Background: Glucose-dependent insulinotropic polypeptide (GIP) is pursued as an anti-obesity target.
Results: The adipocyte GIP receptor (GIPr) promotes high fat diet (HFD)-induced body weight gain by an increase in lean mass rather than fat mass in mice.
Conclusion: The adipocyte GIPr regulates both body weight and body composition.
Significance: Targeting the GIPr may have effects beyond lipid storage and acute glucose metabolism.
Keywords: Adipose Tissue, Glucose Metabolism, Metabolism, Mouse Genetics, Obesity, Glucose-dependent Insulinotropic Polypeptide, Body Composition, Body Weight
Abstract
The glucose-dependent insulinotropic polypeptide receptor (GIPr) has been implicated in high fat diet-induced obesity and is proposed as an anti-obesity target despite an uncertainty regarding the mechanism of action. To independently investigate the contribution of the insulinotropic effects and the direct effects on adipose tissue, we generated transgenic mice with targeted expression of the human GIPr to white adipose tissue or beta-cells, respectively. These mice were then cross-bred with the GIPr knock-out strain. The central findings of the study are that mice with GIPr expression targeted to adipose tissue have a similar high fat diet -induced body weight gain as control mice, significantly greater than the weight gain in mice with a general ablation of the receptor. Surprisingly, this difference was due to an increase in total lean body mass rather than a gain in total fat mass that was similar between the groups. In contrast, glucose-dependent insulinotropic polypeptide-mediated insulin secretion does not seem to be important for regulation of body weight after high fat feeding. The study supports a role of the adipocyte GIPr in nutrient-dependent regulation of body weight and lean mass, but it does not support a direct and independent role for the adipocyte or beta-cell GIPr in promoting adipogenesis.
Introduction
It is well established that peripheral insulin levels and insulin action are of critical importance for maintenance of glucose and lipid homeostasis. Accordingly, the pancreatic beta-cells are highly regulated by numerous factors. Two important regulators are the gut hormones, glucose-dependent insulinotropic polypeptide (GIP)2 and glucagon-like peptide 1 (GLP-1), secreted in response to food intake. In addition to exerting their effects as potent glucose-dependent insulin secretagogues, both hormones also stimulate beta-cell proliferation and inhibit beta-cell apoptosis (1). Unlike GLP-1, GIP has also been proposed to be a key link between overnutrition and obesity by promoting adipogenesis. Thus, orally ingested fat stimulates GIP secretion, and elevated GIP plasma levels have been noted in the obese state (2, 3). Furthermore, GIP was found to promote chylomicron triglyceride clearance in dogs (4) and to lower plasma triglyceride levels after an intraduodenal fat load (5), possibly by increasing lipoprotein lipase activity (6–9). GIP has also been found to inhibit catecholamine- and glucagon-induced lipolysis (10–12) through specific receptor signaling on adipocytes (13–15). Because GIP is a potent stimulator of insulin secretion and plasma GIP and insulin levels are elevated in parallel in the postprandial state (16, 17), interactions between GIP and insulin on lipid metabolism are likely. Thus, GIP has been shown to facilitate uptake of glucose and free fatty acids in cultures of adipocytes or adipose tissue, and this effect was enhanced in the presence of insulin (9, 11, 18, 19). In addition, the lipolytic effect of GIP (12, 20) has been reported to be antagonized by insulin in differentiated 3T3-L1 adipocytes (20). Taken together, substantial evidence points to a direct role of GIP in fat cell metabolism, although net adipogenic effects might require the presence of insulin.
Important in vivo support for the role of GIP in regulating body weight was provided with the finding that mice lacking GIP receptors (GIPr) were protected from high fat diet (HFD)-induced obesity (DIO) (9, 21). More specific support for a primary insulin-independent role of GIP in promoting adiposity was found by intercrossing GIPr−/− mice with mice lacking IRS-1. These relatively insulin-insensitive mice were found to have decreased adiposity compared with the IRS-1 knock-out mice when fed a standard chow (22).
Surprisingly, GLP-1r knock-out mice have been reported to have a similar phenotype as GIPr−/− mice after high fat (HF) feeding (21). Apart from its well established role as an insulin secretagogue, reports on whether GLP-1 directly regulates adipose tissue metabolism are conflicting (23–30). In contrast, GLP-1 is an established satiety factor reducing food intake and, at least in pharmacological doses, body weight gain in rodents and humans (31–36). Furthermore, intracerebroventricular injections of GLP-1 in mice have been shown to reduce adiposity through the sympathetic nervous system, independent of food intake (32). Overall, these extra pancreatic effects antagonize fat storage.
Hence, the physiological importance of the cross-talk between intestine, beta-cells, nervous system, and adipose tissue in the regulation of body weight and adipose tissue mass is still not well defined. To further investigate the contribution of GIPr signaling in beta-cells and adipose tissue to body weight, we generated two transgenic mouse models with expression of the human GIPr targeted to the beta-cells or to adipose tissue and followed their body weight gain under HF feeding. At the end of the study period, body composition and metabolic parameters were investigated. In agreement with previous findings, mice with general ablation of the GIPr had reduced HFD-induced weight gain. As a key finding, we show that this was reversed in mice with targeted expression to white adipose tissue (WAT) but not in mice with targeted expression to pancreatic beta-cells. Surprisingly, total fat masses were similar between all groups, and the mice with general ablation of the GIPr differed predominantly by an absent HFD-induced lean mass gain. This deficiency in HFD-induced lean mass gain was rescued in mice with GIPr signaling in adipose tissue. Hence, these results suggest an important role for the adipocyte GIP receptor in regulating body weight but do not support a direct role for GIP in promoting adipogenesis. Furthermore, isolated preservation of the GIP-dependent part of the enteroinsular axis alone could not be found to promote body weight gain or adipogenesis on a HFD.
EXPERIMENTAL PROCEDURES
Generation of Transgenic Mice
The RIP-II and aP2 promoters in pBluescript SK plasmid (Stratagene) were provided by Dr. P. Herrera and Dr. R. Graves, respectively (37, 38). The human GIPr was cloned in-house and found by sequencing to match the published GIPr. The GIPr cDNA was inserted downstream of the RIP-II and aP2 promoters by PCR. The two plasmids were then purified, linearized, and injected into the pronucleus of fertilized oocytes, harvested from C57BL/6×DBA2 F2 mice, and transferred to pseudopregnant female mice at the two-cell stage using standard procedures. Three lineages of the aP2-hGIPr and two lineages of the RIP-hGIPr were generated. Expression of the human GIPr in founder mice from all lineages was analyzed by quantitative real time (qRT) PCR on liver, pancreas, muscle, and WAT, and one lineage from each strain was selected for further analysis.
GIPr−/− mice were previously published (39) and backcrossed to C57BL/6 for 10 generations. Heterozygous aP2-hGIPr and RIP-hGIPr transgenic mice were backcrossed two generations with C57BL/6 and then further crossed with GIPr−/− for two generations to obtain the genotypes investigated in this study. All mice used in this study were thus backcrossed for four to five generations to C57BL/6 as described under “Results” (see Table 1). Furthermore, using this breeding regimen, all genotypes investigated for each transgenic strain were bred as littermates. As similar data were observed between the control groups, data presented from wild type (nontransgenic and GIPr+/−) and GIPr−/− (nontransgenic and GIPr−/−), respectively, were pooled from mice obtained in the aP2-hGIPr and the RIP-hGIPr breeding regime. Genotyping was performed by PCR on tail genomic DNA before and after each experiment.
TABLE 1.
Representation of the genotype nomenclature and the breeding strategy for intercrossing with GIPr−/− mice and transgenic mice
For intercross 1, each transgenic strain carrying both the hGIPr and the murine GIPr (aP2+/+ and RIP+/+) was intercrossed with GIPr−/− mice giving rise to 50% transgenic and 50% nontransgenic littermates all heterozygous for the murine GIPr. For intercross 2, transgenic and heterozygous animals (aP2+/− or RIP+/−) from the first intercross were bred with GIPr−/− mice for production of all the animals enrolled in experiments. The second intercross gives rise to 50% of littermates carrying the transgenic hGIPr under control of the aP2 promoter or RIP (aP2 or RIP, respectively) and 50% nontransgenic littermates (WT). The distribution of the genetic modification donated from the GIPr−/− mice gives rise to 50% animals heterozygous for the murine GIPr and the other half lacking the murine GIPr. Overall, the second intercross results in four different genotypes as follows: 1) nontransgenic mice heterozygous for the murine GIPr (WT+/−); 2) nontransgenic mice with a whole body ablation of the murine receptor (WT−/−); 3) transgenic mice and heterozygous for the murine GIPr (aP2+/− or RIP+/−), and 4) transgenic mice and whole body ablation of the murine GIPr (aP2−/− or RIP−/−). Results from mice with the genotypes WT+/− and WT−/− from both colonies were pooled.
| Intercross 1 | Intercross 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Breeders | GIPr−/−×aP2+/+ | GIPr−/−×RIP+/+ | GIPr−/−×aP2+/− | GIPr−/−×RIP+/− | ||||
| Offspring | WT+/− | aP2+/− | WT+/− | RIP+/− | WT+/− | aP2+/− | WT+/− | RIP+/− |
| WT−/− | aP2−/− | WT−/− | RIP−/− | |||||
Mice were housed under a light/dark cycle of 12 h with free access to food and water, except where otherwise noted, and bred under specific pathogen-free conditions. All animal experiments were conducted in accordance with institutional guidelines and approved by the Animal Experiments Inspectorate in Denmark.
RNA Preparation and qRT-PCR
Mice (fifth generation), 23–29 weeks of age and fed a standard chow, were used for analysis of expression of the transgenic hGIPr in proximal small intestine, liver, quadriceps muscle, inguinal WAT, epididymal WAT, perirenal WAT, brown adipose tissue, and pancreatic tissue. For isolation of peritoneal macrophages, freshly euthanized mice had their abdominal skin removed and the peritoneal cavity was injected with ∼5 ml of ice-cold PBS; mice were then gently tapped on the abdomen, and the PBS was carefully recovered through a syringe. Macrophages were then pelleted by centrifugation. Total RNA was extracted using TRI Reagent® according to manufacturer's specification. For RNA extraction from fatty tissues, the upper fat phase was separated and removed immediately after homogenization. Total RNA was DNase-treated using Turbo DNA free kit from Applied Biosystems and reverse-transcribed with oligo(dT) primers according to the RevertAid H Minus First Strand Synthesis kit from Fermentas. For analysis of IL-6 expression, total RNA was reverse-transcribed using iScriptTM cDNA synthesis kit (Bio-Rad). qRT-PCR was performed using the following primers: murine GAPDH, 5′-caatgtgtccgtcgtgga-3′ and 5′-gatgcctgcttcaccacc-3′; human GIPr, 5′-gatgcctgcttcaccacc-3′ and 5′-gtagaggacgctgaccagga-3′; murine β-actin primers, 5′- ttctggtgcttgtctcactga-3′ and 5′-cagtatgttcggcttcccattc-3′. For detection of IL-6, a QuantiTect primer assay (Qiagen) was used. Human GIPr expression was calculated using the 2−ΔΔCt method. Normalized relative IL-6 expression levels were calculated using the formula described in Ref. 40.
Western Blot Analysis
Islets were isolated by collagenase digestion and kept in tissue culture for 24 h in RPMI 1640 medium containing 10% newborn calf serum, as described previously (41), and lysed in ice-cold Nonidet P-40 buffer (Invitrogen) supplemented with protease inhibitor mixture (Roche Applied Science). Samples were applied to SDS-PAGE, and mouse anti-β-actin was used for normalization (Santa Cruz Biotechnology). For extraction of WAT proteins, freshly removed inguinal, epididymal, and perirenal WAT were pooled, stored in ice-cold Hanks' buffered saline solution, and immediately lysed using the following protocol. Tissues were dissolved in chloroform/methanol (1:2) by vortexing; chloroform/water (1:1) was added, and samples were centrifuged at 1000 × g for 10 min. The top aqueous phase containing the proteins from the lysed cells was collected. Protein concentration was measured by Bradford assay (Pierce) following the manufacturers' instructions, and 5 μg were applied to SDS-PAGE. Expression of the transgenic human GIPr in the islet and WAT samples was analyzed using a polyclonal rabbit anti-GIPr (Abgent, AP7495a) directed at the N-terminal region of the human receptor.
High Fat Diet Feeding
At 8 weeks of age, male mice (all groups were backcrossed to B57BL/6 for four generations) were fed a HFD or a low fat control diet (CD) made of the same purified dietary components but with varied percentages of lard and soybean oil as the fat source. The HFD had 60% and the CD had 10% kilocalories from fat. The percentages of saturated, monounsaturated, and polyunsaturated fatty acids in the HFD and CD were 37:25, 46:35, and 17:40, respectively (for details see D12492 and D12450B, Research Diets, Inc.). Individual mouse body weights were monitored every 14th day over a period of 28 weeks. For assessment of whole body fat and lean mass, a rodent NMR analyzer was used (EchoMRI 4-in-1 Composition AnalyzerTM, Echo Medical Systems, LLC, Houston, TX). At the end of the study, mice were housed individually and acclimatized for at least 3 days before measurements of food intake were initiated. For determination of food intake, the food was preweighed and reweighed after 24 h. At the end of the experiment mice, were anesthetized and terminally bled into tubes with EDTA. Plasma was collected after centrifugation and stored at −80 °C. Fat pads were excised and weighed. Pancreata were cut in two for protein and histological analysis, respectively. WAT samples for RNA extraction were stored in RNA Later (Ambion) at −80 °C, and liver, pancreas, and WAT samples for histological analysis were fixed in 4% paraformaldehyde.
Oral Glucose Tolerance Test (OGTT)
Mice fed a HFD or CD for 20 weeks were fasted overnight (16–18 h) with free access to water. The mice were given an oral glucose load (2 g/kg) through a gavage tube. Blood samples were taken from a tail vein 0, 30, 60, 90, and 120 min following the glucose load. Blood glucose levels were measured using handheld “plasma-calibrated” glucometer (Accu-Chek Compact Plus, Roche Applied Science). OGTT for measurement of insulin was carried out in a separate experiment at week 21. Blood was collected in pre-chilled EDTA-coated capillary tubes from tail vein 0 and 20 min after glucose administration. Plasma was separated by centrifugation and stored at −80 °C until analysis. For measurements of plasma insulin levels, an ultrasensitive mouse insulin ELISA kit was used (Crystal Chem. Inc.).
Histological Analysis
Paraformaldehyde-fixed pancreas tissue and inguinal and epididymal WAT were embedded in paraffin and cut into 5 μm (pancreas) or 8 μm (WAT) sections using a microtome. Pancreas tissue was immunostained with either glucagon antiserum diluted 1:4800 (ab4304 raised in rabbit) or insulin antiserum diluted 1:12,800 (ab2006-4 raised in guinea pig). The sections were incubated with the primary antibodies overnight at 4 °C, and immunohistochemistry was performed using a biotinylated secondary antibody and a streptavidin biotin conjugate, and the reactions were visualized using diaminobenzidine. Sections were counterstained with hematoxylin. The relative area of immunoreactive cells in three randomly selected islets per section was measured. WAT sections were stained with hematoxylin/eosin, and the diameter of 20 adipocytes per section was measured. Paraformaldehyde-fixed liver tissues were stained with Oil Red O solution for 10 min at room temperature, followed by a light counterstain with hematoxylin. The percentage of Oil Red O staining area was measured in three random visual fields from each slide, and the mean percent ± S.E. was then calculated for each genotype. All measurements were performed on images using the Image Pro software version 7.0.
Protein Extraction from Pancreas
Pancreas tissue was homogenized in 1% trifluoroacetic acid, centrifuged, and further purified on C18 Sep-Pak® cartridges (Waters) following the manufacturer's instructions. Insulin was measured by ELISA (Mercodia, Uppsala, Sweden.) as described by the manufacturer. Glucagon was measured by a radioimmunoassay using the antibody 4305 (42).
Plasma Adipokine Analysis
Plasma content of leptin, total insulin-like growth factor-1 (IGF-1), and total and high molecular weight (HMW) forms of adiponectin were measured by ELISA (Alpco Diagnostics) according to the manufacturer's instruction.
Insulin Tolerance Test (ITT)
Mice enrolled in the study for 23–24 weeks were fasted for 4 h and then received 0.3 units/kg insulin (Actrapid, 100 units/ml, Novo Nordisk) by intraperitoneal injection. A blood sample was taken from a tail vein 0, 30, 60, 90, and 120 min following administration of insulin.
Statistics
Data is presented as mean ± S.E. except where noted. For comparison of means between two groups, a two-tailed unpaired Student's t test was used. For comparison of means between more than two groups, defined by CD or HF diet, a one-way analysis of variance with Dunnett's multiple comparisons test was applied. All groups were compared with WT+/− or WT−/− in two separate post tests. A two-way analysis of variance with Bonferroni post test was applied when comparing groups defined by two factors. AUC was used to compare differences in glucose excursions during OGTT and ITTs. Statistical analysis and AUC calculations were carried out using GraphPad Prism version 5. A p value less than 0.05 was considered statistically significant.
RESULTS
Generation of Transgenic Mice with Expression of Human GIP Receptor under Control of RIP or aP2 Promoter
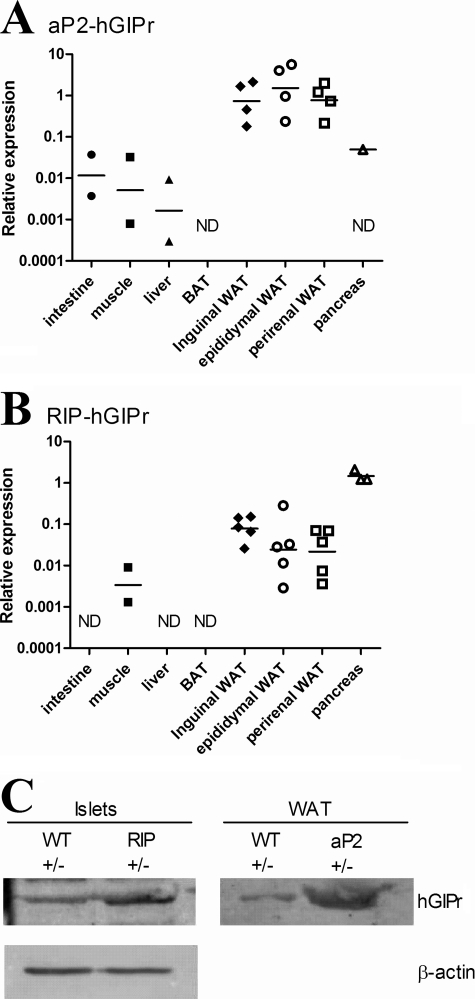
To examine the importance of GIP receptor signaling in WAT and in insulin producing beta-cells on body weight gain, we generated two transgenic mouse strains with expression of the hGIPr under control of the fatty acid-binding protein (aP2-hGIPr) or the rat insulin promoter (RIP-hGIPr). The hGIPr used as the transgene was validated in the 3T3F442A cell line after stable transfection and found to bind its hGIP ligand resulting in cAMP accumulation (not shown). The aP2-hGIPr line chosen for further investigation had a preferential expression of hGIPr mRNA to WAT with low or undetectable levels in whole pancreas (Fig. 1A), whereas RIP-hGIPr mice as expected had a significant pancreatic expression (Fig. 1B). Surprisingly, we also found expression of hGIPr mRNA in WAT under control of the RIP although this was on average at least 10-fold less than compared with mRNA levels detected in the aP2-hGIPr strain. For both strains, only low or undetectable levels of the hGIPr were found in small intestine, skeletal muscle, liver, and brown adipose tissue (Fig. 1, A and B). Potential transgene-driven expressions in macrophages were investigated on peritoneal macrophages in a separate assay, but they were found to be very low and only detectable in some of the samples from aP2-hGIPr transgenic mice and none from the RIP-hGIPr transgenic mice (data not shown). Expression of the transgene at the protein level was confirmed by Western blot analysis on lysates from isolated islets and WAT (Fig. 1C). Use of an antibody directed against the N terminus of human GIPr yielded a band migrating at an apparent mass of 65 kDa, which is in agreement with previous reports detecting the related GIPr in wild type rat tissues (43, 44). To investigate the functions of tissue-restricted GIPr signaling, the two lines were each bred for two generations with the C57BL/6 strain and then further bred with the C57BL/6 backcrossed GIPr−/− mouse, over two further generations to obtain the genotypes investigated in this study (see Table 1 for schematic overview) as follows: 1) nontransgenic and heterozygous for the murine GIPr (WT+/−); 2) nontransgenic with whole body ablation of the murine GIPr (WT−/−); 3) transgenic for the hGIPr under control of the aP2 promoter and heterozygous for the murine GIPr (aP2+/−); 4) transgenic for the hGIPr under control of the aP2 promoter and ablation of the murine GIPr (aP2−/−); 5) transgenic for the hGIPr under control of RIP and heterozygous for the murine GIPr (RIP+/−), and 6) transgenic for the hGIPr under control of RIP and ablation of the murine GIPr (RIP−/−). Hence all mice were backcrossed for four generations from the C57BL/6×DBA/F2 hybrid background in which the founders were created.
FIGURE 1.
Expression of the transgenic human GIPr in the aP2-hGIPr and RIP-hGIPr strains. The relative expression of the transgenic hGIPr in selected tissue are shown for the aP2-hGIPr strain (A) and RIP-hGIPr strain (B). Expressions are relative to GAPDH Ct values in the same sample, and all samples are normalized to an identical calibrator (inguinal WAT cDNA from an aP2-hgipr transgenic mouse). Each replicate is shown by a dot, and the line represents the geometric mean on a logarithmic scale. ND, not detected. C, expression of the transgene at the protein level was confirmed by Western blot analysis on lysates from isolated islets (pool of islets from two to three mice in each group) and WAT (pool of tissue from two mice in each group). Use of an antibody directed against the N terminus of human GIPr yielded a band migrating at an apparent mass of 65 kDa. Equal amount of protein was loaded in the WAT lanes as β-actin was found to precipitate in WAT lysates.
Body Weight after 20 Weeks of HFD or Matched CD
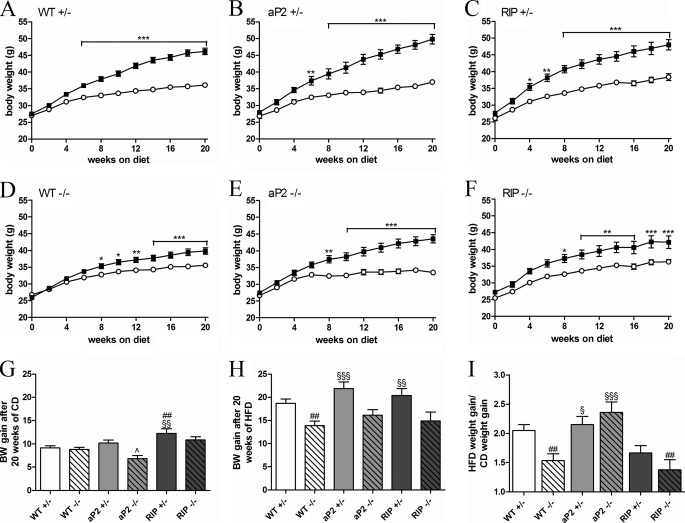
To investigate the effect of GIPr signaling in adipose tissue or pancreatic beta-cells on body weight, male mice were randomly assigned to a purified HFD (60% kcal from fat) or a matched low fat CD (10% kcal from fat) for 20 weeks starting from 8 weeks of age, after which the animals were subjected to a range of physiological experiments for a further 8 weeks (detailed below). As the animals on average suffered a transient 5% weight loss during these experiments, analysis of weight gain was restricted to the data obtained up to week 20 of HF feeding. The longitudinal weight gains are plotted in Fig. 2, A–F, illustrating the weights of the HFD- and CD-fed mice of each genotype. The cumulative weight gains during the study period are also plotted for CD and HFD in Fig. 2, G and H.
FIGURE 2.
Body weight gain when fed a CD or HFD. Male mice were fed a low fat CD or a HFD made of the same purified dietary components. A–F, longitudinal body weights are shown over a period of 20 weeks. Mice fed a CD are shown in white. Mice fed a HFD are shown in black. G and H, cumulative weight gains during the study period are shown in G for each genotype fed a CD and in H for each genotype fed a HFD. I, as variations between groups fed a CD were observed, weight gain obtained after HF feeding was normalized to weight gain obtained when fed a CD. This calculation essentially shows the effect of HFD on body weight gain for each genotype with 1 representing no effect. Data are shown as mean ± S.E. For WT+/− and WT−/−, n = 31–54 per group. For other groups, n = 11–16 per group. *, p < 0.05; **, p < 0.01; ***, p < 0.001, HFD versus CD; ##, p < 0.01 versus WT+/− fed the same diet; §, p < 0.05; §§, p < 0.01; §§§, p < 0.001 versus WT−/− fed the same diet; ∧, p < 0.05; aP2−/− versus aP2+/−.
In the CD groups, RIP+/− mice were found to gain significantly more weight compared with WT+/− indicating an effect of GIPr signaling in beta-cells in regulating body weight. This effect was attenuated in the RIP−/− mice (Fig. 2G). In aP2 transgenic mice fed a low fat diet, the endogenous GIPr (aP2+/−) was found to stimulate weight gain. In agreement with previous observations, this difference was not seen in nontransgenic mice (Fig. 2G).
When fed a HFD WT+/− mice had a significant weight increase compared with control fed mice (Fig. 2A), whereas WT−/− only had a minor weight increase (Fig. 2D). Thus, we could confirm the central findings of the previous reports that investigated GIPr controlled weight gain (9, 21). Both transgenic strains with preserved endogenous murine GIPr signaling had a weight increase comparable with the WT+/− (Fig. 2, B, C and H). Neither the aP2−/− nor the RIP−/− had a significantly higher weight gain during the period of HF feeding when compared with WT−/− (Fig. 2H), although the average aP2−/− mouse gained slightly more weight than the average WT−/− mouse.
As variations were present between groups of animals fed a CD, we decided to control for this factor by normalizing the weight gain obtained on the HFD to the weight gain obtained for animals with the same genotype but fed a CD. This calculation essentially shows the effect of HFD on body weight gain for each genotype (Fig. 2I). According to this analysis, the aP2 promoter-driven GIPr expression increased the aP2−/− weight gain at a comparable level to the endogenous GIPr expression, whereas the RIP-driven expression failed to do so. The measured food intake did not differ significantly between the groups (p = 0.24 for groups on CD and p = 0.56 for groups on HFD), which suggests that differences in body weight gain were independent of food intake (data not shown).
Taken together, these data indicate that GIP receptor signaling in both beta-cells and WAT play a role in regulating body weight. However, only signaling in WAT is essential for HFD-induced body weight gain.
Body Composition and Fat Storage
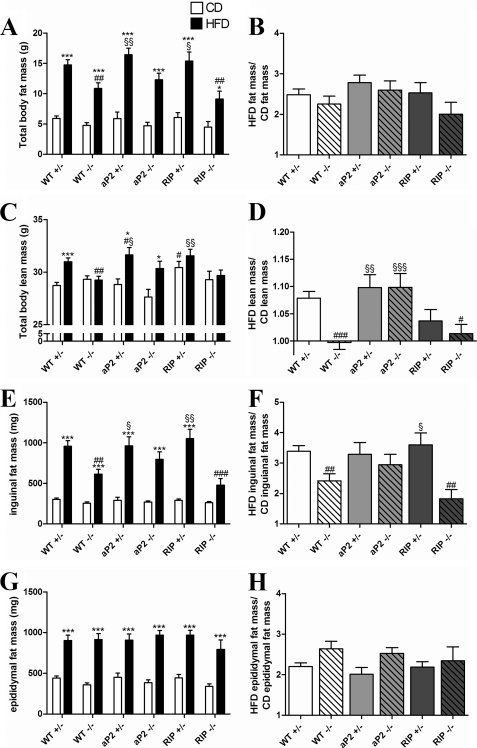
To examine the contribution of fat and lean mass to body weight, we analyzed body composition using a rodent nuclear magnetic resonance (NMR) analyzer. All mice fed a CD had similar body fat mass (Fig. 3A). When fed a HFD WT−/− and RIP−/− had a significantly lower whole body fat mass compared with WT+/− consistent with the observed attenuated HFD-induced weight increase (Fig. 3A). To analyze the sensitivity of mice to DIO, fat mass after HF feeding was normalized to fat mass after CD (Fig. 3B). Surprisingly, despite significant differences in relative HFD-induced body weight gain (Fig. 2I), HFD-induced fat mass was similar in all groups when normalizing to CD (Fig. 3B). To explain the higher body weight gain observed for the RIP+/− mice on CD, we found a significant increase in total lean mass as compared with WT+/− (Fig. 3C). As expected, WT+/− increased their lean mass when fed a HFD (Fig. 3, C and D). This was also observed for aP2+/− and aP2−/− mice but not WT−/−, RIP +/−, or RIP−/− mice (Fig. 3, C and D). The reduced lean mass gain in RIP+/− could possibly be explained by the already increased lean mass of this genotype on a CD.
FIGURE 3.
Analysis of body composition. Total fat (A) and total lean mass (C) was measured by NMR in the 27th week of the study period for each genotype fed a CD (white bar) or a HFD (black bar). At the end of the study, fat pads were excised and weighed. E shows the mass of a single inguinal fat pad for each genotype, and G shows the mass of a single epididymal fat pad. B, D, F, and H show the effect of HFD; masses obtained after HF feeding were for each genotype normalized to the mass obtained when fed a CD. This essentially reflects the effect of the HFD with 1 representing no effect. Data are shown as mean ± S.E. For WT+/− and WT−/−, n = 19–34 per group. In other groups, n = 6–15. *, p < 0.05; ***, p < 0.001; HFD versus CD; #, p < 0.05; ##, p < 0.01; ###, p < 0.001 versus WT+/− fed the same diet; §, p < 0.05; §§, p < 0.01; §§§, p < 0.001 versus WT−/− fed the same diet.
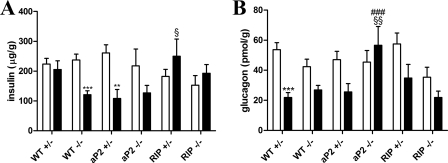
To investigate the contribution of selected WAT depots to the total fat mass, inguinal and epididymal fat pads were removed at the end of the study and weighed. Mass of inguinal fat did not differ in groups fed a CD (Fig. 3E), whereas HFD-induced inguinal fat storage was significantly less in WT−/− and RIP−/− but not in aP2−/− as compared with WT+/− (Fig. 3F). On the contrary, HFD increased epididymal fat pads similarly in all genotypes (Fig. 3, G and H). We also investigated the size distribution of individual adipocytes from epididymal and inguinal fat pads in HFD-fed mice and found only modest differences in adipocyte size, except with regard to the RIP−/− mice that had a significant reduction in inguinal adipocyte size as compared with WT+/− mice (data not shown). Overall, the adipocyte size reflected the size of the fat depot. Analysis of the pancreatic islets was performed by staining individual islets for insulin or glucagon and plotting the fraction of insulin or glucagon-stained area/total area of the islets. Although there were trends to a relative increase in beta-cell area and a relative reduction of α-cell area in the HFD-fed animals, there were no significant genotype-dependent differences (data not shown). However, as this analysis could not address differences in protein content, we decided also to extract glucagon and insulin from pancreatic tissue. These analyses revealed a reduction of insulin content in the WT−/− and aP2 transgenic mice on a HFD, which was rescued by beta-cell GIPr expression (Fig. 4A). Notably glucagon content decreased in all mice except the aP2−/− mice, and aP2+/− mice also had reduced glucagon content (Fig. 4B).
FIGURE 4.
Analysis of pancreatic glucagon and insulin content. Pancreatic tissues were harvested from animals after 28 weeks of feeding a CD (white bar) or a HFD (black bar), and the protein content was extracted. A depicts pancreatic content of insulin as determined by ELISA. B depicts pancreatic content of glucagon as determined by radioimmunoassay. Data are shown as mean ± S.E. For WT+/− and WT−/−, n = 17–22 per group. In other groups, n = 6–10. **, p < 0.01; ***, p < 0.001; HFD versus CD; ###, p < 0.001 versus WT+/− fed the same diet; §, p < 0.05; §§, p < 0.01 versus WT−/− fed the same diet.
Hepatic lipid storage was investigated by Oil Red O staining of liver tissue section. This analysis showed similar and low lipid content (2–7%) in all genotypes on a CD, whereas HF feeding dramatically increased hepatic lipid storage, but only in mice with endogenous GIP receptor expression (20–27% versus 8–9%, data not shown). This supports previous studies suggesting important roles of the GIP receptor in regulating hepatic blood flow and nutrient incorporation (45), but it also suggests that differences in hepatic metabolism do not contribute to the differences in body composition between HF-fed WT−/−, aP2−/−, and RIP−/− mice.
Metabolic Parameters
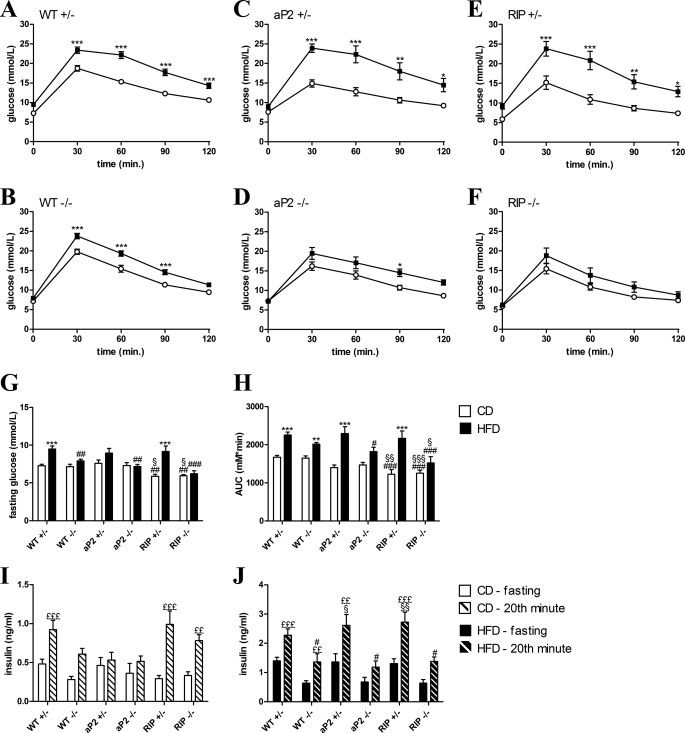
To investigate the effect of the transgenic modulations on metabolic control, we performed oral glucose tolerance test (OGTT) after 20 weeks of low and HF feeding. Mice were fasted overnight with free access to water. As expected, WT+/− and WT−/− mice fed a CD had similar glucose fasting levels, whereas RIP+/− and RIP−/− mice fed a CD had significantly lower fasting glucose levels despite normal fasting insulin levels (Fig. 5, G and I). Consistent with development of insulin resistance, WT+/− mice fed a HFD had significantly elevated fasting glucose levels compared with WT+/− mice fed a CD. In agreement with previous reports, WT−/− mice had unchanged fasting glucose levels, and this was also observed for aP2−/− and RIP−/− mice (Fig. 5G). Surprisingly, WT−/− mice fed a CD had similar glucose excursions as WT+/− (Fig. 5, A, B and H). Expression of the GIPr under the RIP significantly lowered glucose excursions resulting in a significantly improved glucose tolerance when analyzing the AUC for RIP+/− and RIP−/− compared with WT+/− or WT−/− (Fig. 5H). This occurred without a simultaneous increase in glucose-stimulated insulin levels (Fig. 5I). Furthermore, both transgenic strains fed a CD had lower glucose levels 30 min after an oral glucose load (Fig. 5, A–F) but no significant changes in glucose-stimulated insulin release measured 20 min after glucose challenge (Fig. 5I). WT +/− mice significantly increased their AUC when fed a HFD compared with CD, and both fasting and glucose-stimulated insulin release were elevated in agreement with a HFD-induced beta-cell impairment and insulin resistance. Even though the WT−/− mice had a significantly lower body weight than WT+/− mice after 20 weeks of HFD, the glucose excursion did not differ significantly from the WT+/− (Fig. 5, B and H). The beneficial effect of expression of the GIPr in beta-cells or WAT on glucose tolerance that was observed in mice fed a CD was abolished in RIP+/− and aP2+/− mice fed a HFD (Fig. 5, C, E, and H), but it persisted in RIP−/− and aP2−/− mice (Fig. 5, D, F, and H). All mice fed a HFD had elevated fasting and glucose-stimulated insulin levels compared with levels in CD-fed mice, but this reached statistical significance only for mice with preserved murine GIPr expression (Fig. 5, I and J). Furthermore, glucose-stimulated insulin levels were significantly lower in WT−/−, aP2−/−, and RIP−/− fed a HFD compared with WT+/− mice (Fig. 5J).
FIGURE 5.
Oral glucose tolerance tests. Mice fed a CD (white circles) or a HFD (black squares) for 20–22 weeks were given an oral glucose challenge after an overnight fast (16–18 h). Blood samples were taken from a tail vein at the indicated time points for measurement of glucose or insulin. A–F, glucose excursion curves are shown for each genotype fed a CD and HFD. G, fasting glucose levels after an overnight fast (16–18 h). H, AUC are shown for comparison of differences in glucose excursions. OGTTs for measurement of insulin were carried out in a separate experiment. A blood sample was taken 0 and 20 min after an oral glucose load for each genotype fed a CD (I), and a HFD (J). Data are shown mean ± S.E. For WT+/− and WT−/−, n = 19–35 per group. In other groups, n = 6–14. *, p < 0.05; **, p < 0.01; ***, p < 0.001; HFD versus CD; #, p < 0.05; ##, p < 0.01; ###, p < 0.001 versus WT+/− fed the same diet; §, p < 0.05; §§, p < 0.01 versus WT−/− fed the same diet. ££, p < 0.01; £££, p < 0.001 versus insulin fasting levels within the group.
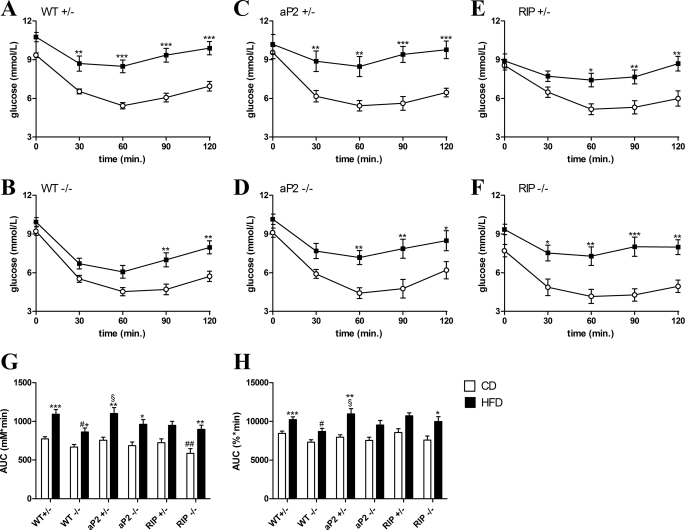
To examine whether the improvements in metabolic control seen in the RIP transgenic mice on CD, or in the mice lacking endogenous GIPr on HFD, reflected changes in insulin sensitivity, we performed insulin tolerance tests after a 4-h fast. As observed for fasting glucose levels, mice expressing the GIPr under control of the RIP showed changes in 4-h postprandial glucose levels (Fig. 6, E and F). RIP−/−, but not RIP+/− mice, fed a CD had significantly lower basal glucose levels than WT+/− mice, whereas both genotypes had significantly lower basal levels after HF feeding (Fig. 6, A, E, and F). These differences naturally affected the AUC glucose (Fig. 6G). To overcome this, glucose excursions shown as percentage of base-line blood glucose were analyzed, and their AUC is shown in Fig. 6H. All mice became significantly insulin-resistant when fed a HFD (Fig. 6, A–F), although, consistent with previous studies, the WT−/− mice are significantly more insulin-sensitive than WT+/− mice after HF feeding (Fig. 6, G and H). Improved insulin sensitivity could even be detected in mice fed the matched CD when comparing the AUC using a Student's t test (p < 0.02), although this did not reach statistical significance in the multiple comparisons test. As the weights were similar in the CD-fed groups, the increased insulin sensitivity in WT−/− mice is probably not exclusively secondary to the lower body weight. On the contrary, mice with exclusive expression of the GIPr under control of the aP2 promoter or RIP were not significantly different from WT+/− or to WT−/− when shown as a percentage of base-line glucose levels (Fig. 6H).
FIGURE 6.
Insulin tolerance test. Mice fed a CD (shown in white) or HFD (shown in black) for 23–24 weeks were fasted 4 h and given 0.3 units/kg insulin by intraperitoneal injection. Blood samples were taken from a tail vein at the indicated time points for measurement of glucose elimination. A–F, glucose excursion curves are shown for each genotype fed a CD and HFD. The AUC are shown for comparison of differences in glucose excursions. G shows the AUC calculated from the glucose concentrations over time, and H shows AUC calculated from glucose excursion curves shown as percentage of base-line blood glucose. Data shown are mean ± S.E. For WT+/− and WT−/−, n = 17–33 per group. In other groups n = 8–12. *, p < 0.05; p < 0.01; ***, p < 0.001, HFD versus CD; #, p < 0.05; ##, p < 0.01 versus WT+/− fed the same diet; §, p < 0.05 versus WT−/− fed the same diet.
Adipokine Expression and Secretion
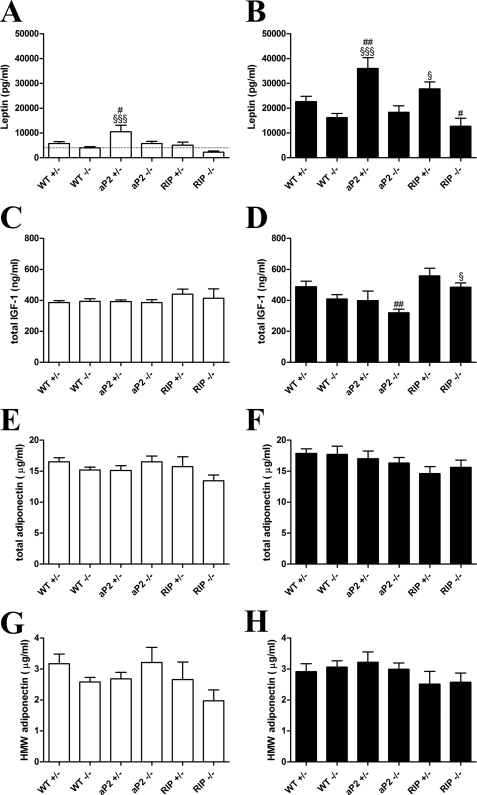
To address whether adipocyte GIPr would lead to secretion of mediators acting on other tissues, we first analyzed the prototypic adipocyte proinflammatory cytokine IL-6 mRNA expression levels in inguinal and epididymal WAT from aP2 transgenic mice and controls. However, IL-6 mRNA levels were similar between groups fed a CD or a HFD, respectively (data not shown). We then assessed the plasma levels of the noninflammatory adipokines leptin and adiponectin as well as the total IGF-1. These analyses revealed no differences in total or HMW adiponectin (Fig. 7, E–H), but adipocyte overexpression of GIPr was found to augment plasma leptin content in the presence of the endogenous GIPr on both CD- and HFD-fed animals (Fig. 7, A and B). Noteworthy, all HFD-fed groups responded with higher leptin levels than their fed counterparts. Total IGF-1 levels were generally similar between groups and only slightly augmented by HFD in WT+/− mice; however; IGF-1 levels were reduced in aP2−/− mice as compared with WT+/− mice (Fig. 7, C and D).
FIGURE 7.
Analysis of plasma adipokine levels. After 28 weeks of feeding a CD (white bar) or a HFD (black bar), animals were bled terminally, and adipokine levels were determined by ELISA as described under “Experimental Procedures.” A and B depict plasma leptin. The dashed line indicates lower detection limit of the assay. C and D depict total plasma IGF-1. E and F depict total plasma adiponectin, and G and H specifically depicts HMW forms of adiponectin. Data are shown as mean ± S.E. For WT+/− and WT−/−, n = 13–20 per group. In other groups n = 7–11. #, p < 0.05; ##, p < 0.01 versus WT+/− fed the same diet; §, p < 0.05; §§§, p < 0.001 versus WT−/− fed the same diet.
DISCUSSION
The main purpose of the study was to investigate the effect of GIPr expression targeted to pancreatic beta-cells or WAT on body weight gain after HF feeding in a physiological model. The central findings of this study are that mice with GIPr expression targeted to WAT have significantly higher HFD-induced body weight gain than mice with a general ablation of the receptor. This was due to an increase in total lean body mass rather than a gain in total fat mass as would have been expected. In addition, GIPr signaling in beta-cells during diminished GIPr signaling in adipose tissue does not seem to be important for regulation of body weight after HF feeding.
The role of the GIPr in regulation of body weight has previously been investigated in the GIPr−/− mouse model. GIPr null mice were reported to be resistant to HFD-induced obesity (9), and this was later reproduced by another research group, although in this study the mice were not completely resistant to HFD-induced weight gain (21). Studies of the GIPr−/− model form the basis for the concept that GIP is an important regulator of body weight in response to HF feeding and support the hypothesis that GIP directly promotes adipogenesis. In addition, different strategies to inhibit GIP function have been investigated. Accordingly, administration of GIPr peptide antagonists, immunization against GIP, or ablation of the intestinal GIP-producing K-cell significantly lower body weight in mouse DIO models (46–48). However, in these physiological models, GIP function has been deduced from whole body ablation of ligand or receptor signaling, making it difficult for a conclusion regarding the tissue-specific effects of the GIPr activation. GIP is a well characterized and potent stimulator of insulin release and has also been suggested to exert insulin-mimetic effects directly in adipose tissue via its specific G protein-coupled receptor, promoting glucose and lipid uptake into adipocytes (9, 11, 12, 15, 18, 19, 49). Recently, GLP-1 receptor knock-out mice were reported to have a phenotype similar to GIPr knock-outs (21). Both single incretin knock-out strains as well as double incretin receptor knock-out mice (DIRKO) had reduced weight gain after 20 weeks of HF feeding. In agreement with a direct role of GIP in adipose tissue, GIP, but not GLP-1, was found to increase resistin plasma levels in a GIP receptor-dependent manner indicating that GIP may modulate the adipokine profile secreted from adipose tissue. This in vivo observation fits well with reports of GIP effects on adipose tissue expressing the GIP receptor (13–15). GLP-1 analogs are, however, known stimulators of satiety, and administration results in weight loss in rodents and humans (31–36). Accordingly, GLP-1r knock-out mice had an increased food intake but maintained low body weight gain after HF feeding (21). Importantly, all three strains (GIPr−/−, GLP-1r−/−, and DIRKO) failed to up-regulate insulin mRNA transcripts and insulin plasma levels under HFD-induced metabolic stress as observed in wild type animals, illustrating the importance of the entero-insular axis in regulating insulin secretion. The importance of incretin-mediated insulin secretion for development of obesity in HF feeding therefore cannot be deduced from these studies. Consequently, to investigate adipocyte and beta-cell-specific mechanisms for the phenotype observed in the HF-fed GIPr knock-out strain, we generated two transgenic mouse models with expression of the hGIPr under control of the aP2 promoter or RIP to target adipose tissue or beta-cells, respectively. These strains were then intercrossed to the GIPr knock-out strain. In agreement with previous studies, we found that WT+/− (for nomenclature see Table 1) and WT−/− fed a CD had similar body weight gains. However, when fed a HFD, WT−/− mice gained significantly less body weight than control mice during the 20-week study period, whereas mice with targeted expression of the GIPr to adipose tissue but otherwise lacking the murine GIPr (aP2−/−) had similar HFD-induced weight gain as WT+/−. This was significantly different from the HFD-induced weight gain in mice with a whole body ablation of the GIPr (WT−/−) indicating an important role for adipocyte expression of the GIPr in regulating body weight (Fig. 2I). In contrast, mice with targeted expression to beta-cells had HFD-induced weight gain similar to WT−/− and significantly different from WT+/− suggesting a phenotype similar to GIPr−/− mice when fed a HFD.
Consistent with a direct role for GIP in promoting adipogenesis, GIP has been demonstrated to enhance glucose and lipid uptake in adipocytes and to increase lipoprotein lipase activity (6, 8, 9, 11, 15, 18–20, 50). Furthermore, GIP has been shown to stimulate lipolysis in a dose-dependent manner but also to facilitate re-esterification in isolated rat adipocytes in a manner similar to insulin (12), whereas other studies in cell culture systems find additive effects of GIP and insulin on acute fat cell metabolism (9, 11, 18, 19). Thus, the net effect of GIP on adipocyte metabolism is unclear. In physiological models, the adipogenic potential of GIP has been estimated from measurements of total fat mass and fat depots by different techniques. In two studies, ovariectomized and aged GIPr null mice fed a standard chow had lower fat mass than wild types, measured by NMR and computed tomography, respectively (51, 52). A role for GIP in nutrient-dependent adipogenesis has been investigated by computed tomography and dual energy x-ray absorption scan. GIPr null mice fed a HFD for 3 weeks significantly increased visceral fat mass compared with control-fed GIPr−/− mice but not to the same level as wild type mice fed a HFD (53). Furthermore, during high fat feeding, mice immunized against GIP had lower total fat mass as measured by dual energy x-ray absorption (47). Other studies have measured weight of excised fat depots finding smaller inguinal, epididymal, and perirenal fat pads in the GIPr−/− mice (21), whereas GIP antagonism in DIO mice has been reported to result in a reduction in subcutaneous WAT but not epididymal and perirenal fat (46); the latter study is very much in line with what we observe in our cohort. Similar results could be seen in humans where GIP did not affect acute whole body lipid metabolism (54), but during a hyperinsulinemic-hyperglycemic clamp, GIP could be seen to increase lipid and glucose uptake in a subcutaneous abdominal fat pad (55). Accordingly, GIP may differentially modulate adiposity depending on type of WAT.
To investigate the importance of tissue-specific GIPr expression on whole body adiposity, we examined total body fat and lean mass by NMR, which, in contrast to previously applied methods, measures all fat depots as well as intraorgan fat droplets. In agreement with a lower body weight, WT−/− and RIP−/− mice had lower total fat mass compared with WT+/− when fed a HFD (Fig. 3A). For analysis of the ability in each strain to alter fat and lean mass in response to a HFD, masses obtained on a HFD were normalized to masses from animals fed a CD (Fig. 3, B and D). Surprisingly, in this analysis WT−/− did not have significantly lower total fat mass compared with WT+/− and did not increase lean mass. Consequently, the body weight gain observed in WT−/− was exclusively due to increases in fat mass to a level similar to WT+/−, and the HFD-induced weight gain difference between WT+/− and WT−/− primarily consists of increased lean mass. This suggests a requirement of GIPr in supporting HFD-induced lean mass gain, and this could be rescued in aP2−/− mice, which had a gain in total fat mass similar to both WT+/− and WT−/− but a HFD-induced gain in lean mass similar to WT+/−. Thus, aP2−/− mice had a body composition similar to WT+/− mice. Like WT−/−, RIP−/− did not significantly change lean mass further indicating a similar impairment in the response to high fat feeding. These results suggest that the adipocyte GIPr supports HFD-induced lean mass gain rather than whole body fat mass. Such an effect could be mediated by any number of secreted hormones and possibly also subject to neuronal regulation. However, several adipokines such as resistin, TNF-α, and IL-6 are usually found to be produced by adipose tissues and to exert systemic effects (56). As IL-6 potently stimulates nutrient uptake in muscle (57), we investigated IL-6 mRNA expression levels as a candidate proinflammatory adipokine regulated by the adipocyte GIPr. However, inguinal and epididymal IL-6 mRNA levels were similarly expressed in WT+/−, WT−/−, and aP2 transgenic mice fed a CD or a HFD, and thus no correlation could be found. Measurements of plasma levels of noninflammatory adipokines did, however, reveal a consistent increase in leptin levels in aP2+/− mice that have an unperturbed enteroinsular axis and thus suggested that adipocyte GIPr were able to synergize with endogenous insulin as has been suggested in cellular studies (9, 11, 18, 19), a synergy that we measure as the insulin-induced secretion of the satiety signal leptin (58). Augmented secretion of leptin also provided a satisfactory explanation as to why augmented adipocyte insulin signaling would not lead to uncontrolled body weight gain in the aP2+/− mice.
Interestingly, although beta-cell GIPr seems dispensable for HFD-induced lean mass gain, we observed that the RIP+/− but not RIP−/− mice fed a CD gained significantly more body weight and lean mass than other groups. Conversely, aP2−/− mice had significantly lower body weight gain than aP2 mice also expressing the endogenous receptor. Overall, this suggests, but does not prove, an interaction between adipocyte GIPr and beta-cell GIPr in promoting lean body mass in mice fed a CD. Taken together, despite the fact that we recapitulate previous findings on GIPr-controlled body weight gain, the data do not support a role for the adipocyte GIPr in promoting adipogenesis as suggested previously. However, even though DIRKO mice were found to have lower total fat mass by NMR after HF feeding, the extent of nutrient-dependent adiposity in GIPr null mice has not, at least to our knowledge, previously been investigated by NMR, a technique measuring all fat, including intraorganic fat droplets, in the body as opposed to computed tomography that recognizes volumes of adipose tissue that easily can be divided into subcutaneous and visceral subtypes. To further investigate the size of fat depots, we excised and weighed inguinal and epididymal fat pads at the end of the study. We found similar epididymal fat masses in all groups (Fig. 3, G and H), whereas WT−/− had significantly lower inguinal fat mass than WT+/− (Fig. 3F). These findings replicate the divergent WAT masses reported by McClean et al. (46) during GIP antagonism but not those by Hansotia et al. (21). The lower inguinal mass was partially reversed in aP2−/− mice, displaying an inguinal fat pad size not significantly different from WT+/− or WT−/−, whereas RIP−/− mice had an inguinal fat mass significantly lower than WT+/−.
As insulin action may significantly contribute to the phenotype investigated, we examined metabolic control by OGTT and ITT. In this study, we did not observe reduced glucose control in WT−/− mice after 20 weeks of low fat feeding, and insulin levels were insignificantly decreased. Previous studies with GIPr−/− mice have reported subtle deterioration in glucose excursion following an OGTT when fed a normal diet (39, 59). However, we performed OGTTs in older mice, and a compensatory mechanism may have evolved. Thus, young adult GIPr−/− mice fed a normal diet have been reported to have increased beta-cell sensitivity to GLP-1 resulting in elevated insulin secretion (59). Furthermore, this is the first study using a purified low fat diet instead of a chow diet with possible metabolic implications (60). After HF feeding, WT−/− mice had lower fasting glucose levels and slightly improved glucose excursions along with a significantly lower glucose-stimulated insulin secretion that characterizes the HF-fed phenotype of the incretin receptor knock-out mice (21, 39).
The transgenic groups were glucose-tolerant without improved insulin sensitivity or insulin secretion. This pattern was, however, disrupted after 20 weeks of HF feeding in transgenic mice also expressing the murine GIPr but preserved in transgenic GIPr knockouts. The observed improved glucose control in HF-fed transgenic knock-out mice was surprising as it took place with similar insulin levels as observed in WT−/− and with an insulin sensitivity between the WT−/− mice and the WT+/− mice. Logically, the improvement would be expected to be due to delayed intestinal uptake or decreased hepatic glucose output; yet mechanisms linking the transgenes to such effects are not clear, and the transgenes do not change hepatic lipid storage. However, it is also possible that the insulin tolerance tests are indiscriminate and that the higher lean mass in the transgenic knock-out mice contributes to increased glucose storage.
Although the aP2+/− and aP2−/− mice had a similar body composition when fed a HFD, they had differences in plasma leptin levels and pancreatic glucagon content. Thus, aP2+/− mice had elevated leptin levels, whereas aP2−/− had elevated glucagon levels. This may not be surprising as leptin is usually secreted in response to nutrients and insulin, and GIPr signaling has been demonstrated to synergize with insulin in adipocytes (8, 15, 61). One effect of increased leptin levels would be to inhibit glucagon expression (62), which would in turn contribute to explain the increased glucagon content seen in the pancreata from aP2−/− mice. Thus, although we could not detect differences in postprandial metabolism that could directly account for the observed changes in body composition after long term exposure to CD or HFD, we found evidence for adipokine secretion that may be acting to influence gene expression and metabolism hours after feeding and GIP release. Thus, one might speculate that postprandial GIPr signaling in adipocytes could induce gene expression in adipocytes that would promote energy mobilization from adipose tissue in the fasted state. If GIPr signalings were to act on adipocytes in such a way it would counteract lean mass and muscle tissue degradation, which would otherwise be degraded to supply amino acids for hepatic gluconeogenesis.
In support of the concept that GIP could facilitate gene expression lasting into the fasted state, we have seen differences in plasma leptin levels in samples collected during the daytime (Fig. 7, A and B). Similarly, others have reported increases in resistin secretion hours after GIP stimulation of adipocytes (61). Both resistin and leptin would antagonize adipocyte insulin sensitivity locally and in the fasted state lead to net energy mobilization (63, 64). Noteworthy, this hypothesis is further supported by the observation of improved insulin sensitivity in WT−/− mice, which could partially be rescued by adipocyte-specific GIPr expression.
Thus, it could be suggested that adipocyte GIPr, in the postprandial state, potentiates insulin signaling but also initiates gene expression leading to local insulin resistance that would facilitate energy mobilization in the fasted state. As insulin resistance is only local, energy utilization in peripheral tissue would not be as impaired.
Nevertheless, whatever the underlying mechanism, the results taken together support an independent role for the adipocyte, but not the beta-cell GIP receptor in the regulation of body weight and lean mass after high fat feeding; yet the study cannot support a direct role for GIP in promoting high fat diet-induced adipogenesis.
Acknowledgments
We thank Dr. P. Herrera for donating the RIP, Dr. R. Graves for donating the promoter of the aP2 gene, and Louise Bruun, Birita Fritleifdôttir Kjærbæk, Jovin Peter Suku, Sofie Pilgaard, Caroline Anna Mikaela Kiaer, and Heidi Paulsen for excellent technical assistance.
This work was supported by the Danish Medical Research Council, the Novo Nordisk Foundation, Fabrikant Vilhelm Pedersen og Hustrus Mindelegat, the Aase and Ejnar Danielsens Foundation, the Augustinus Foundation, the Becket Foundation, and Fonden til Laegevidenskabens Fremme.
- GIP
- glucose-dependent insulinotropic polypeptide
- GIPr
- glucose-dependent insulinotropic polypeptide receptor
- hGIPr
- human glucose-dependent insulinotropic polypeptide receptor
- HFD
- high fat diet
- DIO
- diet-induced obesity
- HF
- high fat
- WAT
- white adipose tissue
- RIP
- rat insulin promoter
- CD
- control diet
- qRT
- quantitative real time
- HMW
- high molecular weight
- OGTT
- oral glucose tolerance test
- ITT
- insulin tolerance test
- AUC
- area under curve.
REFERENCES
- 1. Baggio L. L., Drucker D. J. (2007) Gastroenterology 132, 2131–2157 [DOI] [PubMed] [Google Scholar]
- 2. Falko J. M., Crockett S. E., Cataland S., Mazzaferri E. L. (1975) J. Clin. Endocrinol. Metab. 41, 260–265 [DOI] [PubMed] [Google Scholar]
- 3. Creutzfeldt W., Ebert R., Willms B., Frerichs H., Brown J. C. (1978) Diabetologia 14, 15–24 [DOI] [PubMed] [Google Scholar]
- 4. Wasada T., McCorkle K., Harris V., Kawai K., Howard B., Unger R. H. (1981) J. Clin. Invest. 68, 1106–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ebert R., Nauck M., Creutzfeldt W. (1991) Horm. Metab. Res. 23, 517–521 [DOI] [PubMed] [Google Scholar]
- 6. Eckel R. H., Fujimoto W. Y., Brunzell J. D. (1979) Diabetes 28, 1141–1142 [DOI] [PubMed] [Google Scholar]
- 7. Knapper J. M., Puddicombe S. M., Morgan L. M., Fletcher J. M. (1995) J. Nutr. 125, 183–188 [DOI] [PubMed] [Google Scholar]
- 8. Kim S. J., Nian C., McIntosh C. H. (2007) J. Biol. Chem. 282, 8557–8567 [DOI] [PubMed] [Google Scholar]
- 9. Miyawaki K., Yamada Y., Ban N., Ihara Y., Tsukiyama K., Zhou H., Fujimoto S., Oku A., Tsuda K., Toyokuni S., Hiai H., Mizunoya W., Fushiki T., Holst J. J., Makino M., Tashita A., Kobara Y., Tsubamoto Y., Jinnouchi T., Jomori T., Seino Y. (2002) Nat. Med. 8, 738–742 [DOI] [PubMed] [Google Scholar]
- 10. Dupre J., Greenidge N., McDonald T. J., Ross S. A., Rubinstein D. (1976) Metabolism 25, 1197–1199 [DOI] [PubMed] [Google Scholar]
- 11. Hauner H., Glatting G., Kaminska D., Pfeiffer E. F. (1988) Ann. Nutr. Metab. 32, 282–288 [DOI] [PubMed] [Google Scholar]
- 12. Getty-Kaushik L., Song D. H., Boylan M. O., Corkey B. E., Wolfe M. M. (2006) Obesity 14, 1124–1131 [DOI] [PubMed] [Google Scholar]
- 13. Yip R. G., Boylan M. O., Kieffer T. J., Wolfe M. M. (1998) Endocrinology 139, 4004–4007 [DOI] [PubMed] [Google Scholar]
- 14. Rudovich N., Kaiser S., Engeli S., Osterhoff M., Gögebakan O., Bluher M., Pfeiffer A. F. (2007) Regul. Pept. 142, 138–145 [DOI] [PubMed] [Google Scholar]
- 15. Song D. H., Getty-Kaushik L., Tseng E., Simon J., Corkey B. E., Wolfe M. M. (2007) Gastroenterology 133, 1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deacon C. F., Nauck M. A., Meier J., Hücking K., Holst J. J. (2000) J. Clin. Endocrinol. Metab. 85, 3575–3581 [DOI] [PubMed] [Google Scholar]
- 17. Pederson R. A., Kieffer T. J., Pauly R., Kofod H., Kwong J., McIntosh C. H. (1996) Metabolism 45, 1335–1341 [DOI] [PubMed] [Google Scholar]
- 18. Beck B., Max J. P. (1987) Cell. Mol. Biol. 33, 555–562 [PubMed] [Google Scholar]
- 19. Beck B., Max J. P. (1983) Regul. Pept. 7, 3–8 [DOI] [PubMed] [Google Scholar]
- 20. McIntosh C. H., Bremsak I., Lynn F. C., Gill R., Hinke S. A., Gelling R., Nian C., McKnight G., Jaspers S., Pederson R. A. (1999) Endocrinology 140, 398–404 [DOI] [PubMed] [Google Scholar]
- 21. Hansotia T., Maida A., Flock G., Yamada Y., Tsukiyama K., Seino Y., Drucker D. J. (2007) J. Clin. Invest. 117, 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou H., Yamada Y., Tsukiyama K., Miyawaki K., Hosokawa M., Nagashima K., Toyoda K., Naitoh R., Mizunoya W., Fushiki T., Kadowaki T., Seino Y. (2005) Biochem. Biophys. Res. Commun. 335, 937–942 [DOI] [PubMed] [Google Scholar]
- 23. Bullock B. P., Heller R. S., Habener J. F. (1996) Endocrinology 137, 2968–2978 [DOI] [PubMed] [Google Scholar]
- 24. Ruiz-Grande C., Alarcón C., Mérida E., Valverde I. (1992) Peptides 13, 13–16 [DOI] [PubMed] [Google Scholar]
- 25. Mérida E., Delgado E., Molina L. M., Villanueva-Peñacarrillo M. L., Valverde I. (1993) J. Clin. Endocrinol. Metab. 77, 1654–1657 [DOI] [PubMed] [Google Scholar]
- 26. Valverde I., Mérida E., Delgado E., Trapote M. A., Villanueva-Peñacarrillo M. L. (1993) Endocrinology 132, 75–79 [DOI] [PubMed] [Google Scholar]
- 27. Bertin E., Arner P., Bolinder J., Hagström-Toft E. (2001) J. Clin. Endocrinol. Metab. 86, 1229–1234 [DOI] [PubMed] [Google Scholar]
- 28. Villanueva-Peñacarrillo M. L., Márquez L., González N., Díaz-Miguel M., Valverde I. (2001) Horm. Metab. Res. 33, 73–77 [DOI] [PubMed] [Google Scholar]
- 29. Sancho V., Trigo M. V., González N., Valverde I., Malaisse W. J., Villanueva-Peñacarrillo M. L. (2005) J. Mol. Endocrinol. 35, 27–38 [DOI] [PubMed] [Google Scholar]
- 30. Sancho V., Trigo M. V., Martín-Duce A., Gonz Lez N., Acitores A., Arnés L., Valverde I., Malaisse W. J., Villanueva-Peñacarrillo M. L. (2006) Int. J. Mol. Med. 17, 1133–1137 [PubMed] [Google Scholar]
- 31. Rodriquez de Fonseca F., Navarro M., Alvarez E., Roncero I., Chowen J. A., Maestre O., Gómez R., Muñoz R. M., Eng J., Blázquez E. (2000) Metabolism 49, 709–717 [DOI] [PubMed] [Google Scholar]
- 32. Nogueiras R., Pérez-Tilve D., Veyrat-Durebex C., Morgan D. A., Varela L., Haynes W. G., Patterson J. T., Disse E., Pfluger P. T., López M., Woods S. C., DiMarchi R., Diéguez C., Rahmouni K., Rohner-Jeanrenaud F., Tschöp M. H. (2009) J. Neurosci. 29, 5916–5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang-Christensen M., Larsen P. J., Thulesen J., Rømer J., Vrang N. (2000) Nat. Med. 6, 802–807 [DOI] [PubMed] [Google Scholar]
- 34. Tang-Christensen M., Larsen P. J., Göke R., Fink-Jensen A., Jessop D. S., Møller M., Sheikh S. P. (1996) Am. J. Physiol. 271, R848–R856 [DOI] [PubMed] [Google Scholar]
- 35. Meeran K., O'Shea D., Edwards C. M., Turton M. D., Heath M. M., Gunn I., Abusnana S., Rossi M., Small C. J., Goldstone A. P., Taylor G. M., Sunter D., Steere J., Choi S. J., Ghatei M. A., Bloom S. R. (1999) Endocrinology 140, 244–250 [DOI] [PubMed] [Google Scholar]
- 36. Zander M., Madsbad S., Madsen J. L., Holst J. J. (2002) Lancet 359, 824–830 [DOI] [PubMed] [Google Scholar]
- 37. Higuchi Y., Herrera P., Muniesa P., Huarte J., Belin D., Ohashi P., Aichele P., Orci L., Vassalli J. D., Vassalli P. (1992) J. Exp. Med. 176, 1719–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Graves R. A., Tontonoz P., Platt K. A., Ross S. R., Spiegelman B. M. (1992) J. Cell. Biochem. 49, 219–224 [DOI] [PubMed] [Google Scholar]
- 39. Miyawaki K., Yamada Y., Yano H., Niwa H., Ban N., Ihara Y., Kubota A., Fujimoto S., Kajikawa M., Kuroe A., Tsuda K., Hashimoto H., Yamashita T., Jomori T., Tashiro F., Miyazaki J., Seino Y. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 14843–14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pfaffl M. W. (2001) Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thams P., Capito K. (2001) Diabetologia 44, 738–746 [DOI] [PubMed] [Google Scholar]
- 42. Holst J. J. (1980) Biochem. J. 187, 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lewis J. T., Dayanandan B., Habener J. F., Kieffer T. J. (2000) Endocrinology 141, 3710–3716 [DOI] [PubMed] [Google Scholar]
- 44. Lynn F. C., Pamir N., Ng E. H., McIntosh C. H., Kieffer T. J., Pederson R. A. (2001) Diabetes 50, 1004–1011 [DOI] [PubMed] [Google Scholar]
- 45. Ding K. H., Zhong Q., Xu J., Isales C. M. (2004) Am. J. Physiol. Endocrinol. Metab. 286, E773–E779 [DOI] [PubMed] [Google Scholar]
- 46. McClean P. L., Irwin N., Cassidy R. S., Holst J. J., Gault V. A., Flatt P. R. (2007) Am. J. Physiol. Endocrinol. Metab. 293, E1746–E1755 [DOI] [PubMed] [Google Scholar]
- 47. Fulurija A., Lutz T. A., Sladko K., Osto M., Wielinga P. Y., Bachmann M. F., Saudan P. (2008) PLoS One 3, e3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Althage M. C., Ford E. L., Wang S., Tso P., Polonsky K. S., Wice B. M. (2008) J. Biol. Chem. 283, 18365–18376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Song D. H., Wolfe M. M. (2007) Curr. Opin. Endocrinol. Diabetes Obes. 14, 46–51 [DOI] [PubMed] [Google Scholar]
- 50. Oben J., Morgan L., Fletcher J., Marks V. (1991) J. Endocrinol. 130, 267–272 [DOI] [PubMed] [Google Scholar]
- 51. Isken F., Pfeiffer A. F., Nogueiras R., Osterhoff M. A., Ristow M., Thorens B., Tschöp M. H., Weickert M. O. (2008) Am. J. Physiol. Endocrinol. Metab. 295, E350–E355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamada C., Yamada Y., Tsukiyama K., Yamada K., Yamane S., Harada N., Miyawaki K., Seino Y., Inagaki N. (2007) Biochem. Biophys. Res. Commun. 364, 175–180 [DOI] [PubMed] [Google Scholar]
- 53. Naitoh R., Miyawaki K., Harada N., Mizunoya W., Toyoda K., Fushiki T., Yamada Y., Seino Y., Inagaki N. (2008) Biochem. Biophys. Res. Commun. 376, 21–25 [DOI] [PubMed] [Google Scholar]
- 54. Asmar M., Tangaa W., Madsbad S., Hare K., Astrup A., Flint A., Bülow J., Holst J. J. (2010) Am. J. Physiol. Endocrinol. Metab. 298, E614–E621 [DOI] [PubMed] [Google Scholar]
- 55. Asmar M., Simonsen L., Madsbad S., Stallknecht B., Holst J. J., Bülow J. (2010) Diabetes 59, 2160–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rabe K., Lehrke M., Parhofer K. G., Broedl U. C. (2008) Mol. Med. 14, 741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carey A. L., Steinberg G. R., Macaulay S. L., Thomas W. G., Holmes A. G., Ramm G., Prelovsek O., Hohnen-Behrens C., Watt M. J., James D. E., Kemp B. E., Pedersen B. K., Febbraio M. A. (2006) Diabetes 55, 2688–2697 [DOI] [PubMed] [Google Scholar]
- 58. Cammisotto P. G., Bukowiecki L. J. (2002) Am. J. Physiol. Cell Physiol. 283, C244–C250 [DOI] [PubMed] [Google Scholar]
- 59. Pamir N., Lynn F. C., Buchan A. M., Ehses J., Hinke S. A., Pospisilik J. A., Miyawaki K., Yamada Y., Seino Y., McIntosh C. H., Pederson R. A. (2003) Am. J. Physiol. Endocrinol. Metab. 284, E931–E939 [DOI] [PubMed] [Google Scholar]
- 60. Rajala M. W., Qi Y., Patel H. R., Takahashi N., Banerjee R., Pajvani U. B., Sinha M. K., Gingerich R. L., Scherer P. E., Ahima R. S. (2004) Diabetes 53, 1671–1679 [DOI] [PubMed] [Google Scholar]
- 61. Kim S. J., Nian C., McIntosh C. H. (2007) J. Biol. Chem. 282, 34139–34147 [DOI] [PubMed] [Google Scholar]
- 62. Marroquí L., Vieira E., Gonzalez A., Nadal A., Quesada I. (2011) Diabetologia 54, 843–851 [DOI] [PubMed] [Google Scholar]
- 63. Pérez C., Fernández-Galaz C., Fernández-Agulló T., Arribas C., Andrés A., Ros M., Carrascosa J. M. (2004) Diabetes 53, 347–353 [DOI] [PubMed] [Google Scholar]
- 64. Barnes K. M., Miner J. L. (2009) Curr. Protein Pept. Sci. 10, 96–107 [DOI] [PubMed] [Google Scholar]