Abstract
Compression in the basilar-membrane input–output response flattens the temporal envelope of a fluctuating signal when more gain is applied to lower level than higher level temporal components. As a result, level-dependent changes in gap detection for signals with different depths of envelope fluctuation and for subjects with normal and impaired hearing may reveal effects of compression. To test these assumptions, gap detection with and without a broadband noise was measured with 1 000-Hz-wide (flatter) and 50-Hz-wide (fluctuating) noise markers as a function of marker level. As marker level increased, background level also increased, maintaining a fixed acoustic signal-to-noise ratio (SNR) to minimize sensation-level effects on gap detection. Significant level-dependent changes in gap detection were observed, consistent with effects of cochlear compression. For the flatter marker, gap detection that declines with increases in level up to mid levels and improves with further increases in level may be explained by an effective flattening of the temporal envelope at mid levels, where compression effects are expected to be strongest. A flatter effective temporal envelope corresponds to a reduced effective SNR. The effects of a reduction in compression (resulting in larger effective SNRs) may contribute to better-than-normal gap detection observed for some hearing-impaired listeners.
INTRODUCTION
Detection of temporal gaps in noise markers depends on several factors, including signal level, audibility, and temporal characteristics of the envelope. Compression in the basilar-membrane input–output response effectively flattens the temporal envelope of a fluctuating signal when more gain is applied to lower level than higher level temporal components. As a result, level-dependent changes in gap detection for signals with different depths of envelope fluctuation may reveal effects of compression.
Gap detection is poorer for signals with larger envelope fluctuations (see, e.g., Glasberg and Moore, 1992; Hall and Grose, 1997), which is likely due to increased confusion between the imposed gap and inherent fluctuations of the signal. The “effective” magnitude of envelope fluctuations may differ for subjects with cochlear hearing loss due to reductions in gain and compression (Robles and Ruggero, 2001). To test a related hypothesis, Glasberg and Moore (1992) and Moore et al. (2001) measured gap detection for subjects with normal and impaired hearing for noise markers with varying degrees of envelope fluctuations. Their hypothesis was that loudness recruitment effectively magnifies the amplitude fluctuations in narrow bands of noise and results in poorer gap detection for subjects with cochlear hearing loss than for those with normal hearing. In terms of cochlear mechanics, magnified effective amplitude fluctuations due to loudness recruitment may be explained by a reduction in cochlear compression. Envelope fluctuations were varied due to differences in marker bandwidth and also by external processing of the markers by raising the envelope to a power, N, (Glasberg and Moore, 1992) or by fast-acting compression, similar to that used in some digital hearing aids (Moore et al., 2001). In Glasberg and Moore (1992), poorer gap detection was measured for hearing-impaired than normal-hearing ears, especially for narrower bandwidths and for values of N greater than 1 (i.e., magnified envelope fluctuations). In addition, compressing fluctuations in the markers by raising the envelope to values of N less than 1 resulted in improved gap detection for all subjects, especially for narrower bandwidths (i.e., 50 Hz or less). Similarly, Moore et al. (2001) determined that compression as implemented in some digital hearing aids could also improve hearing-impaired subjects’ detection of gaps in noise with bandwidths up to 50 Hz. Taken together, results confirmed an important role of envelope fluctuations in gap detection.
Hall and Grose (1997) also measured gap detection and loudness growth in normal-hearing subjects for noise markers that differed in bandwidth and thus in prominence of envelope fluctuations. A broadband noise was presented simultaneously with the noise markers at two levels, 0 and 40 dB/Hz. For both broadband noise levels, markers were presented at sensation levels (SLs) of 20, 15, and 10 dB. A larger disparity in gap detection thresholds was predicted between the flatter and fluctuating markers for the higher level background noise compared to the lower level background noise. The investigators assumed that with the higher background resulting in loudness recruitment-like effects, the perceived amplitude fluctuations of the narrower marker would be magnified, making gap detection even more difficult. As expected, their measures of loudness growth confirmed steeper growth in the higher level background noise compared to the lower level background noise. Further, mean gap detection worsened as signal-to-noise (SNR) was reduced for both markers and at both background noise levels. In contrast to their expectations, little difference was observed for gap detection thresholds at the two background noise levels. The authors suggested that the “recruitment-like effect” occurring in masked normal hearing listeners may be functionally dissimilar to the loudness recruitment associated with cochlear hearing loss. However, given the signal presentation levels, results in Hall and Gross (1997) may be consistent with models of cochlear nonlinearities (see, e.g., Glasberg and Moore, 2000). These models assume more linear responses at lower and higher levels and the strongest compression at mid levels, ∼50 dB SPL. The lower and higher levels tested by Hall and Grose (1997) for the fluctuating marker were ∼30–40 and 70–80 dB SPL, respectively, and thus might be expected to result in generally similar basilar-membrane responses. Data were not obtained at mid levels expected to show the strongest compression effects. Although generally similar gap detection was reported at similar marker levels, slightly better performance was reported for the flatter marker in the higher than the lower level background. This was not consistent with their recruitment hypothesis, but was similar to results from Fitzgibbons (1984). In the study by Fitzgibbons, gap detection was measured in three levels of a broadband noise background for an octave-wide noise marker centered at 1000 Hz. Threshold was defined as the SL of the marker needed to detect a gap of fixed duration. As background levels increased, gap detection improved; i.e., there was a decrease in the SL of the marker necessary for detection of the gap. As Hall and Grose (1997) noted, the trend for gap detection to improve with increasing background level was more prevalent in the data from Fitzgibbons (1984) than in their results; however no explanation for this discrepancy was provided.
Most behavioral estimates of cochlear compression involve pure-tone signals, including growth of masking (Oxenham and Plack, 1997) and temporal masking curves (TMC) (Nelson et al., 2001). However, speech is a broadband signal with varying depths of envelope fluctuation. Thus, behavioral estimates of the effects of compression using nonspeech signals with varying bandwidths and depths of envelope fluctuation may more directly relate changes in compression, such as those associated with increased signal level or impaired hearing, to changes in speech understanding. Accordingly, a long-term goal is to use complex signals to estimate compression, rather than pure tones, to explore the relationship between changes in measures of compression and effects on speech understanding. Given the inconclusive results from previous studies with normal-hearing subjects examining level-dependent changes in gap detection for broadband signals (Fitzgibbons, 1984; Hall and Grose, 1997), the current study was designed to include small increases in marker level over a sufficient range of levels to encompass more linear and more compressed responses. In addition, subjects with a range of absolute thresholds were included to assess changes in compression effects with hearing impairment.
Gap detection was measured with 1000-Hz-wide (flatter) and 50-Hz-wide (fluctuating) noise markers as a function of marker level. Adults with normal and impaired hearing listened to markers presented with and without a low-fluctuation broadband noise. As marker level increased, background level also increased, maintaining a fixed SNR to minimize SL effects on gap detection (see, e.g., Florentine and Buus, 1984). Thus, this experiment was designed to hold constant the “acoustic envelope” as marker level increased. The “acoustic envelope” or “acoustic SNR” is defined as the difference in level between the peaks of the marker and the trough imposed by the background noise, prior to cochlear processing. Compression effects are expected to change these peak-to-trough level differences, resulting in the “effective envelope,” or “effective SNR” of the combined marker-plus-background signal. When a background noise is included, the noise determines the SNR and serves as the trough of the envelope, thus limiting the depth of envelope fluctuation. Accordingly, compression effects that reduce the effective SNR also reduce the effective depth of envelope fluctuation.
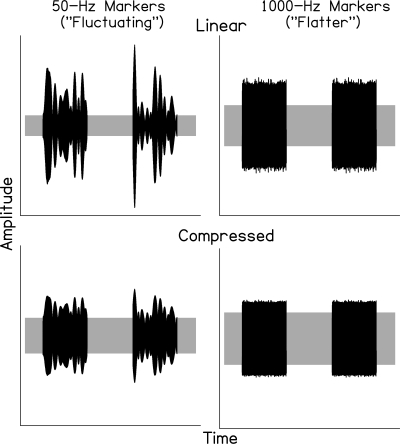
The schematics in Fig. 1 illustrate the temporal envelopes of marker-plus-background signals, including gaps, with and without effects of a basilar-membrane compressor. The fluctuating marker is represented in the left panels by the black waveforms with prominent peaks; the flatter marker is represented in the right panels by the black waveforms with substantially less amplitude fluctuation; in all panels the background noise is represented by the gray rectangles. The top panels illustrate the acoustic envelope of both marker-plus-background signals with no compression. These also illustrate effective envelopes with linear processing, as may occur at high- and low-signal levels. Increasing compression as signals increase from low to mid levels (e.g., from ∼20 to 50 dB SPL) would provide the most gain to the lowest level background noise, less gain to the relatively low-level marker peaks, and the least gain to the highest level marker peaks. As a result, the effective depth of envelope fluctuation and the effective SNR would be reduced for both markers. This is illustrated in the bottom panels of Fig. 1, which show the effect of the basilar-membrane compressor for the background plus fluctuating and flatter markers at the mid levels corresponding to the most compressive segment of the input–output function (i.e., ∼50 dB SPL).1 A reduction in the effective SNR is expected to lead to poorer gap detection, based on reductions in the acoustic SNR that resulted in poorer gap detection under comparable conditions reported by Hall and Grose (1997). Increasing compression would have an additional effect for the fluctuating marker, that is, the reduction in its effective depth of envelope fluctuation could reduce confusion between the imposed gap and the inherent fluctuations in the marker, which would be expected to improve gap detection. Thus, for the fluctuating marker, increasing compression could have effects that result in either better or worse gap detection. It is not known which effect predominates.
Figure 1.
Schematics of markers (black), including gaps and continuous background noise (gray). The fluctuating and flatter markers are shown in the left and right panels, respectively. The temporal envelopes of marker-plus-background signals with and without effects of a basilar-membrane compressor are illustrated in the bottom and top panels, respectively. A formula proposed by Glasberg and Moore (2000) was used for the compression function.1 For ease of viewing, the linear and compressed signals (in the top and bottom panels) have been scaled to equate marker rms.
As signals increase from mid to high levels, the expected effects would be reversed. This can be seen in Fig. 1 by comparing the signals in the bottom panels (reflecting the most compression for mid-level signals) to those in the top panels (reflecting linear processing for high-level signals). Decreasing compression would result in an increasing effective envelope and improving gap detection for both markers. For the fluctuating marker, decreasing compression would have the additional effect of increasing its effective peakiness, resulting in more confusion and poorer gap detection. Finally, level-dependent differences in gap-detection thresholds between normal-hearing and hearing-impaired subjects may reveal effects of reduced cochlear nonlinearities. For example, for the flatter marker, if compression effects result in poorer gap detection at mid levels for normal-hearing subjects, reduced compression for hearing-impaired subjects may result in better gap detection.
The primary goal of this study was to estimate effects of compression using nonspeech signals with varying bandwidths and depths of envelope fluctuation. A secondary goal was to relate these results to a more established estimate of cochlear nonlinearities. Thus, TMCs were also measured and basilar-membrane input–output functions were inferred to compare and correlate with the gap detection results. Although subjects with lower quiet thresholds are assumed to have stronger compression than subjects with higher quiet thresholds, changes in quiet threshold may not accurately reflect changes in compression. Indeed, current evidence is not consistent with regard to the extent to which compression slopes change with increasing hearing loss (see, e.g., Plack et al., 2004; Rosengard et al., 2005; Poling et al., 2011). Therefore, comparing gap detection results to an independent estimate of cochlear compression may help reveal the extent to which differences in compression contribute to differences in gap detection for subjects with similar quiet thresholds.
METHODS
Subjects
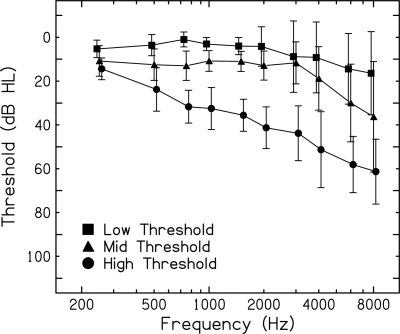
Thirty-eight right-handed subjects participated, spanning a wide range of ages and hearing thresholds. Participants were organized into three groups based on their quiet thresholds, defined as the average detection threshold for the two noise markers (flatter and fluctuating) used in the gap detection task: Low Threshold group (mean age = 35.8 yr, n = 18, 13 females); Mid Threshold group (mean age = 56.8 yr, n = 12, 8 females); and High Threshold group (mean age = 74.5 yr, n = 8, 6 females). Signals were presented to subjects’ right ears to minimize potential effects due to ear differences in gap detection (Sininger and de Bode, 2008). Mean audiometric thresholds [±1 standard deviation (SD)] for the right ears for the three subject groups are shown in Fig. 2. Subjects were paid an hourly wage for their participation.
Figure 2.
Mean (±1 SD) audiograms of right ears for subjects in the Low Threshold (squares), Mid Threshold (triangles), and High Threshold (circles) groups.
Stimuli and apparatus
Gap detection stimuli
The markers were centered at 1000 Hz, with 400 ms duration including gaps and 30 ms raised-cosine external rise and fall ramps. The markers differed in their depths of envelope fluctuation. The 50-Hz-wide Gaussian noise had prominent fluctuations. The 1000-Hz-wide low-fluctuation noise was created with a custom-designed algorithm to produce an envelope with minimal dips and peaks (see, e.g., Kohlrausch et al., 1997,). Because the 1000 Hz bandwidth of the flatter marker is broader than that of the auditory filter centered around 1000 Hz, it is likely that the auditory filter characteristics cause the internal temporal envelope of the marker to be less flat than its acoustic envelope (see, e.g., Kohlrausch et al., 1997). Nevertheless, at the output of the auditory filters around 1000 Hz, the effective depth of envelope fluctuation of the 50 Hz marker is expected to be significantly larger than that of the 1000 Hz marker. In addition, modulation rate increases with increasing marker bandwidth (Rice, 1954), and sensitivity to modulation is better for lower than higher modulation rates (see, e.g., Viemeister, 1979). Thus, the deeper and slower the modulations in the 50-Hz-wide marker are expected to be more salient than the modulations in the 1000-Hz-wide marker.
Gaps were located at the temporal center of each marker and were created by gating the marker off and on with a half-cycle of a raised-cosine function, 20 ms in duration for the fluctuating marker and 8 ms for the flatter marker. Gap duration is defined as the time between the start of gate offset and start of gate onset. For example, for the fluctuating marker, a 20 ms gap occurred when the onset ramp started immediately following the end of the offset ramp. The longer ramp duration was selected for the fluctuating marker to enhance the confusion effect between the imposed gap and the inherent fluctuations in the marker (Grose et al., 2008); the goal was to increase the likelihood that confusion effects and changes in confusion with increasing marker level would be the primary influence on gap detection thresholds for the fluctuating marker. For conditions designed to hold constant the acoustic envelope with increases in marker level, a low-fluctuation background noise was included, which also increased in level. This 2000-Hz-wide background was centered at 1020 Hz and recorded onto a CD for later playback. As described earlier, a schematic of the two markers in the background noise is provided in the top panels of Fig. 1.
Temporal masking curve stimuli
TMCs were measured twice as part of a parallel study (Poling et al., 2011,). The first measure included 37 subjects and a 1000 Hz probe at 10 dB SL. TMCs were measured as a function of the time interval between the masker and the probe, for on-frequency (1000 Hz) and off-frequency (500 Hz) maskers. The second measure included off-frequency TMCs using an 800 Hz masker and a 2000 Hz probe and 12 of the 37 subjects. This measure was added to assess possible compression effects on off-frequency TMCs using lower frequency probes combined with maskers an octave or less below the probe (see, e.g., Lopez-Poveda et al., 2003,; Rosengard et al., 2005,; Lopez-Poveda and Alves-Pinto, 2008). For all TMCs, the masker-probe interval ranged from 0 to 70 ms in 10 ms steps, with a goal of a minimum of seven masker-probe intervals. For cases where masker levels would have exceeded the maximum allowable masker level of 102 dB SPL using 10 ms steps, 5 ms steps were used. Probe and masker durations were 20 and 200 ms, respectively, with 10 ms raised-cosine rise and fall ramps.
Gap detection and temporal masking curve apparatus
Subjects were seated inside a double-walled sound-attenuating booth and registered responses via a button box (TDT RBOX). The signals were digitally generated with custom LABVIEW software (LABVIEW 8.5, National Instruments) and converted to analog using a 16 bit digital-to-analog converter (National Instruments, model 6052E) with a sampling rate of 50 kHz. The generated signals were low-pass filtered at 20 kHz (TDT FT5), attenuated (TDT PA4), and mixed (TDT SM3). The markers were mixed with the background noise (Sony CDR-W66) for gap detection measures; the pure-tone probe and maskers were mixed for the TMCs. For both gap detection and TMC measures, signals were passed through a headphone buffer (TDT HB5) for presentation to the right ear through a TDH-39 (Telephonics) headphone.
Procedures
Marker and background noise levels for gap detection
Gap detection with background noise at fixed signal-to-noise ratios.
Given the goal of measuring effects of level-dependent changes in basilar-membrane compression with a gap detection task, a range of marker levels was desirable. Data were initially obtained from subjects with normal hearing, with levels selected following pilot data collection according to the following criteria: (1) the lowest background level was at least 5 dB above detection threshold for the background, to minimize near-threshold effects; (2) the level of each marker was approximately 10 dB above detection threshold measured in the background noise, to minimize near-threshold effects and differences in marker audibility; and (3) the peak levels of both markers were approximately equal, initially assuming level effects would be revealed as a function of marker peak level. As subjects with hearing loss were recruited, background and marker levels were shifted to higher levels as needed. The initial criterion of equating peak levels between markers was relaxed, as it was found to be not critical. For subjects with hearing loss, the key considerations were to: (1) ensure that the lowest background and marker levels were at least 5 dB above detection threshold for the background and the marker, to minimize near-threshold effects, and (2) limit the highest marker level to avoid loudness discomfort. For all subjects, marker and background levels increased together in 3 dB steps. Individual marker and background levels are provided.2 To facilitate comparison across marker and background noises of different bandwidths and to provide an estimate of signal energy within a critical band, levels are shown as the root mean square (rms) level (dB SPL) within the equivalent rectangular bandwidth (ERB) of an auditory filter centered around 1000 Hz (Glasberg and Moore, 1990).3
Gap detection with no background noise.
For all subjects, marker levels increased in 3 or 6 dB steps and were selected to: (1) be at least 5 dB above detection threshold for the marker, to minimize near-threshold effects; (2) limit the highest marker level to avoid loudness discomfort; (3) include the same range of SPLs for each subject as was included for gap detection with a background noise; and (4) include markers up to at least 30 dB above detection threshold for the marker, to make comparisons across subjects and marker types at relatively high levels where performance typically asymptotes. See Table TABLE I. for the resulting ranges of marker levels.
Table 1.
Minimum and maximum marker levels used to measure gap detection thresholds (with no background noise) for each marker type and subject group.
| Flatter marker | Fluctuating marker | ||||
|---|---|---|---|---|---|
| Subject group | dB SPL | dB SL | dB SPL | dB SL | |
| Low Threshold | Minimum | 9 | 7 | 7 | 6 |
| Maximum | 51 | 53 | 46 | 38 | |
| Mid Threshold | Minimum | 9 | 5 | 19 | 5 |
| Maximum | 57 | 47 | 52 | 34 | |
| High Threshold | Minimum | 27 | 6 | 34 | 6 |
| Maximum | 81 | 42 | 91 | 41 | |
Detection and gap detection
Detection thresholds for both markers and for the background noise were estimated using a three-alternative forced-choice adaptive procedure, which converged on the 70.7% point on the psychometric function (Levitt, 1971). An initial step size of 4 dB was reduced to 2 dB following four reversals. After eight more reversals, the average of the final six reversals was taken as the threshold for that run. Two thresholds were measured for each condition. If the difference between the two estimates exceeded 3 dB, an additional estimate was obtained and the final threshold was taken as the mean of all estimates collected for that condition. The additional run was required for 21%, 24%, and 26% of the estimates for the flatter and fluctuating markers and the background noise, respectively.
Gap detection thresholds were estimated using the same psychophysical procedure as was used for measuring detection thresholds. Gap step size was adjusted by a factor of 1.2. Thresholds were estimated as the geometric mean of the last six reversals in a ten-reversal track. Three thresholds were measured for each marker/background combination. If the SD of the logarithm of the three estimates was greater than 0.2, an additional estimate was obtained and the final threshold was taken as the geometric mean of all estimates collected for that condition. This variability criterion was exceeded for 37% and 55% of the estimates for the flatter and fluctuating markers, respectively. For gap detection without a background noise, two thresholds were measured for each marker level, with a third included if the SD of the logarithm of the two estimates exceeded 0.2. The variability criterion was exceeded for 20% and 37% of the estimates for the flatter and fluctuating markers, respectively. The larger variability in estimates for the fluctuating marker is likely due to the variability in the markers. That is, a new marker with a different temporal envelope was generated after each response within a run.
For each subject, the first marker to be tested (flatter or fluctuating) was selected at random. Detection thresholds for this marker and for the background were obtained first and used to set the range of marker levels for the measurement of gap detection thresholds. The first gap detection condition to be tested (with or without background noise) was selected at random. The order of the levels tested within a condition was also randomized such that thresholds for one set of all marker levels in each condition were obtained prior to obtaining the remaining threshold estimates.
Temporal masking curves
Masker levels at threshold were estimated using the same psychophysical procedure, including step sizes and numbers of reversals, used to measure detection thresholds for the gap signals. The probe level was fixed at 10 dB SL; masker level was varied adaptively, up to a maximum of 102 dB SPL. Three thresholds were measured and averaged for each masker-probe interval, with a fourth included if the SD of the mean of the three estimates exceeded 6 dB (Lopez-Poveda and Alves-Pinto, 2008).
Estimating compression values from TMCs involves the inference of basilar-membrane input–output responses by plotting the level of the off-frequency masker against the level of the on-frequency masker at each masker-probe interval (Nelson et al., 2001,). A three-segment fit was applied to each subject’s inferred basilar-membrane input–output function, corresponding to linear-compressed-linear segments typical of these functions (Yasin and Plack, 2003; Plack et al., 2004). A custom program developed in Matlab (The MathWorks, Natick, MA) using the fminsearch function was used (Plack et al., 2004,), whereby the slopes of the lower and upper segments were fixed at 1.0 (linear response). The slope of the mid-level segment was varied by the fitting procedure to satisfy a least-squares regression criterion. At least five points were required for the fit, with at least three of those points falling in the compressed region. The rms error between fitted and measured values must be <5 dB. Using these criteria, compression slope estimates were obtained for 32 of 37 subjects with the 500 Hz off-frequency masker and for 10 of 12 subjects with the 800 Hz off-frequency masker.
Training for gap detection and temporal masking curves
Prior to data collection, subjects were trained on the gap detection and TMC tasks. For gap detection, training on each condition (flatter and fluctuating marker, with and without background noise) started with a middle-level marker, for which the gap was relatively easy to detect. The highest and lowest marker levels in each condition were included in the initial training session to ensure that the subject would be able to complete the task over the required range of levels. For TMCs, training started with a longer masker-probe interval (i.e., 60 or 70 ms) with the off-frequency masker, to ensure that the probe was relatively easy to hear and that the subject understood the task. For gap detection and TMCs, additional training was provided for a subset of conditions until subjects were familiarized with the tasks. Thresholds obtained during this training were discarded as were the first runs collected in each subsequent day of data collection.
RESULTS AND DISCUSSION
Gap detection with background noise
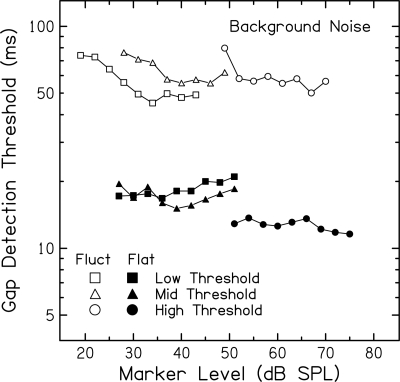
Figure 3 shows mean gap detection thresholds for the three subject groups plotted against marker level. In agreement with previous studies (see, e.g., Glasberg and Moore, 1992; Hall and Grose, 1997), gap detection is better (i.e., thresholds are lower) for the flatter marker than for the fluctuating marker. This difference in gap detection between markers is not the focus of the current study, however, and may be related to several factors (e.g., depth of envelope fluctuation and related confusion, duration of on/off ramps used to create the gaps, bandwidth and associated differences in across-frequency cues and loudness). Of more importance are level-dependent changes in gap detection that may reveal effects of changes in compression on signals with a fixed acoustic SNR. Systematic changes in gap thresholds with level can be seen for both markers and all subject groups. For the flatter marker (closed symbols) thresholds increase (worsen) with increasing level for Low Threshold subjects and decrease (improve) for High Threshold subjects, for whom marker levels are higher. For the fluctuating marker (open symbols), gap thresholds for all subject groups tend to decrease with initial increases in level.
Figure 3.
Mean gap detection thresholds (ms) for subjects in the Low Threshold (squares), Mid Threshold (triangles), and High Threshold (circles) groups plotted as a function of marker level (dB SPL within the ERB centered around 1000 Hz). Results are shown for the flatter (closed) and fluctuating (open) markers presented in background noise at fixed SNRs. More than half the subjects in each group must have measurable thresholds for a mean data point to be included.
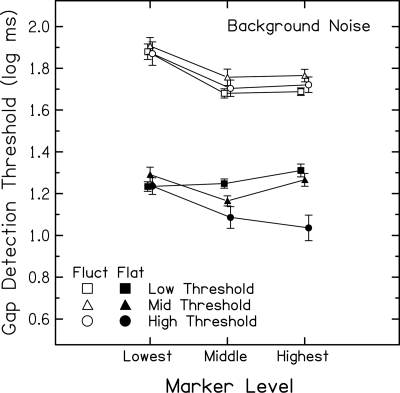
Results in Fig. 3 show absolute marker levels, indicating at what segments along the basilar-membrane input–output function different signal occur. However, because marker levels were selected based on subjects’ quiet thresholds and because subjects with a range of thresholds are included, the mean data in Fig. 3 include missing cells. As a result, statistical analyses could not be performed to assess more fully the effects of interest. To create a complete data set, gap detection thresholds at each subjects’ lowest, middle, and highest marker levels were selected and submitted to repeated measures analyses of variance (ANOVA); mean values [±1 standard error (SE)] are shown in Fig. 4. Results presented in Figs. 34 show similar patterns of increases and decreases in gap detection with increasing marker level. In this and all subsequent data analyses, log transforms of the gap detection thresholds are used to maintain homogeneity of variance. Discussion will focus on the flatter marker first, including individual data shown in Fig. 5. Following that, discussion will return to Figs. 34 to describe results for the fluctuating marker.
Figure 4.
Mean gap detection thresholds (log ms) for subjects in the Low Threshold (squares), Mid Threshold (triangles), and High Threshold (circles) groups plotted against each subject’s lowest, middle, and highest marker level for the flatter (closed) and fluctuating (open) markers presented in background noise at fixed SNRs. For clarity, some data points are offset along the abscissa. Error bars indicate ±1 SE.
Figure 5.
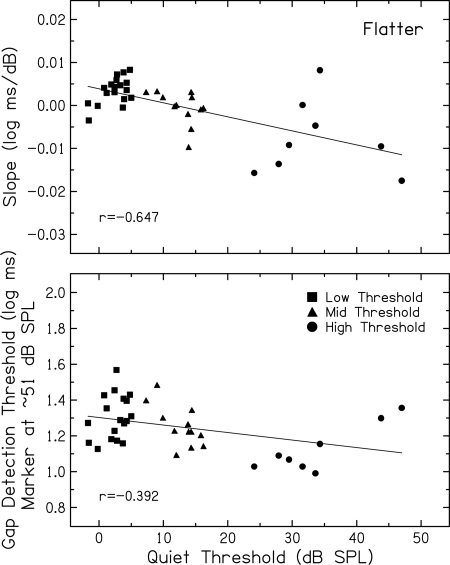
(Top) Slopes of the function relating gap detection threshold to marker level (log ms/dB) for the flatter marker presented in background noise at a fixed SNR plotted against quiet thresholds (dB SPL) for subjects in the Low Threshold (squares), Mid Threshold (triangles), and High Threshold (circles) groups. (Bottom) Same, but the ordinate is gap detection threshold (log ms) at ∼51 dB SPL. Pearson correlation coefficients and linear regression functions are included in each panel.
Flatter marker
Gap thresholds differed across subject groups [F(2,35)= 6.63, p = 0.004] and marker levels [F(2,70) = 9.54, p < 0.001], with the pattern of the level-dependent differences also differing across subject groups [F(4,70) = 9.30, p < 0.001]. This interaction is explored further in the following sections as is the main effect of subject group.
Low Threshold group (closed squares).
Gap detection worsens significantly (thresholds increase) with increases in signal level [post hoc test of linear contrast, F(1,35) = 5.37, p = 0.026]. This is consistent with the effective SNR decreasing as signals increase from low to mid levels as follows. A more linear basilar-membrane response at lower levels (∼30 dB SPL) and more compression at mid levels (∼50 dB SPL), result in bigger increases in response for the background noise than for marker peaks. The resulting reduction in the effective SNR is consistent with poorer gap detection with increases in level.
High Threshold group (closed circles).
Gap detection for this group of subjects with the most hearing loss improves significantly with level [post hoc test of linear contrast, F(1,35) = 16.01, p < 0.001], consistent with increases in the effective SNR as signals increase from mid to high levels (up to ∼80 dB SPL). Attributing changes in gap detection to changes in effective SNR presumes that these subjects have some residual compression. With more compression at mid levels and a more linear response at higher levels, increases in level result in smaller increases in response for the background noise than for marker peaks.
Gap detection for subjects in the High Threshold group is significantly better than for the Mid and Low Threshold groups [post hoc test of contrast between groups across all levels, F(3,33) = 7.56, p < 0.001], with the largest difference between subject groups at the highest marker level and overlapping results at the lowest marker level. This finding is notable as a rare example of better performance for subjects with hearing loss. Better gap detection is consistent with less compression (resulting in less reduction in the effective SNR) for subjects with more hearing loss. These results are also consistent with better-than-normal modulation detection for some hearing-impaired subjects (Glasberg and Moore, 1989; Bacon and Gleitman, 1992; and Moore et al., 1992, Moore et al., 1996).
Mid Threshold group (closed triangles).
The level-dependent pattern of gap detection for Mid Threshold subjects is intermediate between the patterns for the Low and High Threshold groups and appears more similar to that for the Low than High Threshold subjects. In contrast to results for Low Threshold subjects, gap detection for Mid Threshold subjects improves with increases in level from the lowest (∼30 dB SPL) to the middle levels tested (∼40 dB SPL) and then worsens with increases in level from the middle to highest levels tested (∼50 dB SPL) [post hoc test of quadratic contrast, F(1,35) = 28.59, p < 0.001]. This initial improvement in gap detection is not predicted from expected changes in effective SNR due to level-dependent changes in compression, but it may relate to low marker SLs. Indeed, despite strict control to hold constant acoustic SNR with increases in level, the lowest marker levels correspond to lower SLs for subjects in the Mid Threshold group compared to those in the Low Threshold group.
Figure 5 shows correlations between individual subjects’ gap detection measures and quiet thresholds. The top panel of Fig. 5 shows gap detection slopes plotted against quiet thresholds. To quantify level-dependent changes in gap detection, the slope of a straight line fit to all measured gap detection thresholds was calculated for each subject. As indicated by the significant negative correlation (p < 0.001), subjects with lower quiet thresholds have more steeply positive slopes, or gap detection that worsens more as signals increase from low to mid levels. In contrast, subjects with higher quiet thresholds have more steeply negative slopes, wherein gap detection improves more with increases in signal level.
In the bottom panel, a significant negative correlation (p = 0.014) shows a similar systematic relation between subjects’ gap detection thresholds at the marker level closest to 50 dB SPL and their quiet thresholds.4 More specifically, subjects with lower quiet thresholds have poorer gap detection at these mid levels at which compression effects are presumed to be strongest. Further, subjects with higher quiet thresholds have better gap detection at these mid-level markers. Taken together, the patterns of results in both panels are consistent with compression effects given the broad assumption that subjects with lower quiet thresholds exhibit more compression, whereas those with higher quiet thresholds exhibit less compression.
Fluctuating marker
Predicting patterns of gap detection for the fluctuating marker is not straightforward because expected effects are in opposite directions. When signals increase from low to mid (∼50 dB SPL) levels, increasing compression could result in (a) improved gap detection associated with reduced depth of marker fluctuations and reduced confusion between the imposed gap and inherent fluctuations of the marker and (b) poorer gap detection associated with decreasing effective SNR. When signals increase from mid to high levels (up to ∼80 dB SPL), decreasing compression could result in (a) poorer gap detection associated with increased depth of marker fluctuations and increased confusion and (b) improved gap detection associated with increasing effective SNR. Predicting the extent to which gap detection will be better or worse for hearing-impaired subjects with less compression is also not straightforward, given these opposing effects.
As seen in Figs. 34 (open symbols), gap detection significantly improves with increases in level for Low, Mid, and High Threshold groups [F(2,70) = 32.54, p < 0.001]. For Low Threshold and most Mid Threshold subjects, this is consistent with reduced marker peaks and reduced confusion with initial increases above low marker levels (≳20 dB SPL). This assumes the beneficial effects of reduced confusion are initially larger than the detrimental effects of decreasing effective SNR. For High Threshold subjects, this is consistent with increasing effective SNRs with initial increases above mid levels (≳40–60 dB SPL). It also assumes these beneficial effects are larger than the detrimental effects of increasing effective marker peaks and confusion. In contrast to results for the flatter marker, and possibly related to effects canceling out, there are no significant correlations between quiet thresholds and gap detection slopes or thresholds (r values ranging from −0.023 to 0.063, p > 0.05). Similarly, there are no significant differences in gap thresholds across subject groups [F(2,35) = 1.65, p = 0.21] nor did level-dependent differences in gap thresholds differ significantly across subject groups [F(4,70) = 0.34, p = 0.85].
Temporal masking curves
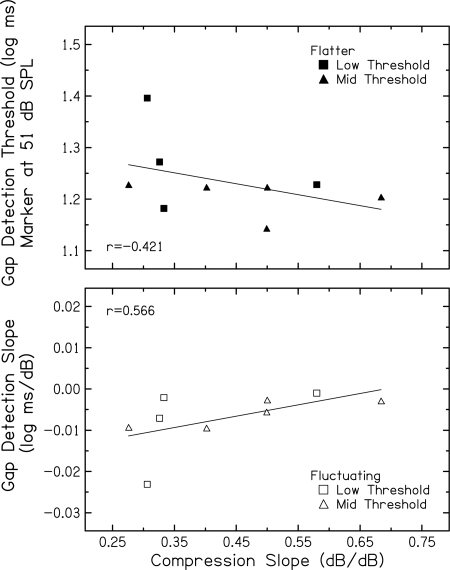
For the 32 subjects in the current study for whom compression slopes could be inferred from TMCs measured with the 500 Hz off-frequency masker, there were no significant correlations between inferred compression slope and any gap measure (e.g., gap detection slope or gap detection threshold for any marker level). This larger set of TMC data was obtained with a 1000 Hz probe for both on- and off-frequency maskers to be most directly comparable to the center frequency of the gap stimuli. Recall that a second set of TMCs was obtained for a smaller number of subjects with an off-frequency condition that included a 2000 Hz probe and 800 Hz masker. Using this linear-reference TMC, the top panel of Fig. 6 shows gap detection thresholds plotted against slopes of the inferred input–output functions for nine of ten Low and Mid Threshold subjects. With only nine subjects, a correlation coefficient of −0.421 is not statistically significant; however, the trend is consistent with predicted compression effects. Gap thresholds shown here are those for the flatter marker at 51 dB SPL, where stronger basilar-membrane compression is expected. Stronger compression is assumed to be associated with more shallow compression slopes and poorer gap detection for the flatter marker, due to more compressed effective SNRs. Indeed, subjects with higher gap detection thresholds tend to have shallower compression slopes.5
Figure 6.
(Top) Gap detection thresholds (log–ms) for the flatter marker at 51 dB SPL plotted against compression slopes (dB/dB) inferred from TMCs for nine subjects in the Low Threshold (squares) and Mid Threshold (triangles) groups. (Bottom) Same, but the ordinate is the slope of the function relating gap detection threshold to marker level (log ms/dB) for the fluctuating marker presented in background noise at a fixed SNR. Pearson correlation coefficients and linear regression functions are included in each panel.
The bottom panel of Fig. 6 shows slopes of the function relating gap detection threshold to marker level for the fluctuating marker plotted against slopes of the input–output functions inferred from TMCs. For these Low and Mid Threshold subjects, fluctuating markers increase from low to mid (≤50 dB SPL) levels. Increasing compression associated with these level increases may result in improving gap detection associated with reduced effective depth of marker fluctuations and reduced confusion. If this beneficial effect of reduced confusion is larger than the detrimental effect of decreasing effective SNR, stronger compression effects could result in bigger improvements in gap detection. Indeed, Fig. 6 (bottom) shows that more steeply negative gap detection slopes tend to be associated with more shallow compression slopes (stronger compression). Thus, although for nine subjects a correlation coefficient of 0.566 is not statistically significant, the trend is consistent with predicted compression effects.
Gap detection without background noise
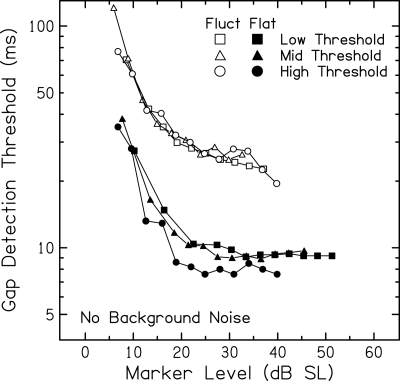
Without background noise, increasing marker level corresponds to increasing marker audibility. Gap detection is known to improve with increases in marker SL until asymptotic performance is achieved (see, e.g., Reed et al., 2009). Figure 7 shows mean gap detection thresholds obtained without background noise plotted against marker SL. As shown by the steep decreases for all functions, there is clear improvement in gap detection as level increases above the lowest SLs for both markers and all subject groups. Across level, gap detection is worse for the fluctuating marker than for the flatter marker, as expected due to the confusion between marker fluctuations and the imposed gap and other factors. As with Fig. 3, mean values include missing cells. To assess level-dependent differences across subject groups, statistical analyses were performed using a subset of gap detection results with no missing data. Results for the flatter marker are discussed first; following that, discussion will return to Fig. 7 for results with the fluctuating marker.
Figure 7.
Mean gap detection thresholds (ms) for subjects in the Low Threshold (squares), Mid Threshold (triangles), and High Threshold (circles) groups plotted as a function of marker sensation level (dB SL) for the flatter (closed) and fluctuating (open) markers presented without background noise. More than half of the subjects in each group must have measurable thresholds for a mean data point to be included.
Flatter marker
Higher SL effects.
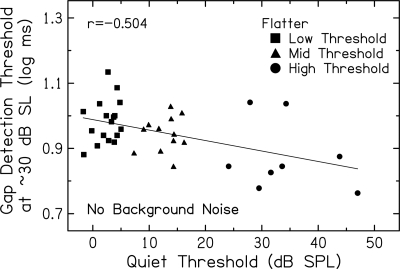
Gap detection thresholds are generally better for subjects with more hearing loss, especially at higher SLs where performance is relatively stable with further increases in level. The highest SL for which gap thresholds were obtained for all subjects was ∼30 dB. Figure 8 shows this significant negative association, with gap detection thresholds obtained at ∼30 dB SL plotted against quiet thresholds (p = 0.001). Similarly, ANOVA results revealed significant differences in gap detection thresholds at ∼30 dB SL across groups [F(2,35) = 6.22, p = 0.005], with significantly better performance for the High Threshold group compared to the Low and Mid Threshold groups [F(1,35)= 9.31, p = 0.004]. These differences in gap detection thresholds may be related to higher SPLs for High Threshold subjects.
Figure 8.
Gap detection thresholds (log ms) for the flatter marker at ∼30 dB SL plotted against quiet thresholds (dB SPL) for subjects in the Low Threshold (squares), Mid Threshold (triangles), and High Threshold (circles) groups. The Pearson correlation coefficient and linear regression function are included.
Lower SL effects.
Subjects with more hearing loss also appear to have steeper improvement in gap detection with initial increases in marker SL (Fig. 7). With more hearing loss and less compression, a given increase in signal level results in a bigger “effective” increase in signal level and a corresponding bigger improvement in gap detection. Although it cannot be tested statistically (due to SLs varying across groups), this finding may reflect stronger compression effects for subjects with better hearing, resulting in smaller effective increases in signal level as marker level increases.
Fluctuating marker
Figure 7 (open symbols) shows no clear differences across subject groups in detection of gaps in the fluctuating marker. As discussed earlier for gap detection with a background noise, compression effects may be more complex for the fluctuating than for the flatter marker. Expected effects of compression are in opposite directions; therefore, the combined effects may obscure level-dependent differences across subject groups. ANOVA results indicate no significant differences across groups in gap detection thresholds at ∼30 dB SL [F(2,35) = 0.09, p = 0.91]. Further, the function relating gap threshold to marker level does not appear to reach asymptote for the range of levels tested. The larger depth of envelope fluctuation and associated confusion effects for the fluctuating marker may require higher SLs for asymptotic performance in quiet compared to the flatter marker.
Effect of age
As reviewed by Reed et al. (2009), when complex markers are included, gap detection may be poorer for older rather than younger subjects. Given the different markers used here and the wide range of subject ages, age was included as a covariate in repeated measures analyses of covariance with threshold as a grouping factor. Age was not a significant factor for gap detection thresholds for the flatter marker, with or without background noise, or for the fluctuating marker, with or without background noise [F(1,34)= 1.28, p = 0.27; F(1,34) = 0.29, p = 0.59; F(1,34) = 0.001, p = 0.98; F(1,34) = 0.031, p = 0.86; respectively].
GENERAL DISCUSSION
This experiment investigated the extent to which changes in gap detection with level are consistent with expected changes in the effective envelopes of the signals due to level-dependent changes in cochlear compression. Subjects with a range of ages and quiet thresholds were included to assess the influence of age and hearing status. Significant level-dependent changes in gap detection were observed, consistent with effects of cochlear compression. For the flatter marker at a fixed acoustic SNR, gap detection worsened with increases in level up to mid levels and improved with additional increases in level. These results can be explained by an effective flattening of the temporal envelope and associated reduction in effective SNR at mid levels, where compression effects are expected to be strongest. Significant effects of quiet threshold on gap detection were observed, whereas significant age effects were not. The effects of a decrease in compression, resulting in larger effective SNRs, may have contributed to better-than-normal gap detection observed for some subjects with hearing loss.
The current results may also be compared to those from two previous studies that examined gap detection using various levels of broadband background noise. In these studies, data were obtained from normal-hearing subjects only, whereas results from the current study are from subjects with a range of thresholds, with results using higher level markers obtained only from subjects with impaired hearing. Consequently, results of previous studies from normal-hearing subjects at higher signal levels may only be compared to current results for hearing-impaired subjects, for which residual compression effects are presumed. As noted in the introduction, in contrast to their expectations, Hall and Grose (1997) found little difference in gap detection with lower and higher background noise levels. However, they did not measure gap detection at mid levels, which may show the strongest compression effects. For the fluctuating marker, the current results are comparable to those of Hall and Grose, i.e., similar gap thresholds at both background levels, which correspond to the background levels paired with fluctuating markers at 31 and 71 dB SPL in the current Fig. 3. In contrast, for the flatter marker, gap thresholds in the current experiment are slightly better at higher than lower background levels (i.e., those paired with markers at 67 and 27 dB SPL in Fig. 3). Although not predicted by their recruitment hypothesis, Hall and Grose also noted slightly better gap detection for the flatter marker in the higher background.
Improving detection of gaps in increasing levels of broadband background noise was also reported by Fitzgibbons (1984). Recall in that study, threshold was defined as the level of a 1000 Hz octave-band noise marker needed to detect a gap of fixed duration. Gap detection was measured in background levels corresponding to those for the flatter marker at 39–74 dB SPL in the current Fig. 3. As background level increased, gap detection improved, generally consistent with the current data and interpretation (i.e., better gap detection at higher levels, for which a more linear response is expected).
Taken together, the data described here provide preliminary support for the use of broadband signals to reflect level-dependent effects of cochlear compression. Moreover, measures of gap detection as a function of broadband marker level may have advantages over other estimates of cochlear compression using pure-tone signals in more directly relating changes in compression to changes in speech understanding in noise, which will be explored in future experiments. These effects are more straightforward for the flatter marker than for the fluctuating marker, for which increasing compression could have effects that result in either better or worse gap detection. Individual subjects’ gap detection thresholds were also less variable for the flatter than fluctuating marker. For repeated estimates of detection of gaps in the flatter marker measured in background noise, the variability criterion was exceeded for approximately one third of the thresholds, whereas for the fluctuating marker, this criterion was exceeded for more than half the thresholds. Thus, even for the flatter marker, for which significant effects were obtained for group mean data, gap detection thresholds may be too variable to provide reliable results for individual subjects. A more robust measure of gap detection may be obtained with refinements to the current procedures, such as increasing the amount of training and number of repetitions averaged for each final threshold estimate.
A considerable range of gap detection thresholds for subjects with similar absolute thresholds was measured here and in previous studies (see, e.g., Glasberg et al., 1987,); one source of this variance could be differences in subjects’ cochlear compression. Preliminary support comes from the previously discussed correlations between gap detection and the independent estimate of compression from TMCs. In general, subjects with lower quiet thresholds exhibit gap detection thresholds that are consistent with more compression, whereas subjects with higher quiet thresholds exhibit gap detection thresholds consistent with less compression (Fig. 5). However, measures of quiet thresholds may not adequately predict the strength of compression effects (see, e.g., Poling et al., 2011,). Following the current results, further research is needed to determine if measures of level-dependent changes in detection of gaps in noise (or other measures using broadband, nonspeech signals) may be useful in relating changes in compression to changes in speech understanding for individual subjects.
ACKNOWLEDGMENTS
This work was supported (in part) by Grant Nos. R01 DC00184 and P50 DC00422 from NIH/NIDCD; the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCRR Grant No. UL1 RR029882; and the James E. and Pamela Knowles Foundation. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant No. C06 RR14516 from the National Center for Research Resources, National Institutes of Health. The authors thank Fu-Shing Lee for assistance with data analysis, Ning-ji He and Bryant Mersereau for signal processing support, Emily Buss for providing advice on and software for generating low-fluctuation noise, Magdalena Wojtczak for helpful suggestions and discussion, and Enrique Lopez-Poveda for providing Matlab files produced by Chris Plack and Vit Drga used for the three-segment TMC fitting procedure. Assistance with data collection by Keeley McKelvey and Gayla Poling is also gratefully acknowledged. Thanks to two anonymous reviewers for their helpful comments on an earlier version of this manuscript.
Footnotes
The formula used for the basilar-membrane compressor (Glasberg and Moore, 2000) provides an estimate of the basilar-membrane input–output function for different amounts of maximum gain (Gmax). Here, maximum gain is assumed to be 40 dB, a value described by Glasberg and Moore as “representative” for frequencies of 1000 Hz and above. Further, in this model, gain changes by 20 dB as signal level increases from 30 to 70 dB, implying compression of ∼2:1. If greater maximum gain and compression values are assumed, as suggested by basilar-membrane input–output functions for higher characteristic frequencies (see, e.g., Ruggero et al., 1997,), as well as by psychoacoustic estimates of basilar-membrane compression using nonsimultaneous maskers (see, e.g., Oxenham and Plack, 1997), the resulting schematics would show larger differences between the linear and compressed waveforms, with even smaller effective SNRs for the compressed waveforms compared to those shown in the bottom panels of Fig. 1.
See supplementary material at http://dx.doi.org/10.1121/1.3643829 E-JASMAN-130-045111 for data tables that provide individual subjects’ quiet thresholds, ages, marker and background noise levels, and corresponding gap detection thresholds.
As an example of how overall levels vary when SPLs within an ERB are equated for signals used in this experiment, for a 50 dB SPL signal, the overall level was 50 dB SPL for the 50-Hz-wide marker, 59 dB SPL for the 1000-Hz-wide marker and 62 dB SPL for the 2000-Hz-wide background noise.
The marker levels closest to 50 dB SPL were 51 dB SPL for 35 subjects, 54 dB SPL for 2 subjects, and 57 dB SPL for 1 subject.
Data from one subject in the High Threshold group were obtained but not included in Fig. 6. The very steep slope estimated for this subject (i.e., >2 dB/dB) was not plausible and was excluded from this analysis.
References
- Bacon, S. P., and Gleitman, R. M. (1992). “Modulation detection in subjects with relatively flat hearing losses,” J. Speech Hear. Res. 35, 642–653. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons, P. J. (1984). “Temporal gap resolution in masked normal ears as a function of masker level,” J. Acoust. Soc. Am. 76, 67–70. 10.1121/1.391008 [DOI] [PubMed] [Google Scholar]
- Florentine, M., and Buus, S. (1984). “Temporal gap detection in sensorineural and simulated hearing impairments,” J. Speech Hear. Res. 27, 449–455. [DOI] [PubMed] [Google Scholar]
- Glasberg, B. R., and Moore, B. C. J. (1989). “Psychoacoustic abilities of subjects with unilateral and bilateral cochlear impairments and their relationship to the ability to understand speech,” Scand. Audiol. Suppl. 32, 1–25. [PubMed] [Google Scholar]
- Glasberg, B. R., and Moore, B. C. J. (1990). “Derivation of auditory filter shapes from notched-noise data,” Hear. Res. 47, 103–138. 10.1016/0378-5955(90)90170-T [DOI] [PubMed] [Google Scholar]
- Glasberg, B. R., and Moore, B. C. J. (1992). “Effects of envelope fluctuations on gap detection,” Hear. Res. 64, 81–92. 10.1016/0378-5955(92)90170-R [DOI] [PubMed] [Google Scholar]
- Glasberg, B. R., and Moore, B. C. J. (2000). “Frequency selectivity as a function of level and frequency measured with uniformly exciting notched noise,” J. Acoust. Soc. Am. 108, 2318–2328. 10.1121/1.1315291 [DOI] [PubMed] [Google Scholar]
- Glasberg, B. R., Moore, B. C. J., and Bacon, S. P. (1987). “Gap detection and masking in hearing-impaired and normal-hearing subjects,” J. Acoust. Soc. Am. 81, 1546–1556. [DOI] [PubMed] [Google Scholar]
- Grose, J. H., Buss, E., and Hall, J. W. (2008). “Gap detection in modulated noise: Across-frequency facilitation and interference,” J. Acoust. Soc. Am. 123, 998–1007. 10.1121/1.2828058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J. W., and Grose, J. H. (1997). “The relation between gap detection, loudness, and loudness growth in noise-masked normal-hearing listeners,” J. Acoust. Soc. Am. 101, 1044–1049. 10.1121/1.418110 [DOI] [PubMed] [Google Scholar]
- Kohlrausch, A., Fassel, R., van der Heijden, M., Kortekaas, R., van de Par, S., and Oxenham, A. J. (1997). “Detection of tones in low-noise noise: Further evidence for the role of envelope fluctuations,” Acta Acust. 83, 659–669. [Google Scholar]
- Levitt, H. (1971). “Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda, E. A., and Alves-Pinto, A. (2008). “A variant temporal masking curve method for inferring peripheral auditory compression,” J. Acoust. Soc. Am. 123, 1544–1554. 10.1121/1.2835418 [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda, E. A., Plack, C. J., and Meddis, R. (2003). “Cochlear non-linearity between 500 and 8000 Hz in listeners with normal hearing,” Acoust. Soc. Am. 113, 951–960. 10.1121/1.1534838 [DOI] [PubMed] [Google Scholar]
- Moore, B. C. J., Glasberg, B. R., Alcántara, J. I., Launer, S., and Kuehnel, V . (2001). “Effects of slow- and fast-acting compression on the detection of gaps in narrow bands of noise,” Br. J. Audiol. 35, 365–374. [DOI] [PubMed] [Google Scholar]
- Moore, B. C. J., Shailer, M. J, and Schooneveldt, G. P. (1992). “Temporal modulation transfer functions for band-limited noise in subjects with cochlear hearing loss,” Br. J. Audiol. 26, 229–237. 10.3109/03005369209076641 [DOI] [PubMed] [Google Scholar]
- Moore, B. C. J., Wojtczak, M., and Vickers, D. A. (1996). “Effect of loudness recruitment on the perception of amplitude modulation,” J. Acoust. Soc. Am. 100, 481–489. 10.1121/1.415861 [DOI] [Google Scholar]
- Nelson, D. A., Schroder, A. C., and Wojtczak, M. (2001). “A new procedure for measuring peripheral compression in normal-hearing and hearing-impaired listeners,” J. Acoust. Soc. Am. 110, 2045–2064. 10.1121/1.1404439 [DOI] [PubMed] [Google Scholar]
- Oxenham, A. J., and Plack, C. J. (1997). “A behavioral measure of basilar-membrane nonlinearity in listeners with normal and impaired hearing,” J. Acoust. Soc. Am. 101, 3666–3675. 10.1121/1.418327 [DOI] [PubMed] [Google Scholar]
- Plack, C. J., Drga, V., and Lopez-Poveda, E. A. (2004). “Inferred basilar-membrane response function for listeners with mild to moderate sensorineural hearing loss,” J. Acoust. Soc. Am. 115, 1684–1695. 10.1121/1.1675812 [DOI] [PubMed] [Google Scholar]
- Poling, G. L., Horwitz, A. R., Ahlstrom, J. B., and Dubno, J. R. (2011). “Individual differences in behavioral estimates of cochlear nonlinearities,” J. Assoc. Res. Otolaryngol., doi: 10.1007/s10162-011-0291-2. [DOI] [PMC free article] [PubMed]
- Reed, C. M., Braida, L. D., and Zurek, P. M. (2009). “Review of the literature on temporal resolution in listeners with cochlear hearing impairment: A critical assessment of the role of suprathreshold deficits,” Trends Amplif. 13, 4–43. 10.1177/1084713808325412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, S. O. (1954). “Mathematical analysis of random noise,” in Selected Papers on Noise and Stochastic Processes, edited by Wax N. (Dover, New York), pp. 133–294. [Google Scholar]
- Robles, L., and Ruggero, M. A. (2001). “Mechanics of the mammalian cochlea,” Physiol. Rev. 81, 1305–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengard, P. S., Oxenham, A. J., and Braida, L. D. (2005). “Comparing different estimates of cochlear compression in listeners with normal and impaired hearing,” J. Acoust. Soc. Am. 117, 3028–3041. 10.1121/1.1883367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero, M., Rich, N., Recio, A., Narayan, S., and Robles, L. (1997). “Basilar-membrane responses to tones at the base of the chinchilla cochlea,” J. Acoust. Soc. Am. 101, 2151–2163. 10.1121/1.418265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sininger, Y. S., and de Bode, S. (2008).”Asymmetry of temporal processing in listeners with normal hearing and unilaterally deaf subjects,” Ear. Hear. 29, 228–238. 10.1097/AUD.0b013e318164537b [DOI] [PubMed] [Google Scholar]
- Viemeister, N. F. (1979). “Temporal modulation transfer functions based upon modulation thresholds,” J. Acoust. Soc. Am. 66, 1634–1680. 10.1121/1.383531 [DOI] [PubMed] [Google Scholar]
- Yasin, I., and Plack, C. J. (2003). “The effects of a high-frequency suppressor on tuning curves and derived basilar-membrane response functions,” J. Acoust. Soc. Am. 114, 322–332. 10.1121/1.1579003 [DOI] [PubMed] [Google Scholar]