Background: Advanced glycation end products (AGEs) play a prominent role in various pathophysiologies.
Results: A new class of lysine amide modifications was established in vivo.
Conclusion: Non-enzymatic Maillard mechanisms participate on amide-AGE formation pathways in vivo.
Significance: Plasma levels of the amide-AGEs were in the same range similar to other established lysine modifications and suggest a comparable impact of non-enzymatic biochemistry on pathophysiologies in the human organism.
Keywords: Carbohydrate Chemistry, Glycation, Oxidative Stress, Post-translational Modification, Protein Acylation, Maillard Reaction, Amide-AGEs, Beta Cleavage, Dicarbonyls, Rearrangement
Abstract
The Maillard reaction in vivo entails alteration of proteins or free amino acids by non-enzymatic glycation or glycoxidation. The resulting modifications are called advanced glycation end products (AGEs) and play a prominent role in various pathologies, including normoglycemic uremia. Recently, we established a new class of lysine amide modifications in vitro. Now, human plasma levels of the novel amide-AGEs N6-acetyl lysine, N6-formyl lysine, N6-lactoyl lysine, and N6-glycerinyl lysine were determined by means of LC-MS/MS. They were significantly higher in uremic patients undergoing hemodialysis than in healthy subjects. Model reactions with N1-t-butoxycarbonyl-lysine under physiological conditions confirmed 1-deoxy-d-erythro-hexo-2,3-diulose as an immediate precursor. Because formation of N6-formyl lysine from glucose responded considerably to the presence of oxygen, glucosone was identified as another precursor. Comparison of the in vivo results with the model experiments enabled us to elucidate possible formation pathways linked to Maillard chemistry. The results strongly suggest a major participation of non-enzymatic Maillard mechanisms on amide-AGE formation pathways in vivo, which, in the case of N6-acetyl lysine, parallels enzymatic processes.
Introduction
The Maillard reaction (non-enzymatic reactions of reducing sugars with amines) in vivo is associated with long term complications of diabetes, uremia, atherosclerosis, and Alzheimer disease as well as with pathophysiologies linked to aging in general. The stable products of this reaction are referred to as advanced glycation end products (AGEs).2 Numerous studies have documented the accumulation of AGEs in tissue proteins but also in the circulation of patients with renal failure, irrespective of the presence of diabetes (1–3). In general, AGEs can accumulate as protein modifications or as AGE-free adducts (4). These free adducts are formed by cellular proteolysis of glycated proteins, direct glycation of amino acids, and digestion of glycated proteins in food. They have high renal clearance and are the major form in which AGEs are excreted from the body in urine and in dialysate in renal replacement therapy (5).
We reported on two groups of Maillard amide-AGEs. A first set N6-{2-[(5-amino-5-carboxypentyl)amino]-2-oxoethyl}lysine and N6-glycoloyl lysine have been established for the reaction of glyoxal with lysine via rearrangement reactions in vitro and in vivo. The formation pathways are directly linked to N6-carboxymethyl lysine (CML) via glyoxalimine structures (6). More recently, we identified a second class of lysine amide modifications N6-acetyl lysine, N6-formyl lysine, N6-lactoyl lysine, and N6-glycerinyl lysine in vitro based on β-dicarbonyl fragmentation of native and oxidized reductone structures. Incubation experiments confirmed the α-dicarbonyl compound 1-deoxy-d-erythro-hexo-2,3-diulose (1-deoxyglucosone, 1-DG) as a direct precursor (7). The essential role of α-dicarbonyls as central intermediates of high reactivity in the complex Maillard chemistry in vitro and in vivo has been manifested over the years. The α-dicarbonyl compounds glyoxal, methylglyoxal and 3-deoxy-d-erythro-hexos-2-ulose (3-deoxyglucosone, 3-DG) were already detected in human plasma (8–11). Even at the low concentrations found in physiological systems, α-dicarbonyls retain potent glycating activity (12).
Acylation of amino acids in vivo within the polypeptide chain was first detected in cell nuclei as a rapid and reversible incorporation of radioactive labeled acetate into histones H3 and H4 (13). This posttranslational acetylation neutralizes the positive charge of the amino acid, changing protein function in diverse ways (14, 15). The modification of core histone tails by histone acetyltransferases or histone deacetylases plays a key role in the regulation of gene expression (16, 17). Despite great biochemical and clinical interest in lysine acetylation, the knowledge of in vivo acetylation sites is limited and subject to current research (18). Piraud et al. (19) presented an ion-pair liquid chromatography/electrospray ionization mass spectrometric analysis for N6-acetyl lysine in human plasma and urine. Tsutsui et al. (20) detected N6-acetyl lysine in the plasma of ddY strain mice, which naturally develop diabetes with age. The lysine adduct was discussed as one of several potential biomarker candidates related to diabetes mellitus.
N6-formyl lysine is formed in vitro by reaction of albumin with trichloroethylene oxide, a major metabolite of 1,1,2-trichloroethylene, one of the most common compounds found in chemical waste dumps (21). In addition, it could be identified in incubation mixtures of various sugars, l-ascorbic acid or l-dehydroascorbic acid with poly-l-lysine and β-lactoglobulin, respectively (22). Jiang et al. (23) observed transfer of formyl groups from 3′-formylphosphate-ended DNA, arising from oxidation of the 5′-position of deoxyribose and subsequent DNA strand breakage by the enediyne antibiotic neocarzinostatin, to histone proteins in human TK6 cells to give N6-formyl lysine.
The aim of the present work was to elucidate formation and relevance of the four amide-AGEs N6-acetyl lysine, N6-formyl lysine, N6-lactoyl lysine, and N6-glycerinyl lysine (structures are shown in Fig. 5) in vivo with regard to non-enzymatic mechanisms within the Maillard reaction. Therefore, the free adducts were analyzed in human blood plasma by means of an LC-MS/MS technique. In model experiments, formation from glucose, the physiological most important sugar, and from various α-dicarbonyl structures as direct precursors was confirmed. Comparison of in vivo with in vitro results allowed us to assess the impact of Maillard chemistry on amide-AGE formation in vivo.
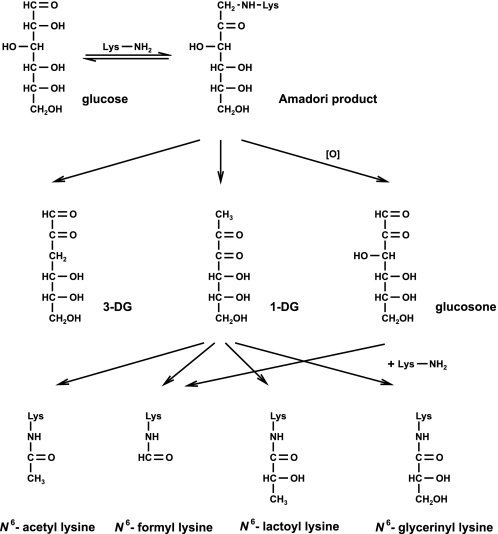
FIGURE 5.
Non-enzymatic degradation pathway of glucose.
EXPERIMENTAL PROCEDURES
Plasma Samples
Written informed consent was obtained from all patients. The study was approved by the Ethics Committee of the Medical Faculty of the Martin Luther University Halle-Wittenberg. Blood samples were obtained from 11 healthy subjects (controls) with normal renal function and 18 non-diabetic patients undergoing hemodialysis (HD patients) using disodium ethylenediaminetetraacetic acid as an anticoagulant. In dialysis patients, samples were obtained predialysis before the mid-week treatment session. Blood samples were centrifuged (2500 × g, 10 min, 4 °C) within 30 min of collection, and the plasma was frozen immediately at −80 °C. Hemodialysis was performed three times weekly for 4–5 h using polysulfone dialyzers and dialysate containing bicarbonate buffer. HbA1c, creatinine, and C-reactive protein were measured by routine methods at the central laboratory of Martin Luther University Clinical Center (Halle/Saale).
Materials
Chemicals of the highest grade available were obtained from Sigma-Aldrich and Thermo Fisher Scientific unless otherwise indicated. 1-deoxy-1-(N6-lysino)-d-fructose (Amadori product) (24), 3-DG (25), d-arabino-hexos-2-ulose (glucosone) (26), 1-DG and 2-ethyl-3-methylquinoxaline (27), CML (28), N6-carboxyethyl lysine (CEL) (29), and N6-lactoyl lysine and N6-glycerinyl lysine (7) were synthesized according to the literature. The identities of target compounds was verified by nuclear magnetic resonance experiments. Furthermore, the elemental composition was confirmed by accurate mass determination.
Model Reactions
In general, incubations were conducted in 0.1 m phosphate buffer, pH 7.4, after sterile filtration in a shaker incubator (New Brunswick Scientific, Nürtingen, Germany) at 37 °C. The reactant concentrations are mentioned in the legends to the corresponding figures and tables. Deaeration and inhibition of metal-catalyzed oxidation chemistry was achieved by using phosphate buffer containing 1 mm diethylenetriaminepentaacetic acid and gassing with argon. Buffer was degassed with helium and stored under argon prior to sample preparation. At various time points aliquots of the reaction mixtures were diluted with 6 m HCl to a final HCl concentration of 3 m. For quantitative removal of the Boc protection group, the samples were kept at room temperature for 30 min. After dilution to appropriate concentrations, the solutions were subjected LC-MS/MS analysis.
Assay of Amide-AGEs, CML, and CEL in Plasma
200 μl of plasma was transferred into a centrifugal filter containing a modified polyethersulfone membrane with 3 kDa molecular weight cut-off (VWR International, Darmstadt, Germany). Centrifugation was carried out at 14,000 × g and 4 °C. The filtrate was diluted on a scale of 1:20 with 0.1% HCl and administered to LC-MS/MS analysis.
Assay of Methylglyoxal in Plasma
Methylglyoxal in plasma samples was analyzed according to a modified method by McLellan et al. (8). Briefly, 500 μl of plasma were incubated with o-phenylenediamine at room temperature in the dark for 24 h under acidic conditions. 2-Ethyl-3-methylquinoxaline was used as internal standard. The filtered supernatant was subjected to LC-MS/MS analysis.
High Performance LC-MS/MS
The HPLC apparatus (Jasco, Gross-Umstadt, Germany) consisted of a pump (PU-2080 Plus) with degasser (LG-2080-02) and quaternary gradient mixer (LG-2080-04), a column oven (Jasco Jetstream II), and an autosampler (AS-2057 Plus). Mass spectrometric detection was conducted on an API 4000 QTrap LC-MS/MS system (Applied Biosystems/MDS Sciex, Concord, ON, Canada) equipped with a turbo ionspray source using electrospray ionization in positive mode: sprayer capillary voltage, 4.0 kV; nebulizing gas flow, 50 ml/min; heating gas, 60 ml/min at 550 °C; and curtain gas, 40 ml/min.
For the detection of the amide-AGEs, CML and CEL, chromatographic separations were performed on a stainless steel column packed with RP-18 material (Vydac CRT, no. 218TP54, 250 × 4.0 mm, RP-18, 5 μm, Hesperia, CA) using a flow rate of 1.0 ml/min. The mobile phase used was water (solvent A) and methanol/water (7:3 (v/v), solvent B). To both solvents (A and B), 1.2 ml/liter heptafluorobutyric acid was added. Analysis was performed at 35 °C column temperature using isocratic elution at 98% A/2% B. For mass spectrometric detection, the multiple reaction monitoring mode was used, utilizing collision-induced dissociation of the protonated molecules with compound specific orifice potentials and fragment-specific collision energies (Table 1).
TABLE 1.
Mass spectrometric parameters for amide-AGE, CML, and CEL quantification
| Retention time | Precursor ion |
Product ion 1a |
Product ion 2b |
Product ion 3b |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m/z | DP | m/z | CE | CXP | m/z | CE | CXP | m/z | CE | CXP | ||
| min | amu | V | amu | eV | V | amu | eV | V | amu | eV | V | |
| N6-acetyl lysine | 11.7 | 189.2 | 30.0 | 126.1 | 18.0 | 10.0 | 84.2 | 31.0 | 5.0 | 143.1 | 14.0 | 10.0 |
| N6-formyl lysine | 8.2 | 175.1 | 25.0 | 112.1 | 20.0 | 13.0 | 84.1 | 35.0 | 7.0 | 129.1 | 15.0 | 13.0 |
| N6-lactoyl lysine | 12.1 | 219.2 | 32.0 | 156.2 | 20.0 | 8.0 | 84.1 | 35.0 | 9.0 | 173.1 | 17.0 | 8.0 |
| N6-glycerinyl lysine | 6.7 | 235.3 | 48.0 | 84.2 | 37.0 | 6.0 | 172.3 | 23.0 | 30.0 | 189.3 | 17.0 | 15.0 |
| CML | 6.9 | 205.1 | 50.0 | 130.1 | 17.0 | 9.5 | 84.1 | 46.0 | 13.0 | |||
| CEL | 11.5 | 219.1 | 54.0 | 130.1 | 18.0 | 11.0 | 84.1 | 33.0 | 7.00 | 173.0 | 18.0 | 15.0 |
a MRM transition used for quantification (quantifier).
b MRM transition used for confirmation (qualifier).
Quantification was performed using the standard addition method. More precisely, increasing concentrations of authentic reference compounds at factors of 0.5, 1, 2, and 3× the concentration of the analyte in the sample were added to separate aliquots of the sample after workup procedure. The aliquots were analyzed, and a regression of response versus concentration was used to determine the concentration of the analyte in the sample. Spikes were run one in approximately every 30 samples. Calibration with this method resolves potential matrix interferences.
To obtain fragmentation spectra of amide-AGEs in plasma workup solutions, target material was first enriched by repeated collection from the above HPLC system. After solvent evaporation in a vacuum concentrator (Savant SpeedVac Plus SC 110 A combined with a Vapor Trap RVT 400, Thermo Fisher Scientific GmbH), the residue was dissolved in water and reinjected, using a collision-induced dissociation experiment. The fragmentation spectra of the authentic references were obtained with the same parameters. For N6-lactoyl lysine, the following parameters were used: declustering potential (DP), 33 V; collision energy (CE), 23 eV; collision cell exit potential (CXP), 8 V; and scan range (m/z), 50–220 (2 s).
For the detection of methylglyoxal quinoxaline, chromatographic separations were performed on a stainless steel column (Knauer, Eurospher 100 C18, 5 μm, 250 × 4.6 mm, Berlin, Germany) using a flow rate of 1.0 ml/min. The mobile phase used was water (solvent A) and methanol/water (7:3 (v/v), solvent B). To both solvents (A and B), 0.6 ml/liter heptafluorobutyric acid was added. Analysis was performed at 35 °C column temperature using gradient elution: 20 (0) to 30 (35) to 100 (65–70) to 20 (75–85); % B (t/min). For mass spectrometric detection, the multiple reaction monitoring mode was used, utilizing a collision-induced dissociation of the protonated molecules with MS parameters as follows: m/z 229.2 → 77.0 (DP, 50 V; CE, 41.0 eV; CXP, 5.0 V, quantifier), m/z 229.2 → 118.1 (DP, 50 V; CE, 30.5 eV; CXP, 7.0 V; qualifier), m/z 229.2 → 65.0 (DP, 50 V; CE, 45.0 eV; CXP, 5.0 V, qualifier). Methylglyoxal quinoxaline was detected at the retention time of tR = 58.1 min. Quantification was performed using the standard addition method with pure authentic reference compounds.
All plasma workup samples and incubations were analyzed in single batches to exclude interassay variations. Intra-assay coefficients of variation were determined by repeated analyses of controls and HD patients. In addition, as shown in Table 2, limit of detection (LOD) and limit of quantification (LOQ) with all steps of the analysis included were estimated according to DIN 32645 (n = 6, confidence level, p = 0.95, k = 3) (30). In model experiments, coefficients of variation < 10% (n = 3) for all target compounds was achieved, and LOD/LOQ was 0.0004/0.001 mmol/mol lysine for acetyl lysine, 0.0013/0.004 mmol/mol lysine for formyl lysine, 0.0007/0.002 mmol/mol lysine for lactoyl lysine, and 0.0135/0.041 mmol/mol lysine for glycerinyl lysine.
TABLE 2.
CV, LOD, and LOQ (all steps of the analysis included) of plasma samples
| CV |
LOD | LOQ | ||
|---|---|---|---|---|
| Controlsa | HD patientsb | |||
| % | pmol/ml | |||
| N6-acetyl lysine | 1–10 | 6–14 | 2.1 | 6.2 |
| N6-formyl lysine | 1–7 | 8–24 | 4.1 | 12.3 |
| N6-lactoyl lysine | 1–13 | 6–16 | 1.7 | 5.1 |
| N6-glycerinyl lysine | 1–20 | 10–18 | 1.3 | 4.0 |
| CML | 1–13 | 8–26 | 5.6 | 16.9 |
| CEL | 1–12 | 7–20 | 5.0 | 14.9 |
a 11 subjects, replicate analyses (n = 2).
b 18 subjects, replicate analyses (n = 6).
Statistical Evaluation
Data are expressed as mean ± S.D. The Student's t test was used for statistical evaluation of significant differences between both groups.
RESULTS
Amide-AGEs in Human Blood Plasma of Uremic Patients versus Healthy Subjects
Plasma was obtained from 11 healthy subjects (controls) with normal renal function and no proteinuria and 18 HD patients. In dialysis patients, samples were obtained predialysis before the mid-week treatment session. Details on the study population are summarized in Table 3. Normal renal function was defined as a serum creatinine level below 102 μmol/liter. After appropriate dilution and separation from the protein residue by a 3 kDa molecular weight cut-off membrane, the plasma samples were subjected to liquid chromatography coupled with mass spectrometric detection using multiple reaction monitoring.
TABLE 3.
Profile of subjects examined in this study
| Healthy subjects | Hemodialysis patients | |
|---|---|---|
| No. of participants | 11 | 18 |
| Age (years) | 66 ± 6 | 69 ± 11 |
| HbA1c (%) | 5.6 ± 0.2 | 6.3 ± 1.7a |
| Serum creatinine (μmol/liter) | 81 ± 8 | 706 ± 222b |
| C-reactive protein (mg/liter) | 1.7 ± 1.1 (<1.0–4.5) | 23 ± 19 (6–70) |
a Not significant.
b p < 0.001 versus healthy subjects.
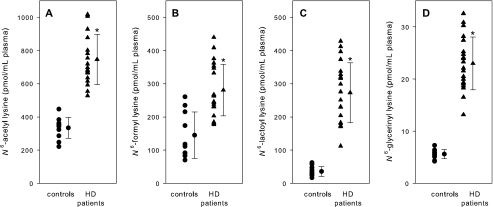
As shown in Fig. 1, plasma levels of all four carboxylic acid amides were significantly higher in HD patients than those in controls (acetyl lysine, 746 ± 151 versus 335 ± 63 pmol/ml plasma; formyl lysine, 281 ± 78 versus 145 ± 70 pmol/ml plasma; lactoyl lysine, 273 ± 90 versus 36 ± 15 pmol/ml plasma; glycerinyl lysine, 23.0 ± 5.1 versus 5.6 ± 0.9 pmol/ml plasma). To compare with well established AGE structures, we measured plasma concentrations of CML and CEL. In accordance with the literature (4), the levels were significantly elevated in HD patients (CML, 2051 ± 760 versus 129 ± 59 pmol/ml plasma; CEL, 2063 ± 785 versus 157 ± 78 pmol/ml plasma).
FIGURE 1.
N6-acetyl lysine (A), N6-formyl lysine (B), N6-lactoyl lysine (C), and N6-glycerinyl lysine (D) levels of plasma from healthy subjects (controls) and HD patients. Data are expressed as mean ± S.D., *, p < 0.001 versus control (A, C, and D), p < 0.01 versus control (B).
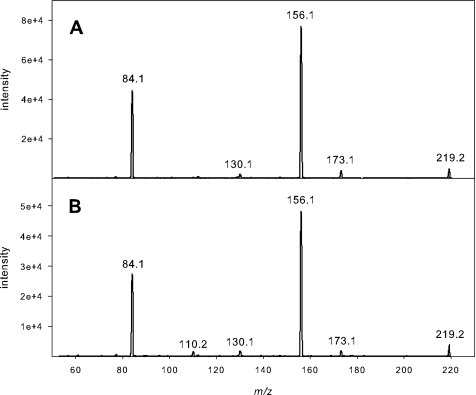
In selected cases, the identity of the detected compounds was confirmed by comparison of the retention time and fragmentation pattern with authentic reference standards. The results for lactoyl lysine are shown in Fig. 2. The quasi molecular ion [M + H]+ at m/z 219.2 is expected to undergo dehydration with immediate decarbonylation to the ion at m/z 173. The subsequent deamination of the immonium fragment renders the most abundant ion at m/z 156. Cyclization of lactoyl lysine to a six-membered ring and elimination of Nϵ functionality yields the ion at m/z 130. m/z 84 presents the pyrrolinium ion.
FIGURE 2.
Verification of N6-lactoyl lysine by collision-induced dissociation of m/z 219.2 [M + H]+, authentic reference (A), and plasma workup of HD patients (B).
Formation of Amide-AGEs in α-Dicarbonyl/N1-t-Boc-lysine Incubations
To identify the direct precursors of the carboxylic acid amides, 1-DG, 3-DG, d-arabino-hexos-2-ulose (glucosone) and methylglyoxal were incubated with N1-t-Boc-lysine under physiological conditions (pH 7.4, 37 °C). To study the impact of oxygen, incubations were conducted under aeration and deaeration. After acidic treatment to remove the Boc protection group, the samples were subjected to LC-MS/MS. Amides were found to be stable under these workup conditions, and artifact formation was excluded by immediate assay of the incubation mixtures at 0 h. The results at 72 h are provided in Table 4. 3-DG is not listed because none of the acylation products could be detected. The data for 1-DG were published in our previous work (7). With methylglyoxal, lactoyl lysine was the sole amide generated and the formation did not respond to aeration. In contrast, aeration revealed significant higher amounts of formyl lysine in glucosone incubations, whereas the levels of acetyl lysine, lactoyl lysine, and glycerinyl lysine were negligible compared with the amounts produced in 1-DG incubations.
TABLE 4.
Comparison of amide concentrations in incubation mixtures of 200 mm glucose, 42 mm 1-desoxyglucoson (1-DG), 42 mm glucosone, and 42 mm methylglyoxal in the presence of 42 mmN1-t-Boc-lysine and in incubation mixtures of 42 mm Amadori product under aerated and deaerated conditions
| Glucosea |
Amadori productb |
1-DGb |
Glucosoneb |
Methylglyoxalb |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aerated | Deaerated | Aerated | Deaerated | Aerated | Deaerated | Aerated | Deaerated | Aerated | Deaerated | |
| mmol/mol lysine | mmol/mol Amadori product | mmol/mol lysine | mmol/mol lysine | mmol/mol lysine | ||||||
| N6-acetyl lysine | 0.46 | 0.35 | 0.004 | 0.007 | 0.78 | 0.75 | 0.05 | 0.04 | < LOD | < LOD |
| N6-formyl lysine | 1.39 | 0.48 | 0.37 | 0.05 | 1.47 | 1.08 | 2.60 | 0.89 | < LOD | < LOD |
| N6-lactoyl lysine | 0.06 | 0.06 | < LOD | < LOD | 0.25 | 0.26 | < LOQ | < LOQ | 0.35 | 0.34 |
| N6-glycerinyl lysine | 0.75 | 0.10 | 0.20 | < LOQ | 0.15 | 0.09 | 0.06 | < LOQ | < LOD | < LOD |
a At 28 days.
b At 72 h.
Formation of Amide-AGEs in Glucose/N1-t-Boc-lysine Incubations
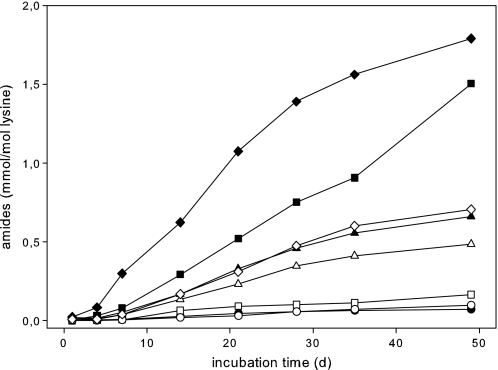
Because glucose is a major source of glycation and glycoxidation in vivo, incubations of glucose with N1-t-Boc-lysine and of 1-deoxy-1-(N6-lysino)-d-fructose, the Amadori product of both, were performed under physiological conditions (pH 7.4, 37 °C) for comparison. The time course of formation of target amides in the glucose-lysine system over a time period of 4 weeks is shown in Fig. 3 and Table 4.
FIGURE 3.
Formation of AGE-amides in incubation mixtures of 200 mm glucose with 42 mmN1-t-Boc-lysine in 0.1 m phosphate buffer, pH 7.4, at 37 °C under aerated (closed symbols) and deaerated conditions (open symbols): N6-acetyl lysine (▴), N6-formyl lysine (♦), N6-lactoyl lysine (●), and N6-glycerinyl lysine (■).
Acetyl lysine and formyl lysine were the dominant amide modifications under deaerated conditions. Both lactoyl lysine and glycerinyl lysine showed considerably lower levels of formation. In contrast to formyl and glycerinyl lysine, the impact of aeration on formation of acetyl and lactoyl lysine was negligible. However, the ratio of glycerinyl to formyl lysine changed dramatically with aeration: 1.50 versus 1.79 mmol/mol lysine compared with 0.16 versus 0.70 mmol/mol lysine with deaeration (incubation time, 48 days). This led to the conclusion that there must be additional oxygen-dependent mechanisms for glycerinyl lysine independent from formation of formyl lysine.
DISCUSSION
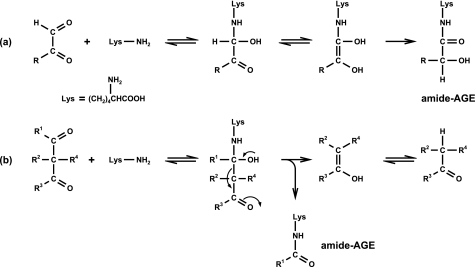
Performing several model incubations, we tried to gain insights into possible formation pathways linked to Maillard chemistry in vivo. N6-{2-[(5-amino-5-carboxypentyl)amino]-2-oxoethyl}lysine and N6-glycoloyl lysine are formed via rearrangement reactions of glyoxal with lysine in contrast to the novel amide-AGEs involving β-dicarbonyl fragmentations (Fig. 4). We already investigated the degradation of the α-dicarbonyl 1-DG in the presence of N1-t-Boc-lysine, identifying 1-DG as a direct precursor of the amide-AGEs (7). Now, glucosone was found to be another potential precursor of formyl lysine, while degradation of 3-DG did not result in any acylation. This is expected because 3-DG in contrast to 1-DG and glucosone cannot be converted to an α-oxoenediol, which is a prerequisite for β-dicarbonyl fragmentations. As shown in Fig. 5, all three C6-dicarbonyl structures are known Maillard degradation products of glucose. Although 1-DG and 3-DG are generated non-oxidatively via enolization and dehydration from the Amadori product, glucosone is formed via autoxidation of glucose or oxidation of the Amadori product or the intermediate Schiff base (31).
FIGURE 4.
Mechanism of amide-AGE formation via rearrangement (a) and β-dicarbonyl cleavage (b).
The results of incubations of glucose with N1-t-Boc-lysine and of the Amadori product of both revealed a very similar formation pattern for acetyl lysine and lactoyl lysine in reaction mixtures with glucose and 1-DG, respectively (Table 4 and Fig. 3), confirming 1-DG as the direct precursor independent from oxidative processes via hydrolytic β-dicarbonyl fragmentation. Although the formation of formyl lysine from 1-DG responded to oxidative conditions, the difference was far more pronounced from glucose or the Amadori product. This pointed to the existence of another precursor emerging from oxidative pathways, which was identified as glucosone. Here, formyl lysine was detected in significant amounts, whereas formation of all other target amides was negligible. These findings were further supported by the same ratio of acetyl to formyl lysine in 1-DG versus glucose incubations under deaerated conditions, i.e. glucosone contributes only under oxidative conditions to formation of formyl lysine from glucose, whereas 1-DG is the sole precursor in absence of oxygen. As formyl lysine, glycerinyl lysine showed a significant discrepancy in glucose versus 1-DG incubations induced by aeration. From the present results the additional reaction pathway leading to glycerinyl lysine from glucose remained unclear as none of the other Maillard intermediates responded. However, it is obvious that oxidation is required and that glucosone is not a relevant precursor.
Comparing the results of in vivo samples with above model experiments, incubations under aerated conditions should simulate the situation in uremia. Uremia has been described as a state of inflammatory stress resulting from either increased oxidation of carbohydrates and lipids (oxidative stress) or inadequate detoxification or inactivation of reactive carbonyl compounds derived from both carbohydrates and lipids by oxidative and non-oxidative chemistry (carbonyl stress) (3). Including only non-diabetic subjects in the present study, the impact of differences in glucose plasma concentrations was avoided.
The comparison gave a very diverse picture. Although all target amides in vivo were significantly increased in uremia (Fig. 1), only formyl and glycerinyl lysine responded to aeration in the glucose-lysine model. In addition, the ratio of concentrations between the single amides was completely different. In vivo, acetyl, formyl, and lactoyl lysine were within the same range, with glycerinyl lysine showing 10-fold lower concentrations. In contrast, glucose incubations showed the smallest concentrations for lactoyl lysine. Given that glycerinyl lysine indeed follows the patterns of the model incubations, this suggests additional pathways for the formation of acetyl, formyl, and lactoyl lysine. An alternative mechanism leading to lactoyl lysine in vivo is the reaction of methylglyoxal with ϵ-amino lysine residues corresponding to our identification of N6-glycoloyl lysine in glyoxal lysine model incubations based on rearrangement reactions. Indeed, when we incubated methylglyoxal with N1-t-Boc-lysine, we found significant amounts of lactoyl lysine independent from the presence of oxygen (Table 4). In vivo, this also explains the 7-fold increase in uremia, as we measured a 4-fold increase in methylglyoxal in such subjects (138 ± 39 versus 496 ± 132 pmol/ml plasma). Concentrations of acetyl lysine were highest in vivo and responded to renal failure by a 2-fold increase. This might be explained by increased cell death triggered by the strong inflammatory processes observed under uremia resulting in accelerated release of acetyl lysine from acetylated histone proteins. In addition, the increased concentration of methylglyoxal in presence of peroxynitrite might also contribute to the acetylation of ϵ-amino lysine residues in vivo. Massari et al. (32) described a mechanism of l-lysine acetylation by a methylglyoxal-peroxynitrite system in vitro. Although the direct reaction of methylglyoxal with proteins probably dominates over that with peroxynitrite, the post-translational acetylation of proteins by radical mechanisms in the presence of methylglyoxal might be a plausible second non-enzymatic pathway to acetyltransferase-catalyzed reactions. Moreover, although much less reactive, H2O2 could replace peroxynitrite in the acetyl-generating reaction from methylglyoxal. The idea to explain the 2-fold increase of formyl lysine in uremia based on the in vitro models is undermined by the fact that neither 1-DG nor glucosone have been detected in vivo so far. An intriguing alternative mechanism might be the with inflammation increased oxidative DNA breakdown to give 3′-formylphosphate-ended DNA fragments as a potential precursor, but again, such species have not been identified in vivo (23).
As a first attempt to assess the importance of the novel amide-AGEs as clinical markers for kidney failure, plasma levels of the well established lysine modifications CML and CEL were measured additionally. In healthy human subjects, concentrations were within the same range. The AGE levels decreased in the following order: acetyl lysine > CEL ≈ formyl lysine ≈ CML > lactoyl lysine > glycerinyl lysine. In uremia, we observed a 13- to 16-fold increase of CEL and CML. Amide-AGEs showed only a 2- to 8-fold increase. Thus, amide-AGEs might be of major importance when compared with other AGE structures identified in vivo so far, even though the impact of uremia on plasma concentrations of CML and CEL is more pronounced.
In conclusion, we previously proposed a formation mechanism for the novel amide-AGEs acetyl, formyl, lactoyl, and glycerinyl lysine via degradation of 1-DG in the presence of lysine (7). Now, glucosone and methylglyoxal were identified as alternative precursors for formyl and lactoyl lysine, respectively. All four amide-AGEs were unequivocally detected in human plasma, formyl, lactoyl, and glycerinyl lysine for the first time. The results strongly suggest a major participation of non-enzymatic Maillard mechanisms on amide-AGE formation pathways in vivo paralleled by enzymatic processes. The pathophysiologic consequences of two to 7-fold increased levels of amide-AGE free adducts in plasma of HD patients is not yet understood and requires further investigation. Also, the question which quantities of AGE-free adducts are derived from the breakdown of AGE-modified proteins or from direct synthesis remains unclear. However, absolute plasma concentration levels were in the same range similar to other established lysine modifications, i.e. CML and CEL, and suggest a comparable impact of non-enzymatic biochemistry on pathophysiologies in the human organism.
Footnotes
- AGE
- advanced glycation end product
- CML
- N6-carboxymethyl lysine
- 1-DG
- 1-deoxy-d-erythro-hexo-2,3-diulose (1-deoxyglucosone)
- 3-DG
- 3-deoxy-d-erythro-hexos-2-ulose (3-deoxyglucosone)
- HD patients
- patients undergoing hemodialysis
- Amadori product
- 1-deoxy-1-(N6-lysino)-d-fructose
- glucosone
- d-arabino-hexos-2-ulose
- CEL
- N6-carboxyethyl lysine
- Boc
- butoxycarbonyl
- DP
- declustering potential
- CE
- collision energy
- CXP
- collision cell exit potential
- LOD
- limit of detection
- LOQ
- limit of quantification.
REFERENCES
- 1. Dawnay A., Millar D. J. (1998) Cell Mol. Biol. 44, 1081–1094 [PubMed] [Google Scholar]
- 2. Friedlander M. A., Wu Y. C., Elgawish A., Monnier V. M. (1996) J. Clin. Invest. 97, 728–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miyata T., van Ypersele de Strihou C., Kurokawa K., Baynes J. W. (1999) Kidney Int. 55, 389–399 [DOI] [PubMed] [Google Scholar]
- 4. Agalou S., Ahmed N., Thornalley P. J., Dawnay A. (2005) Ann. N.Y. Acad. Sci. 1043, 734–739 [DOI] [PubMed] [Google Scholar]
- 5. Thornalley P. J. (2005) Pediatr. Nephrol. 20, 1515–1522 [DOI] [PubMed] [Google Scholar]
- 6. Glomb M. A., Pfahler C. (2001) J. Biol. Chem. 276, 41638–41647 [DOI] [PubMed] [Google Scholar]
- 7. Smuda M., Voigt M., Glomb M. A. (2010) J. Agric. Food Chem. 58, 6458–6464 [DOI] [PubMed] [Google Scholar]
- 8. McLellan A. C., Phillips S. A., Thornalley P. J. (1992) Anal. Biochem. 206, 17–23 [DOI] [PubMed] [Google Scholar]
- 9. Lapolla A., Reitano R., Seraglia R., Sartore G., Ragazzi E., Traldi P. (2005) Mol. Nutr. Food Res. 49, 685–690 [DOI] [PubMed] [Google Scholar]
- 10. Odani H., Shinzato T., Matsumoto Y., Usami J., Maeda K. (1999) Biochem. Biophys. Res. Commun. 256, 89–93 [DOI] [PubMed] [Google Scholar]
- 11. Mirza M. A., Kandhro A. J., Memon S. Q., Khuhawar M. Y., Arain R. (2007) Electrophoresis 28, 3940–3947 [DOI] [PubMed] [Google Scholar]
- 12. Lo T. W., Selwood T., Thornalley P. J. (1994) Biochem. Pharmacol. 48, 1865–1870 [DOI] [PubMed] [Google Scholar]
- 13. Allfrey V. G., Faulkner R., Mirsky A. E. (1964) Proc. Natl. Acad. Sci. U.S.A. 51, 786–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kouzarides T. (2000) EMBO J. 19, 1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang X. J. (2004) Bioessays 26, 1076–1087 [DOI] [PubMed] [Google Scholar]
- 16. Lee K. K., Workman J. L. (2007) Nat. Rev. Mol. Cell Biol. 8, 284–295 [DOI] [PubMed] [Google Scholar]
- 17. Shahbazian M. D., Grunstein M. (2007) Annu. Rev. Biochem. 76, 75–100 [DOI] [PubMed] [Google Scholar]
- 18. Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., Mann M. (2009) Science 325, 834–840 [DOI] [PubMed] [Google Scholar]
- 19. Piraud M., Vianey-Saban C., Petritis K., Elfakir C., Steghens J. P., Bouchu D. (2005) Rapid Commun. Mass Spectrom. 19, 1587–1602 [DOI] [PubMed] [Google Scholar]
- 20. Tsutsui H., Maeda T., Toyo'oka T., Min J. Z., Inagaki S., Higashi T., Kagawa Y. (2010) J. Proteome. Res. 9, 3912–3922 [DOI] [PubMed] [Google Scholar]
- 21. Cai H., Guengerich F. P. (2000) Chem. Res. Toxicol. 13, 327–335 [DOI] [PubMed] [Google Scholar]
- 22. Hasenkopf K., Rönner B., Hiller H., Pischetsrieder M. (2002) J. Agric. Food Chem. 50, 5697–5703 [DOI] [PubMed] [Google Scholar]
- 23. Jiang T., Zhou X., Taghizadeh K., Dong M., Dedon P. C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glomb M. A., Tschirnich R. (2001) J. Agric. Food Chem. 49, 5543–5550 [DOI] [PubMed] [Google Scholar]
- 25. Madson M. A., Feather M. S. (1981) Carbohydr. Res. 94, 183–191 [Google Scholar]
- 26. Bayne S. (1963) Methods Carbohydr. Chem. 421–423 [Google Scholar]
- 27. Glomb M. A., Pfahler C. (2000) Carbohydr. Res. 329, 515–523 [DOI] [PubMed] [Google Scholar]
- 28. Glomb M. A., Monnier V. M. (1995) J. Biol. Chem. 270, 10017–10026 [DOI] [PubMed] [Google Scholar]
- 29. Ahmed M. U., Brinkmann Frye E., Degenhardt T. P., Thorpe S. R., Baynes J. W. (1997) Biochem. J. 324, 565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Materials Testing Standards Committee NA 062 of the Deutsches Institut für Normung (German Institute for Standardization) (1994) DIN 32645; Chemical Analysis: Decision Limit, Detection Limit and Determination Limit; Estimation in Case of Repeatability; Terms, Methods, Evaluation, Beuth Verlag, Berlin [Google Scholar]
- 31. Gobert J., Glomb M. A. (2009) J. Agric. Food Chem. 57, 8591–8597 [DOI] [PubMed] [Google Scholar]
- 32. Massari J., Tokikawa R., Zanolli L., Tavares M. F., Assunção N. A., Bechara E. J. (2010) Chem. Res. Toxicol. 23, 1762–1770 [DOI] [PubMed] [Google Scholar]