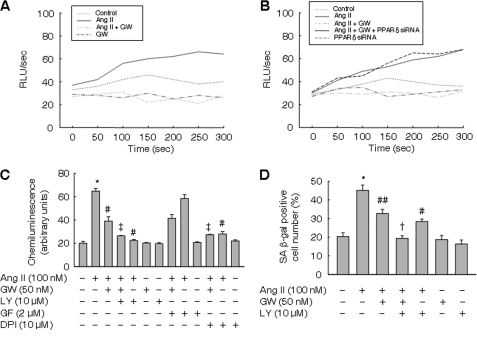
The antioxidant NAC prevented the Ang II-induced premature senescence of VSMCs. Since Ang II has been known to generate ROS by NADPH oxidase (21, 25), we examined the effects of GW501516 on superoxide formation in VSMCs treated with Ang II. While Ang II significantly increased superoxide formation, pretreatment with GW501516 for 24 h significantly suppressed the effect of Ang II (Fig. 3A). Reduced superoxide generation by GW501516 was recovered in the presence of PPARδ siRNA, suggesting a PPARδ-dependent effect of GW501516 on superoxide production (Fig. 3B). Similar results were obtained with another PPARδ siRNA_2 (supplemental Fig. S3).
FIGURE 3.
Ligand-ativated PPARδ or inhibition of PI3K attenuates generation of ROS and cellular senescence induced by Ang II. A-C, superoxide generation in VSMCs. Cells incubated with GW501516 for 24 h were treated with Ang II for 3 h. Whole-cell lysates were prepared for measurement of superoxide by real-time lucigenin chemiluminescence. Data are representative of four separate determinations. The results are expressed as the means ± S.E. (n = 4). D, number of SA β-gal-positive cells. VSMCs pretreated with LY294002 (LY) for 30 min were incubated with GW501516 (GW) for 24 h, followed by treatment with Ang II for 3 days. The results are expressed as the means ± S.E. (n = 4). *, p < 0.01 versus untreated group; #, p < 0.01; ##, p < 0.05 versus Ang II-treated group; †, p < 0.01; ‡, p < 0.05 versus Ang II + GW501516-treated group. GF, GF109203X; DPI, diphenyleneiodonium.
To characterize the signaling pathway involved in Ang II-induced superoxide formation, we examined the effects of kinase inhibitors in cells treated with Ang II. As shown in Fig. 3C, the Ang II-induced production of superoxide was significantly reduced in the presence of LY294002, a phosphatidylinositol 3-kinase (PI3K) inhibitor, but not of GF109203X, a protein kinase C (PKC) inhibitor. Although GF109203X significantly suppressed superoxide production at an early time of Ang II exposure (supplemental Fig. S4), no effect was observed at a longer time of exposure (3 h). These findings suggest that PKC mediates the initial activation of NADPH oxidase, whereas at later time points, activation of PI3K is involved in the enhanced superoxide generation. The effect of LY294002 was further augmented in cells incubated with GW501516 (Fig. 3C). Prior incubation with DPI, a known inhibitor of NADPH oxidase, significantly attenuated the Ang II-induced generation of superoxide in VSMCs. As shown in Fig. 3D, LY294002 significantly reduced the number of SA β-gal-positive cells. To further confirm the role of PI3K/Akt in the GW501516-mediated actions, we investigated the effects of siRNA against Akt in superoxide production and cellular senescence induced by Ang II. The siRNA-mediated down-regulation of Akt further enhanced the GW501516-mediated suppression of superoxide production and SA β-gal-positive cells (supplemental Fig. S5). These results suggest the involvement of PI3K/Akt in GW501516-mediated effects on superoxide formation and cellular senescence induced by Ang II.
Ligand-activated PPARδ Suppresses Activation of Akt Induced by Ang II
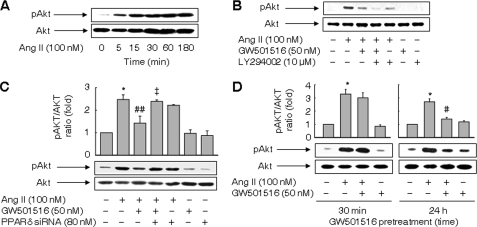
We next focused on the PI3K/Akt pathway implicated in the Ang II-induced superoxide generation and cellular senescence (28). Treatment with Ang II leads to phosphorylation of Akt in a time-dependent manner (Fig. 4A). The activation of Akt by Ang II was markedly reduced in the presence of GW501516 and almost completely abolished in the presence of both GW501516 and LY294002 (Fig. 4B). Down-regulation of PPARδ by siRNA reversed the GW501516-induced decrease in phosphorylated Akt in cells treated with Ang II (Fig. 4C). Similar results were obtained with another PPARδ siRNA_2 (supplemental Fig. S6). These results suggest that PPARδ blocks the activation of Akt induced by Ang II. As crosstalk between PPARδ and Akt has been demonstrated in several cell types (29, 30), we further examined the effects of GW501516 at different pretreatment times. Interestingly, the short time pretreatment (30 min) did not affect the level of phosphorylated Akt, whereas the long time pretreatment (24 h) significantly suppressed the Ang II-induced increase in phosphorylated Akt (Fig. 4D).
FIGURE 4.
Ligand-activated PPARδ suppresses activation of Akt induced by Ang II. A, time-dependent activation of Akt induced by Ang II. B, effects of GW501516 and inhibition of PI3K on Ang II-induced activation of Akt. Cells pretreated with LY294002 for 30 min were incubated with GW501516 for 24 h and exposed to Ang II for 3 h. C, effects of siRNA against PPARδ on Ang II-induced activation of Akt. Cells transfected with PPARδ siRNA were treated with GW501516 for 24 h and exposed to Ang II for 3 h. D, effects of GW501516 on Ang II-induced activation of Akt at different pretreatment times. Cells pretreated with GW501516 for 30 min or 24 h were incubated with Ang II for 3 h. Representative blots from four independent experiments are shown. The results are expressed as the means ± S.E. (n = 4). *, p < 0.01 versus untreated group; #, p < 0.01; ##, p < 0.05 versus Ang II-treated group; ‡, p < 0.05 versus Ang II + GW501516-treated group.
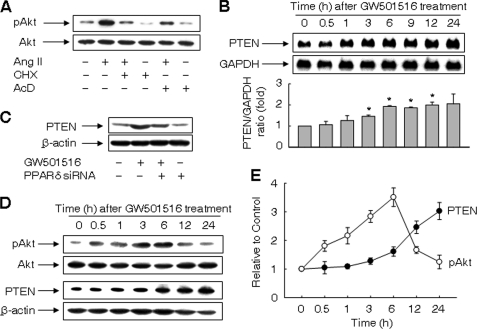
PTEN Is Up-regulated by Ligand-activated PPARδ
We next examined the effects of actinomycin D and cycloheximide on Ang II-induced activation of Akt. The level of phosphorylated Akt was markedly reduced in the presence of actinomycin D or cycloheximide (Fig. 5A). Thus, de novo synthesis of mRNA and protein(s) are involved in the phosphorylation of Akt in Ang II-treated VSMCs.
FIGURE 5.
PTEN is up-regulated by ligand-activated PPARδ. A, effects of cycloheximide (CHX) and actinomycin (AcD) on activation of Akt induced by Ang II. Cells pretreated with 40 μg/ml CHX or 4 μm AcD for 30 min were incubated with Ang II for 3 h. B, time-dependent up-regulation of PTEN mRNA induced by GW501516. Representative blots from four independent experiments and densitometric measurements are shown. The results are expressed as the means ± S.E. (n = 4). *, p < 0.01 versus time zero. C. Effects of siRNA against PPARδ on expression of PTEN. Cells transfected with PPARδ siRNA were incubated with or without GW501516 for 24 h. D and E, time course of activation of Akt and expression of PTEN in VSMCs treated with GW501516. Representative blots from four independent experiments are shown (panel D). Band intensities were quantified using an image analyzer, and fold changes are plotted (panel E).
To investigate the molecular mechanism underlying the PPARδ-mediated inhibition of Akt phosphorylation, we analyzed the expression levels of PTEN, a dual protein/lipid phosphatase that dephosphorylates phosphatidylinositol (3,4,5)-trisphosphate (PIP3) that negatively regulates the PI3K-Akt pathway (31). Treatment with GW501516 significantly increased the level of PTEN transcript in a time-dependent manner (Fig. 5B). The up-regulation of PTEN by GW501516 was suppressed in the presence of siRNA against PPARδ, suggesting the causal role of PPARδ in the up-regulation of PTEN (Fig. 5C). Similar results were obtained with another PPARδ siRNA_2 (supplemental Fig. S7).
We next examined the time course of phosphorylated Akt and PTEN expression. Although we could not detect any change in phosphorylation of Akt at 24 h after GW501516 treatment, activation of Akt was observed at earlier time points with a peak at 6 h after the treatment. On the other hand, the increase in the expression of PTEN was correlated with a decline in phosphorylated Akt (Fig. 5, D and E). These data suggest that PPARδ regulates the activity of Akt via up-regulation of PTEN.
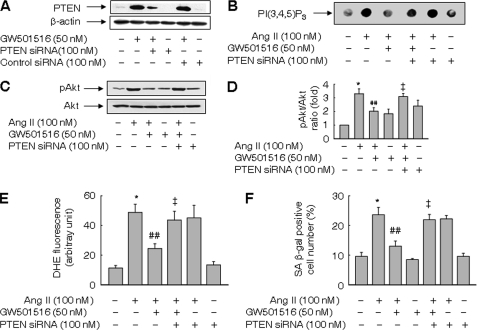
PTEN Is Essential for Effects of PPARδ on Superoxide Generation and Cellular Senescence Induced by Ang II
To clarify the role of PTEN in Ang II-induced cellular senescence, the expression of PTEN was knocked down by siRNA. The level of PTEN in VSMCs was markedly reduced upon transfection with PTEN siRNA in the presence or absence of GW501516 (Fig. 6A). As PTEN is a phosphoinositide 3-phosphatase, we determined the impact of PPARδ knockdown on phospholipid PI(3,4,5)P3 levels. GW501516 suppressed the level of PI(3,4,5)P3 increased by Ang II. By contrast, siRNA against PTEN abolished the effect of GW501516 on PI(3,4,5)P3 (Fig. 6B). Accordingly, induction of PTEN by GW5015416 may account for reduced PI(3,4,5)P3 levels. Knockdown of PTEN also reversed the PPARδ-mediated decline in phosphorylated Akt in Ang II-treated cells (Fig. 6, C and D).
FIGURE 6.
PTEN is essential for effects of PPARδ on superoxide generation and cellular senescence induced by Ang II. A, knockdown of PTEN with siRNA. Cells transfected with either PTEN siRNA or control siRNA were grown for 48 h and treated with GW501516 for 24 h. B, PIP3 levels in cells transfected with PTEN siRNA. Transfected cells were pretreated with GW501516 for 24 h and exposed to Ang II for 3 h. C and D, effects of siRNA against PTEN on Ang II-induced activation of Akt. Representative blots from three independent experiments are shown (panel C). Band intensities were quantified using an image analyzer, and fold changes are plotted (panel D). E, superoxide generation in cells transfected with PTEN siRNA. F, effects of siRNA against PTEN on the number of SA β-gal-positive cells. Cells transfected with PTEN siRNA were pretreated with GW501516 for 24 h, followed by incubation with Ang II for 3 h for superoxide determination, or 3 days for SA β-gal staining. Results are expressed as the means ± S.E. (n = 4). *, p < 0.01 versus untreated group; ##, p < 0.05 versus Ang II-treated group; ‡, p < 0.05 versus Ang II + GW501516-treated group.
Next, we examined the effects of PTEN siRNA on the superoxide generation and cellular senescence induced by Ang II. As demonstrated in Fig. 6E, PTEN siRNA reversed the GW501516-mediated suppression of superoxide generation in Ang II-treated cells. The number of SA β-gal-positive cells reduced by GW501516 was also recovered by transfection with PTEN siRNA (Fig. 6F). These data suggest that PPARδ regulates Ang II-induced superoxide generation and cellular senescence by modulating the expression of PTEN.
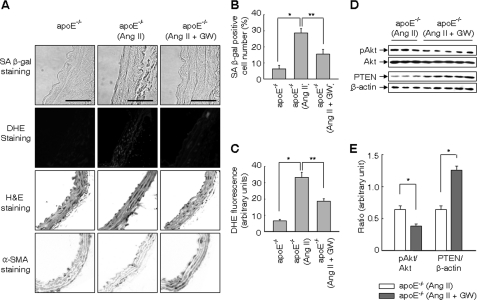
Ligand-activated PPARδ PRevents Ang II-induced Cellular Senescence in apoE-deficient Mice
To verify the findings in cultured cells, we examined cellular senescence in apoE-deficient mice treated with vehicle, Ang II, and/or GW501516 for 4 weeks. Administration of GW501516 significantly attenuated the Ang II-induced increase in SA β-gal-positive cells in the aorta of apoE-deficient mice (Fig. 7, A and B). Most of the cells positive for SA β-gal activity were stained with an antibody against α-smooth muscle actin, indicating that these cells are senescent VSMCs. When the level of superoxide in the vessel was analyzed by dihydroethidium (DHE) staining, DHE fluorescence in Ang II-treated aorta was significantly attenuated in mice administered with GW501618 (Fig. 7, A and C). To ascertain signaling pathways involved in the suppression of Ang II-induced senescence by PPARδ, the expression of PTEN and activation of the PI3K/Akt pathway was examined. Administration of GW501516 significantly decreased the level of phosphorylated Akt in apoE-deficient mice treated with Ang II, while it significantly increased the level of PTEN (Fig. 7, D and E). These results clearly indicate that PPARδ-induced up-regulation of PTEN suppresses Ang II-induced senescence by reducing ROS generation in vascular cells.
FIGURE 7.
Ligand-activated PPARδ prevents Ang II-induced cellular senescence in apoE-deficient mice. A, representative cross-sections of the aorta of vehicle or Ang II-infused apoE-deficient (apoE−/−) mice treated with or without GW501516 (GW) for 4 weeks. Frozen sections were subjected to staining for SA β-gal, DHE, H&E, or α-SMA as described under “Experimental Procedures.” Bars indicate 30 μm. Representative high resolution images from five independent determinations are shown. B and C, number of SA β-gal-positive cells and DHE fluorescence in the vessel. D and E, activation of Akt and expression of PTEN in the vessel. *, p < 0.01; **, p < 0.05.
DISCUSSION
In this study, we demonstrated that ligand-activated PPARδ prevents the Ang II-induced cellular senescence by reducing intracellular ROS generation. This effect of PPARδ was mediated by PTEN, a lipid and protein phosphatase that regulates the level of PIP3 and the PI3K/Akt pathway (31). Administration of a specific ligand of PPARδ, GW501516, in fact increased the expression of PTEN and prevented Ang II-induced cellular senescence in apoE-deficient mice.
Our present data strongly support the hypothesis that PPARδ may be a key target for therapeutic intervention in atherosclerosis by preventing cellular senescence of VSMCs. Previous reports demonstrated that activation of another member of the PPAR family, PPARγ, suppressed the Ang II-induced senescence of endothelial progenitor cells by down-regulation of the AT1 receptor (33). On the other hand, a different line of investigation showed that activation of PPARγ accelerated replicative senescence by inducing p16, a cell cycle inhibitor that prevents entry into the cell cycle (34). Although the role of PPARγ in senescence is controversial, our present data clearly demonstrated that ligand-activated PPARδ prevents cellular senescence induced by Ang II. Because cells with a senescence phenotype have been identified at sites prone to atherosclerosis (22–24), it may be possible to stabilize the plaques by suppressing cellular senescence of VSMCs through PPARδ activation.
Involvement of ROS has been documented in Ang II-induced cellular senescence (21). In the present study, ligand-activated PPARδ significantly decreased ROS generation. Among the enzymes implicated in Ang II-induced ROS formation in vascular cells (25, 35), NADPH oxidase is a main source of ROS generated in VSMCs (36). Increased production of ROS by Ang II was significantly suppressed by LY294002, an inhibitor of PI3K, but not by GF109203X, an inhibitor of protein kinase C. PKC is involved in the initial activation of NADPH oxidase, while activation of the PI3K/Akt pathway leads to translocation of Rac to elicit prolonged oxidase-dependent ROS generation (37). Accordingly, the PI3K/Akt pathway seems to play a dominant role in Ang II-induced ROS generation under the present experimental conditions. DNA damage by ROS was also shown to participate in Ang II-induced cellular senescence (21). In fact, ligand-activated PPARδ reduced the Ang II-induced frequency of γ-H2A.X foci, a marker for DNA strand breakage (38), in the VSMCs. These findings therefore suggest that PPARδ suppresses Ang II-induced cellular senescence by a mechanism relevant to DNA damage.
Up-regulation of PTEN by PPARδ is a key event in the blockade of Ang II-induced cellular senescence by GW501516. The tumor suppressor PTEN, a negative regulator of the PI3K/Akt signaling cascade, modulates a variety of cellular processes including cell growth, survival, proliferation, and migration (39). The involvement of transcription factors such as p53 and Egr-1 has been demonstrated in the regulation of PTEN expression (40, 41). However, the entire picture of the transcriptional regulation of PTEN has been poorly understood. Previous studies reported that PPARδ inhibits the expression of PTEN in keratinocytes (29) and in human non-small cell lung carcinoma (42). On the other hand, induction of PTEN by rosiglitazone, a specific ligand for PPARγ, has been reported (43, 44). The effect of rosiglitazone on PTEN is suggested to take part in various functions of PPARγ such as tumor suppression, anti-inflammatory actions, and cell migration by regulating the PI3K/Akt pathway. Induction of PTEN by rosiglitazone occurs at the transcriptional level via binding of PPARγ to the PPRE located in the 5′-flanking region of the PTEN gene (43). Since the present study has shown that PPARδ regulates the expression of PTEN at the transcriptional level, PPRE in the PTEN gene may also mediate up-regulation of PTEN by PPARδ.
As opposed to the present findings, previous studies demonstrated activation of the PI3K/Akt pathway by PPARδ in several cell types including endothelial progenitor cells (30) and keratinocytes (29). In line with previous studies, GW501516 increased the level of phosphorylated Akt at earlier time points with a peak at 6 h after the treatment. However, we could not detect any change in phosphorylation of Akt at 24 h after GW501516 treatment. Furthermore, no effect on Ang II-induced activation of Akt was observed when cells were pretreated with GW501516 for a short period of 30 min. These findings suggest that the concentration and the time of GW501516 treatment are important determinants for the PPARδ-mediated modulation of the PI3K/Akt pathway. In fact, the specificity and potency of PPARδ on the target genes were reported to differ depending upon the time treated with the ligand and the cell type (45).
In line with the findings obtained in cultured VSMCs, the administration of GW501516 significantly attenuated Ang II-induced cellular senescence in vivo. Reduced levels of phosphorylated Akt and up-regulation of PTEN were demonstrated in the aorta of apoE-deficient mice treated with Ang II. Concomitantly, superoxide generation in the vessel was significantly suppressed in these mice. Various humoral factors involved in the development of atherosclerosis have been shown to increase Akt activity (28). Activation of Akt was observed in human atheromatous lesions, but not in normal arteries (46). Because inhibition of Akt extended the life span of primary cultured human endothelial cells (46), sustained activation of Akt may promote cellular senescence in atheromatous lesions. In this context, PPARδ may serve as an anti-senescent mediator in age-related vascular changes such as atherosclerosis (47).
In conclusion, we demonstrate that ligand-activated PPARδ regulates Ang II-induced cellular senescence in VSMCs by up-regulation of PTEN and ensuing modulation of the PI3K/Akt signaling cascade to reduce cellular superoxide generation. These results have important implications not only for understanding the molecular mechanisms underlying the anti-senescent effect of PPARδ, but also for providing a novel insight into the treatment of atherosclerotic vascular disease.
This work was supported in part by the Mid-career Research Program and MRC Program through an NRF grant funded by MEST (2011-0012427 and 2011-0006200) and Next-Generation BioGreen 21 Program (PJ007980), Rural Development Administration, Republic of Korea.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7.
- PPARδ
- peroxisome proliferator-activated receptor δ
- Ang II
- angiotensin II
- AT1
- Ang II type 1 receptor
- DHE
- dihydroethidium
- NAC
- N-acetyl-l-cysteine
- PI3K
- phosphatidylinositol-3 kinase
- PIP3
- phosphatidylinositol (3,4,5)-trisphosphate
- PKC
- protein kinase C
- PPRE
- PPAR response elements
- PTEN
- phosphatase and tensin homolog deleted on chromosome 10
- ROS
- reactive oxygen species
- SA β-gal
- senescence-associated β-galactosidase
- siRNA
- small interfering RNA
- SIPS
- stress-induced premature senescence
- TGF-β1
- transforming growth factor-β1
- VSMCs
- vascular smooth muscle cells.
REFERENCES
- 1. Ehrenborg E., Krook A. (2009) Pharmacol. Rev. 61, 373–393 [DOI] [PubMed] [Google Scholar]
- 2. Jandeleit-Dahm K. A., Calkin A., Tikellis C., Thomas M. (2009) Curr. Opin. Lipidol. 20, 24–29 [DOI] [PubMed] [Google Scholar]
- 3. Lee C. H., Chawla A., Urbiztondo N., Liao D., Boisvert W. A., Evans R. M., Curtiss L. K. (2003) Science 302, 453–457 [DOI] [PubMed] [Google Scholar]
- 4. Takata Y., Liu J., Yin F., Collins A. R., Lyon C. J., Lee C. H., Atkins A. R., Downes M., Barish G. D., Evans R. M., Hsueh W. A., Tangirala R. K. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 4277–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim H. J., Ham S. A., Kim S. U., Hwang J. Y., Kim J. H., Chang K. C., Yabe-Nishimura C., Kim J. H., Seo H. G. (2008) Circ. Res. 102, 193–200 [DOI] [PubMed] [Google Scholar]
- 6. Kim H. J., Kim M. Y., Jin H., Kim H. J., Kang S. S., Kim H. J., Lee J. H., Chang K. C., Hwang J. Y., Yabe-Nishimura C., Kim J. H., Seo H. G. (2009) Circ. Res. 105, 16–24 [DOI] [PubMed] [Google Scholar]
- 7. Oliver W. R., Jr., Shenk J. L., Snaith M. R., Russell C. S., Plunket K. D., Bodkin N. L., Lewis M. C., Winegar D. A., Sznaidman M. L., Lambert M. H., Xu H. E., Sternbach D. D., Kliewer S. A., Hansen B. C., Willson T. M. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 5306–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y. X., Lee C. H., Tiep S., Yu R. T., Ham J., Kang H., Evans R. M. (2003) Cell 113, 159–170 [DOI] [PubMed] [Google Scholar]
- 9. Li A. C., Binder C. J., Gutierrez A., Brown K. K., Plotkin C. R., Pattison J. W., Valledor A. F., Davis R. A., Willson T. M., Witztum J. L., Palinski W., Glass C. K. (2004) J. Clin. Invest. 114, 1564–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graham T. L., Mookherjee C., Suckling K. E., Palmer C. N., Patel L. (2005) Atherosclerosis 181, 29–37 [DOI] [PubMed] [Google Scholar]
- 11. Hayflick L. (1965) Exp. Cell Res. 37, 614–636 [DOI] [PubMed] [Google Scholar]
- 12. Harley C. B., Futcher A. B., Greider C. W. (1990) Nature 345, 458–460 [DOI] [PubMed] [Google Scholar]
- 13. Holliday R. (1986) Exp. Cell Res. 166, 543–552 [DOI] [PubMed] [Google Scholar]
- 14. Ogryzko V. V., Hirai T. H., Russanova V. R., Barbie D. A., Howard B. H. (1996) Mol. Cell Biol. 16, 5210–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Serrano M., Lin A. W., McCurrach M. E., Beach D., Lowe S. W. (1997) Cell 88, 593–602 [DOI] [PubMed] [Google Scholar]
- 16. Zhu J., Woods D., McMahon M., Bishop J. M. (1998) Genes Dev. 12, 2997–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Zglinicki T., Saretzki G., Döcke W., Lotze C. (1995) Exp. Cell Res. 220, 186–193 [DOI] [PubMed] [Google Scholar]
- 18. Chen Q. M., Prowse K. R., Tu V. C., Purdom S., Linskens M. H. (2001) Exp. Cell Res. 265, 294–303 [DOI] [PubMed] [Google Scholar]
- 19. Hathaway C. A., Heistad D. D., Piegors D. J., Miller F. J., Jr. (2002) Circ. Res. 90, 277–283 [DOI] [PubMed] [Google Scholar]
- 20. Kunieda T., Minamino T., Nishi J., Tateno K., Oyama T., Katsuno T., Miyauchi H., Orimo M., Okada S., Takamura M., Nagai T., Kaneko S., Komuro I. (2006) Circulation 114, 953–960 [DOI] [PubMed] [Google Scholar]
- 21. Herbert K. E., Mistry Y., Hastings R., Poolman T., Niklason L., Williams B. (2008) Circ. Res. 102, 201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ross R., Wight T. N., Strandness E., Thiele B. (1984) Am. J. Pathol. 114, 79–93 [PMC free article] [PubMed] [Google Scholar]
- 23. Bennett M. R., Macdonald K., Chan S. W., Boyle J. J., Weissberg P. L. (1998) Circ. Res. 82, 704–712 [DOI] [PubMed] [Google Scholar]
- 24. Minamino T., Miyauchi H., Yoshida T., Ishida Y., Yoshida H., Komuro I. (2002) Circulation 105, 1541–1544 [DOI] [PubMed] [Google Scholar]
- 25. Griendling K. K., Minieri C. A., Ollerenshaw J. D., Alexander R. W. (1994) Circ. Res. 74, 1141–1148 [DOI] [PubMed] [Google Scholar]
- 26. Lawless C., Wang C., Jurk D., Merz A., Zglinicki T., Passos J. F. (2010) Exp. Gerontol. 45, 772–778 [DOI] [PubMed] [Google Scholar]
- 27. Campisi J. (2001) Trends Cell Biol. 11, S27–S31 [DOI] [PubMed] [Google Scholar]
- 28. Cantley L. C. (2002) Science 296, 1655–1657 [DOI] [PubMed] [Google Scholar]
- 29. Di-Poï N., Tan N. S., Michalik L., Wahli W., Desvergne B. (2002) Mol. Cell 10, 721–733 [DOI] [PubMed] [Google Scholar]
- 30. Han J. K., Lee H. S., Yang H. M., Hur J., Jun S. I., Kim J. Y., Cho C. H., Koh G. Y., Peters J. M., Park K. W., Cho H. J., Lee H. Y., Kang H. J., Oh B. H., Park Y. B., Kim H. S. (2008) Circulation 118, 1021–1033 [DOI] [PubMed] [Google Scholar]
- 31. Stambolic V., Suzuki A., de la Pompa J. L., Brothers G. M., Mirtsos C., Sasaki T., Ruland J., Penninger J. M., Siderovski D. P., Mak T. W. (1998) Cell 1998;95, 29–39 [DOI] [PubMed] [Google Scholar]
- 32.Deleted in proof [Google Scholar]
- 33. Imanishi T., Kobayashi K., Kuroi A., Ikejima H., Akasaka T. (2008) Hypertens. Res. 31, 757–765 [DOI] [PubMed] [Google Scholar]
- 34. Collins C. J., Sedivy J. M. (2003) Aging Cell 2, 145–150 [DOI] [PubMed] [Google Scholar]
- 35. Cai H., Harrison D. G. (2000) Circ. Res. 87, 840–844 [DOI] [PubMed] [Google Scholar]
- 36. Brandes R. P., Kreuzer J. (2005) Cardiovasc. Res. 65, 16–27 [DOI] [PubMed] [Google Scholar]
- 37. Seshiah P. N., Weber D. S., Rocic P., Valppu L., Taniyama Y., Griendling K. K. (2002) Circ. Res. 91, 406–413 [DOI] [PubMed] [Google Scholar]
- 38. Rogakou E. P., Pilch D. R., Orr A. H., Ivanova V. S., Bonner W. M. (1998) J. Biol. Chem. 273, 5858–5868 [DOI] [PubMed] [Google Scholar]
- 39. Chow L. M., Baker S. J., (2006) Cancer Lett. 241, 184–196 [DOI] [PubMed] [Google Scholar]
- 40. Stambolic V., MacPherson D., Sas D., Lin Y., Snow B., Jang Y., Benchimol S., Mak T. W. (2001) Mol. Cell 8, 317–325 [DOI] [PubMed] [Google Scholar]
- 41. Virolle T., Adamson E. D., Baron V., Birle D., Mercola D., Mustelin T., de Belle I. (2001) Nat. Cell Biol. 3, 1124–1128 [DOI] [PubMed] [Google Scholar]
- 42. Han S., Ritzenthaler J. D., Wingerd B., Roman J. (2005) J. Biol. Chem. 280, 33240–33249 [DOI] [PubMed] [Google Scholar]
- 43. Patel L., Pass I., Coxon P., Downes C. P., Smith S. A., Macphee C. H. (2001) Curr. Biol. 11, 764–768 [DOI] [PubMed] [Google Scholar]
- 44. Zhang W., Wu N., Li Z., Wang L., Jin J., Zha X. L. (2006) Cancer Biol. Ther. 5, 1008–1014 [DOI] [PubMed] [Google Scholar]
- 45. Nielsen R., Grontved L., Stunnenberg H. G., Mandrup S. (2006) Mol. Cell Biol. 26, 5698–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miyauchi H., Minamino T., Tateno K., Kunieda T., Toko H., Komuro I. (2004) EMBO J. 23, 212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cooper L. T., Cooke J. P., Dzau V. J. (1994) J. Gerontol. 49, B191–196 [DOI] [PubMed] [Google Scholar]