Abstract
There is no good animal model of large artery injury-induced intimal hyperplasia (IH). Those available are reproducible, providing only a few layers of proliferating cells or have the disadvantage of the presence of a metallic stent that complicates histology evaluation. This study was designed to develop a new, simple model of accelerated IH based on balloon injury in conjunction with disruption of the Internal Elastic Lamina (IEL) in pig external iliac arteries. Iliac artery injury (n = 24) was performed in 12 Yorkshire pigs divided in two groups: Group I (n = 10), overdistention injury induced by an oversized non-compliant balloon; Group II (n = 14), arterial wall disruption by pulling back an isometric cutting balloon (CB) followed by stretching with a compliant Fogarty Balloon (FB). At two weeks, arteries were processed for morphometric analysis and immunohistochemistry (IHC) for smooth muscle cells (SMC) and proliferating cell nuclear antigen (PCNA). When comparing the two groups, at 2 weeks, arteries of group II had a higher incidence of IH (100%vs. 50%, P = 0.0059), increased intimal areas (2.54 ± 0.33 mm2vs. 0.93 ± 0.36 mm2, P = 0.004), increased intimal area/Media area ratios (0.95 ± 0.1 vs. 0.28 ± 0.05; P<0.0001) and decreased lumen areas (6.24 ± 0.44 vs. 9.48 ± 1.56, P=0.026). No thrombosis was noticed in Group II. Neointima was composed by proliferating SMC located with the highest concentration in the area of IEL disruption (IHC). Arterial injury by pulling back CB and FB induces significant IH in pig iliac arteries by two weeks without thrombosis. This model is superior to the classical overdistention non-compliant model and should be useful and cost-effective for preclinical testing of procedures designed to inhibit IH in large peripheral arteries.
Keywords: animal model, intimal hyperplasia, restenosis
Restenosis after successful surgical or endovascular interventions remains a major limitation to long-term therapeutic success in vascular surgery. At least 30% of the patients will experience restenosis during the first year after an infra-inguinal angioplasty (Baril et al. 2010). The proliferative component of restenosis is caused by excessive neointima formation. Therapeutic modalities that may have the potential to prevent intimal hyperplasia (IH) often need to be tested in large animals models (Karas et al. 1992). Mechanical injury to carotid and coronary porcine arteries, by overdistention with an oversized non-compliant balloon (NCB), has been shown to produce a neointimal response virtually identical to human neointima after 4–6 weeks(Touchard & Schwartz 2006; Wang et al. 2006). However, in larger peripheral arteries (i.e. pig iliac arteries), the amount of IH produced in these models remains proportionally lower, resulting in inadequate amount of IH (Ward et al. 2000; Krueger et al. 2006). The only endovascular injury model that has been able to induce significant IH in pig iliac arteries within 4 weeks is an oversized metallic stent injury (Verheye et al. 1999; Harnek et al. 2002; Castro Junior et al. 2006). However, histological evaluation of stented arteries is technically challenging, and immunolabelling of vascular tissues that contain metallic bioprostheses is problematic (Rippstein et al. 2006).
Based on the principle that the degree of mechanical injury might account for variability in IH(Sims 1989), we hypothesize that an arterial denudation model combining cutting balloon (CB) for disruption of the Internal Elastic Lamina (IEL) and a compliant Fogarty balloon (FB) will create a more robust and reproducible arterial wall injury than the classical overdistention NCB injury. We also hypothesize that using this strategy may result in a more significant IH within 2 weeks rather than 4–6 weeks traditionally utilized.
Methods
Animals
The study animals, obtained from a local supplier (Tufts University, Cummings School of Veterinary Medicine, New Grafton, MA), were 12 Yorkshire pigs (average weight, 35 kg). All pigs were fed a normal chow diet without supplements. Experiments involving animals were carried out according to a protocol approved by the institutional Animal Care Committee and the investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Procedures
Pigs were anaesthetized with intramuscular Xylazine (2.2 mg/kg), Telazole (4–5 mg/kg) and Atropine (0.04 mg/kg). After intubation, anaesthesia was maintained with isoflurane (1–2%; Harvard respirator). Vital signs, ECG, heart rate, blood pressure and body temperature were routinely monitored. Animals were draped under sterile conditions. A single dose of heparin (200 UI/kg) and antibiotic (Cefazolin sodium, 40 mg/kg I.V.) was administered preoperatively. A left common carotid cut-down was performed and a 7F introducer sheath was inserted into the descending aorta under fluoroscopic guidance. The diameter of the arteries was determined from fluoroscopy using a sized metallic marker. Subsequently, under fluoroscopy, each external iliac artery (EIA) was catheterized and injured according to protocol. Two groups of injury methods were randomly attributed to a total of 24 external iliac arteries. In Group I (10 EIA), an overdistention injury was performed using a 30-s air inflation of a 70% oversized NCB. In Group II (14 EIA), a disruption of the IEL was accomplished by pulling back an air-inflated isometric CB (Peripheral® Boston Scientific, Natick, MA, USA) followed by an air-inflated 4-F over-the-wire FB (Fogarty Balloon Edwards Lifesciences, Irvine, CA, USA). The retraction distance was 15 mm and was verified under fluoroscopy.
After the procedure, the sheaths and wires were removed and the carotid artery was ligated. For pain management, animals had infiltration of Marcaine (0.5%) with epinephrine into the neck wound and Buprenorphine (0.05–0.1 mg/kg) as needed. Following surgery, animals had free access to a standard normal chow and water while they were maintained in a standard 12-h/light/dark cycle.
Vessel isolation and processing for histology
At 14 days after the procedure, animals were anesthetized and euthanized with Pentobarbital 100 mg/kg I.V. The aorta and iliac arteries were surgically exposed by a trans-peritoneal dissection and perfused through a 7F introducer sheath with saline followed by buffered (pH 7.4) 4% formalin, at 100 mmHg, with the aorta cross-clamped and each iliac artery partially clamped in sequence, for 15 min. Drainage of formalin was performed via distal incisions of each artery. Vessels were stored in 4% formalin overnight, serially dehydrated and embedded in paraffin. Five micrometers cross sections were performed; Verhoeff-van Gieson elastic (Figure 1) and Hematoxylin/Eosin staining were used for morphometric assessments.
Figure 1.
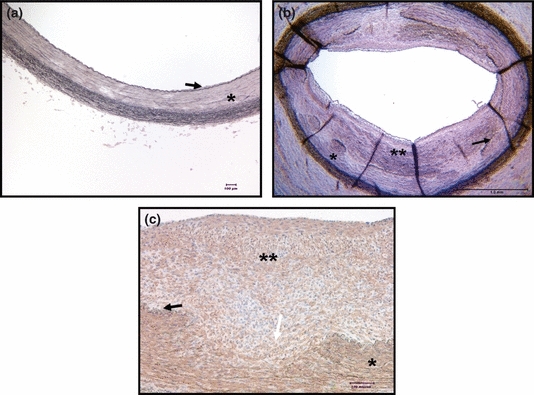
(a) Lawson's elastic van Gieson stain; Non-injured external iliac artery (EIA); magnification ×100. (b) Injured EIA from Group II, Lawson's elastic van Gieson stain, (magnification ×25). (c) Injured EIA from Group II, IHC using anti-smooth muscle α-actin antibody (1:100), Smooth muscle cells are brown stained (magnification ×200), and Hematoxylin counterstaining was used. Black arrow: Internal Elastic Lamina (IEL); Single star: Media; Double star: NeoIntima; White arrow: disrupted IEL. Note the abundant presence of SMC in the neointima.
Vessel injury assessment
All segments of external iliac arteries were serially examined for patency. In the non-thrombosed arteries, the region of the most severe injury was identified and one sample per artery was used for all histological measurements.
The severity of injury to the arteries was assessed as described by Schwartz et al. (1990): 0, IEL intact; 1, rupture of IEL only; 2, media damage; and 3, external elastic lamina (EEL) rupture.
Morphometric measurements
Areas of the adventitia, media, neointima and lumen as well as overall vessel size were measured by computerized planimetry (Spot advanced 4; SPOT Imaging Solutions, Sterling Heights, MI, USA) as has previously been described (Ward et al. 2001). The adventitia is defined as the area between the EEL and periadventitial tissues; overall vessel size is defined as the area circumscribed by the EEL. The media are defined as the region between the EEL and the IEL; when the IEL was missing, it was defined as areas of remnants of medial tissue (i.e. well-organized smooth muscle cells (SMCs) with intervening elastic fibres). The neointima comprises the region between the lumen and the IEL and, when the elastic lamina was missing, the area between the lumen and remnants of medial tissue or the EEL. The lumen area is defined as the region circumscribed by the intima/neointima (lumen border). The ratio between Intimal area and Medial area (I/M ratio) was calculated to quantify IH (Figure 1) (Ward et al. 2001; Krueger et al. 2006).
Immunohistochemical detection of proliferating smooth muscle cells (SMC)
SMCs were characterized by immunohistochemical staining of the α-actin using a monoclonal antibody anti-smooth muscle α-actin (Dako, dilution 1:100; Dako North America Inc., RealCarpinteria, CA, USA) as a primary antibody with a biotinylated secondary antibody incubated with streptavidin-peroxidase, followed by reaction with diaminobenzidine/hydrogen peroxide as substrate-chromogen. (Animal Research Kit peroxidase, Dako Corp) (Groves et al. 1995). The cellular proliferation was evaluated by immunohistochemical detection of proliferating cell nuclear antigen (PCNA) (Groves et al. 1995). After rehydration, antigen retrieval and endogenous peroxidase activity blocking, sections were incubated overnight with a monoclonal anti-PCNA antibody (Santa Cruz Company, 1:100 dilution; Santa Cruz Biotechnology Inc., Delaware Avenue, Santa Cruz, CA, USA) at 4 °C. A biotinylated horse anti-mouse secondary antibody (1:133 dilutions) was then applied at room temperature for 30 min, followed by Biotin-streptavidin complex at room temperature for 30 min. Non-injured arteries and injured arteries exposed only to secondary antibodies served as controls.
Statistical analysis
All data in text and table are presented as mean ± standard error of the mean. GraphPad inStat version 3® (GraphPad Software Inc., La Jolla, CA, USA) was used for all statistical calculations. To identify the variations between the groups, we used a Student's t-test. Chi-squared test was used for qualitative variables. Differences were considered significant at P<0.05.
Results
Technical course
Arterial injury was performed successfully in 24 arteries in 12 animals. All animals survived the expected 2 weeks. No animals experienced observable untoward clinical events after iliac arterial injury. The analyses in this study were based on all the 24 injured arteries (Table 1).
Table 1.
Artery characteristics and arterial responses 2 weeks after arterial injury in pig iliac arteries. Data presented as mean ± standard error of the mean. Group I: overdistention injury using oversized non-compliant balloon inflation. Group II: denudation injury using Cutting Balloon and Fogarty Balloon pull-back
| Group I | Group II | P | |
|---|---|---|---|
| N | 10 | 14 | |
| D art (mm) | 4.7 (0.27) | 5.1 (0.12) | 0.13 |
| Thrombosed | 1 (10%) | 0 | NS |
| IH | 5 (50%) | 14 (100%) | 0.0059 |
| Injury score | 0.9 (0.37) | 2.4 (0.19) | 0.0013 |
| EEL area (mm2) | 17.7 (3,2) | 13.5 (4.4) | 0.0021 |
| Lumen area (mm2) | 9.48 (1.56) | 6.24 (0.44) | 0.026 |
| IH area (mm2) | 0.93 (0.36) | 2.54 (0.33) | 0.0039 |
| I/M ratio | 0.28 (0.05) | 0.95 (0.10) | <0.0001 |
IH, intimal hyperplasia; EEL, external elastic lamina; D art, initial arterial diameter; I/M ratio: intimal area/media area.
Patency and thrombosis
Overall, 4% (1/24) of the injured arteries were thrombosed (Table 1). Overdistention of the artery with an oversized NCB was the only factor that correlated with thrombosis. No artery in the group II developed thrombosis.
Morphological examination
Mean arterial diameter was 4.91 ± 0.14 mm. The two groups were comparable in term of arterial diameter (Table 1). Overall, 79% (19/24) of the injured arteries developed IH after 2 weeks. The occurrence of IH was correlated to an injury score >1 (IEL break), which emphasizes the importance of IEL disruption in IH. The mean number of IEL breaks per artery in Group II was 3.7 ± 0.8. When comparing the two groups, Group II had a higher incidence of IH formation. In non-thrombosed arteries, Group II had a significantly higher injury score than Group I. Intimal areas and Intimal area/Media area ratios were significantly higher in Group II. Lumen areas and areas within the EEL were significantly smaller in Group II (Table 1) (Figure 2). Constrictive remodelling was evaluated by comparing the area within the EEL of an injured segment of the vessel with the one of an uninjured segment of the same vessel. In Group II, the area within the EEL was significantly smaller in the injured part of the artery (13.5 ± 2.95 mm2vs. 20.55 ± 3.5 mm2, P<0.0001, paired Student's t-test), whereas in Group I, this difference was not found (17.68 ± 3.17 mm2vs.17.47 ± 2.17 mm2, P=0.75, paired Student's t-test).
Figure 2.
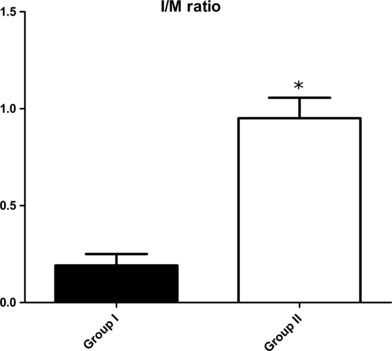
Intimal area/medial area ratio 14 days after injury. Mean (±standard error of the mean) of Group I (n = 10, oversized Cutting Balloon and non-compliant balloon), and Group II (n = 14, pull-back of Cutting Balloon and Fogarty Balloon). Group II > Group I (Student's t-test, P<0.0001).
Immunohistochemestry
Smooth muscle cell staining
After 14 days, all vessels with IH had a positive α-actin staining in the media and neointima, indicating that the cells residing in the neointima area were cells expressing SMC phenotype (Figure 1c).
Cell proliferation staining
No cell proliferation was observed in the control arteries. In the Injured arteries, positive staining was observed in the media and the neointima (Figure 3). Maximum PCNA staining for cell proliferation was observed at the site of IEL disruption.
Figure 3.
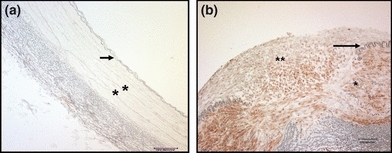
Immuno-histo-chemistry using anti-proliferating cell nuclear antigen (PCNA) antibody. Proliferative cells are brown coloured stained. (a) Non-injured external iliac artery (EIA) (magnification ×100), non-PCNA detected. (b) Injured EIA from Group II (magnification ×200), note the presence in the neointima and the media of proliferative cells, proliferative cells are abundant where the Internal Elastic Lamina (IEL) is disrupted. Black arrow: IEL; single star: Media; double star: NeoIntima; white arrow: disrupted IEL.
Discussion
These experiments describe early arterial IH within two weeks in a large animal model in which proliferation of SMC was characterized. We hypothesized that disruption of the IEL would be crucial to the development of IH. In Group I, the overdistention injury by inflating oversized NCB resulted in minimal IH and one arterial thrombosis. In non-thrombosed arteries, there was minimal IEL disruption. To achieve more effective and reproducible injury by the Schwartz scale, a CB was utilized for cutting of the IEL, and the overdistention with the NCB was replaced by deep endovascular denudation by pulling back a compliant balloon (i.e. Fogarty Balloon). This combination protocol developed a robust IH with a constrictive remodelling in these large diameter arteries without thrombosis within 2 weeks.
Previous endovascular IH models in pig arteries used carotid and coronary arteries. Steele et al. (Steele et al. 1985) first demonstrated that neointimal hyperplasia following NCB injury of the normal pig carotid artery was maximal 2–3 weeks after injury. Other investigators have confirmed the development of similar intimal lesions following overdistension injury in pig coronary arteries after 4–6 weeks (Karas et al. 1992; de Smet et al. 1998). Finally, models using coronary metallic stent implantation (Rodgers et al. 1990; Schwartz et al. 1990, 1992) have been giving rise to even more aggressive intimal responses than balloon injury alone.
Despite these data in coronary and carotid arteries, which are small in diameter, only few studies have evaluated these injuries models on larger, peripheral arteries (Table 2). Those studies showed insufficient hyperplastic responses even 4–8 weeks after injury (Table 2). More precisely, when comparing the response after a similar overdistention injury in similarly sized peripheral and coronary arteries, the IH is significantly lower in the peripheral arteries (Ward et al. 2000). The authors explained this insufficient response in peripheral arteries by focusing on the differences in arterial morphology. Indeed, coronary arteries are morphologically distinct from peripheral arteries even of similar size (elasticity, thickness and fenestration of the IEL) which may affect the extent of injury and the IH induced (Ward et al. 2000). Therefore, as identified in Group I of this study, overdistention injury models do not seem to be appropriate to generate IH in peripheral arteries. This limitation was overcome in this study by directly cutting the IEL with inflation of the CB and pulling it a short distance (Group II). Indeed, when compared with the classical injury models, it induces a significantly more intense IH response within a shorter period of time (Table 2) (Lamawansa et al. 1997; Ward et al. 2000; Harnek et al. 2002; Krueger et al. 2006). Using an atherectomy device, Gonshior et al. created a comparable arterial injury on porcine femoral arteries leading to significant IH after 21 days (Gonschior et al. 1995). However, this injury was carried out by means of a cut-down approach on the target artery as opposed to a purely endovascular technique used in our model (Gonschior et al. 1995).
Table 2.
Comparison between Group II results of the current study and other injury models published in literature
| Current study Group II | Ward et al. Harnek et al. | Lamawansa et al. | Krueger et al. Castro et al. Verheye et al. | |
|---|---|---|---|---|
| Injury method | CB + FB | Balloon overdistention | FB denudation | Stenting |
| Period time | 2 weeks | 4–8 weeks | 4 weeks | 4 weeks |
| D art (mm) | 5.1 | 2.6–6.4 | CIA | Int.IA only |
| Injury score | 2.4 | 0.4 | – | – |
| IH | 100% | ∼40% | – | 38–44% |
| IH area (mm2) | 2.54 | 0.1–1.7 | 1.5 | 1.6–6.4 |
| Medial area (mm2) | 2.88 | 4–6 | 5.7 | 1.1–5.4 |
| I/M ratio | 0.95 | 0.5 | – | 0.9–1.2 |
Int.IA, internal iliac artery; CIA, common iliac artery; IH, intimal hyperplasia; D art, artery diameter; FB, Fogarty Balloon; CB, cutting balloon; IH, intimal hyperplasia; I/M ratio, intimal area/media area.
Many authors point to the importance of IEL disruption in the development of IH (Schwartz et al. 1992; Bonan et al. 1993; Touchard & Schwartz 2006). We also noticed that the pulling back of an inflated, isometric CB creates multiple IEL lacerations in the neighbourhood of four CB blades. This was not identified in a pilot study (data not shown) when the CB was only overinflated and not pulled across the artery, unless there was significant overdistention that resulted in severe vascular injury and often thrombosis. In addition, when the IEL remained intact no IH was detected, similar to the NCB. In contrast, when the IEL was ruptured, neointima filled in between the dissected surfaces of the media. This implies that there are factors unique to the microenvironment around an IEL rupture that lead to an enhanced proliferative response. In fact, the presence of macroscopic thrombus at the site of IEL rupture has been described at 24 h after the injury (Steele et al. 1985). This probably results in the local release of growth factors from the inflammatory response to the thrombus and may prime SMCs for increased proliferation (Groves et al. 1995). The IEL may also act as a physical barrier to cell migration or inhibit paracrine communication between cells of the intima and media. In support of this, IEL has been shown to control the movement of macromolecules (of equivalent size to some growth factors) across the arterial wall (Penn et al. 1994) and may, therefore, limit the permeation of plasma-derived growth factors (e.g. PDGF, FGF-B, insulin-like growth factor) into areas of injury.
Models using oversized metallic stent injury are also based on IEL disruption and therefore proved to be efficient in inducing IH (Verheye et al. 1999; Harnek et al. 2002; Castro Junior et al. 2006). But, characterization of the IH and preclinical studies testing IH inhibition therapeutics is very complicated in arteries with metallic stents. Indeed, conventional paraffin embedding and sectioning procedures have demonstrated limitations in histological study of vessels with stents. In most cases, these procedures require the complete removal of the metallic stent prior to tissue processing, thereby disrupting normal vascular architecture and, in particular, the IH layer. To accurately observe the vascular response, methacrylate resins are employed as the embedding media of choice that compromises immunohistological tissue analysis (Rippstein et al. 2006). In addition, vessel sections need dedicated and very expensive microtomes (Krueger et al. 2006), (Rippstein et al. 2006). With the current protocol of this study, conventional paraffin embedding and sectioning procedures can be used and immunohistological analysis can be easily performed.
Thrombosis is a consequence of a very severe injury to the vessel wall. In this study, thrombosis was only noticed once after significant NCB oversizing. It should be pointed out that previous reported models of porcine iliac artery injury did not give the incidence of arterial thrombosis after injury. Also, in two reported studies, <50% of the injured arteries were analysed for IH (Table 2) (Verheye et al. 1999; Ward et al. 2000).
At 2 weeks, the IH areas and I/M ratio obtained with this model are superior to those obtained using balloon overdistention injuries after 4–6 weeks and comparable to those obtained after metallic stent injuries also after 4 weeks. This is not surprising as it has been shown in the pig carotid injury model that the neointimal response was maximal at 14 days when rupture of the IEL was noticed (Groves et al. 1995).
Therefore, the current model seems very attractive by its feasibility, reproducibility and by the constancy of the IH induced without thrombosis. This constancy can be explained by the fact that a moderate parietal injury was obtained systematically. By having adequate IH in the control groups, this hyperplastic restenosis model will provide a powerful tool for preclinical studies testing new therapies aiming to inhibit IH.
Conclusion
In summary, this endovascular IEL incision and intimal-denudation injury model in clinically relevant peripheral arteries performed by pulling back inflated CB and FB provides a robust and prolific IH without thrombosis within two weeks. The data from this model were superior to the classical overdistention model by the amount of IH induced which was achieved in a shorter period of time. Also, this study emphasizes the importance of the IEL disruption in the development of IH. This novel injury model should be useful to test potential therapies aimed at limiting IH in large diameter peripheral arteries.
This work was supported by the Rita and Monte Goldman Foundation, the Harold and June Geneen Vascular Surgery Research Fund, and the John F Murphy and the Bay State Federal Savings Foundation.
Acknowledgments
None.
Conflicts of interest
Rabih Houbballah, MD: none. Alessandro Robaldo, MD: none. Hassan Albadawi, MD: none. James Titus: none. Glenn M. LaMuraglia, MD: none.
References
- Baril DT, Chaer RA, Rhee RY, Makaroun MS, Marone LK. Endovascular interventions for TASC II D femoropopliteal lesions. J. Vasc. Surg. 2010;51:1406–1412. doi: 10.1016/j.jvs.2010.01.062. [DOI] [PubMed] [Google Scholar]
- Bonan R, Paiement P, Scortichini D, Cloutier MJ, Leung TK. Coronary restenosis: evaluation of a restenosis injury index in a swine model. Am. Heart J. 1993;126:1334–1340. doi: 10.1016/0002-8703(93)90531-d. [DOI] [PubMed] [Google Scholar]
- Castro Junior C, Pereira AH, Pasa MB. Morphometric analysis of the intimal reaction after stent implantation in iliac arteries submitted to angioplasty in pigs. Acta Cir. Bras. 2006;21:139–143. doi: 10.1590/s0102-86502006000300004. [DOI] [PubMed] [Google Scholar]
- Gonschior P, Gerheuser F, Gonschior GM, et al. Experimental directional atherectomy injury in arterial vessels: impact of trauma depth on cellular response. Am. Heart J. 1995;129:1067–1077. doi: 10.1016/0002-8703(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Groves PH, Banning AP, Penny WJ, Lewis MJ, Cheadle HA, Newby AC. Kinetics of smooth muscle cell proliferation and intimal thickening in a pig carotid model of balloon injury. Atherosclerosis. 1995;117:83–96. doi: 10.1016/0021-9150(95)05562-b. [DOI] [PubMed] [Google Scholar]
- Harnek J, Zoucas E, Stenram U, Cwikiel W. Insertion of self-expandable nitinol stents without previous balloon angioplasty reduces restenosis compared with PTA prior to stenting. Cardiovasc. Intervent. Radiol. 2002;25:430–436. doi: 10.1007/s00270-002-1860-x. [DOI] [PubMed] [Google Scholar]
- Karas SP, Gravanis MB, Santoian EC, Robinson KA, Anderberg KA, King SB., III Coronary intimal proliferation after balloon injury and stenting in swine: an animal model of restenosis. J. Am. Coll. Cardiol. 1992;20:467–474. doi: 10.1016/0735-1097(92)90119-8. [DOI] [PubMed] [Google Scholar]
- Krueger KD, Mitra AK, DelCore MG, Hunter WJ, III, Agrawal DK. A comparison of stent-induced stenosis in coronary and peripheral arteries. J. Clin. Pathol. 2006;59:575–579. doi: 10.1136/jcp.2004.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamawansa MD, Wysocki SJ, House AK, Norman PE. Morphometric changes seen in balloon-injured porcine iliac arteries: the influence of sympathectomy on intimal hyperplasia and remodelling. Eur. J. Vasc. Endovasc. Surg. 1997;13:43–47. doi: 10.1016/s1078-5884(97)80049-4. [DOI] [PubMed] [Google Scholar]
- Penn MS, Saidel GM, Chisolm GM. Relative significance of endothelium and internal elastic lamina in regulating the entry of macromolecules into arteries in vivo. Circ. Res. 1994;74:74–82. doi: 10.1161/01.res.74.1.74. [DOI] [PubMed] [Google Scholar]
- Rippstein P, Black MK, Boivin M, et al. Comparison of processing and sectioning methodologies for arteries containing metallic stents. J. Histochem. Cytochem. 2006;54:673–681. doi: 10.1369/jhc.5A6824.2006. [DOI] [PubMed] [Google Scholar]
- Rodgers GP, Minor ST, Robinson K, et al. Adjuvant therapy for intracoronary stents. Investigations in atherosclerotic swine. Circulation. 1990;82:560–569. doi: 10.1161/01.cir.82.2.560. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Murphy JG, Edwards WD, Camrud AR, Vliestra RE, Holmes DR. Restenosis after balloon angioplasty. A practical proliferative model in porcine coronary arteries. Circulation. 1990;82:2190–2200. doi: 10.1161/01.cir.82.6.2190. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J. Am. Coll. Cardiol. 1992;19:267–274. doi: 10.1016/0735-1097(92)90476-4. [DOI] [PubMed] [Google Scholar]
- Sims FH. A comparison of structural features of the walls of coronary arteries from 10 different species. Pathology. 1989;21:115–124. doi: 10.3109/00313028909059547. [DOI] [PubMed] [Google Scholar]
- de Smet BJ, van der Zande J, van der Helm YJ, Kuntz RE, Borst C, Post MJ. The atherosclerotic Yucatan animal model to study the arterial response after balloon angioplasty: the natural history of remodeling. Cardiovasc. Res. 1998;39:224–232. doi: 10.1016/s0008-6363(98)00085-6. [DOI] [PubMed] [Google Scholar]
- Steele PM, Chesebro JH, Stanson AW, et al. Balloon angioplasty. Natural history of the pathophysiological response to injury in a pig model. Circ. Res. 1985;57:105–112. doi: 10.1161/01.res.57.1.105. [DOI] [PubMed] [Google Scholar]
- Touchard AG, Schwartz RS. Preclinical restenosis models: challenges and successes. Toxicol. Pathol. 2006;34:11–18. doi: 10.1080/01926230500499407. [DOI] [PubMed] [Google Scholar]
- Verheye S, Salame MY, Robinson KA, et al. Short- and long-term histopathologic evaluation of stenting using a self-expanding nitinol stent in pig carotid and iliac arteries. Catheter. Cardiovasc. Interv. 1999;48:316–323. doi: 10.1002/(sici)1522-726x(199911)48:3<316::aid-ccd19>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Wang X, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Mouse models of neointimal hyperplasia: techniques and applications. Med. Sci. Monit. 2006;12:RA177–RA185. [PubMed] [Google Scholar]
- Ward MR, Kanellakis P, Ramsey D, Jennings GL, Bobik A. Response to balloon injury is vascular bed specific: a consequence of de novo vessel structure? Atherosclerosis. 2000;151:407–414. doi: 10.1016/s0021-9150(99)00407-4. [DOI] [PubMed] [Google Scholar]
- Ward MR, Kanellakis P, Ramsey D, Funder J, Bobik A. Eplerenone suppresses constrictive remodeling and collagen accumulation after angioplasty in porcine coronary arteries. Circulation. 2001;104:467–472. doi: 10.1161/hc3001.091458. [DOI] [PubMed] [Google Scholar]


