Abstract
OTHER THEMES PUBLISHED IN THIS IMMUNOLOGY IN THE CLINIC REVIEW SERIES
Allergy, Metabolic Diseases, Cancer, Autoinflammatory Diseases, Type 1 diabetes and viruses.
Herpes virus infections are chronic and co-exist with acquired immune responses that generally prevent severe damage to the host, while allowing periodic shedding of virus and maintenance of its transmission in the community. Herpes simplex viruses type 1 and 2 (HSV-1, HSV-2) are typical in this regard and are representative of the viral subfamily Alphaherpesvirinae, which has a tropism for neuronal and epithelial cells. This review will emphasize recent progress in decoding the physiologically important CD8+ and CD4+ T cell responses to HSV in humans. The expanding data set is discussed in the context of the search for an effective HSV vaccine as therapy for existing infections and to prevent new infections.
Keywords: antigen, epitope, herpes simplex virus, lymphocyte, vaccine
Introduction
Currently, there is neither a preventative vaccine nor a cure for herpes. Caused by infection with herpes simplex viruses HSV-1 and HSV-2, the two common forms – oro-facial and genital herpes – are known for painful, recurrent erosions in skin or mucosal tissues. Once acquired, herpes simplex virus (HSV)-1 and HSV-2 are thought to take up permanent residence in neurones for the remainder of the life of their host, where they transition periodically from latent to lytic stages, leaving their resident neurone(s) to reinfect the epithelium and potentially transit to new hosts. Symptoms or lesions may or may not be present. The ability of HSV to cause primary and recurrent infections and shedding without symptomatic disease allows many herpetic infections to go undiagnosed and untreated. The cycle of transmission is maintained. Although current anti-HSV medications reduce the severity and duration of symptomatic HSV reactivation, they are less effective in preventing person-to-person transmission even when taken on a daily basis [1].
Reduction of HSV infections would have worldwide public health benefit. The World Health Organization estimated that more than 16% of the global population between 15 and 49 years of age (500 million people) was infected with HSV-2 in 2003 and that approximately 23·6 million new infections occurred that year [2]. This is a concern, because HSV can produce deadly infections of the central nervous system and disseminated disease in immune-compromised adults and newborns [3] and especially because infection with HSV appears to elevate the risk of acquiring HIV-1 [4]. HSV-suppressive medications can reduce genital and plasma levels of human immunodeficiency virus (HIV)-1 [5,6], but they have not yet been shown to lower rates of acquisition or transmission of HIV, perhaps because they do not completely suppress recurrent HSV. HSV attracts large numbers of HIV-susceptible cells to infected areas which persist after viral clearance, such that incomplete HSV suppression may still allow a critical level of these cells to be recruited to the genital epithelium [7–10]. The high global prevalence and health risks associated with HSV infections provide the rationale for a vaccine that will either prevent new infections or reduce the intensity of shedding and, therefore, the infectivity of people in the chronic disease phase. There is also strong patient demand and much commercial interest in a therapeutic vaccine for established HSV infections to reduce lesions, shedding and infectivity. The bar is admittedly high for immunotherapy of chronic infections, but the threshold has been reached for the related alphaherpes virus, varicella zoster virus (VZV) [11].
Towards vaccine development, researchers are interested in exploiting T cells for their long-term and specific immunological memory, and their demonstrated ability in vitro and in animals to control virus replication. T cells are known to participate in protective immunity after vaccination and to correlate with viral reactivation in animal manipulation and explant models [12–14]. For instance, mice lacking CD4+ and/or CD8+ T cells have diminished control of HSV-2 even after pre-immunization with attenuated (thymidine kinase-deficient) HSV-2, highlighting the importance for both T cell subsets in protection against HSV [15,16]. Effector CD8+ T cells surround HSV-1-infected ganglia and control HSV-1 latency at the ganglion in mice [13,17–19]. They also surround nerve termini in HSV-2-infected genital epithelium and accompany CD4+ T cells in viral clearance from genital lesions [20–23]. Developing a vaccine that induces T cell activity is therefore a logical approach in the fight against HSV. To achieve success in this venture, we must understand which specific viral proteins are recognized by T cells and, among these, which antigens are recognized in a high proportion of people (population prevalence). Ideally, we would find correlates between disease severity and T cell responses and use these measures as desired outcomes for a vaccine. T cell end-points include: the global number of responder cells, the identity of antigenic targets, the number of T cells activated by specific proteins or epitopes (immunodominance), the diversity of the T cell response and the memory/effector and homing phenotypes of HSV-specific T cells. Our review focuses on the first factor; current knowledge of T cell targets, with a summary of data concerning population prevalence, immunodominance and phenotype.
HSV genes and proteins
HSV-1 and HSV-2 are highly related viruses. There are partial genome sequences for a few laboratory-adapted and wild-type strains [e.g. NCBI population sets (PopSet): 125657058, HSV-1 US4; PopSet: 30960860, HSV-1 US4-US6; PopSet: 224552497, HSV-1 UL23; PopSet: 261863287, HSV-2 UL23; http://www.ncbi.nlm.nih.gov/popset], in addition to complete genome sequences for three HSV-1 strains (17, F and H129; GenBank: X14112, GU734771 and GU734772) and one HSV-2 strain (HG52; GenBank: Z86099). Each virus contains about 80 genes for structural (capsid, tegument, envelope) and non-structural (enzymes, regulatory) proteins arranged in four regions in the viral genome designated unique long (UL), unique short (US), repeat long (RL) and repeat short (RS). The structure of HSV and the specific properties of these proteins are reviewed elsewhere [24].
HSV capsids have typical icosahedral symmetry and are formed from at least eight proteins [25,26]. Capsid or capsid-component proteins are attractive vaccine constituents, because most are abundant and introduced into the cytoplasm upon virion entry, thereby accessing the major histocompatibility complex (MHC) class I pathway before HSV down-regulates protein synthesis or inhibits antigen processing. Caspid proteins, in the form of virus-like particles (VLP), are the basis of the successful vaccine against sexually transmitted human papillomavirus (HPV). They mediate protection by inducing neutralizing antibodies [27]. Like HPV, HSV capsids have self-assembly properties and VLPs can be generated from recombinant proteins [28]. However, unlike HPV, the viral envelope surrounding the capsid on HSV (below) renders anti-capsid antibody responses less likely to have neutralizing activity. Nevertheless, the high prevalence of T cell responses among herpes-infected individuals to HSV capsid proteins [29,30] (albeit in small study populations) continues to make capsid proteins attractive in the vaccine arena.
A structural layer termed the tegument occupies a position between the envelope and capsid of the herpes particle and contains more than 20 proteins. Preformed tegument proteins are again injected into the cytoplasm upon viral entry, some at high copy number, where they may mediate immune evasion [e.g. the host shut-off (vhs) protein encoded by UL41] or transactivate viral promoters after transit to the nucleus [virion protein (VP)16 encoded by UL48]. Selected tegument proteins are known to be both population-prevalent and immunodominant targets of HSV-specific T cells, and are thus potential vaccine candidates [30–39].
Eleven proteins with characteristics of transmembrane proteins are encoded in the HSV genome. Many localize to inner nuclear membranes through which viral budding occurs, allowing their incorporation into the viral envelope, and some sort to other locations, including endosomes, endoplasmic reticulum, the Golgi complex and the plasma membrane [40]. Particular envelope glycoproteins [e.g. glycoprotein (g)B, gD, and the gH/gL complex] have received attention as subunit vaccine candidates because neutralizing antibodies target their extracellular domains. Others are attractive because antibodies negate their immune evasive effects (e.g. complement binding by gC and the Fc receptor activity of gE) [41,42]. The presence of antibody responses to most envelope glycoproteins implies the presence of cognate protein-specific CD4+ T cell responses, which have been confirmed by many studies [31,43–47]. Only more recently have studies focused on CD8+ T cells [48,49], addressing the potential of using an envelope-based vaccine to broadly stimulate useful CD4+ and CD8+ T cell responses in addition to antibodies.
Non-structural proteins encoded by HSV-1 and HSV-2 include enzymes (e.g. ribonucleotide reductase, DNA polymerase, thymidine kinase), regulatory proteins (e.g. DNA-binding proteins) and proteins expressed with immediate–early kinetics [e.g. infectious cell protein (ICP)0, ICP4, ICP22, ICP27, and ICP47] that have roles in gene regulation, viral replication or immune subversion. These typically are not rational vaccine targets candidates for antibodies; however, they are recognized by T cells [30,37,50–54]. The immediate–early proteins have a theoretical advantage in that their recognition might occur quickly after viral infection, allowing cytotoxic T lymphocytes (CTL) to lyse infected cells before the production of daughter virus.
Proteins in each structural and functional class are known virulence factors or are required for viral replication and are thus indirectly important in vaccinology. Mutant viruses lacking such proteins display marked attenuation or an inability to replicate in normal cells, and have been advanced as candidate whole-virus vaccines [55,56]. These approaches have persistent safety and manufacturing concerns, but are conceptually attractive because of their potential to elicit broadly targeted antibody, CD4+ T cell and CD8+ T cell responses.
HSV T cell epitope discovery
Mapping of T cell epitopes within HSV proteins is under way for both humans and inbred mice. When performed using samples from infected humans, epitope mapping reveals proteins recognized by T cells, presumably modulated by immune subversion mechanisms in the infection context in the natural host [57]. Murine epitope identification is most relevant for preclinical vaccine and basic pathogenesis studies [58–61]. While it is unlikely that specific epitopes will be shared between mice and humans, given differences in MHC peptide binding motifs, preferences at the open reading frame (ORF) level may arguably transfer across species. For example, three ORFs (UL27, UL29, UL39) that account for the bulk of the CD8+ T cell response in C57BL/6 mice are also well represented in the human CD8+ T cell response, in terms of both traditional immunodominance and number of distinct epitopes [29,31,37,53]. To bridge the gap between species, transgenic ‘humanized’ mice and rabbits that carry human leucocyte antigen (HLA) molecules are beginning to enter use in the HSV system [48].
Reports of T cell epitopes – identified by means of flow cytometry, enzyme-linked immunospot (ELISPOT) or cellular proliferation assays – for both wild-type and transgenic mice and for human subjects and for both HSV-1 and HSV-2 are summarized in Table 1. Of note, the density of epitope data from each study probably reflects not simply the biology of infection, but the rationale for the study and the experimental approach taken. Some highly focused studies have investigated limited antigen sets in great detail, while other data sets reflect surveys of larger but still incomplete subsets of viral antigens. Studies that limit antigen complexity typically can investigate relatively large numbers of people. In contrast, studies that have interrogated T cell responses to the entire HSV proteome have been performed in only a small number or subjects. The focused studies typically emphasize ORFs used in vaccines (e.g. gD) or antigens of convenience readily available in formats compatible for detailed testing (e.g. VP16 available as recombinant protein, or the immediate early proteins available as vaccinia recombinants) [32,62]. Our group has performed studies of representative immediate–early, capsid, tegument and envelope proteins using overlapping peptide sets, with reagent cost and peripheral blood mononuclear cell (PBMC) requirements setting practical upper limits on the number of ORFs investigated. The criteria for selecting ORF subsets have included previously documented T cell responses, a vaccine track record or a role for neutralizing antibody [29,30,53,54]. Whole proteome studies were accomplished using cost-efficient antigen formats (such as expression-cloning libraries of HSV genomic fragments or full-length ORFs) or by in silico screening of predicted viral proteomes [37,61,63,64], and have not yet used proteome-spanning peptide sets. In short, there are clearly gaps in the current data set, and conclusions are preliminary. Regardless, the data prove that humans and mice mount T cell responses against many HSV ORFs, including structural and non-structural proteins.
Table 1.
T cell epitope discovery and responses to herpes simplex virus (HSV) open reading frames (ORFs) in humans, mice and rabbits
| T cells to HSV-1 | T cells to HSV-2 | ||||
|---|---|---|---|---|---|
| ORF | CD4+ | CD8+ | CD4+ | CD8+ | Publication(s) |
| RL1 (ICP34.5) | |||||
| RL2 (ICP0) | Hu | Hu | Hu | [30,37,38,50,54,92] | |
| UL1 (gL) | Hu | [37] | |||
| UL2 | |||||
| UL3 | |||||
| UL4 | |||||
| UL5 | |||||
| UL6 | |||||
| UL7 | Hu | [50] | |||
| UL8 | |||||
| UL9 | Mo-W | [53] | |||
| UL10 (gM) | |||||
| UL11 | Hu | [92] | |||
| UL12 | |||||
| UL13 | Hu | [37] | |||
| UL14 | |||||
| UL15 | |||||
| UL16 | |||||
| UL17 | |||||
| UL18 (VP23) | |||||
| UL19 (VP5) | Hu | Hu | [30,54,92] | ||
| UL20 | |||||
| UL21 | Hu | Hu | [32,37,50] | ||
| UL22 (gH) | Mo-W | [53] | |||
| UL23 (TK) | Hu | [78] | |||
| UL24 | |||||
| UL25 (VP26) | Hu, Mo-T | Hu | [30,37,50,92] | ||
| UL26 (VP21) | Hu | Hu | [37,50] | ||
| UL26.5 (VP22a) | |||||
| UL27 (gB) | Hu | Hu, Mo-W | Hu | Hu | [31,37,53,80,92,93] |
| UL28 (ICP18.5) | Mo-W | [53] | |||
| UL29 (ICP8) | Hu, Mo-W | Hu | [30,37,53] | ||
| UL30 (DNA pol) | |||||
| UL31 | Hu | [37] | |||
| UL32 | |||||
| UL33 | |||||
| UL34 | |||||
| UL35 (VP26) | |||||
| UL36 (VP1/2) | |||||
| UL37 | Hu | [37] | |||
| UL38 (VP19c/ICP32) | |||||
| UL39 (ICP6/ICP10) | Hu, Mo-W | Hu | Hu | [30,37,52–54] | |
| UL40 | Hu, Mo-W | [37,53] | |||
| UL41 (VHS) | Hu, Mo-W | [37,53] | |||
| UL42 | |||||
| UL43 | |||||
| UL44 (gC) | Mo-W | [53] | |||
| UL45 | |||||
| UL46 (VP11/12) | Hu | Hu, Mo-W | Hu, Mo-W | [30,37,39,50,92] | |
| UL47 (VP13/14) | Hu | Mo-W | Hu, Mo-W | [37–39,50] | |
| UL48 (VP16) | Hu | Hu | [32–35,37,94] | ||
| UL49 (VP22) | Hu | Hu, Mo-W | Hu, Mo-W | [30,32,37–39,50,54,92] | |
| UL49A (gN) | |||||
| UL50 (dUTPase) | Hu | [32] | |||
| UL51 | |||||
| UL52 | |||||
| UL53 (gK) | Mo-W | Hu; Mo-W | [37,53,95,96] | ||
| UL54 (ICP27) | Hu | Hu, Mo-W | [37,50,51,97] | ||
| UL55 | |||||
| UL56 | |||||
| US1 (ICP22) | Hu | [37] | |||
| US2 | |||||
| US3 | |||||
| US4 (gG) | Mo-W | [53] | |||
| US5 (gJ) | |||||
| US6 (gD) | Rb-W, Rb-T, Hu | Rb-T, Hu, Mo-T | Hu, Mo-W | Hu, Mo-W, Mo-T | [31,39,43–45,48–50,54,84] |
| US7 (gI) | Hu | [37] | |||
| US8 (gE) | Hu | [50] | |||
| US8A | |||||
| US9 | Hu | [50] | |||
| US10 | |||||
| US11 | |||||
| US12 (ICP47) | |||||
| RS1 (ICP4) | Hu | Hu | Hu | [37,54,92] | |
ORF names per annotation of HSV-1 (NC_001806) and HSV-2 (NC_001798) complete genome sequences. Hu: human; Mo-T: transgenic mouse with human major histocompatibility complex (MHC); Mo-W: mouse with wild-type (mouse) MHC; Rb-T: transgenic rabbit with human MHC; Rb-W: rabbit with wild-type (rabbit) MHC.
During the past 10 years, the number of known defined (minimal) epitopes, epitope-containing regions and antigenic ORFs have blossomed, such that a list-style review is neither useful nor practical. Many of these epitopes are now catalogued in the Immune Epitope Database (IEDB; http://www.immuneepitope.org/), in addition to their listings within specific manuscripts cited in Table 1. Herein, we will illustrate selected epitopes and epitope-containing regions in an immunological and clinical context, speculating on their relevance in the design of therapeutic or preventative vaccines against HSV or for understanding immune correlates of protection. Amino acid numbers of epitopes are based on full-length protein sequences from HSV-1 strain 17 and HSV-2 strain HG52 prior to signal sequence cleavage.
Immunodominant and population prevalent responses
Vaccine design requires knowledge of the population prevalence of responses to candidate antigens. ORF-length or other long vaccine antigens must contain epitopes suitable for HLA binding by most people, while epitopes under consideration for inclusion in a polyvalent or multi-epitope vaccine should incite population-prevalent responses in people with the HLA-type or superfamily under consideration. Our surveys show prevalent T cell responses to several HSV ORFs [29,30,65] as studied with ORF-covering peptide sets. For some ORFs, population-prevalent minimal epitopes have been defined among HLA-appropriate persons.
In most ethnic groups, HLA-A*0201 is one of the most abundant of the HLA-A alleles: within the United States, 28% Caucasian, 20% Hispanic, 12% African Americans and 9% Asian-Pacific Islanders possess the HLA-A*0201 allele, with high frequencies also observed for some native American tribes [66–71]. Discovery of HLA A*0201-restricted epitopes should, thus, benefit generation of a vaccine with high population coverage. Prevalent CD8+ T cell responses to HLA-A*0201-restricted epitopes have been reported for glycoprotein B (UL27) and tegument protein VP13/14 (UL47) of HSV-2 [63]. The population prevalence of HSV-2-infected HLA-A*0201-bearing people for an epitope in gB2 (HSV2:gB442–451) is about 50%, while 82% respond to HSV2:UL47551–559. This implies that the latter epitope or ORF may be more suitable for vaccines, at least among A*0201-bearing people.
In direct ex vivo assays, CD8+ T cell responses to one immunodominant epitope in HSV-2 UL25 (HSV2:UL25372–380) were detectable in approximately 20% of HSV-2 seropositive subjects carrying HLA-A2; up to 0·5% CD8+ T cells from these individuals bound an HLA-A*0201 tetramer containing this epitope [30]. Although restriction of T cell recognition has only been demonstrated formally for the HLA-A*0201 allelic variant, HSV2:UL25372–380 has strong affinity for HLA-A*0201, -A*0202, -A*0203 and -A*0206. Its amino acid sequence is completely conserved in HSV-1 (HSV1:UL25367–375) and is recognized by HLA A*0201-restricted CD8+ T cells isolated from HSV-1-infected people. We recently vaccinated HHD-II mice – an HLA-A*0201 transgenic strain severely deficient in endogenous murine MHC class I expression [72]– with a prototype HSV-1 UL25 vaccine formulated as a full-length gene driven by a constitutively active cytomegalovirus (CMV) promoter in a pDEST-based backbone. The vaccine (100 µg) was administered intramuscularly three times every 2 weeks. Two weeks after the third dose HHD-II mice, but not control C57BL/6 mice, showed abundant and high-avidity splenocyte T cell responses to the HSV1:UL25367–375 synthetic peptide. Mice vaccinated with an empty vector did not respond (Fig. 1) and responses were absent in HLA-A*0201-negative mice. Coverage of the frequent HLA-A*0201 and both viral types is a rationale for inclusion of UL25 – or at least the HLA-A*0201 epitope – in a vaccine. Similarly, Chentoufi et al. focused on HSV-1 gD (gD1), which is highly homologous to HSV-2 gD (gD2), in the context of HLA-A*0201, by observing T cell responses to peptides selected using HLA peptide binding prediction algorithms. Using a variety of readouts, including HLA-transgenic animals, evidence for several population-prevalent epitopes was obtained with some being more protective against HSV-1 keratitis in the transgenic models [48,49].
Fig. 1.
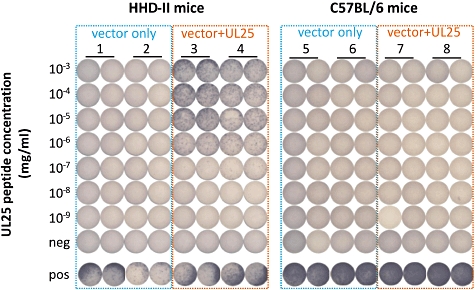
Transgenic HHD-II [human leucocyte antigen (HLA)-A*0201-bearing] mice were immunized using the herpes simplex virus (HSV)-1 UL25 gene in a standard plasmid backbone or with plasmid alone (mice 1–4). Wild-type C57BL/6 mice were treated with the same UL25 construct or vector control (mice 5–8). Isolated splenocytes were tested in duplicate for reactivity against an immunodominant, type-common HLA-A*0201-restricted epitope from HSV UL25 (HSV1:UL25367–375). Only UL25 immunized HHD-II mice were reactive to peptide in interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay. Cells from all mice responded to the positive (pos) control antigen concanavalin A, but not to the dimethylsulphoxide (DMSO)-negative (neg) control.
Among HLA-B alleles, HLA-B*0702 is one of the most abundant. Within the United States, it is the most frequent HLA-B allele in people of European ancestry (14%) and among the top five HLA-B alleles for those of African (7%) and Hispanic (5%) descent, while slightly less frequent in Americans of Asian-Pacific origin (3%) [66]. As with HLA-A*0201, inclusion of epitopes restricted by HLA-B*0702 is rational for a vaccine targeting CD8+ T cell responses.
Tegument protein VP22 (encoded by UL49) is abundant in proline residues, a characteristic of HLA-B*07-restricted epitopes [73,74]. Accordingly, several minimal HLA-B*0702-restricted epitopes are known for HSV UL49 products (HSV2:UL4949–57, HSV2:UL4982–90, HSV2:UL4999–108, HSV2:UL49131–140 and HSV1:UL49291–290) [30,37]. Among these, HSV2:UL4949–57 stands out as both population-prevalent and immunodominant in HLA-B*07-carrying subjects [30,38]. The immunodominance of HSV2:UL4949–57 was validated by direct ex vivo tetramer-staining of up to 0·6% of unmanipulated CD8+ PBMC binding the relevant tetramer [75]. This is the highest reactivity observed to any HSV-1 or HSV-2 epitope, albeit there are probably many undiscovered epitopes that may be equally immunodominant in the proper HLA context. Incorporation of this target into a vaccine would cover most individuals bearing HLA-B*0702, with an important caveat discussed below.
T cell escape variations
A good vaccine will be effective against multiple viral strains. HSV isolates show non-synonymous (amino acid-altering) nucleotide differences in some genes. These variations can be shown to alter virulence in animal models, albeit there is little evidence for clinically ‘hotter’ strains of HSV-1 or HSV-2 in human epidemiology studies. Controversially, these variations could alter HSV recognition by immune cells [76]. Dudek et al. [76] showed recently in a mouse model that whole virus-based vaccines with an HSV-2 laboratory strain backbone gave lower protection against a low-passage clinical African HSV-2 strain than against a laboratory-adapted strain, and speculated that this could be related to changes in immune epitopes. For the highly conserved HSV-2 US6 gene, which encodes gD, only three amino acid (aa) differences (0·8% of 393 aa) have been found between isolates, making it ideal for a global vaccine component [39]. In contrast, HSV-2 isolates from South America, North America and Africa show 22 aa substitutions (5·9% of 376 aa) in the UL23 gene, which encodes viral thymidine kinase (TK) [77]. TK is known to be a CD8+ T cell target [78], but this level of variability decreases its attractiveness as a vaccine component.
Recently, our sequencing efforts showed three CD8+ T cell epitopes we identified in HSV-2 UL49 (above) span regions of nucleotide polymorphism that generate non-synonymous changes. Interesting, one mutation prevents an epitope variant from binding HLA-B*0702 [39](Table 2). We hypothesize that viral evolution could allow HSV to escape detection by CD8+ T cells. In the case of the immunodominant HLA-B*0702-restricted HSV2:UL4949–57 epitope, ∼ 70% of wild-type strains have the sequence RPRGEVRFL, while ∼29% have the sequence RPMREVRFL [79]. Both the major and the minor variants bind well to HLA B*0702, but differences occur in T cell responsiveness to the variants. A T cell clone derived from an HLA-B*0702-bearing subject infected with the majority sequence HSV-2 strain could not lyse HLA-B*0702-bearing antigen-presenting cells (APC) pulsed with the minority peptide in a standard cytotoxicity assay, superficially looking like immune escape (Fig. 2). However, when we studied HLA-B*0702-bearing people infected with the minor variant strain – using the minor variant peptide and interleukin (IL)-2/IL-7 to restimulate PBMC to create a short-term T cell line [38]– we recovered CD8+ T cells that selectively lysed APC pulsed with this variant (Fig. 2). As the T cell receptor (TCR) repertoire can recognize the minor variant peptide, it remains mysterious as to why viral sequences should vary within this immunodominant epitope but not achieve typical immune escape. As a practical matter, vaccines that include only the majority forms of these variable epitopes are likely to generate T cells that miss some viral strains. Further research is needed to determine the overlap between T cell targets and HSV coding variants.
Table 2.
Human leucocyte antigen (HLA)-B*0702 binding capacity of herpes simplex virus (HSV)-2 VP22 (UL49) epitope variants identified in 120 sampled genes
| Epitope | Sequences | Variant frequency | IC50 (nm) | Probable HLA-B*0702 binder? |
|---|---|---|---|---|
| HSV2:UL4949-57 | RPRGEVRFL | 93/120 (78%) | 0·23 | Yes |
| RPMREVRFL | 26/120 (22%) | 2·1 | Yes | |
| HSV2:UL4982-90 | RPRRSASVA | 110/120 (92%) | 0·64 | Yes |
| RPRHSASVA | 10/120 (8%) | 0·59 | Yes | |
| HSV2:UL49131-140 | APATPATDPA | 59/120 (49%) | 196 | Yes |
| ASATPATDPA | 61/120 (51%) | > 70 000 | No |
Fig. 2.
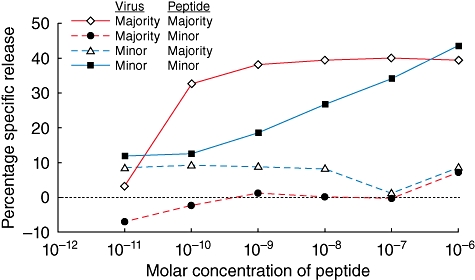
T cell clones or bulk cultures were tested for cytotoxicity in standard 51Cr release assays to major (RPRGEVRFL) or minor (RPMREVRFL) variant epitopes from UL49 (amino acids 49–57) of HSV-2 (see text). Subject A (red lines) was infected with the major variant virus and subject B (blue lines) was infected with the minor variant. A previously reported (5491·48) CD8+ T cell clone [38] of subject A reacted strongly to the major epitope but not to the minor epitope, suggesting that the minor variant represents a possible T cell escape mutation. Conversely, bulk T cells of subject B responded only to the minor epitope, indicating that specific T cell recognition of this epitope variant is possible.
Immune correlates of protection
Epitope discovery has facilitated investigation of HSV-specific T cells in the search for immune correlates of protection against HSV. Some studies suggest that T cell epitope specificities vary with different clinical presentation of HSV. For example, a recent study identified three CD4+ T cell epitope-containing regions within gB that were recognized differentially by T cells from asymptomatic versus symptomatic HSV-1 infected people [80]. T cells responsive to peptides HSV1:gB166–180 and HSV1:gB666–680 were found only in asymptomatic individuals, whereas those reactive to peptide HSV1:gB661–675 were found only in subjects with symptoms. As all three peptides induced CD4+ T cells with similar functional characteristics, the mechanisms driving differences in disease presentation are unclear. One possibility is a link to HLA restriction. Epitopes associated specifically with the asymptomatic seropositive phenotype, if confirmed, are rational components of a preventative or therapeutic vaccine.
A small number of individuals, termed ‘immune seronegative’ (IS), display persistent T cell immunity to HSV without generation of antibody or detectable viral shedding [81]. It is unclear if IS people have been sensitized by a subinfectious inoculum, have had peripheral lytic HSV infection that was cleared before latent infection was established, have latently infected neurones from which HSV cannot reactivate or if some other immune or host mechanism is at work [81]. Regardless, the prospect that IS people possess T cells that offer superior control of HSV has prompted more detailed investigations. The prevalent targets of IS T cells thus far appear to be non-structural ORFs – ICP10 (UL39), ICP0 (RL2), ICP4 (RS1) and ICP8 (UL29) – rather than the full range of capsid, tegument and glycoproteins that HSV-2 seropositive subjects additionally target at high frequency. However, a relatively small number of people have been studied and the entire ORFeome was not surveyed. Reactivity against HSV genes that are expressed with immediate–early (RL2 and RS1) or early (UL39 and UL29) expression kinetics may reflect a benefit in early inhibition of viral replication, but this requires verification. A small number of CD8+ and CD4+ T cell epitope-containing regions are defined for IS subjects; however, some epitopes show overlap with routine HSV seropositive people and there is not enough information to know if any epitopes are unique to IS people at this time.
Other correlates of protection might be found in the magnitude, function and phenotypic characteristics of HSV-reactive T cells. Recent studies describe CD4+ T cells reactive to the same gD epitopes that are less abundant in males than in females, correlating with the apparent differential efficacy of a gD vaccine in women [82]. CD4+ T cells exposed to three immunodominant peptides from HSV-1 gD (HSV1:gD74–107, HSV1:gD102–129, HSV1:gD146–176) from HSV-seropositive women were higher in number – determined by interferon (IFN)-γ production and proliferation – than in cells from demographically matched men [44]. The same gender-specific differences were observed for CD4+ T cells of HSV-1 infected transgenic (HLA-DR1/HLA-DR4-bearing) BALB/c mice. Moreover, when used for immunization, the gD peptides protected significantly more female than male mice from lethal ocular HSV-1 infection. Why there would be differences between genders in T cell reactivity to gD is unclear, and the clinical relevance of this observation has been cast into doubt by the failure of a recent Phase III clinical trial to confirm efficacy of the adjuvanted gD2-subunit vaccine in a large study of women [83].
In a separate study, distinct epitopes in gD of HSV-1 appeared to activate discrete helper T cell subsets in BALB/c mice. Some CD4+ T cell epitope-containing regions predominantly induce Th1 (HSV1:gD25–53, HSV1:gD74–107, HSV1:gD121–148, HSV1:gD171–204, HSV1:gD253–282 and HSV1:gD357–382) or both Th1 and Th2 subsets (HSV1:gD225–259), whereas others (HSV1:gD47–77, HSV1:gD102–129) induce Th2 responses alone [84]. Immunization with T helper type 1 (Th1)-specific peptides protect mice against lethal HSV-1 challenge, whereas Th2-specific peptides do not, suggesting that Th1 cells are better for protection against HSV. The same study showed that the multiple epitopes co-operated synergistically to elevate T cell responses above levels anticipated by response magnitudes to single epitopes. These observations signify the importance of both individual epitope characteristics and the number of concordant epitope recognition events in controlling the magnitude and mechanistic properties of T cells responding to HSV [84].
Murine studies also revealed an association between the epitope repertoire and adjuvants or cytokines in the context of gD-based vaccines. Splenic T cells of BALB/c mice immunized with a gD2 vaccine reacted to five epitope-containing regions of gD [45]. When accompanied with an alum adjuvant, responses to one peptide (HSV2:gD130–144) were diminished and responses to a sixth region (HSV2:gD310–324) were gained, whereas T cells responded to only two regions (HSV2:gD270–284 and HSV2:gD358–372) when IL-12 was administered with the gD vaccine, although the HSV2:gD358–372 peptide was immunodominant in all three formulations. Further work is required to appreciate the benefit of altering the breadth or specific targeting of T cell epitopes in protection from infection. Once understood, immune modulation by adjuvants and cytokines during vaccination could be a means to increase vaccine efficacy by improving epitope targeting.
Finally, immune correlates for protection against ocular herpes were examined using transgenic (HLA-A*0201-bearing) rabbit immunized with a lipopeptide vaccine containing human CD4+ (HSV1:gD74–107) and CD8+ (HSV1:gD78–86, HSV1:gD95–103, HSV1:gD303–311) T cell epitopes [44,49]. Differences in T cell magnitude and function were observed in rabbits following ocular infection with HSV-1 [48]. Rabbits with mild corneal herpetic disease had higher levels of epitope-specific (tetramer-binding) and IFN-γ-producing CD8+ T cells in the trigeminal ganglion, draining lymph node and conjunctiva relative to those with severe disease. These data suggest that vaccines that induce a higher magnitude of IFN-γ-secreting CD8+ T cells may be more protective against infection with HSV.
Tissue location and homing
T cells must be able to traffic from lymph nodes to the site of an infection to be most effective. Programming of T cells to home to preferred anatomical locations is thought to occur in localized lymphoid tissues, and is impacted by the route of immunization during vaccination [85]. Evidence is growing to confirm that HSV epitope-specific T cells do possess qualities that direct them to the skin, an area of much current research [86].
The expression of cutaneous lymphocyte antigen (CLA) is a characteristic of T cells that home to the skin [87]. CLA-expression has been described on 50–70% CD8+ T cells and ∼20% CD4+ T cells reactive to HSV-2, supporting their ability to home to inflamed skin [88]. CD8+ T cells specific for epitopes within a range of tegument (e.g. HSV2:UL46354–362, HSV2: UL47551–559, HSV2:UL47289–298, HSV2:UL4949–57, HSV2:UL7174–186), capsid (e.g. HSV2:UL25405–413 and HSV2:UL26475–483), glycoprotein (e.g. HSV2:gD365–373, HSV2:gE518–526) and non-structural (e.g. ICP0743–751) proteins of HSV-2 express CLA [50,75,88]. Furthermore, in-situ staining proved the HSV-specific cells locate to the skin. CD8+ T cells reactive to HSV-2 multimers specific for an array of HSV-2 proteins can be identified at the site of genital lesions and linger at those sites after lesion healing [20].
Differences in tissue locations may occur between T cells that recognize different ORFs. Recent studies in mice suggest that higher proportions of CD8+ T cells specific for immunodominant epitopes in ribonucleotide reductase (UL39 and UL40), ICP8 (UL29) and gB (UL27) reside at the trigeminal ganglion relative to the spleen, whereas CD8+ T cells that target ‘late’ ORFs have lower frequencies at the ganglion [53]. As CD8+ T cell are known to maintain viral latency at the mouse ganglion [13,17], we may speculate that they do this by reacting early during reactivation. Whether this represents an immune correlate or is merely related to differential viral protein expression is uncertain. HSV-1-reactive CD8+ T cells also localize to infected human trigeminal ganglia [18], but we await data concerning their fine specificity and a comparison to the pattern of antigen recognition in the blood and skin.
Concluding remarks
Both vaccine design and pathogenesis research benefit from the identification of T cell epitopes and have motivated the epitope mapping studies summarized in this review. The emerging picture is that T cells target many proteins of HSV-1 and HSV-2 to effectively resolve, albeit temporarily, localized recurrent disease in otherwise healthy individuals. Consequently, there seem to be many options to choose from for inclusion in subunit vaccines that generate memory T cells against HSV.
For vaccine design, it is important to include targets that generate adaptive immunity in a large frequency of the population, while eliciting responses that provide optimal protection against infection or even reactivation. To date, the first challenge has proved amenable to brute force analysis, while the latter still remains unsolved. Envelope glycoproteins gB and gD and the tegument protein VP16 provide reasonable population coverage for eliciting CD4+ T cell responses [32,44,46,65,80]. With regard to CD8+ T cells, there is an emerging consensus that tegument proteins, the larger immediate early regulatory proteins such as ICP0 and ICP4, and the large enzyme UL39 are reasonable candidates for activating CD8+ T cells [30,65]. Larger surveys of more people with defined HSV severity using the entire predicted proteome are still required to consolidate this impression.
Several studies have used infected tissues – e.g. skin lesions, uterine cervical swabs and corneal specimens [36,38,89]– as a source of biologically pre-enriched HSV-specific T cells and proceeded to define epitopes and antigens. It remains unclear whether the entire T cell repertoire disclosed by more comprehensive blood-based studies are represented passively in these infected tissues or the dorsal root ganglia (which also contain HSV-specific T cells in humans [18]) or if tissue T cells have preferential specificity for certain epitopes or antigens. Distinct epitopes have certainly been identified at different anatomical sites, such as genital skin, in the eye, at the ganglion or in the blood, but a complete survey comparing epitope specificities of T cells that surround latently infected ganglia or active lesion sites or that circulate in the blood or lymph may cast light on which epitopes are important at different stages of infection/reactivation. Is targeting of immediate–early ORFs at the ganglion better for halting viral activation, for example? Recent studies in mice suggest differing epitope specificities are evident between tissue sites. The induction of appropriate homing potential via vaccination may also be very important. To date, however, it is unknown if eliciting ganglion-homing is achievable, and whether it would be helpful or harmful. Researchers await the availability of specific adjuvants or delivery platforms capable of inducing tissue resident memory T cells [90].
Despite a good deal of effort, there are few hard data for immune correlates of disease severity. Reports associating mild herpetic disease with CD4+ T cell epitopes in gB and CD8+ T cell epitopes in gD are tantalizing, but require confirmation. Some epitopes drive T cell responses in many individuals with the appropriate HLA allele, such that their inclusion in a polyepitope vaccine may be reasonable. In addition, some adjuvant formulations can alter which epitopes are targeted, illustrating a potential means for manipulating T cells to recognize epitopes that provide the most promising reaction. A greater understanding of the most promising epitopes, the responses they drive and their correlation with better immune protection will help to produce the best vaccine against HSV.
The limitation of most of these extant studies is the incomplete coverage of viral ORFs. Many proteins are understudied or unstudied, due largely to the specimen requirements and technical issues in obtaining a complete ORFeome-covering antigen set at a reasonable cost. Thus, at this time it is difficult to interpret ORF specificities and their meaning in the context of better outcomes with complete confidence. Locally, our research group is addressing this shortfall by screening for T cell responses to viruses with large genomes using a whole ‘ORFeome approach’[37,91]. Hopefully, the answer to which ORFs offer the most protective epitopes will soon be within our reach.
Acknowledgments
We thank Dr Joseph Blattman for help with HHDII mice. We also thank staff and study subjects at University of Washington Virology Research Clinic for providing human samples. Funding was provided by NIH grants AI30731 and AI081060 (to D.M.K.) and NIAID contract HHSN272200900042C (to A.S.).
Disclosure
Dr Koelle is a consultant to Immune Design Corporation and a co-inventor on patents owned by the University of Washington concerning herpes simplex virus vaccines. All other authors have nothing to disclose.
References
- 1.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 2.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008;86:805–12. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James SH, Kimberlin DW, Whitley RJ. Antiviral therapy for herpesvirus central nervous system infections: neonatal herpes simplex virus infection, herpes simplex encephalitis, and congenital cytomegalovirus infection. Antiviral Res. 2009;83:207–13. doi: 10.1016/j.antiviral.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 5.Zuckerman RA, Lucchetti A, Whittington WL, et al. HSV suppression reduces seminal HIV-1 levels in HIV-1/HSV-2 co-infected men who have sex with men. AIDS. 2009;23:479–83. doi: 10.1097/QAD.0b013e328326ca62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayaud P, Legoff J, Weiss HA, et al. Impact of acyclovir on genital and plasma HIV-1 RNA, genital herpes simplex virus type 2 DNA, and ulcer healing among HIV-1-infected African women with herpes ulcers: a randomized placebo-controlled trial. J Infect Dis. 2009;200:216–26. doi: 10.1086/599991. [DOI] [PubMed] [Google Scholar]
- 7.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–71. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–19. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J, Hladik F, Woodward A, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–92. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson KE, Redd AD, Quinn TC, et al. Effects of HIV-1 and herpes simplex virus type 2 infection on lymphocyte and dendritic cell density in adult foreskins from Rakai, Uganda. J Infect Dis. 2011;203:602–9. doi: 10.1093/infdis/jiq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanford M, Keating GM. Zoster vaccine (Zostavax): a review of its use in preventing herpes zoster and postherpetic neuralgia in older adults. Drugs Aging. 2010;27:159–76. doi: 10.2165/10489140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Noisakran S, Carr DJ. Lymphocytes delay kinetics of HSV-1 reactivation from in vitro explants of latent infected trigeminal ganglia. J Neuroimmunol. 1999;95:126–35. doi: 10.1016/s0165-5728(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 13.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–66. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheridan BS, Cherpes TL, Urban J, Kalinski P, Hendricks RL. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J Virol. 2009;83:2237–45. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN-gamma-secreting CD4(+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol. 2001;82:845–53. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- 16.Parr MB, Parr EL. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J Virol. 1998;72:2677–85. doi: 10.1128/jvi.72.4.2677-2685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–71. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verjans GM, Hintzen RQ, van Dun JM, et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci USA. 2007;104:3496–501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derfuss T, Arbusow V, Strupp M, Brandt T, Theil D. The presence of lytic HSV-1 transcripts and clonally expanded T cells with a memory effector phenotype in human sensory ganglia. Ann NY Acad Sci. 2009;1164:300–4. doi: 10.1111/j.1749-6632.2009.03871.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Koelle DM, Cao J, et al. CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101:1500–8. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobbs ME, Strasser JE, Chu CF, Chalk C, Milligan GN. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J Virol. 2005;79:14546–54. doi: 10.1128/JVI.79.23.14546-14554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham AL, Turner RR, Miller AC, Para MF, Merigan TC. Evolution of recurrent herpes simplex lesions. An immunohistologic study. J Clin Invest. 1985;75:226–33. doi: 10.1172/JCI111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arvin A, Campadelli-Fiume G, Mocarski E, et al. human herpesviruses: biology, therapy, and immunoprophylaxis. 1st edn. Cambridge: Cambridge University Press; 2007. [PubMed] [Google Scholar]
- 25.Thomsen DR, Roof LL, Homa FL. Assembly of herpes simplex virus (HSV) intermediate capsids in insect cells infected with recombinant baculoviruses expressing HSV capsid proteins. J Virol. 1994;68:2442–57. doi: 10.1128/jvi.68.4.2442-2457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toropova K, Huffman JB, Homa FL, Conway JF. The herpes simplex virus 1 UL17 protein is the second constituent of the capsid vertex-specific component required for DNA packaging and retention. J Virol. 2011;85:7513–22. doi: 10.1128/JVI.00837-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kemp TJ, Hildesheim A, Safaeian M, et al. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine. 2011;29:2011–14. doi: 10.1016/j.vaccine.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henson BW, Johnson N, Bera A, Okoye ME, Desai KV, Desai PJ. Expression of the HSV-1 capsid protein VP19C in Escherichia coli: a single amino acid change overcomes an expression block of the full-length polypeptide. Protein Expr Purif. 2011;77:80–5. doi: 10.1016/j.pep.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosken N, McGowan P, Meier A, et al. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol. 2006;80:5509–15. doi: 10.1128/JVI.02659-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laing KJ, Magaret AS, Mueller DE, et al. Diversity in CD8(+) T cell function and epitope breadth among persons with genital herpes. J Clin Immunol. 2010;30:703–22. doi: 10.1007/s10875-010-9441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koelle DM, Corey L, Burke RL, et al. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol. 1994;68:2803–10. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koelle DM, Frank JM, Johnson ML, Kwok WW. Recognition of herpes simplex virus type 2 tegument proteins by CD4 T cells infiltrating human genital herpes lesions. J Virol. 1998;72:7476–83. doi: 10.1128/jvi.72.9.7476-7483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwok WW, Liu AW, Novak EJ, et al. HLA-DQ tetramers identify epitope-specific T cells in peripheral blood of herpes simplex virus type 2-infected individuals: direct detection of immunodominant antigen-responsive cells. J Immunol. 2000;164:4244–9. doi: 10.4049/jimmunol.164.8.4244. [DOI] [PubMed] [Google Scholar]
- 34.Novak EJ, Liu AW, Gebe JA, et al. Tetramer-guided epitope mapping: rapid identification and characterization of immunodominant CD4+ T cell epitopes from complex antigens. J Immunol. 2001;166:6665–70. doi: 10.4049/jimmunol.166.11.6665. [DOI] [PubMed] [Google Scholar]
- 35.Kwok WW, Gebe JA, Liu A, et al. Rapid epitope identification from complex class-II-restricted T-cell antigens. Trends Immunol. 2001;22:583–8. doi: 10.1016/s1471-4906(01)02038-5. [DOI] [PubMed] [Google Scholar]
- 36.Verjans GM, Dings ME, McLauchlan J, et al. Intraocular T cells of patients with herpes simplex virus (HSV)-induced acute retinal necrosis recognize HSV tegument proteins VP11/12 and VP13/14. J Infect Dis. 2000;182:923–7. doi: 10.1086/315759. [DOI] [PubMed] [Google Scholar]
- 37.Jing L, Haas J, Dann G, Dong L, Laing K, Wald ADK. Comprehensive evaluation of the CD8 responses to HSV-1 in humans. J Immunol. 2011;186:105.41. [Google Scholar]
- 38.Koelle DM, Chen HB, Gavin MA, Wald A, Kwok WW, Corey L. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J Immunol. 2001;166:4049–58. doi: 10.4049/jimmunol.166.6.4049. [DOI] [PubMed] [Google Scholar]
- 39.Muller WJ, Dong L, Vilalta A, et al. Herpes simplex virus type 2 tegument proteins contain subdominant T-cell epitopes detectable in BALB/c mice after DNA immunization and infection. J Gen Virol. 2009;90:1153–63. doi: 10.1099/vir.0.008771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aubert M, Chen Z, Lang R, et al. The antiapoptotic herpes simplex virus glycoprotein J localizes to multiple cellular organelles and induces reactive oxygen species formation. J Virol. 2008;82:617–29. doi: 10.1128/JVI.01341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awasthi S, Lubinski JM, Friedman HM. Immunization with HSV-1 glycoprotein C prevents immune evasion from complement and enhances the efficacy of an HSV-1 glycoprotein D subunit vaccine. Vaccine. 2009;27:6845–53. doi: 10.1016/j.vaccine.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hook LM, Huang J, Jiang M, Hodinka R, Friedman HM. Blocking antibody access to neutralizing domains on glycoproteins involved in entry as a novel mechanism of immune evasion by herpes simplex virus type 1 glycoproteins C and E. J Virol. 2008;82:6935–41. doi: 10.1128/JVI.02599-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim M, Taylor J, Sidney J, et al. Immunodominant epitopes in herpes simplex virus type 2 glycoprotein D are recognized by CD4 lymphocytes from both HSV-1 and HSV-2 seropositive subjects. J Immunol. 2008;181:6604–15. doi: 10.4049/jimmunol.181.9.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Castelli FA, Zhu X, Wu M, Maillere B, BenMohamed L. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin Vaccine Immunol. 2008;15:1436–49. doi: 10.1128/CVI.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper D, Mester JC, Guo M, et al. Epitope mapping of full-length glycoprotein D from HSV-2 reveals a novel CD4+ CTL epitope located at the transmembrane–cytoplasmic junction. Cell Immunol. 2006;239:113–20. doi: 10.1016/j.cellimm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Mikloska Z, Cunningham AL. Herpes simplex virus type 1 glycoproteins gB, gC and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J Gen Virol. 1998;79(Pt 2):353–61. doi: 10.1099/0022-1317-79-2-353. [DOI] [PubMed] [Google Scholar]
- 47.Johnson RM, Lancki DW, Fitch FW, Spear PG. Herpes simplex virus glycoprotein D is recognized as antigen by CD4+ and CD8+ T lymphocytes from infected mice. Characterization of T cell clones. J Immunol. 1990;145:702–10. [PubMed] [Google Scholar]
- 48.Chentoufi AA, Dasgupta G, Christensen ND, et al. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol. 2010;184:2561–71. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chentoufi AA, Zhang X, Lamberth K, et al. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol. 2008;180:426–37. doi: 10.4049/jimmunol.180.1.426. [DOI] [PubMed] [Google Scholar]
- 50.Koelle DM, Liu Z, McClurkan CL, et al. Immunodominance among herpes simplex virus-specific CD8 T cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci USA. 2003;100:12899–904. doi: 10.1073/pnas.2131705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nugent CT, McNally JM, Chervenak R, Wolcott RM, Jennings SR. Differences in the recognition of CTL epitopes during primary and secondary responses to herpes simplex virus infection in vivo. Cell Immunol. 1995;165:55–64. doi: 10.1006/cimm.1995.1186. [DOI] [PubMed] [Google Scholar]
- 52.Salvucci LA, Bonneau RH, Tevethia SS. Polymorphism within the herpes simplex virus (HSV) ribonucleotide reductase large subunit (ICP6) confers type specificity for recognition by HSV type 1-specific cytotoxic T lymphocytes. J Virol. 1995;69:1122–31. doi: 10.1128/jvi.69.2.1122-1131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.St Leger AJ, Peters B, Sidney J, Sette A, Hendricks RL. Defining the herpes simplex virus-specific CD8+ T cell repertoire in C57BL/6 mice. J Immunol. 2011;186:3927–33. doi: 10.4049/jimmunol.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Posavad CM, Remington M, Mueller DE, et al. Detailed characterization of T cell responses to herpes simplex virus-2 in immune seronegative persons. J Immunol. 2010;184:3250–9. doi: 10.4049/jimmunol.0900722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brehm M, Samaniego LA, Bonneau RH, DeLuca NA, Tevethia SS. Immunogenicity of herpes simplex virus type 1 mutants containing deletions in one or more alpha-genes: ICP4, ICP27, ICP22, and ICP0. Virology. 1999;256:258–69. doi: 10.1006/viro.1999.9653. [DOI] [PubMed] [Google Scholar]
- 56.Halford WP, Puschel R, Gershburg E, Wilber A, Gershburg S, Rakowski B. A live-attenuated HSV-2 ICP0 virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine. PLoS ONE. 2011;6:e17748. doi: 10.1371/journal.pone.0017748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tigges MA, Koelle D, Hartog K, Sekulovich RE, Corey L, Burke RL. Human CD8+ herpes simplex virus-specific cytotoxic T-lymphocyte clones recognize diverse virion protein antigens. J Virol. 1992;66:1622–34. doi: 10.1128/jvi.66.3.1622-1634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 59.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–30. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 60.Ramachandran S, Davoli KA, Yee MB, Hendricks RL, Kinchington PR. Delaying the expression of herpes simplex virus type 1 glycoprotein B (gB) to a true late gene alters neurovirulence and inhibits the gB-CD8+ T-cell response in the trigeminal ganglion. J Virol. 2010;84:8811–20. doi: 10.1128/JVI.00496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mo A, Musselli C, Chen H, et al. A heat shock protein based polyvalent vaccine targeting HSV-2: CD4(+) and CD8(+) cellular immunity and protective efficacy. Vaccine. 2011;29:8530–41. doi: 10.1016/j.vaccine.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Mikloska Z, Ruckholdt M, Ghadiminejad I, Dunckley H, Denis M, Cunningham AL. Monophosphoryl lipid A and QS21 increase CD8 T lymphocyte cytotoxicity to herpes simplex virus-2 infected cell proteins 4 and 27 through IFN-gamma and IL-12 production. J Immunol. 2000;164:5167–76. doi: 10.4049/jimmunol.164.10.5167. [DOI] [PubMed] [Google Scholar]
- 63.Koelle DM, Magaret A, McClurkan CL, et al. Phase I dose-escalation study of a monovalent heat shock protein 70-herpes simplex virus type 2 (HSV-2) peptide-based vaccine designed to prime or boost CD8 T-cell responses in HSV-naive and HSV-2-infected subjects. Clin Vaccine Immunol. 2008;15:773–82. doi: 10.1128/CVI.00020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bi J, Yang H, Yan H, Song R, Fan J. Knowledge-based virtual screening of HLA-A*0201-restricted CD8(+) T-cell epitope peptides from herpes simplex virus genome. J Theor Biol. 2011;281:133–9. doi: 10.1016/j.jtbi.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 65.Braun RP, Payne LG, Dong L. Characterization of the IFN-gamma T-cell responses to immediate early antigens in humans with genital herpes. Virol J. 2006;3:54. doi: 10.1186/1743-422X-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maiers M, Gragert L, High-resolution KW. HLA alleles and haplotypes in the United States population. Hum Immunol. 2007;68:779–88. doi: 10.1016/j.humimm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 67.Ellis JM, Henson V, Slack R, Ng J, Hartzman RJ, Katovich Hurley C. Frequencies of HLA-A2 alleles in five U.S. population groups. Predominance of A*02011 and identification of HLA-A*0231. Hum Immunol. 2000;61:334–40. doi: 10.1016/s0198-8859(99)00155-x. [DOI] [PubMed] [Google Scholar]
- 68.Klitz W, Gragert L, Maiers M, et al. Four-locus high-resolution HLA typing in a sample of Mexican Americans. Tissue Antigens. 2009;74:508–13. doi: 10.1111/j.1399-0039.2009.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams R, Chen YF, Endres R, et al. Molecular variation at the HLA-A, B, C, DRB1, DQA1, and DQB1 loci in full heritage American Indians in Arizona: private haplotypes and their evolution. Tissue Antigens. 2009;74:520–33. doi: 10.1111/j.1399-0039.2009.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tu B, Mack SJ, Lazaro A, et al. HLA-A, -B, -C, -DRB1 allele and haplotype frequencies in an African American population. Tissue Antigens. 2007;69:73–85. doi: 10.1111/j.1399-0039.2006.00728.x. [DOI] [PubMed] [Google Scholar]
- 71.Mack SJ, Tu B, Lazaro A, et al. HLA-A, -B, -C, and -DRB1 allele and haplotype frequencies distinguish Eastern European Americans from the general European American population. Tissue Antigens. 2009;73:17–32. doi: 10.1111/j.1399-0039.2008.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pascolo S. HLA class I transgenic mice: development, utilisation and improvement. Exp Opin Biol Ther. 2005;5:919–38. doi: 10.1517/14712598.5.7.919. [DOI] [PubMed] [Google Scholar]
- 73.Lund O, Nielsen M, Kesmir C, et al. Definition of supertypes for HLA molecules using clustering of specificity matrices. Immunogenetics. 2004;55:797–810. doi: 10.1007/s00251-004-0647-4. [DOI] [PubMed] [Google Scholar]
- 74.Sidney J, Peters B, Frahm N, Brander C, Sette A. HLA class I supertypes: a revised and updated classification. BMC Immunol. 2008;9:1. doi: 10.1186/1471-2172-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koelle DM, Liu Z, McClurkan CM, et al. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. J Clin Invest. 2002;110:537–48. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dudek TE, Torres-Lopez E, Crumpacker C, Knipe DM. Evidence for differences in immunologic and pathogenesis properties of herpes simplex virus 2 strains from the United States and South Africa. J Infect Dis. 2011;203:1434–41. doi: 10.1093/infdis/jir047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watson-Jones D, Wald A, Celum C, et al. Use of acyclovir for suppression of human immunodeficiency virus infection is not associated with genotypic evidence of herpes simplex virus type 2 resistance to acyclovir: analysis of specimens from three phase III trials. J Clin Microbiol. 2010;48:3496–503. doi: 10.1128/JCM.01263-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berger C, Flowers ME, Warren EH, Riddell SR. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107:2294–302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dong L, Li P, Oenema T, McClurkan CL, Koelle DM. Public TCR use by herpes simplex virus-2-specific human CD8 CTLs. J Immunol. 2010;184:3063–71. doi: 10.4049/jimmunol.0903622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chentoufi AA, Binder NR, Berka N, et al. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J Virol. 2008;82:11792–802. doi: 10.1128/JVI.00692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Posavad CM, Wald A, Hosken N, et al. T cell immunity to herpes simplex viruses in seronegative subjects: silent infection or acquired immunity? J Immunol. 2003;170:4380–8. doi: 10.4049/jimmunol.170.8.4380. [DOI] [PubMed] [Google Scholar]
- 82.Stanberry LR, Spruance SL, Cunningham AL, et al. D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 83.Cohen J. Immunology. Painful failure of promising genital herpes vaccine. Science. 2010;330:304. doi: 10.1126/science.330.6002.304. [DOI] [PubMed] [Google Scholar]
- 84.BenMohamed L, Bertrand G, McNamara CD, et al. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J Virol. 2003;77:9463–73. doi: 10.1128/JVI.77.17.9463-9473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–22. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–20. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 87.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 88.Koelle DM, Gonzalez JC, Johnson AS. Homing in on the cellular immune response to HSV-2 in humans. Am J Reprod Immunol. 2005;53:172–81. doi: 10.1111/j.1600-0897.2005.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koelle DM, Schomogyi M, Corey L. Antigen-specific T cells localize to the uterine cervix in women with genital herpes simplex virus type 2 infection. J Infect Dis. 2000;182:662–70. doi: 10.1086/315749. [DOI] [PubMed] [Google Scholar]
- 90.Bedoui S, Whitney PG, Waithman J, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–95. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 91.Jing L, McCaughey SM, Davies DH, et al. ORFeome approach to the clonal, HLA allele-specific CD4 T-cell response to a complex pathogen in humans. J Immunol Methods. 2009;347:36–45. doi: 10.1016/j.jim.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Posavad CM, Magaret AS, Zhao L, Mueller DE, Wald A, Corey L. Development of an interferon-gamma ELISPOT assay to detect human T cell responses to HSV-2. Vaccine. 2011;29:7058–66. doi: 10.1016/j.vaccine.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Danke NA, Koelle DM, Kwok WW. Persistence of herpes simplex virus type 2 VP16-specific CD4+ T cells. Hum Immunol. 2005;66:777–87. doi: 10.1016/j.humimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 95.Osorio Y, Mott KR, Jabbar AM, et al. Epitope mapping of HSV-1 glycoprotein K (gK) reveals a T cell epitope located within the signal domain of gK. Virus Res. 2007;128:71–80. doi: 10.1016/j.virusres.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mott KR, Chentoufi AA, Carpenter D, BenMohamed L, Wechsler SL, Ghiasi H. The role of a glycoprotein K (gK) CD8+ T-cell epitope of herpes simplex virus on virus replication and pathogenicity. Invest Ophthalmol Vis Sci. 2009;50:2903–12. doi: 10.1167/iovs.08-2957. [DOI] [PubMed] [Google Scholar]
- 97.Haynes JR, Arrington J, Dong L, Braun RP, Payne LG. Potent protective cellular immune responses generated by a DNA vaccine encoding HSV-2 ICP27 and the E. coli heat labile enterotoxin. Vaccine. 2006;24:5016–26. doi: 10.1016/j.vaccine.2006.03.046. [DOI] [PubMed] [Google Scholar]


