Abstract
The ability to produce exopolysaccharides (EPS) is widespread among lactobacilli including Lactobacillus rhamnosus, the commonly used probiotic bacteria. Exopolysaccharides are a major component of the bacterial biofilm with a well-documented impact on adherence of bacteria to host cells. However, their immunoregulatory properties are unknown. The aim of this study was to examine the immunostimulatory potential of EPS derived from L. rhamnosus KL37. We investigated the effect of EPS on the production of inflammatory mediators by mouse peritoneal macrophages and compared it with the effect of Lipopolysaccharide (LPS). Exopolysaccharides, at concentrations higher than those of LPS, stimulated production of both pro-inflammatory (TNF-α, IL-6, IL-12) and anti-inflammatory (IL-10) cytokines. Interestingly, analysis of the balance of TNF-α/IL-10 production showed a potential pro-inflammatory effect of EPS. Furthermore, our data demonstrate that exposure of macrophages to LPS induced a state of hyporesponsiveness, as indicated by reduced production of TNF-α after restimulation with either LPS or EPS (‘cross-tolerance’). By contrast, EPS could make cells tolerant only to subsequent stimulation by the same stimulus. We also examined the relationship between TNF-α production and activation of mitogen-activated protein kinases (MAPKs) by EPS and LPS. Pretreatment of macrophages with specific inhibitors of p38 and ERK MAPKs reduced TNF-α production induced by both stimuli to the same extent. In conclusion, these data demonstrate that EPS can effectively stimulate production of inflammatory mediators by macrophages in vitro. However, to predict whether EPS could be clinically useful as an immunomodulatory agent, further in vivo studies with highly purified EPS are necessary.
Keywords: cytokines, exopolysaccharides, Lactobacillus rhamnosus, macrophages, probiotics
Probiotics are live bacteria exhibiting health-promoting activities. Recently, an increasing number of clinical studies report that probiotics may be useful in the prevention/treatment of atopic diseases and gastrointestinal infections in children (Vaarala 2003; Shida & Nanno 2008). The commonly used probiotics in human medicine are various species of lactic acid bacteria including different strains of Lactobacillus rhamnosus (L. rhamnosus) (Marcone et al. 2008; Prescott et al. 2008; Szymański et al. 2008).
It has been postulated that the observed clinical effects of probiotic bacteria depend on their immunoregulatory properties (e.g. anti-inflammatory properties) and are strain specific (Christensen et al. 2002; Matsumoto et al. 2005). However, probiotic lactic acid bacteria have been shown to stimulate both pro-inflammatory (Th1) and anti-inflammatory responses (Th2, Treg) (Matsuguchi et al. 2003; Mohamadzadeh et al. 2005). Therefore, the selection of the correct probiotic bacterial strain seems to be crucial to achieve the desired therapeutic effect.
Interestingly, beneficial (anti-inflammatory) effects have been achieved not only using live bacteria, but also using dead bacteria, bacterial cell-wall components (peptydoglycan, lipoteichoic acids) and bacterial DNA (Dalpke et al. 2002; Rachmilewitz et al. 2002; Matsuguchi et al. 2003). Zhang et al. (2005) reported that both live and heat-killed L. rhamnosus GG may ameliorate inflammation by decreasing TNF-α-induced IL-8 production by epithelial Caco-2 cells. Moreover, lipoteichoic acid (LTA), a major component of the cell wall of lactobacilli, activates macrophages and dendritic cells through TLR2, in a strain-specific manner (Matsuguchi et al. 2003). The great structural diversity in the LTAs derived from different bacteria may give rise to a variety of immunoregulatory properties.
Much less is known about the immunoregulatory potential of lactobacilli-derived exopolysaccharides (EPS). Exopolysaccharides are a key component of the biofilm matrix of many biofilm-forming bacteria, including Lactobacillus species (Watnick & Kolter 2000; Laws et al. 2001; Ciszek-Lenda 2011), although biofilms contain a whole variety of proteins, glycoproteins, glycolipids and extracellular DNA (Flemming et al. 2007).
Nevertheless, it is well established that EPS plays an important role in bacteria immune evasion, resistance towards antibacterial agents and bacterial adhesion properties (Vuong et al. 2004; Ruas-Madiedo et al. 2006).
More recently, it has been reported that EPS-producing probiotics significantly attenuate experimental colitis, in a dose-dependent manner (Sengül et al. 2006). However, the target(s) and the presence of EPS-specific receptor(s) are still not established, and it has proved difficult to define the common biological properties of lactic acid bacteria EPS because of its extraordinary structural diversity.
In a previous study, we have shown that exopolysaccharide-producing Lactobacillus strains (L. reuteri 115, L. johnsonii 142, L. animalis 148) induce cytokine production by macrophages in a strain-specific manner (Marcinkiewicz et al. 2007). In preliminary studies, crude EPS isolated from L. rhamnosus KL37 also ameliorated the development of collagen-induced arthritis in mice. Bacterial exocellular polymeric substances may therefore exert different immunoregulatory effects.
The main aim of this study was to further evaluate the immunoregulatory potential of EPS derived from the high-exopolysaccharide producer, L. rhamnosus KL37. We have examined the stimulatory effects of EPS on the release of inflammatory mediators by thioglycollate-induced mouse peritoneal macrophages in vitro, a well-studied model of the in vivo inflammatory response (Shnyra et al. 1998; Kaji et al. 2010). The immunoregulatory potential of EPS was compared with that of Lipopolysaccharide (LPS), to establish whether EPS may become good candidates for clinical use as the active components of probiotic bacteria.
Materials and methods
Mice
Inbred CBA/J mice (8–12 weeks of age, 18–22 g) were maintained in the Animal Breeding Unit, Department of Immunology, Jagiellonian University Medical College, Cracow. All mice were housed in the laboratory room with water and standard diet ad libitum. The authors were granted permission by the Local Ethics Committee to use mice in this study.
EPS isolation
Exopolysaccharide was obtained from L. rhamnosus KL37 strain. The strain was isolated from the faeces of the human newborns and then stored at −70 °C in MRS broth supplemented with 10% glycerol. Bacteria were cultivated in supplemented MRS liquid broth (Oxoid, Cambridge, UK) under anaerobic conditions at 37 °C for 48 h. Cells were harvested by centrifugation at 7300 g (4 °C, 30 min) and washed twice with phosphate buffer solution (PBS). Bacterial mass was suspended in water (10 ml) and sonicated three times for 5 min, in an ice bath. After centrifugation at 4000 g (30 min, 4 °C), the supernatant was centrifuged twice at 16,300 g at 4 °C for 1 h and then precipitated with five volumes of cold ethanol (−20 °C, overnight). The precipitated material was recovered by centrifugation at 16,300 g, 4 °C for 20 min and freeze-dried. Purification of EPS was performed by gel filtration on a column in TSK HW-50 (1.6 × 100 cm) in 0.05 M aqueous pyridine acetate buffer (pH 5.6). The eluate was monitored with a Knauer differential refractometer. The first fraction, which eluted in the void volume, contained EPS and was the subject of the present investigation.
Cells
Peritoneal mouse macrophages (Mφ) were induced by intraperitoneal injection of 2.0 ml of thioglycollate or paraffin oil (both Sigma, St. Louis, MO, USA). Cells were collected 72 h later by washing out the peritoneal cavity with 5 ml of DPBS (Dubelco's phosphate buffer solution) containing 5 U heparin/ml (Polfa, Warsaw, Poland). Cells were centrifuged, and red blood cells were lysed by osmotic shock using lysing buffer (155 mM NH4Cl, 10 mM NaHCO3, 0.1 mM EDTA). Osmolarity was restored by addition of 2 × concentrated PBS. For each experiment, at least three mice were used as donors of peritoneal macrophages.
Cell culture and treatment
Macrophages (Mφ) were cultured in 24-well flat-bottom cell culture plates at 5 × 105 per well in RPMI 1640 medium (JR Scientific Inc., Woodland, CA, USA) supplemented with 5% FBS, and gentamicin 50 mg/ml (Krka, Novo Mesto, Slovenia), at 37 °C in an atmosphere of 5% CO2. After 1 h, non-adherent cells were removed and the adherent cells (Mφ) were stimulated with indicated concentrations of EPS (1–100 μg/ml), LPS (0.0001–0.1 μg/ml) (Sigma-Aldrich, Steinham, Germany) or 107 cfu/ml of whole heat-killed bacteria, L. rhamnosus KL37 or Escherichia coli 0111:B4. After 24 h, culture supernatants were collected and frozen at −80 °C until used. All groups were investigated in duplicates, if not stated otherwise.
Flow cytometric analysis of thioglycollate-induced peritoneal exudate cells
Macrophages (Mφ) induced by intraperitoneal injection of thioglycollate were harvested as described earlier, washed with PBS containing 2% FBS and 0.02% sodium azide and stained with allophyocyanin-conjugated anti-mouse F4/80 monoclonal antibody (eBioscience, Frankfurt, Germany) in combination with: PE-conjugated anti-mouse CD11b (BD Pharmingen, San Diego, CA, USA) and biotin-conjugated anti-mouse Gr1 (BioLegend, San Diego, CA, USA). Non-specific binding of antibodies was blocked by 2.4G2 monoclonal antibody (BD Pharmingen). Isotype-matched controls (all from BD Pharmingen) were included. Cells were incubated with antibodies 40 min, at 4 °C in the dark, washed twice and suspended in PBS containing 2% FBS and 0.05% sodium azide. For the detection of biotinylated antibodies incubation with FITC-conjugated streptavidin (BD Pharmingen) followed for 30 min, at 4 °C in the dark. To exclude dead cells, propidium iodide (PI; Sigma-Aldrich) was added just before analysis. Cells were analysed on Becton Dickinson FACSCalibur with CellQuest Pro Software (BD Biosciences, San Jose, CA, USA).
Cell culture and priming effect
To study polysaccharide-induced priming effect, macrophages were incubated in medium with increasing concentrations of EPS (0.1–30 μg/ml) or LPS (0.00001–0.01 μg/ml) for 6 h. Cells then were washed with PBS, and new medium was added before the second stimulation with either EPS (30 μg/ml) or LPS (0.1 μg/ml). After further 18 h, culture supernatants were collected and frozen at −80 °C until used. The cytokine production was determined by ELISA.
Cell culture and inhibitors of MAP kinases
To investigate cell signalling induced by EPS, Mφ were precultured with SB 203580, the inhibitor of MAP kinase p38 and PD 98059, the inhibitor of Erk-MEK1/2 kinase (both Calbiochem, NY, USA), at concentrations 10 and 20 μM, respectively, 30 min before stimulation with LPS (0.1 μg/ml) or EPS (100 μg/ml). After 20 h, culture supernatants were collected and frozen at −80 °C until used.
Cytokines determination
Cytokine concentrations in culture supernatants were measured using sandwich ELISA as described previously (Marcinkiewicz et al. 2007). For IL-6, IL-10 and IL-12 microtiter plates (Corning, NY, USA) were coated overnight with rat antibodies against a mouse cytokine (capture antibody). For IL-6, rat anti-mouse IL-6 and biotinylated rat anti-mouse IL-6 (both BD Pharmingen) mAbs were used as capture and detecting antibodies. Recombinant mouse IL-6 (PeproTech, Rocky Hill, New York, USA) was used as a standard. For IL-10, rat anti-mouse IL-10 and biotinylated rat anti-mouse IL-10 mAbs were used as capture and detecting antibodies. Recombinant mouse IL-10 was used as a standard (all reagents from BD Pharmingen). For IL-12p40, rat anti-mouse IL-12 (p40rp70) (BD Pharmingen) mAb and biotinylated rat anti-mouse IL-12(p40) (Endogen, Woburn, MA, USA) mAb were used as capture and detecting antibodies. Recombinant mouse IL-12 (Genzyme, Cambridge, UK) was used as a standard. For TNF-α, hamster anti-mouse/rat TNF-α and biotinylated rabbit anti-mouse/rat TNF-α (both BD Pharmingen) mAbs were used as capture and detecting antibodies. Recombinant mouse TNF-α (Sigma-Aldrich) was used as a standard. After blocking the plates with 3% skimmed milk (4% albumin for IL-10) for 2 h, standards and tested supernatants were added and incubated overnight. Finally, biotinylated antibodies against the same cytokine were added for 1 h. The ELISA was developed using horseradish peroxidase conjugated with streptavidin (Vector, Burlingame, CA, USA) followed with o-phenylenediamine and H2O2 (both Sigma-Aldrich). The reaction was stopped with 3 M H2SO4. The optical density of each sample was measured at 492 nm in a microplate reader; 0.05% Tween-20 in phosphate buffer was used as a washing solution.
Nitrite (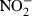 ) determination
) determination
Nitric oxide, quantified by the accumulation of nitrite as a stable end product, was determined by a microplate assay (Ding et al. 1988). Briefly, 100 μl of sample supernatants were incubated with an equal volume of Griess reagent [1% sulphanilamide in 2 M HCl (Sigma-Aldrich) and 0.1%N-1-naphthylenediamine dihydrochloride in deionized water (POCH, Gliwice, Poland)] at room temperature for 10 min. The absorbance at 550 nm was measured with a microplate reader. Nitrite concentration was calculated from a sodium nitrite standard curve.
PGE2 determination
PGE2 concentration in supernatants was determined by Prostaglandin E2 Monoclonal EIA kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instruction.
Statistical analysis
Statistical significance of differences between groups was analysed using one-way anova, followed, if significant, by an LSD test for post hoc comparison. Results are expressed as mean ± SEM values. A P-value <0.05 was considered statistically significant. Analysis was performed using Graphpad Prism v. 5.01 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
The stimulatory effect of EPS isolated from L. rhamnosus KL37 and whole bacterial cells on cytokine production by peritoneal macrophages
Previously, we have shown that various strains of lactobacilli effectively stimulate the production of inflammatory mediators from oil-induced mouse peritoneal macrophages (Marcinkiewicz et al. 2007). All these bacteria are strong producers of EPS. In this study, to examine immunostimulatory potential of EPS, mouse peritoneal macrophages were cultured either with EPS derived from L. rhamnosus KL37 or with the whole killed bacteria cells and cytokine production was analysed. The effect was compared with the effect of killed E. coli bacteria and LPS. As shown in Table 1, both pro-inflammatory (TNF-α, IL-6, IL-12) and anti-inflammatory (IL-10) cytokines were released from oil-induced macrophages in response to dead L. rhamnosus KL37 bacteria. In contrast, EPS derived from these bacteria was less effective than whole bacteria or LPS. In addition, the balance of macrophage TNF-α/IL-10 and IL-12/IL-10 production induced by EPS differs from that induced by whole bacteria (see Table 1). Interestingly, EPS induced more TNF-α and IL-12 than IL-10, suggesting its pro-inflammatory (Th1-type) immunoregulatory potential.
Table 1.
The stimulatory effect of EPS isolated from Lactobacillus rhamnosus KL37 and the whole bacterial cells on cytokine production by peritoneal macrophages
| TNF-α† | IL-6† | IL-10† | IL-12p40† | TNF-α vs IL-10‡ | IL-12p40‡ vs IL-10 | |
|---|---|---|---|---|---|---|
| Escherichia coli | 1413 ± 655** | 29,181 ± 5593*** | 857 ± 88*** | 3652 ± 847*** | 1.6:1 | 4:1 |
| LPS | 1085 ± 241** | 23,195 ± 1749*** | 104 ± 8*** | 1820 ± 328 ** | 10:1 | 17:1 |
| L. rhamnosus | 2107 ± 562*** | 15,659 ± 56*** | 1231 ± 396*** | 1006 ± 426*** | 1.7:1 | 0.8:1 |
| EPS | 469 ± 119* | 11,685 ± 2068*** | 77 ± 22* | 1243 ± 233** | 6:1 | 16:1 |
| Untreated | 167 ± 22 | 2073 ± 308 | 9 ± 4 | 385 ± 55 | 18:1 | 43:1 |
Cytokines were analysed by ELISA in supernatants collected from 24 h cultures of oil-induced peritoneal macrophages (5 × 105 per well) stimulated with exopolysaccharides (EPS) (100 μg/ml), Lipopolysaccharide (LPS) (1 μg/ml) or killed bacteria (107 cfu per well). Data are mean ± SEM values of three independent experiments.*P < 0.05, **P < 0.005, ***P < 0.001, treated vs. untreated macrophages.
Level of cytokines is expressed in pg/ml.
The balance was calculated from data shown in this table.
In subsequent experiments, we used thioglycollate-induced mouse peritoneal macrophages as more appropriate for our experimental model. As examined by flow cytometry, more than 75% of these cells express were F4/80, which is a marker of mature macrophages, 75% were CD11b+ and 18% were Gr1+. Moreover, analysis of cytokine production in response to EPS showed that thioglycollate-induced peritoneal macrophages were significantly more effective than oil-induced macrophages (see Table 2).
Table 2.
Cytokine production in response to EPS by peritoneal macrophages
| TNF-α* | IL-6* | IL-10* | IL-12p40* | |
|---|---|---|---|---|
| Thio-induced macrophages | 3587 ± 547 | 21302 ± 1081 | 101 ± 37 | 2057 ± 217 |
| Oil-induced macrophages | 318 ± 74 | 6135 ± 1526 | 31 ± 8 | 869 ± 207 |
Cytokines were analysed by ELISA in supernatants collected from 24 h cultures of oil-induced or thioglycollate-induced peritoneal macrophages (5 × 105 per well) stimulated with exopolysaccharides (EPS) at concentration of 30 μg/ml. Data are mean ± SEM values of three independent experiments.
Level of cytokines is expressed in pg/ml.
The dose-dependent effect of EPS on cytokine release from peritoneal macrophages
In vitro, thioglycollate-induced peritoneal macrophages spontaneously produce negligible amounts of TNF-α, IL-6, IL-12p40 and undetectable amounts of IL-10. Upon in vitro stimulation of these macrophages with EPS, a substantial release of both pro- and anti-inflammatory cytokines was observed (Figure 1). EPS stimulated the release of cytokines in a dose-dependent manner. At concentrations above 3 μg/ml, EPS induced a massive release of cytokines (>10-fold increase). At lower concentrations (0.01–1 μg/ml), EPS had no effect on cytokine production (data not shown). In response to EPS, macrophages produced much more pro-inflammatory cytokines (TNF-α, IL-6) than anti-inflammatory cytokines (IL-10). The ratio of TNF-α/IL-10 was above 30:1, indicating a pro-inflammatory pattern of cytokines secreted by macrophages incubated with EPS.
Figure 1.
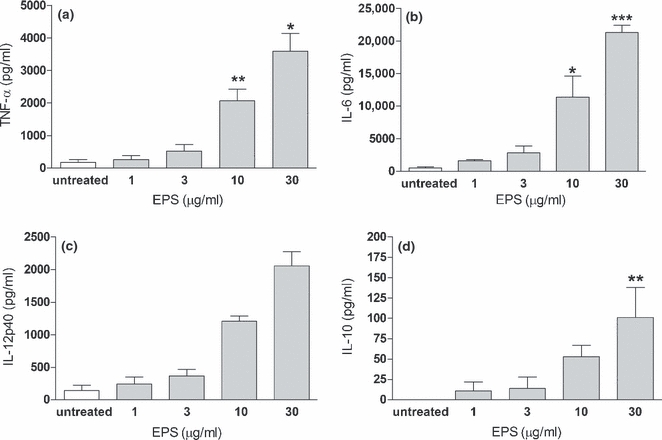
Dose-dependent effect of exopolysaccharides (EPS) on cytokine secretion from peritoneal macrophages. TNF-α (a), IL-6 (b), IL-12p40 (c) and IL-10 (d) were analysed by ELISA in supernatants collected from 24 h cultures of peritoneal macrophages (5 × 105 per well) stimulated with indicated concentrations of EPS. Data are mean ± SEM values of three independent experiments. *P < 0.05, **P < 0.005, ***P < 0.001, EPS-treated vs. untreated macrophages.
Kinetics of EPS-induced cytokine release from macrophages in vitro
To examine the kinetics of cytokine production by macrophages, the cells were incubated with EPS for different periods of time. The concentration of cytokines was measured in supernatants collected after 6, 24 and 48 h. The cytokine release from macrophages stimulated with EPS was time dependent (Figure 2). Rapid release of TNF-α was observed during the first 6 h of the culture. The level of TNF-α slightly decreased after 24 h and declined significantly after 48 h of stimulation with EPS (Figure 2a). IL-6 production was maximal at 24 h and decreased after 48 h (Figure 2b). A similar pattern of cytokine secretion was seen for IL-10 (Figure 2d). Interestingly, when supernatants were removed after 6 h and replaced with the culture medium without EPS, neither TNF-α nor IL-10 was detectable in supernatants collected after 24 h (data not shown). In contrast, the level of IL-12p40 induced by EPS continued to increase until the end of the culture (Figure 2c).
Figure 2.
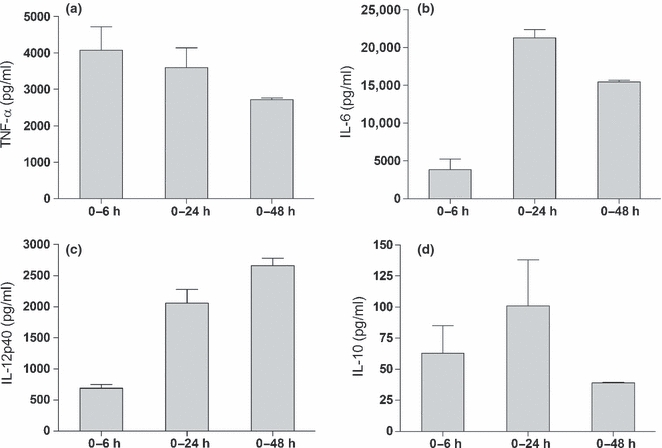
Kinetics of the EPS-induced secretion of cytokines. Mouse peritoneal macrophages (5 × 105 per well) were stimulated with exopolysaccharides (EPS) (30 μg/ml). The amounts of TNF-α (a), IL-6 (b), IL-12p40 (c) and IL-10 (d) in culture supernatants were estimated after 6, 24 or 48 h of incubation with EPS. Data are mean ± SEM values of three independent experiments.
The relative potency of EPS and LPS in induction of pro-inflammatory mediators
Lipopolysaccharide, the main component of cell walls of Gram-negative bacteria, is known to be a strong inducer of inflammatory mediators from immune cells (Erroi et al. 1993; Zhang et al. 2008). To examine whether EPS has comparable immunoregulatory properties, peritoneal macrophages were stimulated with EPS or LPS. As shown in Figure 3, LPS is a much stronger inducer of cytokines and inflammatory mediators than EPS. LPS, even at a concentration of 0.01 μg/ml, induced substantial amounts of all mediators tested (TNF-α, IL-10, 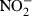 and PGE2). In contrast, EPS required 100-fold higher concentrations (at least 3 μg/ml). EPS induced the production of all cytokines in a dose-dependent manner. LPS induced the production of IL-10 and NO in a dose-dependent fashion, while production of TNF-α was maximal at 0.01 μg/ml and declined at higher concentrations. Importantly, the balance of pro-/anti-inflammatory cytokines induced by EPS was different from that induced by LPS. Namely, the ratio of TNF-α/IL-10 was approximately 5:1 for the highest concentration of LPS used. On the other hand, regardless of the EPS concentration, the ratio of TNF-α/IL-10 was always above 30:1. These data suggest pro-inflammatory (‘Th1 type’) properties of crude EPS isolated from L. rhamnosus KL37.
and PGE2). In contrast, EPS required 100-fold higher concentrations (at least 3 μg/ml). EPS induced the production of all cytokines in a dose-dependent manner. LPS induced the production of IL-10 and NO in a dose-dependent fashion, while production of TNF-α was maximal at 0.01 μg/ml and declined at higher concentrations. Importantly, the balance of pro-/anti-inflammatory cytokines induced by EPS was different from that induced by LPS. Namely, the ratio of TNF-α/IL-10 was approximately 5:1 for the highest concentration of LPS used. On the other hand, regardless of the EPS concentration, the ratio of TNF-α/IL-10 was always above 30:1. These data suggest pro-inflammatory (‘Th1 type’) properties of crude EPS isolated from L. rhamnosus KL37.
Figure 3.
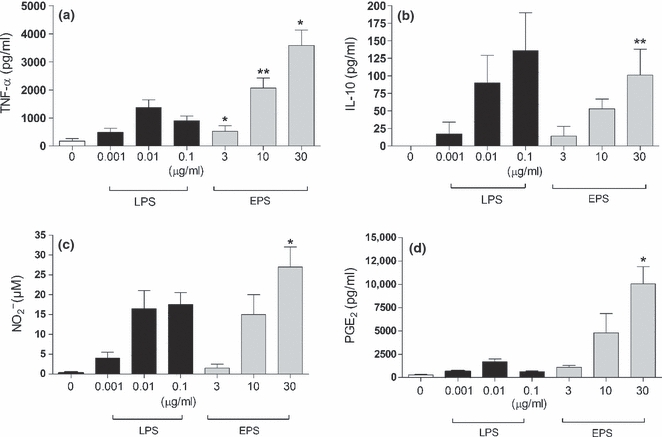
Comparison of EPS and lipopolysaccharide (LPS) capacity to induce the production of inflammatory mediators. Mouse peritoneal macrophages (5 × 105 per well) were stimulated with indicated concentrations of either EPS (grey bars) or LPS (black bars). After 24 h, supernatants were collected and the amounts of TNF-α (a), IL-10 (b), 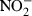 (c) and PGE2 (d) were determined as described in Methods. Data are mean ± SEM values of three independent experiments. *P < 0.05; **P < 0.005, EPS-treated vs. untreated macrophages.
(c) and PGE2 (d) were determined as described in Methods. Data are mean ± SEM values of three independent experiments. *P < 0.05; **P < 0.005, EPS-treated vs. untreated macrophages.
The priming effect of EPS and LPS on cytokine production by peritoneal macrophages
It is well known that priming (pretreatment) of macrophages with very low doses of LPS attenuates the effect of subsequent LPS stimulation (Zhang & Morrison 1993; Randow et al. 1995). To examine the ability of EPS to induce a similar effect, macrophages were preincubated with either LPS (0.00001–0.01 μg/ml) or EPS (0.01–30 μg/ml) for 6 h and then were re-stimulated with LPS (0.1 μg/ml) (Figure 4) or EPS (30 μg/ml, the effective stimulatory concentration of EPS) (Figure 5). Priming of macrophages with LPS at a concentration of 0.01 or 0.001 μg/ml resulted in significantly diminished production of TNF-α and increased production of IL-10 in response to LPS re-stimulation. In contrast, pretreatment of macrophages with EPS did not alter the cytokine production (TNF-α, IL-10) induced by subsequent stimulation with LPS (Figure 4).
Figure 4.
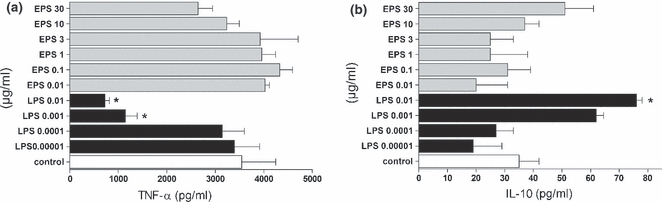
Priming effect of LPS and EPS on TNF-α (a) and IL-10 (b) production by peritoneal macrophages re-stimulated with LPS. The cells (5 × 105 per well) were preincubated for 6 h with indicated concentrations of EPS and LPS, washed twice and re-stimulated with LPS (0.1 μg/ml). After 18 h, supernatants were collected and the amounts of cytokines were estimated by ELISA. Data are mean ± SEM values of three independent experiments. Control group (white bars) – not-primed macrophages, re-stimulated only with LPS. *P < 0.05 control macrophages vs. macrophages primed with LPS 0.01 and 0.001 μg/ml.
Figure 5.
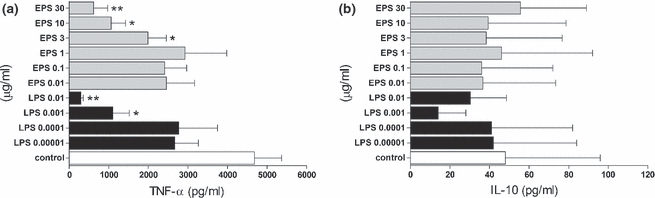
Priming effect of LPS and EPS on TNF-α (a) and IL-10 (b) production by peritoneal macrophages re-stimulated with EPS. The cells (5 × 105 per well) were preincubated for 6 h with indicated concentrations of EPS and LPS, washed twice and re-stimulated with EPS (30 μg/ml). After 18 h, supernatants were collected and the amounts of cytokines were measured by ELISA. Data are mean ± SEM values of three independent experiments. Control group (white bars) – not-primed macrophages, re-stimulated only with EPS. *P < 0.05 control macrophages vs. macrophages primed with LPS 0.001 μg/ml, EPS 3 and10 μg/ml; **P < 0.005 control macrophages vs. macrophages primed with LPS 0.01 μg/ml and EPS 30 μg/ml.
We also tested the effect of LPS and EPS priming on the secretion of cytokines after re-stimulation with EPS. As shown in Figure 5, a statistically significant reduction in TNF-α production was observed in macrophages pretreated with LPS, even when used at very low concentrations. Interestingly, macrophage priming with EPS also reduced TNF-α production after subsequent stimulation with EPS (Figure 5a). In contrast, no significant changes between the levels of IL-10 produced by macrophages pretreated with either EPS or LPS were observed after re-stimulation with EPS (Figure 5b).
The role of MAP kinases in the EPS-triggered cytokine production
It is well known that MAP kinases (MAPKs), including p38 and ERK, are involved in the synthesis of cytokines in response to LPS (Dong et al. 2002). Signalling pathways engaged during the response to EPS remain unknown. To investigate the functional role of p38 and ERK pathways in EPS-induced cytokine production, macrophages were pretreated with selective inhibitors of the p38 (SB 203580) and Erk-MEK1/2 (PD 98059) pathways, prior to stimulation with EPS or LPS. The effect of these inhibitors on TNF-α production is shown in Figure 6. Inhibition of either p38 or Erk-MEK1/2 MAPK pathways significantly reduced TNF-α production by macrophages stimulated with both LPS and EPS. When the two inhibitors were used simultaneously, the production of TNF-α was completely abolished. These data suggest that both MAPKs, p38 and Erk-MEK1/2 (to less extent), are involved in cytokine production by macrophages stimulated with EPS.
Figure 6.
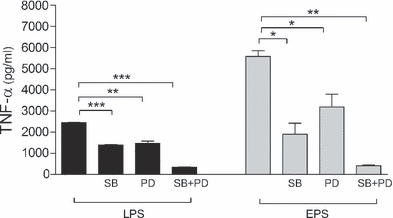
Effects of inhibitors specific for MAPK pathway on the production of TNF-α by macrophages. Peritoneal macrophages (5 × 105 per well) were pretreated with SB 203580 (p38 inhibitor, 10 μM) or/and PD 98059 (Erk-MEK1/2 inhibitor, 20 μM) for 30 min and then stimulated with lipopolysaccharide (LPS) (0.1 μg/ml) or exopolysaccharides (EPS) (100 μg/ml) for 24 h. The levels of TNF-α in supernatants were determined by ELISA. Data are mean ± SEM values of three independent experiments. Black bars – LPS treated, grey bars – EPS treated. **P < 0.005; ***P < 0.001.
Discussion
Lactobacillus rhamnosus is one of most commonly used bacteria in probiotic therapies. In clinical studies, L. rhamnosus GG significantly reduced incidence of respiratory infections, reduced duration of diarrhoea and ameliorated symptoms of atopic dermatitis (Hojsak et al. 2010; Nermes et al. 2011; Ferrie & Daley 2011). Such a wide spectrum of beneficial clinical effects is difficult to explain without understanding the mechanisms responsible for cross-talk between lactobacilli, their secreted products and host cells. The immunostimulatory properties of probiotic bacteria are of special interest.
Lactobacilli strongly induce the production of inflammatory mediators in a strain-specific manner (Christensen et al. 2002; Marcinkiewicz et al. 2007). Mouse peritoneal macrophages and macrophage cell lines showed a strong response to lactobacilli species in vitro. The relative potency of different components of L. casei on TNF-α production by RAW264.7 macrophages was found to be protoplast > cell wall > polysaccharide–peptidoglycan complex. Importantly, it has been demonstrated that purified LTA, a component of L. casei protoplast, markedly induced TNF-α production by RAW264.7 cells (Matsuguchi et al. 2003). These data suggest that LTA, at least from some Lactobacillus strains, is a potent TLR2 ligand and a key molecule responsible for immunostimulation by these bacteria (Lehner et al. 2001; Kaji et al. 2010). Recently, an increasing number of reports postulate a crucial role of biofilm components in bacteria–host interactions (Servin & Coconnier 2003; Sengül et al. 2011). However, very little is known about immunostimulatory properties of EPS, the major components of lactic bacteria biofilm (Vu et al. 2009).
In this study, we addressed the issue whether EPS derived from lactobacilli bacteria can stimulate production of inflammatory mediators from mouse macrophages. For this investigation, we have selected L. rhamnosus KL37–derived EPS because the structure of EPS-KL37 is known (Lipiński et al. 2003). L. rhamnosus growing in basal minimum medium may secrete massive amounts of EPS reaching maximum concentrations of 500 μg/ml (Ruas-Madiedo & de los Reyes-Gavilán 2005). In addition, our preliminary studies showed its promising therapeutic effect on the development of collagen-induced arthritis in mice.
In the present study, we have demonstrated that EPS, at concentrations ranging 3–30 μg/ml, effectively induces the production of macrophage cytokines, especially TNF-α, IL-6 and IL-12. This finding supported by analysis of the balance of TNF-α to IL-10 suggests that EPS has an overall proinflammatory activity. However, its stimulatory potential was significantly lower than that of intact bacterial cells and lower than that of LPS. Importantly, effective concentrations of EPS were similar to immunostimulatory concentrations of LTA, the major TLR2 ligand of lactic bacteria (Vidal et al. 2002; Kim et al. 2007), suggesting EPS may also be active in vivo (Vu et al. 2009). Whole bacteria were stronger inducers of anti-inflammatory IL-10 than EPS, suggesting intact bacteria and EPS may have opposing effect on macrophage polarization.
The overall, contribution of EPS to the production of inflammatory mediators in vivo is difficult to predict. Exopolysaccharides as the exocellular component of bacteria biofilm may not participate in the activation of immune cells by bacterial cell-wall components (Vidal et al. 2002). Indeed, EPS may even screen bacteria from the immune cells in vivo.
In the present study, we also demonstrate some new immunomodulatory properties of EPS such as EPS desensitization of macrophages. It is well documented that exposure of macrophages to LPS induces a state of hyporesponsiveness (tolerance) to subsequent stimulation with LPS (Zhang & Morrison 1993). Recently, it has been shown that similar effect may be induced by LTA, the component of lactobacilli bacteria (Lehner et al. 2001). In the present study, we addressed an issue whether EPS impact on macrophages (‘macrophage priming’) can also induce desensitization of macrophages. We have shown that pretreatment of macrophages with LPS results in decreased production of TNF-α after re-stimulation with LPS. Interestingly, the production of TNF-α was also reduced after re-stimulation with EPS. A similar effect, named ‘cross-tolerance’, was seen after exposure of mouse macrophages to LPS and subsequent stimulation with other stimuli (Lehner et al. 2001). It is noteworthy that the LPS priming of macrophages resulted in a decreased production of pro-inflammatory TNF-α and enhanced production of anti-inflammatory IL-10. IL-10 may contribute to the down-regulation of TNF-α production by macrophages exposed to bacteria for the second time. In contrast to LPS, EPS priming did not induce macrophage ‘cross-tolerance’. The molecular mechanisms responsible for the differential activation of macrophages by LPS and EPS have not been elucidated to date. However, some mechanisms that underlie the induction of LPS tolerance have been postulated. For example, LPS may lead to down-regulation of TLR4 expression and reduced activation of MAPKs (Martin et al. 2001; Dowling et al. 2008). Activation of MAPKs is one of the crucial signal transduction events that control cytokine production (Dong et al. 2002). Little is known regarding active components of probiotics involved in MAPK activation. Our results clearly indicate that inhibitors of both ERK and p38 MAPKs inhibit the production of TNF-α induced by LPS and EPS. Interestingly, IL-10 production was also markedly reduced by MAPKs inhibitors, whereas the production of IL-12p40 was even enhanced (data not shown). These data are in agreement with the recent study of Kaji et al. (2010) who showed that selective blockade of ERK MAPK activation induced by L. plantarum resulted in a decrease in IL-10 and a simultaneous increase in IL-12 production. However, the molecular mechanism of this effect is unclear.
In conclusion, several questions regarding the biological properties of EPS still remain to be answered. Specific receptor(s) for EPS is/are still not defined. Although our present data clearly show the immunostimulatory potential of crude EPS, to use EPS in humans, further in vivo studies using highly purified EPS are necessary. Furthermore, because of the heterogeneity of bacterial EPS, the strain-specific biological properties of EPS have to be taken into consideration.
Acknowledgments
We are very grateful to Prof Benjamin Chain from UCL for critical reading and valuable advices in preparation of the final version of this manuscript. This study was supported by the grants of Jagiellonian University College of Medicine: No. K/PBP/000288 and No. K/ZDS/000684.
References
- Christensen HR, Frøkiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 2002;168:171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- Ciszek-Lenda M. Biological functions of exopolysaccharides from probiotic bacteria. Centr. Eur. J. Immunol. 2011;36:51–55. [Google Scholar]
- Dalpke AH, Frey M, Morath S, Hartung T, Heeg K. Interaction of lipoteichoic acid and CpG-DNA during activation of innate immune cells. Immunobiology. 2002;206:392–407. doi: 10.1078/0171-2985-00189. [DOI] [PubMed] [Google Scholar]
- Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J. Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu. Rev. Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- Dowling D, Hamilton CM, O'Neill SM. A comparative analysis of cytokine responses, cell surface marker expression and MAPKs in DCs matured with LPS compared with a panel of TLR ligands. Cytokine. 2008;41:254–262. doi: 10.1016/j.cyto.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Erroi A, Fantuzzi G, Mengozzi M, et al. Differential regulation of cytokine production in lipopolysaccharide tolerance in mice. Infect. Immun. 1993;61:4356–4359. doi: 10.1128/iai.61.10.4356-4359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrie S, Daley M. Lactobacillus GG as treatment for diarrhea during enteral feeding in critical illness: randomized controlled trial. J. Parenter. Enteral. Nutr. 2011;35:43–49. doi: 10.1177/0148607110370705. [DOI] [PubMed] [Google Scholar]
- Flemming HC, Neu TR, Wozniak DJ. The EPS matrix: the “house of biofilm cells”. J. Bacteriol. 2007;189:7945–7947. doi: 10.1128/JB.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojsak I, Abdović S, Szajewska H, Milosević M, Krznarić Z, Kolacek S. Lactobacillus GG in the prevention of nosocomial gastrointestinal and respiratory tract infections. Pediatrics. 2010;125:1171–1177. doi: 10.1542/peds.2009-2568. [DOI] [PubMed] [Google Scholar]
- Kaji R, Kiyoshima-Shibata J, Nagaoka M, Nanno M, Shida K. Bacterial teichoic acids reverse predominant IL-12 production induced by certain Lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J. Immunol. 2010;184:3505–3513. doi: 10.4049/jimmunol.0901569. [DOI] [PubMed] [Google Scholar]
- Kim HG, Gim MG, Kim JY, et al. Lipoteichoic acid from Lactobacillus plantarum elicits both the production of interleukin-23p19 and suppression of pathogen-mediated interleukin-10 in THP-1 cells. FEMS Immunol Med Microbio. 2007;49:205–214. doi: 10.1111/j.1574-695X.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- Laws A, Gu Y, Marshall V. Biosynthesis, characterization, and design of bacterial exopolysaccharides from lactic acid bacteria. Biotechnol. Adv. 2001;19:597–625. doi: 10.1016/s0734-9750(01)00084-2. [DOI] [PubMed] [Google Scholar]
- Lehner MD, Morath S, Michelsen KS, Schumann RR, Hartung T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J. Immunol. 2001;166:5161–5167. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- Lipiński T, Jones C, Lemercinier X, et al. Structural analysis of the Lactobacillus rhamnosus strain KL37C exopolysaccharide. Carbohydr. Res. 2003;338:605–609. doi: 10.1016/s0008-6215(02)00526-8. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz J, Ciszek M, Bobek M, et al. Differential inflammatory mediator response in vitro from murine macrophages to lactobacilli and pathogenic intestinal bacteria. Int. J. Exp. Pathol. 2007;88:155–164. doi: 10.1111/j.1365-2613.2007.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcone V, Calzolari E, Bertini M. Effectiveness of vaginal administration of Lactobacillus rhamnosus following conventional metronidazole therapy: how to lower the rate of bacterial vaginosis recurrences. New Microbiol. 2008;31:429–433. [PubMed] [Google Scholar]
- Martin M, Katz J, Vogel SN, Michalek SM. Differential induction of endotoxin tolerance by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. J. Immunol. 2001;167:5278–5285. doi: 10.4049/jimmunol.167.9.5278. [DOI] [PubMed] [Google Scholar]
- Matsuguchi T, Takagi A, Matsuzaki T, et al. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin. Diagn. Lab. Immunol. 2003;10:259–266. doi: 10.1128/CDLI.10.2.259-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Hara T, Hori T, et al. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin. Exp. Immunol. 2005;140:417–426. doi: 10.1111/j.1365-2249.2005.02790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamadzadeh M, Olson S, Kalina WV, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc. Natl Acad. Sci. USA. 2005;8:2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermes M, Kantele JM, Atosuo TJ, Salminen S, Isolauri E. Interaction of orally administered Lactobacillus rhamnosus GG with skin and gut microbiota and humoral immunity in infants with atopic dermatitis. Clin. Exp. Allergy. 2011;41:370–377. doi: 10.1111/j.1365-2222.2010.03657.x. [DOI] [PubMed] [Google Scholar]
- Prescott SL, Wickens K, Westcott L, et al. Supplementation with Lactobacillus rhamnosus or Bifidobacterium lactis probiotics in pregnancy increases cord blood interferon-gamma and breast milk transforming growth factor-beta and immunoglobin A detection. Clin. Exp. Allergy. 2008;38:1606–1614. doi: 10.1111/j.1365-2222.2008.03061.x. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D, Karmeli F, Takabayashi K, et al. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology. 2002;122:1428–1441. doi: 10.1053/gast.2002.32994. [DOI] [PubMed] [Google Scholar]
- Randow F, Syrbe U, Meisel C, et al. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J. Exp. Med. 1995;181:1887–1892. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas-Madiedo P, de los Reyes-Gavilán CG. Invited review: methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J. Dairy Sci. 2005;88:843–856. doi: 10.3168/jds.S0022-0302(05)72750-8. [DOI] [PubMed] [Google Scholar]
- Ruas-Madiedo P, Gueimonde M, Margolles A, de los Reyes-Gavilán CG, Salminen S. Exopolysaccharides produced by probiotic strains modify the adhesion of probiotics and enteropathogens to human intestinal mucus. J. Food. Prot. 2006;69:2011–2015. doi: 10.4315/0362-028x-69.8.2011. [DOI] [PubMed] [Google Scholar]
- Sengül N, Aslím B, Uçar G, et al. Effects of exopolysaccharide-producing probiotic strains on experimental colitis in rats. Dis. Colon Rectum. 2006;49:250–258. doi: 10.1007/s10350-005-0267-6. [DOI] [PubMed] [Google Scholar]
- Sengül N, Iºık S, Aslım B, Uçar G, Demirbağ AE. The effect of exopolysaccharide-producing probiotic strains on gut oxidative damage in experimental colitis. Dig. Dis. Sci. 2011;56:707–714. doi: 10.1007/s10620-010-1362-7. [DOI] [PubMed] [Google Scholar]
- Servin AL, Coconnier MH. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract. Res. Clin. Gastroenterol. 2003;17:741–754. doi: 10.1016/s1521-6918(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Shida K, Nanno M. Probiotics and immunology: separating the wheat from the chaff. Trends Immunol. 2008;29:565–573. doi: 10.1016/j.it.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Shnyra A, Brewington R, Alipio A, Amura C, Morrison DC. Reprogramming of lipopolysaccharide-primed macrophages is controlled by a counterbalanced production of IL-10 and IL-12. J. Immunol. 1998;160:3729–3736. [PubMed] [Google Scholar]
- Szymański H, Armańska M, Kowalska-Duplaga K, Szajewska H. Bifidobacterium longum PL03, Lactobacillus rhamnosus KL53A, and Lactobacillus plantarum PL02 in the prevention of antibiotic-associated diarrhea in children: a randomized controlled pilot trial. Digestion. 2008;78:13–17. doi: 10.1159/000151300. [DOI] [PubMed] [Google Scholar]
- Vaarala O. Immunological effects of probiotics with special reference to lactobacilli. Clin. Exp. Allergy. 2003;33:1634–1640. doi: 10.1111/j.1365-2222.2003.01835.x. [DOI] [PubMed] [Google Scholar]
- Vidal K, Donnet-Hughes A, Granato D. Lipoteichoic acids from Lactobacillus johnsonii strain La1 and Lactobacillus acidophilus strain La10 antagonize the responsiveness of human intestinal epithelial HT29 cells to lipopolysaccharide and gram-negative bacteria. Infect. Immun. 2002;70:2057–2064. doi: 10.1128/IAI.70.4.2057-2064.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu B, Chen M, Crawford RJ, Ivanova EP. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules. 2009;14:2535–2554. doi: 10.3390/molecules14072535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong C, Kocianova S, Voyich JM, et al. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 2004;279:54881–54886. doi: 10.1074/jbc.M411374200. [DOI] [PubMed] [Google Scholar]
- Watnick P, Kolter R. Biofilm, city of microbes. J. Bacteriol. 2000;182:2675–2679. doi: 10.1128/jb.182.10.2675-2679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Morrison DC. Lipopolysaccharide structure–function relationship in activation versus reprogramming of mouse peritoneal macrophages. J. Leukoc. Biol. 1993;54:444–450. doi: 10.1002/jlb.54.5.444. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li N, Caicedo R, Neu J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J. Nutr. 2005;135:1752–1756. doi: 10.1093/jn/135.7.1752. [DOI] [PubMed] [Google Scholar]
- Zhang D, Chen L, Li S, Gu Z, Yan J. Lipopolysaccharide (LPS) of Porphyromonas gingivalis induces IL-1beta, TNF-alpha and IL-6 production by THP-1 cells in a way different from that of Escherichia coli LPS. Innate Immun. 2008;14:99–107. doi: 10.1177/1753425907088244. [DOI] [PubMed] [Google Scholar]


