Background: The mechanism initiating pathological corneal neovascularization (CoNV) remains unclear.
Results: After injury, substantial CoNV occurs during an initial, VEGFR-2-dependent phase, prior to influence from inflammatory cells.
Conclusion: Pathological CoNV can be pharmacologically uncoupled from inflammatory cell recruitment and may be coordinated by VEGF from repair epithelial cells.
Significance: This work reveals a window in which angiogenesis and inflammation can be selectively targeted during injury repair.
Keywords: Angiogenesis, Cornea, Epithelium, Inflammation, Vascular Endothelial Growth Factor (VEGF)
Abstract
Pathological neovascularization occurs when a balance of pro- and anti-angiogenic factors is disrupted, accompanied by an amplifying inflammatory cascade. However, the interdependence of these responses and the mechanism triggering the initial angiogenic switch have remained unclear. We present data from an epithelial debridement model of corneal neovascularization describing an initial 3-day period when a substantial component of neovascular growth occurs. Administration of selective inhibitors shows that this initial growth requires signaling through VEGFR-2 (vascular endothelial growth factor receptor-2), independent of the accompanying inflammatory response. Instead, increased VEGF production is found prominently in repair epithelial cells and is increased prior to recruitment of neutrophil/granulocytes and macrophage/monocytes. Consequently, early granulocyte and monocyte depletion has little effect on corneal neovascularization outgrowth. These data indicate that it is possible to pharmacologically uncouple these mechanisms during early injury-driven neovascularization in the cornea and suggest that initial tissue responses are coordinated by repair epithelial cells.
Introduction
The mammalian cornea has evolved as an avascular tissue to provide the clearest refractive optical quality and light access to the interior of the eye. However, the cornea also has a second key function as the external barrier between the sensitive structures inside the eye and the external environment (1). These dual roles can be somewhat at odds with each other as typical tissue inflammatory and repair responses can compromise the functional clarity of the cornea. The cornea limits the extent of these responses by maintaining an avascular or “angiogenic privileged” state (2), which minimizes the influx of platelets, inflammatory cells, and cytokines in response to an infection or injury that produces a lasting scar. This principle contributes to the success of refractive surgeries, high graft survival rates of corneal transplants, and corneal wound repair (3–5). However, some injuries or infections can induce pathological corneal angiogenesis (CoNV),3 an overwhelming inflammatory and neovascular response, resulting in rapid sprouting and growth of capillaries from the limbal vascular plexus into the central cornea, which can lead to permanent corneal opacity (6–8). Models of CoNV have been prominently used in the angiogenesis field, helping to explore the general relationship between inflammatory processes and vascular growth.
Recent work suggests that the avascular state in the cornea is achieved and maintained by a balance of pro- and anti-angiogenic factors, including thrombospondins, endostatin, pigment epithelium-derived factor, and tissue inhibitors of metalloproteases (reviewed in Ref. 7). The most prominent of these factors is vascular endothelial growth factor-A (VEGF), which is constitutively expressed at low levels in the corneal epithelium, endothelium, and limbal vascular endothelial cells and has been reported in infiltrating monocytes, neutrophils, and repair epithelium during CoNV (9–13). Secreted VEGF interacts with the greatest affinity to receptor tyrosine kinases VEGFR-1 and 2. Although VEGFR-2 is expressed in limbal vascular endothelial cells and is critical for angiogenic growth (14, 15), soluble VEGFR-1 can act as an anti-angiogenic VEGF decoy, or sink, to help maintain normal corneal clarity (16, 17). This complex environment has been hypothesized to result in a “buffered” system, which maintains an avascular tissue most of the time, but which can still respond when necessary to infection or injury.
In addition to its pro-angiogenic role, VEGF is also an important chemotactic factor for inflammatory cells, particularly monocytes/macrophages, through binding to VEGFR-1 and promotes corneal lymphangiogenesis (10, 18). Consequently, inhibition of VEGF protein has been shown to result in reduced inflammatory cell recruitment after corneal injury, and deletion of various chemokines and receptors has resulted in a reduced neovascular response (9, 10, 12, 19–21). These observations have generated a concept of CoNV pathogenesis in which inflammation and angiogenesis are intimately linked in an amplification cascade. However, in the midst of this cellular milieu, the mechanism and source of the trigger for the initial angiogenic switch have remained unclear.
Here we present data from a corneal epithelial debridement model, which results in rapid and dense angiogenesis from limbal vessels. We show that a substantial component of the CoNV growth occurs over an initial 3-day period, which requires VEGFR-2 signaling independent of the accompanying inflammatory response. Instead, increased VEGF is found prominently in repair epithelial cells, prior to recruitment of granulocytes and monocytes. Consequently, early granulocyte and monocyte depletion has little effect on CoNV. These data indicate that it is possible to pharmacologically uncouple these mechanisms during early injury-driven neovascularization and suggest that initial responses are coordinated by repair epithelial cells.
EXPERIMENTAL PROCEDURES
Corneal Epithelial Debridement
All animal experiments were approved by the Animal Care and Use Committee at the Novartis Institutes for Biomedical Research. Corneal neovascularization was induced in female C57/Bl6 mice between 6 and 8 weeks old by transient removal of the corneal epithelium by gentle mechanical scraping, including removal of the limbal cells (9, 22, 23). Briefly, mice were anesthetized by i.p. administration of 250 mg/kg of Avertin. All eyes were locally anesthetized by topical application of 0.5% proparacaine solution (Bausch and Lomb, Rochester, NY) for 1 min. Topical anesthetic was blotted away with sterile gauze. Sterile phosphate-buffered saline (PBS) was applied to keep the eyes moist during surgery. Surgery was performed under a standard laboratory dissecting microscope. The eyes were proptosed with serrated forceps, and the corneal epithelium was removed with a sterile disposable scalpel using central brushing motions following the corneal surface. Antibiotic ointment was applied to the debrided eyes, and the animals were allowed to recover on a warming pad. In some experiments, suspensions of dexamethasone, the VEGFR antagonists, HGO452, sunitinib, and pazopanib,(synthesized by the NIBR Department of Chemistry), or vehicle were administered as a 3.5-μl drop topically at a half hour prior to surgery, and dosing was generally continued twice daily for the timeframe indicated. Requests for HGO452 should be referred to the NIBR Ophthalmology Group. The vehicle used for both compounds was 1% ethanol, 5% PEG 400, and 2.5% Pluronic detergent in phosphate buffer, pH 7.4.
Preparation of Corneas for Analysis of Neovascularization
For measurement of neovascular areas, animals were generally sacrificed on the 6th day after debridement, unless otherwise described. Prior to euthanasia, all animals were injected with 100 μl of the vascular label FITC-concanavalin A (Vector Laboratories, Burlingame, CA) intravenously. 10 min after injection, the animals were euthanized by CO2 asphyxiation. Eyes were enucleated with curved forceps so as to avoid fresh corneal damage and fixed in 4% paraformaldehyde for 2 h at room temperature under constant rocking. After fixation, eyes were transferred to 1× PBS and were stored at 4 °C until dissection. Corneas were isolated from the rest of the eye tissues under a dissecting microscope. 2 mm of sclera was left beyond the limbal vessels to ensure that all corneal vessels were captured. Four radial cuts were made in each cornea to facilitate flat-mounting. Corneas were mounted epithelial side up with VECTASHIELD HardSet mounting medium (Vector Laboratories). Fluorescent images of each cornea were captured using an Axiocam MR3 camera on an AxioImage M1 microscope (Zeiss, Thornwood, NY). The area of corneal vasculature was quantified with the AxioVision version 4.5 software (Zeiss).
Histology and Immunofluorescence
All histology and immunofluorescence images are representative of at least three independent experiments, unless otherwise noted. For standard histology, enucleated eyes were fixed with neutral buffered formalin for at least 2 days. Fixed tissues were embedded in paraffin, sectioned at a thickness of 5 μm, and stained with hematoxylin and eosin (H&E) according to established protocols.
Alternatively, for immunofluorescence, enucleated eyes were frozen in OCT (Sakura, Torrance, CA) on dry ice. Serial sections were cut at a thickness of 6 μm. After warming to room temperature, the sections were fixed in cold acetone for 10 min and washed in PBS. Blocking was performed for 1 h at room temperature in 1% BSA, 5% goat serum in PBS. Sections were incubated with primary antibodies diluted in 1% BSA in PBS overnight at 4 °C. Sections were washed with 1× PBS and incubated with secondary antibodies diluted in 1% BSA in PBS for 1 h at room temperature. After washing again, sections were cover-slipped with VECTASHIELD HardSet mounting medium with DAPI (Vector Laboratories) and imaged as described above. Primary antibodies used were: rat anti-mouse GR-1 (BioLegend, San Diego, CA), rat anti-mouse F4/80 (Abcam, Cambridge, MA), goat anti-mouse VEGF (R&D systems, Minneapolis, MN), and goat anti-cytokeratin 12 (Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies used were: Alexa Fluor 647, goat anti-rat (Invitrogen), and Alexa Fluor 594 rabbit anti-goat (Invitrogen). Integrated density of staining was calculated using the ImageJ software (24).
Real-time RT-PCR
Following euthanasia, corneas were removed from the eyes, submerged in RNAlater solution (Ambion, Austin, TX), and stored at 4 °C. RNA was extracted from the corneas with the RNeasy fibrous tissue kit (Qiagen, Valencia, CA) as per the manufacturer's instructions. Total RNA was isolated from homogenized tissues using RNeasy micro columns (Qiagen) including a DNase I treatment step. 1 μg of DNase-treated RNA was reverse-transcribed in a 50-μl reaction mixture (TaqMan Gold RT-PCR reagents, Applied Biosystems, Foster City, CA), and the final volume of cDNA was adjusted to 500 μl. 5 μl of this cDNA was used for each 20-μl total volume TaqMan PCR reaction. Expression of the VEGF was determined on an ABI Prism 7900HT (Applied Biosystems) using a TaqMan gene expression assay in a multiplex reaction with TaqMan mouse GAPDH endogenous control reagents (Applied Biosystems). Raw data were analyzed using the Sequence Detector software, and results were reported as VEGF mRNA expression relative to GAPDH.
ELISAs
A mouse myeloperoxidase (MPO) ELISA (Hycult Biotechnology) was used to quantify corneal MPO content. Total protein was isolated as follows. After euthanasia, corneas were dissected from the eye and flash-frozen on dry ice. 100 μl of 1× lysis buffer (Cell Signaling) plus 1 mm PMSF were added to each frozen sample. Corneas were then homogenized at 30 Hz in a TissueLyser (Qiagen) for 2 min. Total protein concentrations were determined by the BCA protein assay (Pierce Biotechnology) as per the manufacturer's instructions. The MPO ELISA was performed as per the manufacturer's instructions. Concentrations for additional proteins were determined by multiplex ELISA analyses through the Searchlight Testing Service (Aushon Biosystems, Waltham, MA). Total protein extraction and quantification were performed as described above. Protein samples were diluted to 400 μg/ml with PBS. Samples were then shipped frozen on dry ice for Searchlight analysis to determine concentrations of VEGFR-2, VCAM-1, ICAM-1, VEGF, IL-1β, TNF-α, TGF-β1, MCP-1, and MMP-9.
GR-1 Depletion Model
Depletion of GR-1 positive granulocytes and monocytes was induced by i.p. injection of the antibody RB6-8C5 (Southern Biotech, catalog number 1900-01, Birmingham, AL), essentially as outlined in Refs. 25 and 26. Briefly, C57BL/6 mice were injected intraperitoneally with 250 μg of either RB6-8C5 or control rat IgG2b (BD Pharmingen). In some experiments, corneal epithelial debridement followed after 1 h, with repeated injections of antibody given at 6 and 24 h. At selected time points, animals were euthanized by CO2 asphyxiation, and whole blood and/or eyes were collected for further analyses. Measurement of the corneal neovascular area and MPO ELISAs were performed as described above. Analyses of neutrophil and monocyte counts were performed with a HemaVet multispecies hematology system (Drew Scientific Inc., Waterbury, CT).
RESULTS
Corneal Epithelial Debridement Elicits Rapid and Dense Angiogenic and Inflammatory Response within Days
Mechanical debridement of the corneal epithelium has been established as a pathophysiologically relevant model of inflammatory angiogenesis in a variety of species (9, 22, 23). However, most studies are assessed after 7–14 days, and we were unable to uncover a published murine time course and characterization. Therefore, initial experiments were conducted to clarify the progression of angiogenesis and epithelial repair. The corneal epithelium was removed by gentle scraping under anesthesia, taking care to completely debride the limbus. At successive time points, animals were perfused with the vascular label, FITC-concanavalin A, and euthanized. Ten eyes were collected per time point on days 3, 6, 10, 17, and 20 after the procedure. Corneas were then dissected, mounted, and imaged for total neovascular area, normalized to control (non-debrided) limbal vessel areas. Notably, substantial and dense vessel outgrowth had already taken place by the 3rd day after surgery, oriented toward the central cornea (Fig. 1, A and B). Although total neovascular area continued to increase through day 20, there was a concomitant increase in variability of growth at later time points (Fig. 1B).
FIGURE 1.
Epithelial debridement induces rapid and consistent corneal angiogenesis and inflammation by 3 days. A, representative FITC-concanavalin A-injected corneal flat mounts at various times over 20 days following epithelial debridement. B, quantification of total vascular area per cornea normalized to control (undebrided) limbal vessel areas (n = 10 corneas/group). Error bars indicate S.E. NV Area, neovascular area. C, H&E-stained corneal sections showing the progression of re-epithelialization over a matter of days. The images are all oriented with the limbus to the left (L) and the central cornea to the right (C). Note the repair epithelium at the wound edge (arrowhead) accompanied by underlying inflammatory infiltrates. By day 7, the cornea completely resurfaced with a stratified epithelium (E) but includes the presence of new shallow stromal vessels (S) (arrows). The presence of inflammatory cells was reduced by this time (20×).
Some eyes were also sectioned and stained with H&E at various time points over 7 days to characterize the wound repair response (Fig. 1C). At 6 h following surgery, the epithelium was absent. By days 2 and 3, repair epithelial cells were migrating from the limbus toward the central cornea, generally completing a continuous layer by day 4. By day 7, the cornea had completely resurfaced with a new stratified epithelium, accompanied by the appearance of underlying shallow blood vessels (Fig. 1C). Inflammatory cells were also concentrated under the migrating epithelial wound edge by day 3 and were reduced in number after the epithelium formed a complete layer (Fig. 1C).
VEGFR-2 Inhibition following Debridement Blocks CoNV with Minimal Effects on Inflammation
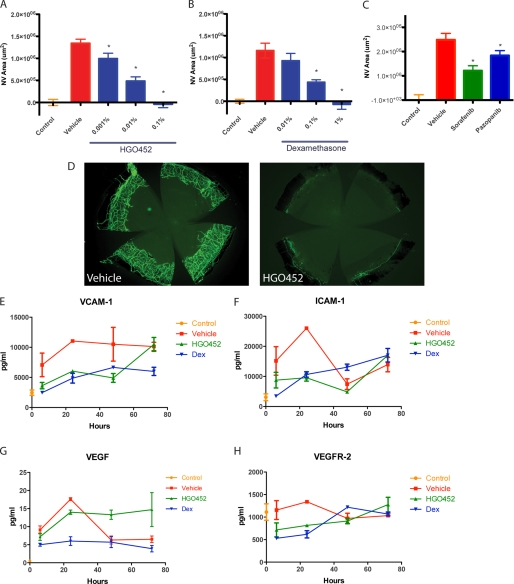
Specific inhibitors were administered to test the dependence of this model on VEGFR-2 signaling. HGO452 is a small molecule compound that potently inhibits VEGFR-2 kinase activity with a biochemical IC50 of 3 nm and cellular IC50 of <1 nm. It shows at least an order of magnitude selectivity over the related kinases RET, c-Abl, and fibroblast growth factor receptor (FGFRs) 1–3 and at least 100-fold selectivity over a panel of 50 other kinases (supplemental Table 1) (27). HGO452 was dosed twice daily, topically to the cornea, over 6 days in debrided animals in concentrations from 0.001% to 0.1%. CoNV quantification resulted in significant and dose-dependent inhibition of neovascularization with an ED50 concentration of only 0.009% and complete inhibition at 0.1% as compared with vehicle (Fig. 2, A and D). A pharmacokinetic study was completed with this compound at a dose concentration of 0.04% suspension (0.016 mg/kg), resulting in a corneal Cmax of 171 nm and area under the curve of 200 nm·h over 24 h (supplemental Fig. 1). Unfortunately, we could not obtain the corneal pharmacokinetic at the same dosing concentration as the efficacy studies because it fell below the limit of detection. However, extrapolation to the ED50 dose of 0.009% corresponds to a maximum concentration of only 38 nm HGO452. At this concentration, only VEGFR-2 would be maximally inhibited and the influence of off-target effects would be minimized. Plasma levels were almost undetectable.
FIGURE 2.
VEGFR-2 inhibition potently blocks corneal neovascularization and early angiogenesis markers in a dose-dependent manner. A, quantification of corneal neovascular areas (NV Area) after topical treatment with increasing doses of the VEGFR-2 antagonist, HGO452, resulting in a dose response with an ED50 of 0.009% (n = 10, *, p < 0.05). B, CoNV areas after topical treatment with Dex, resulting in a dose response with an ED50 of 0.1% drug (n = 10, *, p < 0.05). C, CoNV areas after treatment with 0.3% VEGFR-2 antagonists sorafenib or pazopanib showing significant inhibition as compared with vehicle (n = 8, *, p < 0.05). D, representative images from the experiment in A showing the potent inhibitory effects of 0.1% HGO452. E–H, corneal concentrations of vascular markers measured by multiplex ELISA over the initial 3 days of dosing for: control (no debridement), vehicle-treated, 1% Dex, or 0.1% HGO452. Concentrations of VCAM-1, ICAM-1, and VEGFR-2 itself were reduced for both Dex and HGO452. Concentrations of VEGF were reduced with Dex, but not HGO452 (n = 8). Error bars in panels A–C and E–H indicate S.E.
As a positive control, the synthetic corticosteroid, dexamethasone (Dex), was dosed. Dex is used clinically as a topical anti-inflammatory agent for treatment of corneal diseases but also has direct anti-angiogenic properties, being effective in multiple models of ocular neovascularization (28–33). To confirm the efficacy of Dex, debrided eyes were treated topically, twice daily, with concentrations from 0.01 to 1%, over 6 days. A significant and dose-dependent inhibition of CoNV was observed as compared with vehicle, with an ED50 concentration of 0.1% and complete inhibition with a 1% concentration (Fig. 2B).
To further confirm the dependence of neovascularization in this model on VEGFR-2 signaling, two marketed receptor tyrosine kinase inhibitors with prominent VEGFR-2 activity were also dosed. Sorafenib and pazopanib are kinase inhibitors that target VEGFR-2 with IC50 values of 90 and 30 nm, respectively (34, 35). Each produced significant CoNV inhibition when dosed topically at 0.3% (Fig. 2C). Therefore, each compound was efficacious in the model, but HGO452 proved more potent than either sorafenib or pazopanib, suggesting that its higher potency toward VEGRF-2 is responsible for its increased efficacy with respect to inhibition of CoNV.
For further characterization, select vascular and inflammatory markers from debrided corneas were analyzed over the critical first 3 days by multiplex ELISA, after administration of HGO452, Dex, or vehicle (Figs. 2, E–H, and 3). For these studies, the compounds were activity-matched in dose to produce a near complete inhibition of neovascularization: 1% Dex and 0.1% HGO452. Concentrations of VCAM-1 and ICAM-1 were inhibited to similar degrees by administration of Dex or HGO452 as compared with vehicle (Fig. 2, D and E). Dex treatment also resulted in reduction of VEGF concentrations, but HGO452 resulted in sustained VEGF concentrations that may reflect a positive feedback mechanism in response to receptor blockade (Fig. 2G). Concentrations of VEGFR-2 itself were inhibited by both Dex and HGO452 (Fig. 2H). Therefore, administration of each compound resulted in reduced vascular markers, consistent with CoNV area measurements.
FIGURE 3.
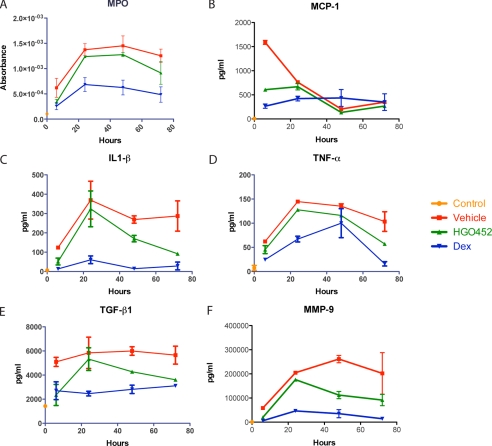
Inhibition of VEGFR-2 signaling has minimal effects on early corneal inflammation. A, ELISA results from a corneal debridement time course for the neutrophil marker MPO showing reduced concentrations after treatment with 1% Dex, but not 0.1% HGO452, over 3 days following debridement (n = 8). B–F, multiplex ELISA results from corneal debridement time courses for proinflammatory cytokines MCP-1, IL-1β, TNF-α, TGF-β1, and the protease MMP-9 all displayed reduced concentrations after treatment with 1% Dex, but not 0.1% HGO452, over 3 days following debridement (n = 8). Error bars in all panels indicate S.E.
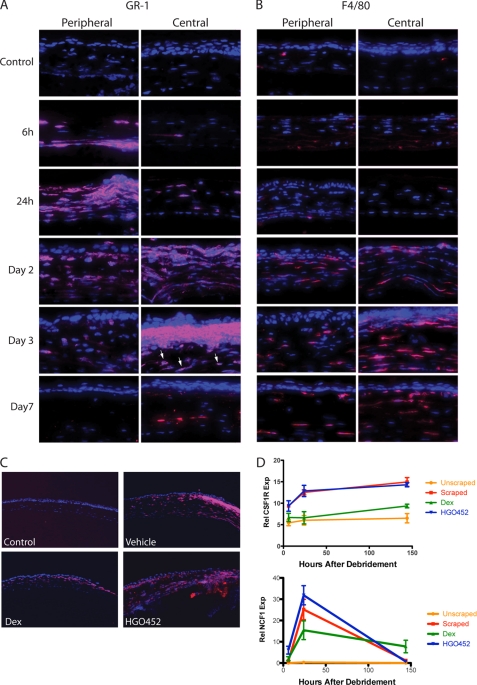
To assess the effect of each compound on inflammation, corneal lysates from treated animals were tested by ELISA for additional markers over the same time course. Concentrations of MPO, IL-1β, TNF-α, TGF-β1, and MMP-9 all showed marked reductions after Dex treatment, but there was little effect from HGO452 (Fig. 3, A–F). Immunofluorescence staining was also performed on sections from a time course of debrided corneas for the neutrophil/granulocyte marker GR-1. Some GR-1-positive cells appeared in the peripheral stroma by 6 h after surgery and migrated centrally under the repair epithelium, largely disappearing by day 7 (Fig. 4A). We also observed GR-1 staining in larger stromal cells, which did not appear until 2–3 days after surgery (Fig. 4A). GR-1 epitopes have been demonstrated to reside on other inflammatory cell lineages besides granulocytes, including dendritic cells, lymphocytes, and monocytes/macrophages (36–38). These larger cells stained positive for F4/80, indicating the infiltration of macrophages into the stroma (Fig. 4B). Dex-treated corneas stained fewer cells with GR-1 as compared with vehicle or HGO452 (Fig. 4C). This observation was corroborated by real-time RT-PCR of corneal lysates probed for the macrophage marker CSF1R (colony-stimulating factor 1 receptor), and neutrophil marker NCF1 (neutrophil cytosolic factor 1) (Fig. 4D).
FIGURE 4.
Neutrophils and macrophages migrate into debrided cornea from limbus over several days and are not influenced by VEGFR-2 inhibition. A, representative corneal sections probed with the granulocyte marker, GR-1 (red), at various time points following debridement. Shallow stromal staining was notable by 6 h in the periphery and increased centrally with time, diminishing by day 7. This antibody also later recognized larger, macrophage-like cells in the stroma (e.g. day 3, central arrows) (40×). B, similar sections stained for the macrophage marker, F4/80, identifying cells throughout the corneal stroma that appear on day 2 and remain on day 7 (40×). C, GR-1-stained sections 48 h after debridement. Control (undebrided) sections showed no staining, vehicle-dosed sections showed prominent staining, and staining is reduced after treatment with 1% Dex, but remains robust after treatment with 0.1% HGO452 (10×). D, real-time RT-PCR results from corneas probed for the macrophage marker CSF1R and the neutrophil marker NCF1, and treated with vehicle, Dex, or HGO452. Increases in these markers were inhibited by Dex, but HGO452 had little effect. Error bars indicate S.E. Rel NCF1 Exp, relative NCF1 expression; Rel CSF1R Exp, relative CSF1R expression.
CoNV in this model is therefore dependent on VEGFR-2 activity, but blockade of this signal has minimal effect on concentrations of pro-inflammatory cytokines or prominent recruitment of neutrophils and later macrophages. This result was interesting as inhibition of secreted VEGF has been previously shown to result in reduced inflammatory cell recruitment (10).
During Initial Debridement Response, VEGF Is Largely Produced by Repair Epithelium, Not Neutrophils or Macrophages
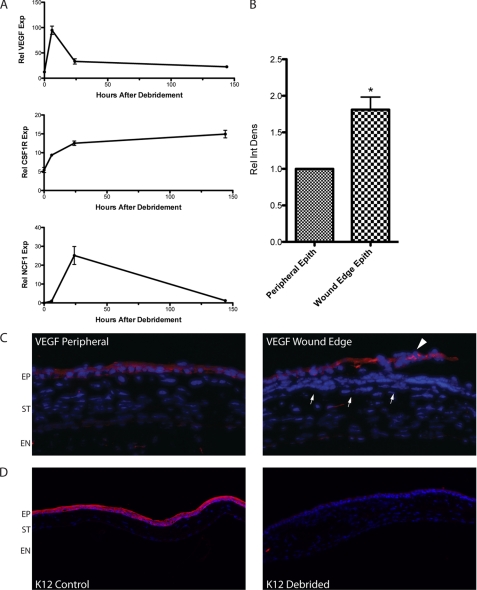
Expression and localization of VEGF in healing corneas were also analyzed, focusing on the first 3 days, coincident with the initial burst of angiogenesis (Fig. 1). Time courses of cDNAs were prepared from whole corneas for real-time RT-PCR and probed for VEGF, CSF1R (macrophages), and NCF1 (neutrophils). VEGF message rapidly increased following debridement by 10-fold, sharply peaking at 6 h. In comparison, CSF1R message gradually increased, continuing to rise until the last time point at 6 days. NCF1 message remained low at 6 h but sharply spiked at 24 h. Therefore, strongly increased VEGF transcription is present in the cornea prior to increases in macrophages or neutrophils (Fig. 5A).
FIGURE 5.
VEGF expression increases prior to macrophage or neutrophil recruitment and is localized to repair epithelium. A, quantitative RT-PCRs from debrided corneas over 6 days probed for VEGF, the macrophage marker CSF1R, and the neutrophil marker NCF1. VEGF expression sharply peaked by 6 h, whereas CSF1R slowly increased over 3 days, and NCF1 peaked at 24 h (n = 4). Rel VEGF Exp, relative VEGF expression; Rel NCF1 Exp, relative NCF1 expression; Rel CSF1R Exp, relative CSF1R expression. B, relative integrated density (Rel Int Dens) of VEGF staining quantified from the 100-μm wound edge epithelial (Epith) cells showed an increase as compared with adjacent epithelial regions on the same section from days 1–3 (n = 4, *, p < 0.05). Error bars in panels A and B indicate S.E. C, representative images from B. Prominent epithelial staining for VEGF protein was present in peripheral repair epithelium and weak in keratocytes and endothelial cells on day 2. Staining was consistently more intense in wound edge epithelial cells from the same section (arrowhead, 20×). VEGF staining was absent or very weak in inflammatory cells at this time (arrows) (20×). EP, epithelium; ST, stroma; EN, endothelium. D, staining for the corneal epithelial keratin 12 (K12) is strongly present in control (naive) corneas, but not in the resurfaced repair epithelium at 7 days following debridement (10×).
Sections from 1- and 2-day debrided and control corneas were then probed with a VEGF antibody to localize the source of increased protein. Control corneas displayed faint constitutive staining in the epithelium, stromal keratocytes, and endothelium as reported previously (13). In debrided corneas, there was prominent staining in the repair epithelium, concentrated in the leading edge cells on day 2 (Fig. 5C). This increase was quantified by integrated intensity analyses of equal 100-μm areas of wound edge epithelial cells, normalized to peripheral cell staining from the same section (n = 4 independent experiments). Using this methodology, a 2-fold increase in VEGF staining intensity was observed in wound edge epithelial cells (Fig. 5B). We did not observe strong staining in inflammatory cells at this time (Fig. 5C), although signal was more apparent in various stromal cells at later time points (supplemental Fig. 2). Previous studies have suggested that damage or removal of limbal epithelial stem cells can result in an epithelium that fails to express corneal epithelial keratins and eventually develops goblet cells and other markers of the vascularized conjunctiva (22, 23). Re-epithelialized eyes from day 7 were sectioned and probed for keratin 12, a mouse corneal epithelial marker that is not expressed in the conjunctiva (39). Control corneal epithelium strongly stained for keratin 12, but no staining was detected on resurfaced repair epithelium 7 days after debridement (Fig. 5D). These results suggest that a distinct repair epithelium resurfaces the debrided cornea and secretes VEGF.
Depletion of Initial Neutrophil and Macrophage Response Has Little Effect on CoNV in Debrided Corneas
Based on time course studies, VEGFR-2-dependent neovascularization, and VEGF localization, we hypothesized that the majority of early inflammatory cells appearing in the cornea have little influence on CoNV initiation. As the most prominent infiltrating cells over the first 3 days were neutrophils, with a smaller number of macrophages, we addressed this question by depleting these cells in the epithelial debridement model. Systemic depletion of both cell types by injection of the RB6-8C5 GR-1 antibody has been previously reported (26, 36, 40).
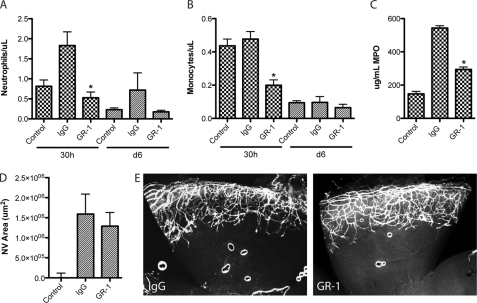
Intraperitoneal injection of three serial doses of anti-GR-1 was performed to produce sustained depletion over the first few days as compared with IgG isotype control (Fig. 6, supplemental Fig. 3).Blood neutrophil and monocyte counts were measured at 30 h, and both were significantly reduced in GR-1-injected animals. This effect was maintained as a trend until day 6 (Fig. 6, A and B). As an assessment of local corneal depletion, MPO levels were measured by ELISA and were similarly significantly reduced (Fig. 6C). In contrast, there was no significant change in neovascular area between IgG and anti-GR-1 groups (Fig. 6, D and E). These results suggest that early inflammatory cell recruitment following corneal debridement contributes little to CoNV growth.
FIGURE 6.
Early depletion of neutrophils/granulocytes and macrophages/monocytes does not inhibit corneal angiogenesis following debridement. A, blood neutrophil counts following corneal debridement after administration of three serial doses of GR-1. Neutrophils were strongly inhibited at 30 h as compared with IgG control, and this trend was maintained to day 6 (n = 4, *, p < 0.05). B, blood monocyte counts from the same samples. Monocytes were also strongly inhibited at 30 h as compared with IgG control (n = 4, *, p < 0.05). C, corneal MPO concentrations 30 h after debridement were significantly reduced as compared with IgG control (n = 8, *, p < 0.05). D, quantification of neovascular area (NV Area) showed no significant difference between GR-1- and IgG-injected groups (n = 8). Error bars in panels A–D indicate S.E. E, representative images from D showing substantial neovascular growth in GR-1 and control corneas by day 6.
DISCUSSION
Pathological angiogenesis in the cornea is proposed to result from an inflammatory amplification cascade in which macrophages, and to some extent neutrophils, play an intimate role in inducing and maintaining a neovascular response (10–12, 19, 20). Here we present data using a corneal injury model in which the angiogenic and inflammatory components have been pharmacologically uncoupled over an initial 3-day period. We propose that the epithelial repair response during this period may be a more critical signal for triggering the angiogenic switch than inflammatory cell recruitment.
These conclusions are based on several lines of reasoning. A careful time course of neovascularization established that substantial growth was completed by day 3. This result is interesting, given that most published assessments of corneal neovascularization models are conducted after 7–14 days. The initial burst of angiogenesis is also coincident with epithelial resurfacing of the cornea. Topical administration of a VEGFR-2 inhibitor, HGO452, was able to completely block neovascular growth and increases in angiogenesis markers VCAM, ICAM, and VEGFR-2 itself over the first 3 days. Dex had a similar effect, albeit at 10-fold higher doses. However, unlike Dex, VEGFR-2 blockade had little effect on a panel of inflammatory markers over the same time period, including IL-1β, TNF-α, TGF-β1, MCP-1, MPO, and MMP-9, or on recruitment of neutrophils/granulocytes and monocyte/macrophages. Inhibition of VEGF-mediated chemotaxis in the cornea has been shown to result in reduced inflammatory cell recruitment, particularly through binding to VEGFR-1 (10). Therefore, the initial neovascular growth is VEGFR-2-dependent, but this pathway has minimal effect on inflammation or inflammatory cell recruitment.
Based on these data, we speculated on the early role of inflammatory cells in this model and the source of VEGF protein. Analyses of macrophage and neutrophil marker expression in debrided corneas over time showed that these signals increase after the acute VEGF signal, and macrophages generally did not appear until the end of the 3-day period. Immunofluorescence staining for VEGF protein in corneal sections during the first 3 days also failed to indicate any signal in inflammatory cells but was prominent in the repair epithelium. Staining was particularly intense in the wound edge epithelium, which was increased in comparison with peripheral epithelium.
We hypothesized that not only could angiogenesis be inhibited through VEGFR-2 without effect on pro-inflammatory signals, but that initial granulocyte and monocyte recruitment could be inhibited without influencing the early CoNV response. We had administered Dex as a positive control for its demonstrated efficacy in various models of neovascularization (29–32) and for its broad anti-inflammatory properties. However, it has also been described as an angiostatic steroid with direct effects on angiogenesis through a mechanism that is still unclear (28, 30–33). To address this point, we used an alternate strategy to specifically deplete inflammatory cell recruitment in the cornea during the first few days. The GR-1 antibody, RB6-8C5, was initially thought to specifically deplete neutrophils, but has now been recognized to also deplete populations of macrophages/monocytes, dendritic cells, and lymphocytes (36–38). Early depletion of granulocytes and monocytes had no significant effect on neovascular growth.
These results provide insight into the mechanism triggering CoNV and can be integrated with data that have indicated macrophage (10, 20, 21, 41) and neutrophil (11, 12) involvement in amplifying this response. One interpretation of the current data is that the mechanisms driving debridement-induced CoNV differ from other injuries, such as alkali burn or suture. However, the general profile of inflammatory cell appearance and markers following debridement is consistent with other models. Also, prominent epithelial VEGF staining after several types of corneal injury has been previously noted (9, 11, 12). The methods used to deplete macrophages and neutrophils have generally been evaluated after 7–14 days, when their involvement in promoting and amplifying angiogenesis may be greater. The present data are consistent with this theory as we observed that F4/80/CSF1R-positive macrophage signals increased throughout the first week. Additionally, some methods of inflammatory cell depletion may be open to other interpretations. Gong and Koh (12) dosed anti-GR-1 at 3-day intervals, over a week, to specifically inhibit neutrophils in a burn-induced CoNV model. However, our data suggest this sparse dosing regimen would not have led to consistent depletion over this period (supplemental Fig. 3) and may have also depleted macrophages. Additionally, clodronate has been used by several groups to selectively deplete macrophages in the cornea (10, 21), but has since been shown to be directly anti-angiogenic (42).
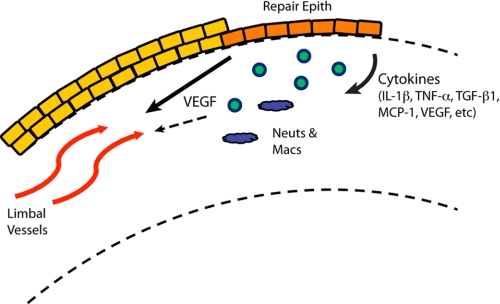
We propose a two-phase perspective in which the repair epithelium in the cornea coordinates initial angiogenic and inflammatory responses that become interdependent over time (Fig. 7). The coordination of injury responses by repair epithelial cells is an emerging theme in a variety of tissues, such as the lung epithelium, skin keratinocytes, gastrointestinal epithelium, and corneal epithelium (3, 5, 43–48). In each case, epithelial cell activities involve secretion of growth factors and inflammatory cytokines, activation of extracellular matrix remodeling enzymes, and alteration of cell adhesion molecules. Conversely, a key role for uninjured corneal epithelial cells has been established in the maintenance of angiogenic privilege (16, 17, 49). We showed that the repair epithelium, following debridement, no longer expresses the corneal epithelial marker, keratin 12, suggesting that the epithelial environment may become more like the vascularized conjunctiva or skin and permissive to vascularization. It will be interesting to test this perspective further with direct manipulation of epithelial cell function and thorough investigation of the early responses in alternative injury models.
FIGURE 7.
Model of CoNV initiation. Repair epithelial cells (Repair Epith) at the wound edge secrete pro-inflammatory cytokines, including VEGF, leading to angiogenesis from limbal vessels and recruitment of neutrophils (Neuts) and macrophages (Macs). These processes are initially separate, but may become interdependent over time.
Clinically, corneal neovascularization can result from a range of insults, including infection, burns, transplants, and extended contact lens wear (6, 7). There is yet to be a large scale epidemiological study combining all of these causes; however, a survey of patients reporting to the General Eye Service of the Massachusetts Eye and Ear Infirmary for the year of 1996 found corneal neovascularization in 4.14% of cases (8), representing a potentially sizeable number when extrapolated to the general population. Development of pharmacologic tools that can selectively inhibit different aspects of these injuries may enable more appropriate therapies tailored to avoid unnecessary side effects.
Supplementary Material
Acknowledgments
We thank Margaret McLaughlin for helpful comments and suggestions, Guido Bold for synthesis of HGO452, Gerald Artman for subsequent production, Vinayak Hosagrahara for PK support, and Meihua Ju for technical support.
This work was supported by the Novartis Institutes for Biomedical Research (NIBR).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1–3.
- CoNV
- corneal neovascularization
- CSF1R
- colony-stimulating factor 1 receptor
- Dex
- dexamethasone
- ICAM-1
- intercellular adhesion molecule 1
- MCP-1
- monocyte chemotactic protein 1
- NCF1
- neutrophil cytosolic factor 1
- MMP-9
- matrix metalloproteinase 9
- MPO
- myeloperoxidase
- VCAM-1
- vascular cell adhesion molecule 1
- VEGFR
- VEGF receptor.
REFERENCES
- 1. Sivak J., Sivak B. (2000) in Vertebrate Eye Development (Fini M. E., ed.) pp. 1–13, Springer-Verlag, Berlin, Heidelberg [Google Scholar]
- 2. Cursiefen C., Maruyama K., Jackson D. G., Streilein J. W., Kruse F. E. (2006) Cornea 25, 443–447 [DOI] [PubMed] [Google Scholar]
- 3. Fini M. E., Stramer B. M. (2005) Cornea 24, S2–S11 [DOI] [PubMed] [Google Scholar]
- 4. Küchle M., Cursiefen C., Nguyen N. X., Langenbucher A., Seitz B., Wenkel H., Martus P., Naumann G. O. (2002) Graefes Arch. Clin. Exp. Ophthalmol. 240, 580–584 [DOI] [PubMed] [Google Scholar]
- 5. Sivak J. M., Fini M. E. (2002) Prog. Retin. Eye Res. 21, 1–14 [DOI] [PubMed] [Google Scholar]
- 6. Chang J. H., Gabison E. E., Kato T., Azar D. T. (2001) Curr. Opin. Ophthalmol. 12, 242–249 [DOI] [PubMed] [Google Scholar]
- 7. Zhang S. X., Ma J. X. (2007) Prog. Retin. Eye Res. 26, 1–37 [DOI] [PubMed] [Google Scholar]
- 8. Lee P., Wang C. C., Adamis A. P. (1998) Surv. Ophthalmol. 43, 245–269 [DOI] [PubMed] [Google Scholar]
- 9. Amano S., Rohan R., Kuroki M., Tolentino M., Adamis A. P. (1998) Invest. Ophthalmol. Vis. Sci. 39, 18–22 [PubMed] [Google Scholar]
- 10. Cursiefen C., Chen L., Borges L. P., Jackson D., Cao J., Radziejewski C., D'Amore P. A., Dana M. R., Wiegand S. J., Streilein J. W. (2004) J. Clin. Invest. 113, 1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gan L., Fagerholm P., Palmblad J. (2004) Acta Ophthalmol. Scand. 82, 557–563 [DOI] [PubMed] [Google Scholar]
- 12. Gong Y., Koh D. R. (2010) Cell Tissue Res. 339, 437–448 [DOI] [PubMed] [Google Scholar]
- 13. Philipp W., Speicher L., Humpel C. (2000) Invest. Ophthalmol. Vis. Sci. 41, 2514–2522 [PubMed] [Google Scholar]
- 14. Olsson A. K., Dimberg A., Kreuger J., Claesson-Welsh L. (2006) Nat. Rev. Mol. Cell Biol. 7, 359–371 [DOI] [PubMed] [Google Scholar]
- 15. Ozaki H., Seo M. S., Ozaki K., Yamada H., Yamada E., Okamoto N., Hofmann F., Wood J. M., Campochiaro P. A. (2000) Am. J. Pathol. 156, 697–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ambati B. K., Nozaki M., Singh N., Takeda A., Jani P. D., Suthar T., Albuquerque R. J., Richter E., Sakurai E., Newcomb M. T., Kleinman M. E., Caldwell R. B., Lin Q., Ogura Y., Orecchia A., Samuelson D. A., Agnew D. W., St Leger J., Green W. R., Mahasreshti P. J., Curiel D. T., Kwan D., Marsh H., Ikeda S., Leiper L. J., Collinson J. M., Bogdanovich S., Khurana T. S., Shibuya M., Baldwin M. E., Ferrara N., Gerber H. P., De Falco S., Witta J., Baffi J. Z., Raisler B. J., Ambati J. (2006) Nature 443, 993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cursiefen C., Chen L., Saint-Geniez M., Hamrah P., Jin Y., Rashid S., Pytowski B., Persaud K., Wu Y., Streilein J. W., Dana R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11405–11410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luttun A., Tjwa M., Moons L., Wu Y., Angelillo-Scherrer A., Liao F., Nagy J. A., Hooper A., Priller J., De Klerck B., Compernolle V., Daci E., Bohlen P., Dewerchin M., Herbert J. M., Fava R., Matthys P., Carmeliet G., Collen D., Dvorak H. F., Hicklin D. J., Carmeliet P. (2002) Nat. Med. 8, 831–840 [DOI] [PubMed] [Google Scholar]
- 19. Ambati B. K., Anand A., Joussen A. M., Kuziel W. A., Adamis A. P., Ambati J. (2003) Invest. Ophthalmol. Vis. Sci. 44, 590–593 [DOI] [PubMed] [Google Scholar]
- 20. Ambati B. K., Joussen A. M., Kuziel W. A., Adamis A. P., Ambati J. (2003) Cornea 22, 465–467 [DOI] [PubMed] [Google Scholar]
- 21. Lu P., Li L., Liu G., van Rooijen N., Mukaida N., Zhang X. (2009) Cornea 28, 562–569 [DOI] [PubMed] [Google Scholar]
- 22. Joussen A. M., Poulaki V., Mitsiades N., Stechschulte S. U., Kirchhof B., Dartt D. A., Fong G. H., Rudge J., Wiegand S. J., Yancopoulos G. D., Adamis A. P. (2003) Invest. Ophthalmol. Vis. Sci. 44, 117–123 [DOI] [PubMed] [Google Scholar]
- 23. Kruse F. E., Chen J. J., Tsai R. J., Tseng S. C. (1990) Invest. Ophthalmol. Vis. Sci. 31, 1903–1913 [PubMed] [Google Scholar]
- 24. Rasband W. S. (2011) ImageJ, United States National Institutes of Health, Bethesda, MD [Google Scholar]
- 25. Tepper R. I., Coffman R. L., Leder P. (1992) Science 257, 548–551 [DOI] [PubMed] [Google Scholar]
- 26. Conlan J. W., North R. J. (1994) J. Exp. Med. 179, 259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sousa D., Cepeda R., Woolfenden A., Demirs J., Jaffee B., Cherry A., Sivak J. M. (2010) Invest. Ophthalmol. Vis. Sci. 42 ARVO E-Abstr. 5697 [Google Scholar]
- 28. Folkman J., Ingber D. E. (1987) Ann. Surg. 206, 374–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishibashi T., Miki K., Sorgente N., Patterson R., Ryan S. J. (1985) Arch. Ophthalmol. 103, 708–711 [DOI] [PubMed] [Google Scholar]
- 30. Leibowitz H. M., Kupferman A., Stewart R. H., Kimbrough R. L. (1978) Am. J. Ophthalmol. 86, 418–423 [DOI] [PubMed] [Google Scholar]
- 31. Nakao S., Hata Y., Miura M., Noda K., Kimura Y. N., Kawahara S., Kita T., Hisatomi T., Nakazawa T., Jin Y., Dana M. R., Kuwano M., Ono M., Ishibashi T., Hafezi-Moghadam A. (2007) Am. J. Pathol. 171, 1058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Proia A. D., Hirakata A., McInnes J. S., Scroggs M. W., Parikh I. (1993) Exp. Eye Res. 57, 693–698 [DOI] [PubMed] [Google Scholar]
- 33. Raizman M. (1996) Arch. Ophthalmol. 114, 1000–1001 [DOI] [PubMed] [Google Scholar]
- 34. Bukowski R. M., Yasothan U., Kirkpatrick P. (2010) Nat. Rev. Drug Discov. 9, 17–18 [DOI] [PubMed] [Google Scholar]
- 35. Wilhelm S., Carter C., Lynch M., Lowinger T., Dumas J., Smith R. A., Schwartz B., Simantov R., Kelley S. (2006) Nat. Rev. Drug Discov. 5, 835–844 [DOI] [PubMed] [Google Scholar]
- 36. Daley J. M., Thomay A. A., Connolly M. D., Reichner J. S., Albina J. E. (2008) J. Leukoc. Biol. 83, 64–70 [DOI] [PubMed] [Google Scholar]
- 37. Han Y., Cutler J. E. (1997) J. Infect. Dis. 175, 1169–1175 [DOI] [PubMed] [Google Scholar]
- 38. Tvinnereim A. R., Hamilton S. E., Harty J. T. (2004) J. Immunol. 173, 1994–2002 [DOI] [PubMed] [Google Scholar]
- 39. Tanifuji-Terai N., Terai K., Hayashi Y., Chikama T., Kao W. W. (2006) Invest. Ophthalmol. Vis. Sci. 47, 545–551 [DOI] [PubMed] [Google Scholar]
- 40. Czuprynski C. J., Theisen C., Brown J. F. (1996) Infect. Immun. 64, 3946–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mwaikambo B. R., Sennlaub F., Ong H., Chemtob S., Hardy P. (2006) Invest. Ophthalmol. Vis. Sci. 47, 4356–4364 [DOI] [PubMed] [Google Scholar]
- 42. Rose K., Finger I. E., Ferenz K. B. (2011) Biomed. Pharmacother. 65, 46–51 [DOI] [PubMed] [Google Scholar]
- 43. Crosby L. M., Waters C. M. (2010) Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L715–L731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fuchs E., Horsley V. (2008) Genes Dev. 22, 976–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hassell J. R., Birk D. E. (2010) Exp. Eye Res. 91, 326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karrasch T., Jobin C. (2009) Z. Gastroenterol 47, 1221–1229 [DOI] [PubMed] [Google Scholar]
- 47. Sivak J. M., West-Mays J. A., Yee A., Williams T., Fini M. E. (2004) Mol. Cell. Biol. 24, 245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. West-Mays J. A., Dwivedi D. J. (2006) Int. J. Biochem. Cell Biol. 38, 1625–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Poulaki V., Mitsiades N., Kruse F. E., Radetzky S., Iliaki E., Kirchhof B., Joussen A. M. (2004) Am. J. Pathol. 164, 1293–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.