Abstract
OTHER THEMES PUBLISHED IN THIS IMMUNOLOGY IN THE CLINIC REVIEW SERIES
Allergy, Metabolic Diseases, Cancer, Autoinflammatory Diseases, Type 1 diabetes and viruses.
Invariant natural killer T (iNKT) cells have been shown to play a key role in the regulation of immunity in health and disease. However, iNKT cell responses have also been found to influence both rejection and the induction of tolerance following transplantation of allogeneic cells or organs. Although a number of mechanisms have been identified that lead to iNKT cell activation, how iNKT cells are activated following transplantation remains unknown. This review will attempt to identify potential mechanisms of iNKT cell activation in the context of transplantation by applying knowledge garnered from other disease situations. Furthermore, we put forward a novel mechanism of iNKT cell activation which we believe may be the dominant mechanism responsible for iNKT activation in this setting, i.e. bystander activation by interleukin-2 secreted by recently activated conventional T cells.
Keywords: NKT cells, rejection, tolerance, transplantation
Introduction
Natural killer T (NKT) cells represent a small population of T lymphocytes that are reactive to lipid antigens, presented in the context of the non-polymorphic major histocompatibility complex (MHC)-class I-like molecule, CD1d [1]. Although there appears to be a number of subtypes of NKT cells, which can be determined through their T cell receptor (TCR) usage, cytokine production, expression of specific surface molecules and reactivity, the most extensively characterized are the invariant NKT (iNKT) cells [2]. iNKT cells are defined by the expression of a semi-invariant TCR composed of a Vα14-Jα18 chain associated with Vβ8·2, Vβ7, Vβ2 chains in mice and Vα24-Jα18 associated with Vβ11 in humans [3–5]. The distribution of iNKT cells is varied, accounting for 30% of the total T cell population in the liver and <1% in the spleen, lymph nodes and blood in mice and at slightly lower levels in humans [6,7]. Although iNKT cells represent a relatively low frequency of T cells, their limited TCR diversity means that they respond at high frequency following activation.
iNKT cells have been shown to play an important role in health and disease being involved in both the induction of immunity to pathogens and malignancies in addition to the maintenance of tolerance to self-antigens. For example, mice deficient in NKT cells are susceptible to the development of chemically induced tumours, whereas wild-type mice are protected [8]. Indeed, these experimental findings correlate with clinical data showing that patients with advanced cancer have decreased iNKT cell numbers in peripheral blood [9]. In addition, in studies using non-obese diabetic (NOD) mice that develop a spontaneous form of type 1 diabetes (T1D) mediated by autoreactive T cells, iNKT cells can alter the kinetic of disease onset and severity of disease. In this model, such mice have been found to contain reduced numbers of NKT cells and either activation or increasing the number of iNKT cells in NOD mice affords a degree of protection from T1D [10–12].
The prototypical iNKT cell agonist is the marine sponge-derived α-galactosylceramide (α-GalCer), although a broad spectrum of endogenous and exogenous ligands have now been characterized [13–19]. The use of α-GalCer has provided important insight into how iNKT cell activation can regulate immunity, and collectively these studies have demonstrated that iNKT cells occupy an important immunological niche providing a bridge between the innate and adaptive arms of the immune system [2]. For example, iNKT cell activation is associated with key steps in ensuring effective immune responses through the maturation of dendritic cells (DCs), activation of NK cells and B cells, enhancing T cell responses and suppression of myeloid-derived suppressor cells (MDSC) and interleukin (IL)-10-producing neutrophils [20–29]. Although many of these mechanisms have been defined using potent iNKT cell agonists, such as α-GalCer, the intricacies of iNKT cell activation, in the absence of such super-agonists, have largely been restricted to iNKT cell responses in the context of cancer, autoimmunity and pathogenic infections [30–32]. In this review we will discuss the mechanisms of iNKT cell activation in these disease situations and then relate these potential modes of activation to iNKT cell responses following transplantation.
iNKT cells are activated following transplantation
In mice, the absence of NKT cells does not alter the kinetics of rejection of fully MHC mismatched cardiac, skin or islet (placed under the kidney capsule) transplants [33–35], which may be due to the potent conventional T cell alloimmune response overshadowing any contribution to rejection made by iNKT cells. However, there is ample evidence to suggest that iNKT cells are activated and participate in responses to transplanted tissue. For example, iNKT cells have been shown to infiltrate both cardiac and skin allografts prior to rejection and have been found in expanded numbers in peripheral lymphoid tissue following transplantation [36–38]. Furthermore, an increase in the number of CD4+ iNKT cells has been reported in patients undergoing an acute cardiac rejection episode compared to those patients with stable transplants [39].
The impact of iNKT cell activation following transplantation
iNKT cells are not only activated, but also influence the ensuing immune response to allografts being either pro-rejection or facilitating the induction of tolerance, depending on the model studied (Table 1; reviewed in [40]). For example, it has been found consistently that animals deficient in either NKT cells or iNKT cells are resistant to the induction of tolerance by co-stimulatory/co-receptor molecule blockade [33,38,41]. Importantly the adoptive transfer of NKT cells into such mice restores tolerance which is dependent on interferon (IFN)-γ, IL-10 and/or CXCL16 [33,37,38,41,42]. In addition, iNKT cells have proved to be essential for the induction of tolerance to corneal allografts and have been demonstrated to prevent graft-versus-host disease (GVHD) in an IL-4-dependent manner [43–45].
Table 1.
Summary of the involvement of invariant natural killer T (iNKT) cells in transplant tolerance (a) and rejection (b). It should be noted that in one study in islet transplantation iNKT cells were not shown to contribute to either transplant tolerance or rejection [35]
| Species | Transplant | Tolerance regimen | Mechanism of action | Ref. |
|---|---|---|---|---|
| (a) | ||||
| Mice | Heart | Anti-LFA-1/ICAM-1 or anti-B7-1/B7-2 | IFN-γ production? | [33] |
| Anti-CD154 mAb | iNKT cells require CXCL16/CXCR6 interactions to traffic to allograft | [38] | ||
| Anti-CD154 mAb | IL-10 production | [41] | ||
| Mice | Skin | None | IL-10 production | [37] |
| Mice | Heart | Anti-CD4, CD8, CD154 mAb | Not determined | * |
| Mice | Islet (rat xenograft) | Anti-CD4 mAb | IFN-γ production but the dose of CD4 is critical for tolerance induction | [42] |
| Mice | Cornea | None | Enhanced regulatory T cell function | [43] |
| Mice | Bone marrow | None | Prevent GVHD. IL-4-dependent | [44,45] |
| (b) | ||||
| Mice | Islet | None | IFN-γ production promotes PMN recruitment to graft | [46] |
| None | Islet destruction only found when islets injected into portal vein | [34] | ||
| Rapamycin | Not determined | [34] | ||
| Mice | Skin | Anti-CD4, CD8, CD154 mAb | Not determined | * |
| Mice | Skin | None | Induction of a Th17 response | ** |
GVHD: graft-versus-host disease; mAb: monoclonal antibody; Th: T helper; IFN: interferon; PMN: polymorphonuclear; IL: interleukin; LFA: leucocyte function antigen; ICAM: intercellular adhesion molecule.
Jukes J-P, Zhao Z & Jones ND, unpublished observations
Janes SE & Jones ND, manuscript in preparation.
However, following transplantation iNKT cell responses do not always promote allograft acceptance, as we have shown that the activation of iNKT cells can promote the rejection rather than acceptance of skin allografts (Janes SE, unpublished observation). Furthermore, transplantation of islets into the liver via the portal vein resulted in the activation of iNKT cells, IFN-γ secretion and islet destruction mediated by neutrophils [34,46]. However, this iNKT cell response appears to be unique to intraliver delivery of islets, as the same response was not seen upon islet transplantation under the kidney capsule [34]. These data may suggest that iNKT cells require a degree of inflammation or ischaemia to become activated, as Li et al. has shown that following ischaemia–reperfusion injury of the kidney iNKT cells are recruited rapidly, produce IFN-γ and promote neutrophil recruitment [47]. Furthermore, iNKT cell responses may alter depending on the type of transplant carried out, for example, following either vascularized (heart) or non-vascularized (skin) grafts, as the alloantigen drains to iNKT cells residing in the spleen or axillary lymph nodes, respectively. Doisne et al. have shown since that NK1·1–iNKT cells resident in peripheral lymph nodes preferentially produce IL-17 under inflammatory conditions [48]. Moreover, we have shown that iNKT cells produce IL-17 following skin transplantation and promote graft rejection (Janes SE & Jones ND, manuscript in preparation). However, such iNKT cell responses can be manipulated, as Oh et al. have shown that manipulating iNKT cells to release IL-10, through multiple injection of α-GalCer, can also prolong skin graft survival [37].
In summary, the activation of iNKT cells following transplantation of allogeneic cell or tissue transplants can alter the ensuing alloimmune response resulting either in enhanced rejection or, more often than not, the facilitation of the induction of tolerance. However, how iNKT cells are activated in this context remains incompletely understood.
TCR-mediated activation of iNKT cells
iNKT cells express a semi-invariant TCR that confers reactivity to glycolipids presented in the context of CD1d, which is the most extensively characterized mechanism of iNKT cell activation. However, in response to infection, the immune system relies upon a complex network of signals through the activation of receptors for pathogen-associated molecular patterns, such as the Toll-like receptors (TLRs), expressed on antigen-presenting cells (APC), consequently promoting antigen-specific T cell responses [49]. During such responses iNKT cells have been shown to respond through the recognition of microbial-derived lipid antigens, or through APC-derived cytokines following TLR ligation, in combination with and without the presentation of self- or microbial-derived lipids (Fig. 1).
Fig. 1.
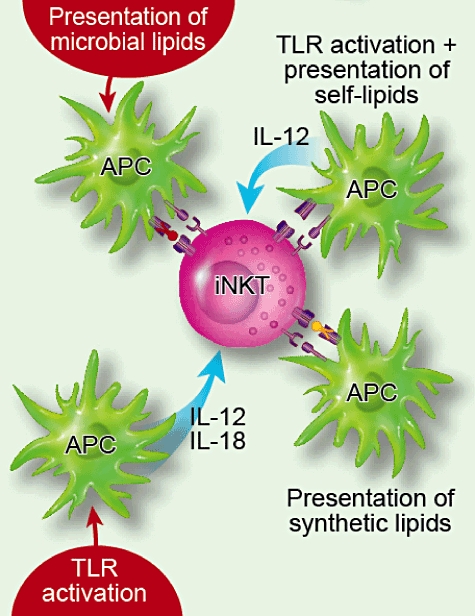
Invariant natural killer T (iNKT) cells can become activated through a number of directly or indirect pathways. The ‘direct’ pathway acts through the recognition of synthetic or microbial ligands presented in the context of CD1d. In contrast, the ‘indirect’ pathways relies upon the release of cytokines from antigen-presenting cells (APC) that have been activated through Toll-like receptor (TLR) ligation in the presence or absence of recognition of CD1d–self-glycolipid complexes. In addition, iNKT cell–APC interactions can be influenced by ligation of a number of co-stimulatory molecules; for example, CD28, CD40L, OX40, GITR, ICOS, 41BB (CD137).
There are several types of bacterial antigens that can directly stimulate iNKT cells when bound to CD1d; for example, Sphingomonas spp., Borrelia burgdorferi and Leishmania donovani, acting independently of TLR-mediated activation of APC [14–17,50,51]. Interestingly, the composition of the cell wall of Gram-negative S. capsulata which lacks the TLR ligand, lipopolysaccharide (LPS), has a combination of immunogenic (e.g. α-glucuronosylceramide) and non-immunogenic glycosphingolipids (e.g. tetrasaccharide-containing glycosphingolipids), the balance of which is proposed to determine the immune response to infection [15,16,52].
iNKT cells have also been shown to be activated during viral infection, as De Santo et al. demonstrated that both NKT (CD1d−/−) and iNKT (Jα18−/−) cell-deficient mice were highly susceptible to influenza compared with wild-type mice [26]. In this model iNKT cells were found to suppress the expansion of MDSC which were expanded in CD1d and Jα18−/− mice [26]. Importantly, although the exact mechanism of iNKT cell activation was not determined, the authors suggest that iNKT cells required TCR–CD1d interactions, as the adoptive transfer of iNKT cells to Jα18−/− but not CD1d−/−mice suppressed MDSC expansion following infection with PR8 [26].
In addition to responses to pathogen-derived glycolipids, iNKT cells may respond to endogenously produced glycosphingolipids such as isoglobotrihexosylceramide (IGb3) and lysophosphatidylcholine (LPC), as well as further unidentified self-non-glycosphingolipids ligands [18,19,53–55]. While it appears that in the steady state peripheral iNKT cells are not activated by self-glycolipids [56], it has been suggested that TLR signalling attenuates alpha-galactosidase A expression (that degrades potential glycolipid antigens), resulting in the accumulation and enhanced presentation of self-glycolipids and subsequent activation of iNKT cells [57]. This pathway for self-glycolipid recognition appears important for NKT activation by pathogens such as Salmonella enteric, which does not contain agonist glycolipids [58–60]. In such instances endogenous glycolipid ligands may be increased which, in addition to the release of APC-derived cytokines such as IL-12, results in iNKT cell activation [58–61].
Although iNKT cells have been shown to be involved in both tumour immunosurveillance [62,63] and the control of autoimmunity [12,64,65], few studies have sought to determine how iNKT cells may be activated in these diseases. However, Falcone and colleagues showed that by increasing the expression of CD1d on islets (using a β-cell promoter) type 1 diabetes failed to develop in NOD mice, which was attributed to a recruitment of iNKT cells to the islets and biased IL-4 cytokine production [66]. Interestingly, in a model of collagen-induced arthritis one further pathway of TCR-mediated iNKT cell activation has been described in which iNKT cells were found to respond to an endogenous collagen peptide (PPGANGNPGPAGPPG; mCII707–721) rather than to a glycolipid [67]. This peptide was shown to be presented by CD1d and resulted in the activation of iNKT cells [67]. Because Lui and colleagues were able to demonstrate successfully that mCII707–721vaccination was able to provide benefit in a number of autoimmune diseases (i.e. antigen-induce airway inflammation, collagen-induced arthritis and experimental autoimmune encephalitis), it is possible that TCR-mediated iNKT cell activation may be required in the context of autoimmune responses [67].
Taken together, these data suggest that following transplantation iNKT cells may be activated by glycolipids presented by donor APC or recipient APC that have received inflammatory signals resulting in the presentation of self-glycolipids. However, there is little evidence to support a role for TCR-mediated activation of iNKT cells following transplantation. In fact, Oh et al. have shown that where iNKT cells were required for prolonged allograft survival, this process was not dependent on CD1d expression by the donor [37]. We have found similarly that iNKT cells expand in lymph nodes following fully allogeneic skin transplantation regardless of whether or not the donor expresses CD1d (Jukes et al. in press). Furthermore, the transfer of iNKT cells into CD1d knock-out mice was found to restore iNKT cell-mediated graft prolongation, suggesting that iNKT cell function in this model was not dependent on recognition of glycolipids presented by recipient APC [37].
iNKT cell activation by cytokines
In contrast to iNKT cell activation through direct TCR-mediated signals, following the recognition of glycolipid, iNKT cells can also be activated directly by proinflammatory cytokines. iNKT cells express the IL-12 receptor constitutively and have been shown to respond directly to IL-12 leading to tumour clearance [68]. Furthermore, Nagarajan and Kronenberg found that APC exposed to Escherichia coli lipopolysaccharides (LPS) resulted in the release of IL-12 and IL-18 [69]. NKT cells exposed to both IL-12 and IL-18 produced IFN-γ, thus amplifying innate-derived signals via TLR to promote immunity [69]. Furthermore, NKT responses in the context of mouse cytomegalovirus (MCMV) were shown to be dependent on TLR-9-dependent activation of DCs, resulting in the subsequent activation of iNKT cells by IL-12 and IFN- α/β[70,71]. Importantly, iNKT cell activation and IFN-γ secretion were found to be independent of CD1d-glycolipid recognition, as iNKT cells were activated similarly in the presence of a blocking CD1d monoclonal antibody (mAb) or following transfer to CD1d−/− hosts.
Brigl and colleagues have recently reaffirmed this pathway of activation by investigating the dominant signals received by iNKT cells following exposure to microbes that contain previously defined iNKT cell ligands (Sphingomonas yanoikuyae, Streptococcus pneumoniae) [72]. Interestingly, these studies found that following infection iNKT cell activation was dominated by activation through the IL-12/signal transducer and activator of transcription-4 (STAT-4) pathway rather than through TCR-mediated recognition [72].
The process of transplantation results in surgical trauma and ischaemia reperfusion injury that results in the activation of DCs and the expression of proinflammatory cytokines [73]. Indeed, transplantation has been shown to be accompanied by the release of a number of endogenous TLR ligands such as heat shock proteins and oligosaccharides of hyaluronan that can result in TLR-4-dependent DC maturation [74,75]. Therefore, the transplantation process itself may result in the activation of iNKT cells by IL-12/IL-18/IFN-α/β following TLR ligation of APC. However, although no study to date has addressed this possibility directly, we and others have shown that iNKT cells expand in the draining lymph node following allogeneic but not syngeneic transplantation (Jukes et al. in press; [37]). Furthermore, iNKT cells were found to have only infiltrated allogeneic and not syngeneic skin and heart transplants (Fig. 2; data not shown). We would argue that if iNKT cells were activated after transplantation by proinflammatory cytokines either directly or in combination with self-glycolipid recognition then activation should have been evident after syngeneic transplantation. This does not rule out the impact of innate derived signals in shaping the ensuing iNKT cell response, but only that it is unlikely to be the primary driving force behind iNKT cell activation following transplantation.
Fig. 2.
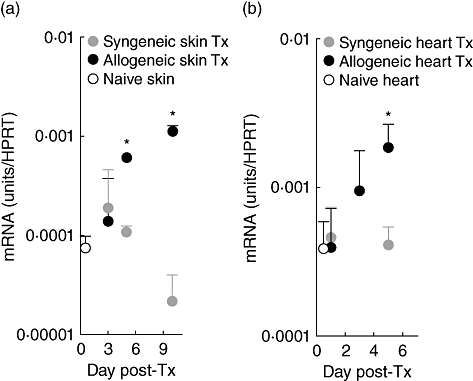
Invariant natural killer T (iNKT) cells are recruited to allogeneic but not syngeneic skin and heart grafts in multiple mouse strain combinations. CBA (syngeneic) or C57BL/10 (allogeneic) skin grafts were analysed by quantitative real-time polymerase chain reaction (qRT–PCR) for Vα14Jα18 mRNA expression (to quantify the infiltration of iNKT cells) on days 3, 5 and 10 post-transplant (a). In addition, heart grafts taken from B6 (H2b) recipients of either syngeneic (C57BL/6) or allogeneic (BALB/c) grafts were analysed for the expression of Vα14Jα18 mRNA on days 1, 3 and 5 post-transplant (b). Results are expressed as mRNA [units/hypoxanthine guanine phosphoribosyl transferase (HPRT)] with naive skin or heart used to give baseline expression. n = 3 per time-point. All error bars indicate mean ±standard deviation.*P ≤ 0·05.
Bystander iNKT cell activation during adaptive immune responses
We and others have been able to reveal that iNKT cells are activated following transplantation of allogeneic tissue. This led us to question whether iNKT cells may receive activating signals from the adaptive as well as the innate immune system. Although iNKT cells died when cultured with allogeneic stimulator cells, we found that the addition of conventional alloreactive T cells to such cultures resulted in iNKT cell activation, proliferation and cytokine production both in vitro and in vivo (Jukes et al. in press). The mechanism of iNKT cell activation was independent of TCR recognition, but rather operated through the sequestration of IL-2 derived from activated T cells (Jukes et al. in press).
This study now provides a further pathway by which iNKT cells may be activated following transplantation (as well as in the context of cancer immunosurveillance and autoimmunity). This highlights the potential of iNKT cells to aid in the process of tolerance or rejection, and taken together with our recent data may suggest that the adaptive immune response could simply ‘tap’ into the reservoir of iNKT cell-derived cytokines, to aid in the successful generation of either anti- or proinflammatory immune responses.
Although IL-2 may activate iNKT cells, clearly the already described innate environmental cues may influence the subsequent behaviour of iNKT cells in terms of expansion and cytokine production. Therefore, conditions that lead to allograft tolerance may promote the expansion of iNKT cells with suppressive potential, such as the recently described forkhead box protein 3 (Foxp3+) [76] or IL-10-producing [41] iNKT cells. In particular, iNKT cells and regulatory T cells (Tregs) may unite in depleting the microenvironment of IL-2, encouraging the production of anti-inflammatory cytokines (such as IL-10, TGF-β), thereby promoting allograft survival (Fig. 3). Alternatively, if iNKT cells respond to IL-2 under proinflammatory conditions then immunity may be amplified by the secretion of proinflammatory cytokines by iNKT cells (Fig. 3).
Fig. 3.
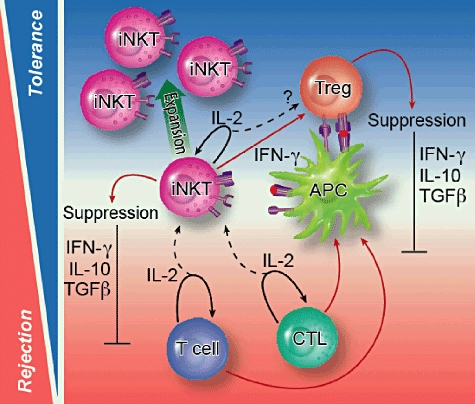
Mechanisms by which invariant natural killer T (iNKT) cells are activated following transplantation; iNKT cells have been implicated in both the induction of transplantation tolerance and rejection, although the mode of activation remains largely unknown. We have recently described a novel mechanism of bystander iNKT cell activation following sequestration of interleukin (IL)-2 produced by activated conventional T cells (dotted black arrow). This pathway of activation is independent of CD1d–glycolipid/T cell receptor (TCR) interactions and is associated with the production of effector cytokine release [i.e. interferon (IFN)-γ]. We hypothesize that IL-2-mediated iNKT cell activation may be important in creating a microenvironment that promotes transplant tolerance, as regulatory T cells and iNKT cells may act synergistically to deplete IL-2 from the local microenvironment in addition to suppressing alloreactive T cell responses via the release of anti-inflammatory cytokines and/or interaction with APC bearing alloantigen. It is conceivable that such responses may be further enhanced through iNKT cell–APC interactions via cytokine production (i.e. IL-12) and co-stimulatory molecules (CD28, CD40L, OX40, GITR, ICOS, 41BB).
Can iNKT cells aid transplant tolerance?
Because transplant patients remain on lifelong immunosuppression to prevent immune-mediated rejection it is important to explore multiple avenues of research that may release such a burden of global immunosuppression, while maintaining graft function. Our interest in iNKT cells is to understand not only how they are involved in the immune response to allografts but also how they may be used as a therapeutic ‘stepping-stone’ to tolerance. Although many new synthetic iNKT cell agonists have been described which could be used to amplify iNKT cell responses following transplantation, the factors that govern whether iNKT cell activation may promote or attenuate the induction of tolerance are incompletely defined. Understanding not only how iNKT cells are activated but also how accessory signals integrate to dictate the class and strength of the iNKT cell response will be critical in aiding the development of potential therapeutic strategies involving the manipulation of these cells. Although there are many questions remaining regarding iNKT cell responses following transplantation, dissecting how iNKT cells respond in different microenvironments may allow us to ‘tap’ into the potential ability of iNKT cells to amplify pathways that promote tolerance.
Disclosure
The authors have no conflicting financial interests.
References
- 1.Balk SP, Bleicher PA, Terhorst C. Isolation and characterization of a cDNA and gene coding for a fourth CD1 molecule. Proc Natl Acad Sci USA. 1989;86:252–6. doi: 10.1073/pnas.86.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–68. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budd RC, Miescher GC, Howe RC, Lees RK, Bron C, MacDonald HR. Developmentally regulated expression of T cell receptor beta chain variable domains in immature thymocytes. J Exp Med. 1987;166:577–82. doi: 10.1084/jem.166.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceredig R, Lynch F, Newman P. Phenotypic properties, interleukin 2 production, and developmental origin of a ‘mature’ subpopulation of Lyt-2- L3T4- mouse thymocytes. Proc Natl Acad Sci USA. 1987;84:8578–82. doi: 10.1073/pnas.84.23.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowlkes BJ, Kruisbeek AM, Ton-That H, et al. A novel population of T-cell receptor alpha beta-bearing thymocytes which predominantly expresses a single V beta gene family. Nature. 1987;329:251–4. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- 6.Hammond KJ, Pelikan SB, Crowe NY, et al. NKT cells are phenotypically and functionally diverse. Eur J Immunol. 1999;29:3768–81. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara S, Nieda M, Kitayama J, et al. CD8(+)NKR-P1A(+) T cells preferentially accumulate in human liver. Eur J Immunol. 1999;29:2406–13. doi: 10.1002/(SICI)1521-4141(199908)29:08<2406::AID-IMMU2406>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13:459–63. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- 9.Motohashi S, Kobayashi S, Ito T, et al. Preserved IFN-alpha production of circulating Valpha24 NKT cells in primary lung cancer patients. Int J Cancer. 2002;102:159–65. doi: 10.1002/ijc.10678. [DOI] [PubMed] [Google Scholar]
- 10.Baxter AG, Kinder SJ, Hammond KJ, Scollay R, Godfrey DI. Association between alphabetaTCR+CD4−CD8− T-cell deficiency and IDDM in NOD/Lt mice. Diabetes. 1997;46:572–82. doi: 10.2337/diab.46.4.572. [DOI] [PubMed] [Google Scholar]
- 11.Poulton LD, Smyth MJ, Hawke CG, et al. Cytometric and functional analyses of NK and NKT cell deficiencies in NOD mice. Int Immunol. 2001;13:887–96. doi: 10.1093/intimm/13.7.887. [DOI] [PubMed] [Google Scholar]
- 12.Lehuen A, Lantz O, Beaudoin L, et al. Overexpression of natural killer T cells protects Valpha14- Jalpha281 transgenic nonobese diabetic mice against diabetes. J Exp Med. 1998;188:1831–9. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita M, Motoki K, Akimoto K, et al. Structure–activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38:2176–87. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 14.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–86. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 15.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 16.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Li Y, Kinjo Y, et al. Lipid binding orientation within CD1d affects recognition of Borrelia burgorferi antigens by NKT cells. Proc Natl Acad Sci USA. 2010;107:1535–40. doi: 10.1073/pnas.0909479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox D, Fox L, Tian R, et al. Determination of cellular lipids bound to human CD1d molecules. PLoS ONE. 2009;4:e5325. doi: 10.1371/journal.pone.0005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox LM, Cox DG, Lockridge JL, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eberl G, MacDonald HR. Rapid death and regeneration of NKT cells in anti-CD3epsilon- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9:345–53. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 21.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermans IF, Silk JD, Gileadi U, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–7. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 23.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–18. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silk JD, Hermans IF, Gileadi U, et al. Utilizing the adjuvant properties of CD1d-dependent NKT cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114:1800–11. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galli G, Nuti S, Tavarini S, et al. Innate immune responses support adaptive immunity: NKT cells induce B cell activation. Vaccine. 2003;21(Suppl 2):S48–54. doi: 10.1016/s0264-410x(03)00200-7. [DOI] [PubMed] [Google Scholar]
- 26.De Santo C, Salio M, Masri SH, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–48. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Santo C, Arscott R, Booth S, et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol. 2010;11:1039–46. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, Brenner MB. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3:1163–8. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- 29.Carnaud C, Lee D, Donnars O, et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–50. [PubMed] [Google Scholar]
- 30.Cohen NR, Garg S, Brenner MB. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 31.Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med. 2009;9:4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- 32.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seino KI, Fukao K, Muramoto K, et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci USA. 2001;98:2577–81. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toyofuku A, Yasunami Y, Nabeyama K, et al. Natural killer T-cells participate in rejection of islet allografts in the liver of mice. Diabetes. 2006;55:34–9. [PubMed] [Google Scholar]
- 35.Beilke JN, Kuhl NR, Van Kaer L, Gill GRG. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat Med. 2005;11:1059–65. doi: 10.1038/nm1296. [DOI] [PubMed] [Google Scholar]
- 36.Maier S, Tertilt C, Chambron N, et al. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/− mice. Nat Med. 2001;7:557–62. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 37.Oh K, Kim S, Park SH, et al. Direct regulatory role of NKT cells in allogeneic graft survival is dependent on the quantitative strength of antigenicity. J Immunol. 2005;174:2030–6. doi: 10.4049/jimmunol.174.4.2030. [DOI] [PubMed] [Google Scholar]
- 38.Jiang X, Shimaoka T, Kojo S, et al. Cutting edge: critical role of CXCL16/CXCR6 in NKT cell trafficking in allograft tolerance. J Immunol. 2005;175:2051–5. doi: 10.4049/jimmunol.175.4.2051. [DOI] [PubMed] [Google Scholar]
- 39.Galante NZ, Ozaki KS, Cenedeze MA, et al. Frequency of Valpha24+Vbeta11+ NKT cells in peripheral blood of human kidney transplantation recipients. Int Immunopharmacol. 2005;5:53–8. doi: 10.1016/j.intimp.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Jukes JP, Wood KJ, Jones ND. Natural killer T cells: a bridge to tolerance or a pathway to rejection? Transplantation. 2007;84:679–81. doi: 10.1097/01.tp.0000280551.78156.ac. [DOI] [PubMed] [Google Scholar]
- 41.Jiang X, Kojo S, Harada M, Ohkohchi N, Taniguchi M, Seino KI. Mechanism of NKT cell-mediated transplant tolerance. Am J Transplant. 2007;7:1482–90. doi: 10.1111/j.1600-6143.2007.01827.x. [DOI] [PubMed] [Google Scholar]
- 42.Ikehara Y, Yasunami Y, Kodama S, et al. CD4(+) Valpha14 natural killer T cells are essential for acceptance of rat islet xenografts in mice. J Clin Invest. 2000;105:1761–7. doi: 10.1172/JCI8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonoda KH, Taniguchi M, Stein-Streilein J. Long-term survival of corneal allografts is dependent on intact CD1d-reactive NKT cells. J Immunol. 2002;168:2028–34. doi: 10.4049/jimmunol.168.4.2028. [DOI] [PubMed] [Google Scholar]
- 44.Zeng D, Lewis D, Dejbakhsh-Jones S, et al. Bone marrow NK1.1(−) and NK1.1(+) T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189:1073–81. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leveson-Gower DB, Olson JA, Sega EI, et al. Low doses of natural killer T cells provide protection from acute graft-versus-host disease via an IL-4-dependent mechanism. Blood. 2011;117:3220–9. doi: 10.1182/blood-2010-08-303008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasunami Y, Kojo S, Kitamura H, et al. Valpha14 NKT cell-triggered IFN-gamma production by Gr-1+CD11b+ cells mediates early graft loss of syngeneic transplanted islets. J Exp Med. 2005;202:913–18. doi: 10.1084/jem.20050448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Huang L, Sung SS, et al. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia–reperfusion injury. J Immunol. 2007;178:5899–911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 48.Doisne JM, Becourt C, Amniai L, et al. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor (gamma)t+ and respond preferentially under inflammatory conditions. J Immunol. 2009;183:2142–9. doi: 10.4049/jimmunol.0901059. [DOI] [PubMed] [Google Scholar]
- 49.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 50.Amprey JL, Im JS, Turco SJ, et al. A subset of liver NKT cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med. 2004;200:895–904. doi: 10.1084/jem.20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattner J, Savage PB, Leung P, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–15. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinjo Y, Pei B, Bufali S, et al. Natural Sphingomonas glycolipids vary greatly in their ability to activate natural killer T cells. Chem Biol. 2008;15:654–64. doi: 10.1016/j.chembiol.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou D, Mattner J, Cantu C, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–9. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 54.Yuan W, Kang SJ, Evans JE, Cresswell P. Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J Immunol. 2009;182:4784–91. doi: 10.4049/jimmunol.0803981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pei B, Speak AO, Shepherd D, et al. Diverse endogenous antigens for mouse NKT cells: self-antigens that are not glycosphingolipids. J Immunol. 2011;186:1348–60. doi: 10.4049/jimmunol.1001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moran AE, Holzapfel KL, Xing Y, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–89. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Darmoise A, Teneberg S, Bouzonville L, et al. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 2010;33:216–28. doi: 10.1016/j.immuni.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–7. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 59.Paget C, Mallevaey T, Speak AO, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 60.Salio M, Speak AO, Shepherd D, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci USA. 2007;104:20490–5. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salio M, Cerundolo V. Linking inflammation to natural killer T cell activation. PLoS Biol. 2009;7:e1000226. doi: 10.1371/journal.pbio.1000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smyth MJ, Thia KY, Street SE, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–8. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–27. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson SB, Kent SC, Patton KT, et al. Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature. 1998;391:177–81. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 65.Mars LT, Novak J, Liblau RS, Lehuen A. Therapeutic manipulation of iNKT cells in autoimmunity: modes of action and potential risks. Trends Immunol. 2004;25:471–6. doi: 10.1016/j.it.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Falcone M, Facciotti F, Ghidoli N, et al. Up-regulation of CD1d expression restores the immunoregulatory function of NKT cells and prevents autoimmune diabetes in nonobese diabetic mice. J Immunol. 2004;172:5908–16. doi: 10.4049/jimmunol.172.10.5908. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Teige A, Mondoc E, Ibrahim S, Holmdahl R, Issazadeh-Navikas S. Endogenous collagen peptide activation of CD1d-restricted NKT cells ameliorates tissue-specific inflammation in mice. J Clin Invest. 2011;121:249–64. doi: 10.1172/JCI43964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui J, Shin T, Kawano T, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 69.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–13. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 70.Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–6. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wesley JD, Tessmer MS, Chaukos D, Brossay LN. K cell-like behavior of Valpha14i NKT cells during MCMV infection. PLoS Pathog. 2008;4:e1000106. doi: 10.1371/journal.ppat.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brigl M, Tatituri RV, Watts GF, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208:1163–77. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldstein DR. Toll like receptors and acute allograft rejection. Transpl Immunol. 2006;17:11–15. doi: 10.1016/j.trim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 74.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 75.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Monteiro M, Almeida CF, Caridade M, et al. Identification of regulatory Foxp3+ invariant NKT cells induced by TGF-beta. J Immunol. 2010;185:2157–63. doi: 10.4049/jimmunol.1000359. [DOI] [PubMed] [Google Scholar]


