Abstract
Objectives
To refine the definition of the Malignant MRI profile in acute stroke patients using baseline DWI and PWI findings from the pooled DEFUSE/EPITHET database.
Methods
Patients presenting with acute stroke within 3-6 hours from symptom onset were treated with tPA or placebo. Baseline and follow-up DWI and PWI images from both studies were reprocessed using the same software program. An ROC curve analysis was used to identify Tmax and DWI volumes that optimally predicted poor outcomes (mRS 5-6) at 90 days in patients who achieved reperfusion.
Results
Sixty-five patients achieved reperfusion and 46 did not reperfuse. ROC analysis identified a PWI (Tmax>8s) volume of >85 mL as the optimal definition of the Malignant profile. Eighty-nine percent of the Malignant profile patients had poor outcome with reperfusion versus 39% without reperfusion (p=0.02). Parenchymal hematomas occurred more frequently in Malignant profile patients who experienced reperfusion vs. no reperfusion (67% vs. 11%, p<0.01). DWI analysis identified a volume of 80 mL as the best DWI threshold, but this definition was less sensitive than PWI-based definitions.
Conclusions
Stroke patients likely to suffer parenchymal hemorrhages and poor outcomes following reperfusion can be identified from baseline MRI findings. The current analysis demonstrates that a PWI threshold (Tmax>8s) of approximately 100 mL is appropriate for identifying these patients. Exclusion of Malignant profile patients from reperfusion therapies may substantially improve the efficacy and safety of reperfusion therapies.
Clinical Trial Registration Information
EPITHET study is registered on www.clinicaltrials.gov, unique identifier NCT00238537.
Keywords: Acute Stroke, DWI, PWI, MRI, Thrombolysis, tPA, Reperfusion
Introduction
It is well established that the benefit of intravenous thrombolysis for treatment of acute ischemic stroke declines with longer onset to treatment times.1 Regression of the ischemic penumbra over time is the predominant hypothesis to explain this decline; however, reperfusion injury and hemorrhage may also contribute. Early reperfusion following ischemic stroke can cause additional injury to the ischemic arterial wall and microvasculature leading to cerebral edema and brain hemorrhage.2 Although the risk factors for reperfusion injury have not been well established, it has been hypothesized that patients with large volumes of severely ischemic tissue are at greatest risk.3 Support for this hypothesis has been provided by both the DEFUSE4 and EPITHET5 trials.
The “Malignant profile” was first described by the DEFUSE group as an MRI pattern associated with symptomatic intracranial hemorrhage and poor outcome following reperfusion.4 Criteria for the Malignant profile were empirically defined as a baseline lesion on diffusion-weighted MRI (DWI) greater than 100mL and/or a lesion on perfusion-weighted MRI (PWI) of more than 100mL using Tmax delay of more than 8 seconds. In the EPITHET study,5 patients with the Malignant profile had a greater infarct growth, a lower frequency of reperfusion and were less likely to have good neurological and functional outcomes compared with patients who did not have the malignant profile.
These data suggest that excluding Malignant profile patients may improve outcomes in clinical trials that are evaluating the efficacy of reperfusion therapies. The goal of this study was to attempt to refine the definition of the Malignant profile using baseline DWI and PWI findings from the pooled DEFUSE/EPITHET database.
Methods
The design, methodology, and primary results of DEFUSE and EPITHET studies have been previously reported.4, 5 Briefly, DEFUSE was a prospective multinational trial. Patients with acute stroke with National Institutes of Health stroke scale score (NIHSS) greater than 5 were enrolled if they could be treated with tPA within 3-6 hours after symptom onset. MRI scans, including DWI, PWI and MR angiography (MRA), were obtained before treatment and repeated 3-6 hours after initiation of thrombolytic therapy and at 30 days. EPITHET was a phase II prospective randomized, double blinded, placebo-controlled, multinational trial with similar entry criteria to DEFUSE. Patients were randomized to treatment with intravenous tPA or placebo. MRI scans, including DWI, PWI and MRA, were obtained before treatment and repeated at day 3-5 and day 90.
MRI protocols and postprocessing
In both studies: DWI images were obtained with b-values of 0 and 1000 s/mm2, dynamic susceptibility PWI images were acquired with gradient-echo imaging after a bolus of intravenous gadolinium. The software used to create the PWI maps differed between studies and resulted in systematic differences in PWI volumes.6 In addition, the criteria used for identification of DWI lesions also differed. Therefore, we uniformly reprocessed all DWI and PWI images from both studies using the same methodology. We used an in-house developed software package called RAPID (Rapid Analysis of Perfusion Imaging Data).7
In this study, the DWI lesion was defined by RAPID as pixels with ADC values below 615×10-6 mm2/s or with DWI values above 2.7 standard deviation of the average value in the brain.7 The PWI lesion was defined as pixels with Tmax delay greater than a specific threshold. Optimal cut-off volumes for Tmax thresholds of >6s, >8s and >10s were tested to determine an optimized definition of a malignant PWI lesion. Based on the original definition of the Malignant profile in DEFUSE, the pre-specified primary analysis was a Tmax threshold of >8s. A threshold of Tmax>6s was used to define the baseline and early follow-up PWI volumes that were used to determine reperfusion status.
Using the interactive software package Medical Image Processing Analysis and Visualization (MIPAV),8 the segmented lesions were reviewed by a single investigator trained as a stroke neurologist. Lesion masks, corrected for artifacts and erroneous locations, were saved and corrected volumes were recorded. Final lesion masks were adjudicated by the group of investigators.
A reanalysis of all MRA results from both studies was also performed by the group of investigators to assess the presence and degree of obstruction (complete occlusion, partial obstruction, and normal) in major intracerebral arteries.
Definition of Reperfusion
The pre-specified thresholds for defining reperfusion used in the original study design of each trial were applied. For DEFUSE patients, reperfusion was defined as a >30% reduction in volume of Tmax>6s on the 3-6 hours follow-up scan. For EPITHET patients a >90% reduction in volume of Tmax>6s was required on the day 3-5 scan. To achieve early reperfusion, at least a 10 mL reduction in PWI lesion volume was required for all patients.
Assessment of Brain Hemorrhage
Hemorrhagic transformation/parenchymal hematoma was adjudicated by a blinded committee (EPITHET) or a neuroradiologist (DEFUSE) as part of the primary data analysis for each of the individual studies using ECASS criteria.9 The original adjudications from each study were used for this analysis.
Optimal Identification of the Malignant Profile
A receiver operating characteristic (ROC) curve analysis was used to identify PWI and DWI volumes that optimally predicted poor outcomes (modified Rankin scale [mRS] 5-6) at 90 days in patients who achieved reperfusion. Patients who did not have a technically adequate baseline DWI and PWI were excluded. Patients with baseline Tmax>6s volumes <10mL were also excluded because by definition they could not achieve reperfusion.
Optimal PWI volume thresholds were compared with the optimal DWI volume threshold to identify the single parameter that best defined the Malignant profile. To assess if combinations of DWI and PWI volume thresholds were superior to single parameter definitions, ROC curves were created from probability functions derived from logistic regression analyses using both PWI and DWI volumes as independent predictors.
To determine if the parameter chosen to define the Malignant profile was an independent predictor of poor outcomes, a multivariate analysis was performed. The analysis was first performed for the entire group of patients, then subsequently for patients in whom reperfusion status could be assessed. Finally, an analysis was performed to identify any parameter in which the interaction term between that variable and reperfusion was significantly associated with poor outcome. Any interaction between reperfusion status and study (DEFUSE vs. EPITHET) was also assessed.
Statistical analysis
Continuous variables were compared using Student t-test or Mann-Whitney U test. Categorical data were analyzed using χ2 or Fisher's exact tests. Related proportions were compared using McNemar test. The optimal PWI or DWI volume limits for the ROC analysis were pre-specified to be able to predict poor outcome with specificity of at least 0.95, i.e. with a minimal possible false positive rate. For volumes that achieved this degree of specificity, the optimal volume cut-off was identified based on maximizing sensitivity. Odds ratios for poor outcome with the Malignant vs. non-Malignant profiles were estimated for the reperfused and not reperfused groups of patients and compared using Breslow-Day statistic. Criterion for entering a variable into the multivariate logistic regression analysis was significance at α<0.1 in univariate analysis. We used a backward stepwise procedure to remove variables from the model. All tests were two-tailed and statistical significance was defined at α<0.05.
Results
One hundred and seventy-five patients were enrolled in DEFUSE and EPITHET studies. Of these, one withdrew consent, 63 were excluded from the ROC analysis due to inability to determine reperfusion status: 34 had technically inadequate baseline and/or post-treatment PWI scans, and 29 had a small PWI lesion at baseline. Sixty-five patients achieved reperfusion and 46 did not reperfuse. There was no difference in the rate of poor outcome between placebo and tPA (23% vs. 25% p=0.737). Baseline characteristics are shown in the Table 1.
Table 1.
Baseline characteristics for all patients in which reperfusion status could be determined (N=111)
| Age, mean(SD) | 72(15) |
| Gender, female | 56(51%) |
| Hypertension | 69(62%) |
| Diabetes | 29(26%) |
| Hyperlipidemia | 35(32%) |
| History of Smoking | 40(36%) |
| Atrial Fibrillation | 41(37%) |
| Admission Glucose, mg/dL, mean(SD) | 139(51) |
| SBP baseline, mm Hg, mean(SD) | 146(20) |
| DBP baseline, mm Hg, mean(SD) | 76(14) |
| NIHSS baseline, median(IQR) | 14(9-17) |
| Time to baseline MRI, min mean(SD) | 257(51) |
| Time to treatment, min mean(SD) | 301(46) |
| Evidence of Obstruction on MRA | 81(79%)* |
| Degree of Obstruction, median(IQR) | 0(0-1)† |
| Received tPA | 71(64%) |
not done in 2 and not technically adequate in 7 patients
degree of obstruction categories: 0=complete occlusion, 1=partial occlusion, 2=normal
For the pre-specified primary PWI analysis (Tmax>8s), the ROC analysis (Figure 1) identified a volume of >85 mL as the optimal definition of the Malignant profile. Using this criterion to define the Malignant profile, 27/111 (24%) patients with known reperfusion status qualified as malignant. This threshold predicted poor outcome with high true positive rate in the group of patients who reperfused (Table 2) and provided good differentiation from the group of patients who did not reperfuse: in the reperfused vs. not reperfused groups, 8/9 (89%) vs. 7/18 (39%) of these patients had a poor outcome, p=0.019. In contrast, among patients without the Malignant profile there was a trend for reperfusion to be associated with a smaller chance of poor outcome: 5/56 (9%) had poor outcome in the reperfusion group vs. 7/28 (25%) without reperfusion, p=0.094. The odds ratio for having a poor outcome in optimally defined Malignant vs. non-malignant patients was 82 (95% CI 8.4-792) in patients who reperfused vs. 1.9 (95% CI 0.5-6.8) in patients who did not reperfuse, p=0.002. When adjusted for treatment with tPA vs. placebo these ORs were 94 (8.5-1036) vs. 2.5 (0.6-10) respectively. The analyses performed using Tmax>6s and >10s thresholds identified volumes of >120 mL and >65mL as optimal (Table 2).
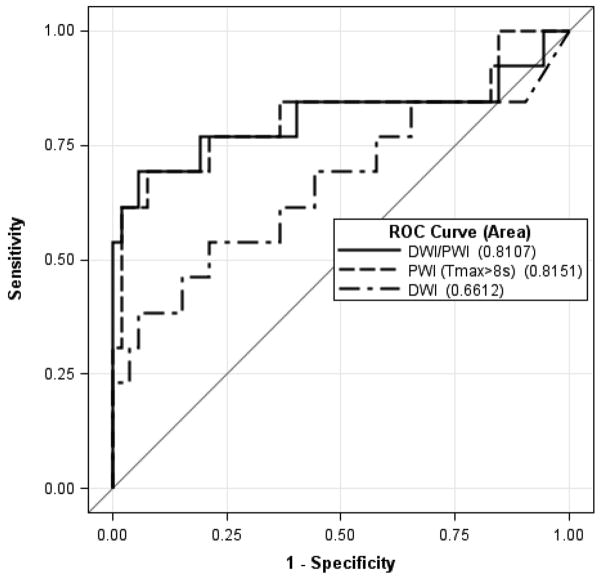
Figure 1.
A receiver operating characteristic curves and areas under the curve (AUC) to identify PWI (Tmax>8s delay) and DWI volumes as well as their combination that optimally predicted poor outcomes (mRS 5-6) at 90 days in patients who achieved reperfusion.
Table 2.
Malignant lesion definitions and their efficacy for prediction of poor outcome (mRS 5-6 at 90 days).
| Malignant lesion definition | Reperfusi on status | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | OR (95% CI) | p-value of OR difference |
|---|---|---|---|---|---|---|
| DWI>80 ml | positive | 0.23(0.06-0.54) | 0.98(0.88-0.999) | 0.75(0.22-0.99) | 15.3(1.4-162) | 0.226 |
| negative | 0.21(0.06-0.51) | 0.91(0.74-0.98) | 0.50(0.14-0.86) | 2.6(0.5-15) | ||
| Tmax>6sec >120 ml | positive | 0.62(0.32-0.85) | 0.98(0.88-0.999) | 0.89(0.51-0.99) | 82(8.4-792) | 0.001 |
| negative | 0.43(0.19-0.70) | 0.66(0.47-0.81) | 0.35(0.15-0.61) | 1.4(0.4-5.2) | ||
| Tmax>8sec >85 ml | positive | 0.62(0.32-0.85) | 0.98(0.88-0.999) | 0.89(0.51-0.99) | 82(8.4-792) | 0.002 |
| negative | 0.50(0.24-0.76) | 0.66(0.47-0.81) | 0.39(0.18-0.64) | 1.9(0.5-6.8) | ||
| Tmax>10sec >65 ml | positive | 0.54(0.26-0.80) | 0.98(0.88-0.999) | 0.88(0.47-0.99) | 60(6.2-570) | 0.010 |
| negative | 0.50(0.24-0.76) | 0.72(0.53-0.86) | 0.44(0.21-0.69) | 2.6(0.7-9.4) |
PPV – positive predictive value
OR – odds ratio
The DWI analysis identified a volume of 80 mL as optimal; however, this threshold was less sensitive (0.23) compared with the PWI threshold (0.62; p = 0.06). The overall performance of DWI to predict outcome was less accurate when compared to PWI although the difference was not statistically significant: areas under the curve (AUC) 0.66 vs. 0.82, p=0.203. No combination of DWI and PWI thresholds performed better than PWI alone (Figure 1).
The baseline MRA was of adequate technical quality for analysis in 22 of the 27 patients identified as malignant based on the optimized definition. A complete ICA occlusion was present in 12 and partial ICA occlusion in 2; the other 8 had either a complete (n=6) or partial (n=2) M1 occlusion. Among the malignant patients who reperfused, 67% developed a parenchymal hematoma (either a PH1 or PH2) on an early follow-up scan. In contrast, among the non-reperfusers only 11% developed a parenchymal hematoma (all PH1), p=0.006.
Among patients with the Malignant profile, the baseline characteristics were similar between those who reperfused vs. those who did not reperfuse (Table 3). Baseline volume of (Tmax>8s) >85mL was verified to be an independent predictor of poor outcome among all patients (Table 4); other independent predictors of poor outcome were older age, and higher baseline NIHSS. The only predictor of poor outcome that had a significant interaction between the “parameter times reperfusion”, was baseline volume of (Tmax>8s) >85mL. Adjusted for age, this was a powerful independent predictor of poor outcome in patients who reperfused (OR=78.6 [3.2-1937]; p=0.039 for interaction factor with reperfusion status), but was not significantly predictive of poor outcome in the non reperfused patients (OR 4.2 [0.86-20.4], p= 0.077). There was no interaction between reperfusion status and study group (DEFUSE vs. EPITHET).
Table 3.
Baseline characteristics for Malignant profile patients: reperfused vs. not reperfused groups.
| Reperfused N=9 |
Not reperfused N=18 |
p-value | |
|---|---|---|---|
| Age, median(IQR) | 82(80-85) | 64(55-74) | 0.002* |
| Gender, female | 3(33%) | 4(22%) | 0.653 |
| Hypertension | 6(67%) | 13(72%) | 1.000 |
| Diabetes | 2(22%) | 6(33%) | 0.676 |
| Hyperlipidemia | 2(22%) | 5(28%) | 1.000 |
| History of Smoking | 3(33%) | 10(56%) | 0.420 |
| Atrial Fibrillation | 3(33%) | 6(33%) | 1.000 |
| Admission Glucose, mg/dL, median(IQR) | 129(108-142) | 138(114-222) | 0.425 |
| SBP baseline, mm Hg, median(IQR) | 165(125-179) | 140(135-154) | 0.381 |
| DBP baseline, mm Hg, median(IQR) | 81(67-89) | 78(63-90) | 0.757 |
| NIHSS baseline, median(IQR) | 18(12-22) | 17(14-20) | 0.699 |
| Time to baseline MRI, min median(IQR) | 245(205-261) | 225(188-280) | 0.746 |
| Time to treatment, min median(IQR) | 290(197-310) | 276(240-340) | 0.777 |
| Evidence of Obstruction on MRA | 6(100%)† | 16(100%)‡ | 1.000 |
| Degree of Obstruction, median(IQR)§ | 0(0-0) | 0(0-0) | 0.912 |
| Received tPA | 7(78%) | 6(33%) | 0.046* |
significant at α<0.05
not technically adequate in 3 patients
not technically adequate in 2 patients
degree of obstruction categories: 0=complete occlusion, 1=partial occlusion, 2=normal
Table 4.
Univariate and multivariate predictors of poor outcome (90-day mRS=5-6 vs. mRS<5) based on baseline characteristics and dichotomized DWI and Tmax>8sec baseline lesion volumes
| Variables | Univariate analysis with all patients | Multivariate analysis with all patients having technically adequate baseline DWI and PWI | |
|---|---|---|---|
| (N=174) | (N=161) | ||
| p | p | OR(95%CI) | |
| Age | <0.001 | 0.001 | 1.48(1.17-1.88)* |
| Systolic BP | 0.070 | ||
| Baseline NIHSS | <0.001 | 0.010 | 1.13(1.03-1.24)* |
| Time to treatment | 0.049 | ||
| Evidence of Obstruction on MRA1 | 0.004 | ||
| Degree of obstruction MRA1 | 0.004 | ||
| DWI baseline volume >80 mL | 0.001 | ||
| Tmax>8sec baseline volume >85 mL | <0.001 | <0.001 | 8.9(2.9-27.5) |
odds ratios (OR) are estimated for 5 years increase of age and 1 score increase of NIHSS.
Discussion
This analysis confirms that patients who have poor outcomes associated with reperfusion can be identified from baseline MRI findings. The original definition of the Malignant profile (DWI and/or PWI Tmax>8s thresholds of 100 mL) was based on observations from a very small sample of patients.4 The current analysis affirms that a threshold of approximately 100 mL is appropriate and suggests that PWI may perform better than DWI for identification of the Malignant profile.
The volume of the severe perfusion deficit (Tmax>8 sec) >85mL was an independent predictor of poor outcome in patients who reperfused, but was not a significant predictor in patients who did not reperfuse. These findings suggest that reperfusion may lead to detrimental effects in this select patient population. An optimized definition of the Malignant profile based only on DWI lesion size identified patients who had poor outcomes regardless of whether reperfusion was achieved.
If these findings can be verified prospectively they will have very important clinical and research consequences. Including patients who have very poor prognostic signs that are independent of reperfusion may dilute the power of a study to establish the benefit of a reperfusion therapy. In contrast, treating patients who are at increased risk of harm from reperfusion can directly offset the benefits of an otherwise effective reperfusion therapy.
PWI findings may be a more sensitive predictor of reperfusion related injury than DWI because a large and severe PWI lesion may be a better predictor of serious injury to the ischemic arterial wall. Reperfusion of injured microvasculature may cause breakdown of the blood brain barrier, leading to vasogenic edema and potentially to symptomatic hemorrhage. This hypothesis is supported by our finding that parenchymal hematomas occurred significantly more frequently in malignant profile patients who experienced reperfusion compared with those who did not reperfuse. Another reason why the early severe PWI lesions may be more sensitive than DWI lesions for identification of the malignant patients is that expansion of DWI lesions into areas of severe hypoperfusion evolves over time. Therefore, the early DWI lesion can underestimate the volume of severe hypoperfusion. The EPITHET investigators demonstrated that very low cerebral blood volume is a stronger predictor of the risk of hemorrhagic transformation than DWI volume.10 The UCLA-Samsung Stroke collaborators observed that aggressive treatment and severe hypoperfusion, but not DWI volume, were associated with hemorrhagic transformation.11
In a retrospective study reported by Yoo et al,12 among patients undergoing intra-arterial therapy, baseline DWI lesion volume of greater than 70 mL was identified as a threshold to define a futile group. All six patients in the futile group had a poor clinical outcome (defined as mRS≥3 at 3 months) including a high mortality rate despite a 50% recanalization rate. These patients had also a significantly larger mean transit time PWI lesion volumes.
There are some limitations to our study. Post-treatment MRI scans were done at different time-windows in DEFUSE and EPITHET studies, therefore we were not able to define reperfusion identically for each study population. In addition, reperfusion was ascertained at a relatively late time-point in EPITHET. The sample size of Malignant profile patients is small, and particularly small in the subgroup with reperfusion. Due to this limitation, 95% CI for ORs were very wide. The Malignant profile patients with reperfusion were older than the patients who did not achieve reperfusion but had identical median baseline NIHSS scores. Not all patients could be included in the primary analysis because of technically inadequate PWI scan at either baseline or early follow-up. We used only one ADC threshold to identify DWI lesions, however the threshold used was optimized in a prior study and produces volumes that are similar to adjacent thresholds.7 Using other ADC thresholds or alternative methods for quantifying DWI lesions might yield better results and should be considered for further study. Other considerations for further research include analyses of the performance of total PWI plus DWI lesion volume or the union of PWI and DWI lesions voxels on coregistered maps.
Summary
In conclusion, based on data from the pooled DEFUSE/EPITHET dataset, a baseline Tmax>8sec PWI lesion greater than 85 mL defines patients who respond unfavorably to reperfusion; they experienced worse clinical outcomes and higher rates of parenchymal hematoma with reperfusion. If validated in prospective studies, exclusion of Malignant profile patients from reperfusion therapies may substantially improve the efficacy and safety of reperfusion therapies.
Acknowledgments
Sources of Funding: The DEFUSE study was supported by the National Institutes of Health (National Institute of Neurological Disorders and Stroke, RO1 NS39325). The EPITHET study was supported by National Health and Medical Research Council, Australia; National Stroke Foundation, Australia; and Heart Foundation of Australia. The pooled DEFUSE-EPITHET project received funding from the National Institute of Neurological Disorders and Stroke (K23 NS051372, PI M.G.L., and K24 NS044848, PI G.W.A.).
Footnotes
Disclosures: Drs. Mlynash, Lansberg, De Silva, Lee, Christensen, Straka, Campbell, Bammer, Olivot, and Desmond report no disclosures. Dr. Donnan received research support from Boehringer Ingelheim and Sanofi-Aventis and has served on advisory boards for Boehringer Ingelheim, Servier, Sanofi-Aventis and Bristol-Myers Squibb. Dr. Davis served on an advisory board for Boehringer Ingelheim. Dr. Albers has consulted for Genentech and Lundbeck.
References
- 1.Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment: Pooled analysis of Atlantis, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 2.Maier CM, Hsieh L, Crandall T, Narasimhan P, Chan PH. Evaluating therapeutic targets for reperfusion-related brain hemorrhage. Ann Neurol. 2006;59:929–938. doi: 10.1002/ana.20850. [DOI] [PubMed] [Google Scholar]
- 3.von Kummer R, Meyding-Lamade U, Forsting M, Rosin L, Rieke K, Hacke W, Sartor K. Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. AJNR Am J Neuroradiol. 1994;15:9–15. [PMC free article] [PubMed] [Google Scholar]
- 4.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 5.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, Chalk JB, Fink JN, Kimber TE, Schultz D, Hand PJ, Frayne J, Hankey G, Muir K, Gerraty R, Tress BM, Desmond PM. Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (EPITHET): A placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 6.Lansberg MG, Lee J, Christensen S, Straka M, Silva DAD, Mlynash M, Campbell BC, Bammer R, Olivot JM, Davis SM, Donnan GA, Albers GW on behalf of the DEFUSE-EPITHET investigators. Two different studies with one consistent message. Stroke. 2010;41:e295. Abstract P157. [Google Scholar]
- 7.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. Journal of Magnetic Resonance Imaging. 2010;32:1024–1037. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAuliffe MJ, Lalonde FM, McGarry D, Gandler W, Csaky K, Trus BL. Medical image processing, analysis and visualization in clinical research. Computer-Based Medical Systems (CBMS) 2001:381–386. [Google Scholar]
- 9.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Hoxter G, Mahagne MH, Hennerici M, Group ES. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: The european cooperative acute stroke study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 10.Campbell BC, Christensen S, Butcher KS, Gordon I, Parsons MW, Desmond PM, Barber PA, Levi CR, Bladin CF, De Silva DA, Donnan GA, Davis SM. Regional very low cerebral blood volume predicts hemorrhagic transformation better than diffusion-weighted imaging volume and thresholded apparent diffusion coefficient in acute ischemic stroke. Stroke. 2010;41:82–88. doi: 10.1161/STROKEAHA.109.562116. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Bang OY, Liebeskind DS, Ovbiagele B, Kim GM, Chung CS, Lee KH, Saver JL for the U-SSC. Impact of baseline tissue status (diffusion-weighted imaging lesion) versus perfusion status (severity of hypoperfusion) on hemorrhagic transformation. Stroke. 2010;41:e135–142. doi: 10.1161/STROKEAHA.109.563122. [DOI] [PubMed] [Google Scholar]
- 12.Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. MRI-based selection for intra-arterial stroke therapy: Value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]