Abstract
OTHER THEMES PUBLISHED IN THIS IMMUNOLOGY IN THE CLINIC REVIEW SERIES
Metabolic Diseases, Host Responses, Cancer, Autoinflammatory Diseases, Type 1 diabetes and viruses.
Allergen-specific immunotherapy is an effective clinical treatment for hypersensitivity to many allergens. Studies of basophils during immunotherapy have provided insight into underlying immune mechanisms and support the potential use of basophil activation as a biomarker of clinical outcomes. This review examines the evidence for different pathways of basophil modulation associated with various forms of immunotherapy. Better understanding the molecular mechanisms of basophil activation and desensitization and the relationship between suppression of these effector cells to clinical outcomes holds promise for further development and improvement in potential therapies for allergic diseases.
Keywords: basophil, desensitization, immunotherapy
Introduction
Basophils are a rare population of peripheral leucocytes which play an important role as effector cells in allergic disease. Characterized by their high surface expression of the tetrameric form of the high-affinity immunoglobulin (Ig)E receptor (FcεRI), they can be stimulated in an IgE-dependent manner to release a number of pro-allergic inflammatory mediators, including histamine, leukotriene C4 and T helper type 2 (Th2) cytokines [interleukin (IL)-4, IL-13].
The earliest assessment of human basophil activation focused on the measurement of ex vivo mediator release, such as histamine and leukotriene C4 [1]. The subsequent discovery of surface markers correlating with basophil activation has driven a shift in the predominant diagnostic methodology toward the use of flow cytometry.
Assessment of basophil reactivity
Clinical studies utilizing flow cytometry for measurement of markers of basophils activation have primarily focused on 2 markers, CD63 and CD203c. CD63 is a tetraspanin protein localized predominantly to the membranes of late endosomes of many cell types, including modified late endosomes that are the secretory granules of basophils. The dramatic increase in CD63 surface membrane expression upon basophil activation was shown to correlate closely with histamine release [2,3]. This correlation holds for both IgE and non-IgE mediated stimulation when the outcome of basophil activation is ‘anaphylactic degranulation’– complete fusion of secretory vesicles with the plasma membrane – but not with incomplete or ‘piecemeal degranulation’[4]. Anaphylactic degranulation results in a predominantly bimodal CD63 expression (see Fig. 1). Another marker, CD203c, or the type II transmembrane ectoenzyme E-NPP3 [5], is basophil-specific and expressed constitutively on the cell surface, although it is also up-regulated with activation. In contrast to CD63, increases in surface CD203c are generally more rapid, more transient and can be seen with stimuli that result in activation without anaphylactic degranulation, such as IL-3 [6,7] (see Fig. 1). Additional surface markers, such as CD69, have also been used to study basophil activation, although not as extensively as CD63 and CD203c [8].
Fig. 1.
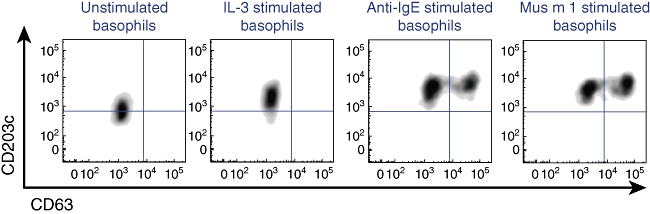
Markers of basophil activation. Basophils, identified on scatter characteristics and as CD123+CRTH2+ HLA-DR– cells, from a mouse allergic donor demonstrate up-regulation of CD203c and increased frequency of CD63hi with activation.
The use of basophil activation markers as a diagnostic measure of allergic disease has emerged as an investigative tool, known as the basophil activation test (BAT). Clinical applications for the BAT in the diagnosis of hypersensitivity to drugs, food, Hymenoptera venom and environmental allergens have been reviewed elsewhere [9,10], and these studies hold promise for the use of BAT as an additional clinical tool.
This review will discuss assessing alterations in basophil activation in clinical immunotherapy trials [11,12], its correlation to clinical outcomes, and its kinetics. We will discuss possible intrinsic and extrinsic mechanisms of modulation. Intrinsic mechanisms reflect the internal processes in basophils that may impact activation, whereas extrinsic mechanisms refer to factors outside the individual basophils which may impact their activation.
Measuring basophil activation and its suppression
One important aspect of allergen-induced basophil degranulation is the allergen dose–response curve, which has several important aspects that significantly influence the interpretation of clinical studies discussed in this article. The dose–response curve of IgE-mediated human basophil stimulation with increasing doses of antigen is generally very broad (often greater than 5 log difference) and is often significantly bell-shaped (i.e. having both sub- and supraoptimal dose ranges) (see Fig. 2). In addition, there is a large degree of variability of basophil sensitivity and maximal responsiveness among different allergic donors to the same allergen. Investigators have used specific characteristics of the dose–response curve, including the maximal activation (basophil reactivity, CDmax) as well as the effective dose at 50% of the maximal activation [50% effective dose (ED50) or basophil sensitivity, CDsens], in comparisons between individual donors [3,9,13]. We therefore propose calculating the area under the curve (AUC; see Fig. 2) as an alternate method of comparing basophil responses.
Fig. 2.
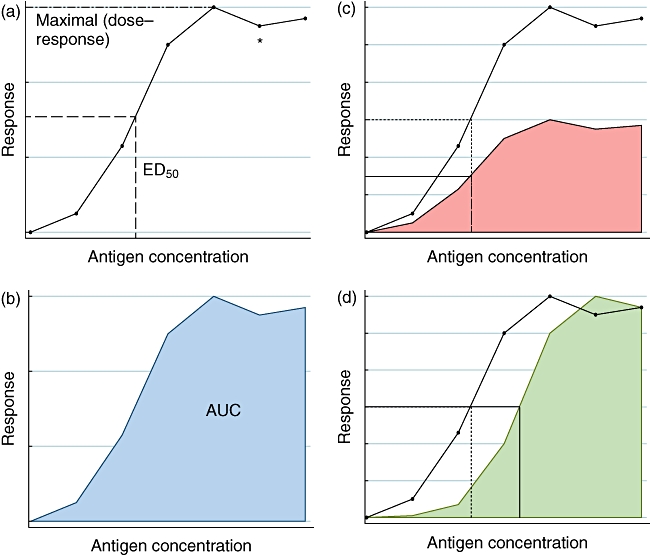
Characteristics of the basophil dose–response curve. Plotting of immunoglobulin (Ig)E-mediated basophil activation with increasing antigen doses leads to a dose–response curve as above. A. The maximal dose response is also known as basophil reactivity, and the effective dose at 50% of the maximal dose response (ED50) is also referred to as basophil sensitivity. *Refers the supraoptimal part of the dose–response curve. B. Another method of comparison of basophil curves could use the area under the curve (AUC). C. Variation in basophil maximal dose response between donors with similar basophil reactivity. D. Variation in basophil reactivity between donors with similar maximal dose response.
Clinical studies of basophil activity during immunotherapy
Allergen-specific immunotherapy effectively improves clinical symptoms of IgE-mediated, type I hypersensitivity to a variety of allergens [12,14]. The underlying mechanism of this clinical efficacy has been speculated to relate to the suppression of allergic effector cells resulting in decreased release of immediate effector molecules. Suppression of basophil activation has been seen in several routes of immunotherapy administration, including subcutaneous, sublingual and oral immunotherapy [15–17]. These studies have used traditional, cluster and rush protocols [15,18,19] to study a diversity of allergens, including Hymenoptera venom, environmental and food allergens [15,17,20]. Factors highlighted by these studies include the correlation of basophil suppression in patients undergoing immunotherapy with clinical improvement and the kinetics of basophil suppression during immunotherapy.
Correlation with clinical outcomes
Suppression of basophil activation in patients treated with immunotherapy has been shown to correlate with treatment efficacy. For example, histamine release from in vitro antigen-stimulated peripheral mononuclear cells has been found to be higher in patients treated with bee venom immunotherapy who react to post-immunotherapy sting challenge in comparison to those who tolerated the challenge [21]. In a study of 21 patients undergoing bee venom immunotherapy for longer than 3 years, the five patients who failed the sting challenge had the highest CD63hi percentage of in vitro antigen-stimulated basophils [22]. Similarly, in a study of venom allergic patients who underwent 2–7 years of immunotherapy, those who failed sting challenge had significantly higher in vitro antigen-stimulated basophil CD63 up-regulation than those who passed sting challenge [23]. In 17 immunotherapy patients with yellow-jacket or honeybee allergy, patients with a clinical history of systemic reactions had a higher CD203c up-regulation post-sting challenge as well as in vitro antigen-stimulated basophil CD203c up-regulation when compared to patients with a history of large local reactions [8]. This study is unique in its comparison of the in vivo antigen stimulation via sting challenge, and the in vitro antigen stimulation via antigen stimulation of peripheral basophils, to demonstrate consistent CD203c changes. CD63 did not follow the pattern of CD203c up-regulation in this study; however, its assessment was limited due to the near absence of a bimodal CD63 expression of basophils (see Fig. 1 and below).
Correlation of basophil activation with increased side effects during immunotherapy has been described in studies of Hymenoptera venom hypersensitivity. In patients undergoing modified rush immunotherapy (RIT) to wasp, those who had side effects had a greater percentage of CD63hi basophils after in vitro antigen stimulation than those who tolerated the immunotherapy [24]. The correlation with side effects during immunotherapy was not reproducible in a subgroup analysis of another study in which 57 Hymenoptera venom-allergic patients underwent immunotherapy [25–27]. Differences in the basophil activation testing parameters may account for these contradictory results. Both studies also used a limited time-frame for side effect monitoring during immunotherapy, which may have created an artificial bias. Further studies aimed specifically at assessment of side effects throughout the duration of immunotherapy may substanciate this correlation.
Suppression of other basophil effector functions, such as the secretion of Th2 cytokines IL-4 and IL-13, has also been studied. In a study by Plewako et al. in 14 patients undergoing RIT with cat or birch extracts, CD203c expression as well as histamine, IL-4 and IL-13 release were seen to be decreased early in treatment, starting during the build-up phase of therapy [28]. Notably, the authors also found that the side-effect symptom score during the immunotherapy correlated with a higher percentage of antigen-stimulated IL-4- and IL-13-producing basophils before the start of treatment as well as histamine release from antigen-stimulated peripheral blood mononuclear cells. There was continued suppression of basophil CD203c expression and a decreased percentage of IL-4/IL-13-positive basophils through the immunotherapy.
The correlation between clinical symptom scores and basophil activation has also been seen in patients who received immunotherapy with other allergens. Patients who underwent RIT to Japanese cedar pollen demonstrated in vitro antigen-stimulated basophil CD203c suppression at 1 month after the initiation of immunotherapy, with continued suppression through the duration of the year-long study [20,29]. Here, quality of life and symptom score assessment pre- and post-immunotherapy demonstrated significant improvement in this study.
However, some studies did not demonstrate any change in basophil activation markers with immunotherapy. For example, a placebo-controlled trial with five-grass pollen sublingual therapy did not find any difference of in vitro induced CD203c expression after 4 months of treatment, despite symptomatic improvement in subjects' rhinoconjunctivitis [30]. Similarly, in 25 patients who underwent a modified RIT protocol with wasp venom and tolerated a subsequent sting challenge at 6 months, only two patients had a decrease in their percentage of CD63hi basophils after in vitro antigen stimulation [31]. However, the use of only two antigen concentrations and the imposition of a CD63hi cut-off to define a categorical response, based on BAT sensitivity and specificity for diagnosis of hypersensitivity, may have biased this study's findings. In another double-blind, placebo-controlled study of patients with Myrmecia pilosula hypersensitivity, there was no difference in basophil activation between immunotherapy and placebo patients [32].
In summary, some studies demonstrate correlations between clinical outcomes and basophil reactivity. However, more work is needed to better understand which basophil activation readouts are best and whether there are consistent aspects of study design (e.g. dose, timing, route, etc) for which basophils may be more or less suited as biomarkers.
Mechanisms of basophil suppression in immunotherapy
Kinetics of basophil suppression
Studies describing the kinetics of basophil suppression have provided some of the first insights into the mechanisms of that suppression. In 1996, Jutel et al. demonstrated that histamine release from antigen-stimulated peripheral mononuclear cells was decreased in bee-allergic patients after undergoing the build-up phase of ultra-RIT [33], suggesting that the early tolerance induced by the immunotherapy resulted in basophil suppression. Since then, the onset of basophil suppression during immunotherapy has been studied in clinical trials with a variety of immunotherapy schedules and routes.
In one peanut oral immunotherapy trial (OIT), during which dose escalation occurred over months, the onset of basophil suppression in immunotherapy-treated patients occurred during the first 4 months of therapy compared to baseline values before initiation of OIT, and persisted through the immunotherapy period [17]. Similarly, Ebo et al. noted that in patients treated with RIT for Vespula vulgaris hypersensitivity noted that basophil CD63 up-regulation was not significantly different from baseline at 5 days, but was significantly decreased at 6 months [15].
Another study of RIT in 48 patients with Hymenoptera venom hypersensitivity did not find any change of in vivo basophil CD63 expression between the pre- and post-build-up phase; however, in 20 of those patients who were examined 1 week after completion of RIT, there was a significant decline in CD63 basophil expression [34]. An important methodological difference of this study is the measurement of in vivo activation, which may influence the ability to detect differences in basophil activation.
Interestingly, Mikkelsen et al. studied serial basophil activation in patients undergoing a mix of cluster and traditional subcutaneous immunotherapy to Vespula vulgaris, with a 7–11-week build-up phase. Suppression of in vitro antigen-stimulated basophil activation was seen at 3 weeks and returned subsequently to the initial baseline, where it remained at weeks 7 and at the time of maintenance initiation [18]. As clinical outcomes of immunotherapy were not reported, the absence of sustained basophil suppression could be attributed to a lack of clinical efficacy.
Comparison of the kinetics of basophil suppression with immunotherapy is hampered by the need for serial measurements through the build-up phases of immunotherapy, as basophil suppression may be an early phenomenon during the course of immunotherapy. This may be particularly true if the immunotherapy protocol involves daily exposure to antigen, which may be anergy-inducing, versus intermittent allergen dosing, which may not have this effect at all.
Extrinsic changes during immunotherapy
The serological changes that occur during immunotherapy are likely a primary mechanism affecting basophil and other effector cell activation. As degranulation is dependent on antigen-stimulated specific IgE cross-linking on the surface of basophils, modulation of basophil activation has been speculated to correlate with levels of specific IgE. Evidence for early transient increase in specific IgE has been seen in oral and sublingual immunotherapy [16,17,30] with subsequent decrease in specific IgE after 1 or more years of immunotherapy [15–17], although some studies have not seen significant change [20,29,35].
Factors that influence IgE-mediated basophil activation (and therefore may also impact the suppression of basophil activation) include surface density of the high-affinity IgE receptor (FcεRI), fraction of membrane-bound-specific IgE (which is influenced by the ratio of specific to total IgE in the serum) [36], the clonality of the antigen-specific IgE, biochemical properties of the allergen and intrinsic basophil sensitivity [9]. For instance, an elegant set of studies devised by Christensen et al. utilized recombinant specific IgE with predetermined affinity to Der p 2 to demonstrate the effect of clonality of IgE on basophil activation [37]. This paper demonstrates that both the affinity and composition of the surface allergen-specific IgE impacts basophil degranulation. The inconsistent changes in specific IgE levels associated with clinically effective immunotherapy suggest that other immunotherapy-induced changes are mechanistically more important.
An early and sustained increase in allergen-specific IgG4 has been detected more reproducibly [16,17,20,28,29,34,38–40], although older studies did not find an association between IgG and clinical improvement [41,42]. Direct suppression of basophil activation by allergen-specific IgG4 could occur by either blocking IgE-allergen binding and/or signalling via inhibitory IgG receptors. Several studies have shown that IgG4-containing serum from patients post-immunotherapy can suppress basophil activation [18,35,40,43,44] or decrease of β-hexosaminidase release from rat basophilic leukaemia (RBL) cells [39].
One model of blocking IgE-allergen interaction with IgG generated recombinant IgG with Phl p 2 epitope specificity from a human grass-allergic donor's IgE [45], to demonstrate in vitro inhibition of IgE-grass pollen complex binding to CD23 of B cells as well as decreased histamine release from antigen-stimulated basophils. Subsequent studies of both subcutaneous and sublingual grass-pollen immunotherapy have shown the induction of allergen-specific IgG antibodies with IgE-allergen blocking capability and their persistence even after cessation of immunotherapy [46,47].
Using another mechanistic approach, Uermosi and colleagues devised a chimeric Fel d 1 IgG antibody to demonstrate decreased degranulation of basophils from patients with cat allergies [48]. Suppression was effective with either IgG1 or IgG4 and was increased further with the use of two or three different epitope specificities of the IgG antibodies. This increased suppression was speculated to be due to more efficient FcγRIIB cross-linking. Interestingly, previous work suggests that IgG epitope specificity is affected by immunotherapy and increases suppressive activity after immunotherapy [49].
IgG-mediated basophil suppression includes signalling through low-affinity IgG receptors (FcγIIRA and FcγIIRB), which are expressed on the surface of circulating basophils. Stimulation of these receptors can induce inhibitory signalling through their ITIMs. Using cat-specific IgG from serum of cat allergic patients on subcutaneous immunotherapy, Cady et al. demonstrated that suppression of CD203c expression on basophils acts via inhibitory receptors FcγIIRA and FcγIIRB [50]. Moreover, co-stimulation of FcεRI and FcγIIR on basophils results in suppression of basophil activation and increase in SHP-1 levels [51]. Another study utilized a chimeric IgG antibody to bind both Fcε and Fcγ receptors on basophils, which decreased antigen-specific basophil degranulation from atopic donors [52]. Furthermore, a chimeric fusion protein of Fcγ-Fcε that bound both FcγIIR and FcεRI was found to decrease human basophil activation as well as Syk phosphorylation in vitro[53]. This type of inhibitory mechanism has been used in an antigen-specific manner by Zhu et al., who devised a chimeric fusion of Fcγ to cat allergen Fel d 1 designed to aggregate FcγRIIB and FcERI to demonstrate a decrease in histamine release from basophils of cat-allergic patients [54].
The above studies suggest that extrinsic factors, such as IgG4 and FcgRII stimulation, may contribute to the suppression of basophil activation during immunotherapy.
Intrinsic basophil changes during suppression
As noted above, suppression of basophil activity with immunotherapy has been evidenced by suppression of markers of basophil activation. IgE-dependent basophil activation begins with cross-linking of antigen-specific surface IgE, with subsequent recruitment and phosphorylation of tyrosine kinases Lyn and Syk, leading to activation of Phosphoinositide 3-kinase (PI3K) and phospholipase C activation. Intracellular calcium mobilization from inositol triphosphate (IP3) generation leads to secretion and/or de novo synthesis of basophil allergic mediators, including histamine, cytokines and leukotrienes.
Variability in human basophil mediator release has been linked to levels of these intracellular signalling molecules. About 10–20% of the human population have ‘non-releaser basophils’, which do not secrete histamine to anti-IgE stimulation [55]. Basophil histamine release has been correlated with expression levels of Syk and phosphatidylinositol 5′ phosphatase (SHIP) expression in the human population [56]. A composite characteristic of basophil activation can be summarized using the term ‘intrinsic basophil sensitivity’ to refer to the intracellular signalling characteristics of patients' basophils.
Additional studies on pathways of signal termination of antigen-stimulated IgE-FcεRI signalling pathways substantiated the role of syk and actin-mediated pathways [57]. However, these studies also suggest that the pathways of signal self-termination may not be the same as those of anergy, or desensitization, in basophils. Several approaches to study basophil anergy or desensitization have been employed in vivo. The use of suboptimal antigen stimulation, which would not stimulate maximal mediator release from basophils for longer periods of time (24 h), resulted in reduced Syk but not Lyn levels [58]. Another approach was to stimulate basophils in vitro in calcium-free conditions, which would inhibit mediator release. When basophils were stimulated in a calcium-free environment in the presence of actin inhibitors mediator release was unchanged, suggesting that although actin-mediated pathways may play a role in antigen-IgE-FcεRI signal termination, they do not impact basophil anergy [59].
The in vitro induction of basophil desensitization most similar to the clinical model of immunotherapy uses repeated antigen stimulation, which was performed by Lund et al. to demonstrate grass-specific desensitization of basophils from grass allergic donors [60]. A similar model of antigen-induced anergy suggested that Syk and PI3 are not involved in mechanisms of anergy [61]. A study inducing desensitization by using increasing antigen concentrations to stimulate antigen-specific IgE-sensitized bone-marrow-derived mast cells suggested the internalization of FcεRI–IgE-antigen complexes is impaired during the process. This is contrary to previous speculations about possible FcεRI internalization as a mechanism for decreasing antigen sensitivity of allergen effector cells [62]. Whether this applies to human basophils remains to be studied, as FcεRI endocytosis has been reported in these basophils [63]. Hence, further studies on intrinsic changes in basophil activation during immunotherapy are needed and may highlight potential avenues for therapeutic intervention.
Conclusions
Clinical studies suggest that there is down-regulation of basophil activity during the course of allergen-specific immunotherapy. There is also some evidence for the use of in vitro basophil activation tests to monitor clinical outcome measures, including clinical efficacy and side effects. Other measures, such as the quantity of allergen-specific IgE, do not reflect the complexity of the in vivo allergic response, as there are multiple factors regulating the activity of allergic effector cells, including the ratio of specific to total IgE, which has also been referred to as ‘IgE-specific activity’[64].
While advances in multiparametric flow cytometry, improvement in basophil isolation techniques and a better understanding of basic basophil physiology has greatly enhanced our ability to study this rare population of immunological cells, our use of basophil activation as a biomarker continues to be limited by several factors. The size of the clinical studies is often limited; the largest studies mentioned above contain fewer than 50 patients. As there is considerable heterogeneity between individual responses to immunotherapy as well as basophil reactivity, these studies are limited considerably by their size.
Furthermore, the ongoing challenge in assessment of basophil activation is the method of comparison between donors using either the basophil sensitivity or basophil reactivity measures. Depending on the basophil dose–response curve, utilization of a limited allergen dose range and comparison of extrapolated parameters can lead to a bias towards negative results. A more precise comparison of dose–response curves might use the AUC to reflect basophil reactivity in these large clinical studies (see Fig. 2).
Although our understanding of intracellular signalling pathways in basophils is incomplete, further measurement of these parameters in large clinical studies may provide more valid insight into the modulation of this cell type during immunotherapy.
In recent years, basophils have been shown to be able to augment the initiation of allergic responses in murine models, and may play a larger role in the allergic response than mere allergen-induced secretion of immediate mediators. Further exploration into the effect of immunotherapy on basophil functions may shed greater light on the modification of the allergic response induced by immunotherapy.
Disclosure
Studies on basophil activation by mouse allergen are supported by funding from NIAID R01 AI081845.
References
- 1.Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med. 1989;321:228–32. doi: 10.1056/NEJM198907273210405. [DOI] [PubMed] [Google Scholar]
- 2.Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991;88:328–38. doi: 10.1016/0091-6749(91)90094-5. [DOI] [PubMed] [Google Scholar]
- 3.Metzelaar MJ, Wijngaard PL, Peters PJ, Sixma JJ, Nieuwenhuis HK, Clevers HC. CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J Biol Chem. 1991;266:3239–45. [PubMed] [Google Scholar]
- 4.MacGlashan D., Jr Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin Exp Allergy. 2010;40:1365–77. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buhring HJ, Seiffert M, Giesert C, et al. The basophil activation marker defined by antibody 97A6 is identical to the ectonucleotide pyrophosphatase/phosphodiesterase 3. Blood. 2001;97:3303–5. doi: 10.1182/blood.v97.10.3303. [DOI] [PubMed] [Google Scholar]
- 6.Monneret G, Gutowski MC, Bienvenu J. Detection of allergen-induced basophil activation by expression of CD63 antigen using a tricolour flow cytometric method. Clin Exp Immunol. 1999;115:393–6. doi: 10.1046/j.1365-2249.1999.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hennersdorf F, Florian S, Jakob A, et al. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res. 2005;15:325–35. doi: 10.1038/sj.cr.7290301. [DOI] [PubMed] [Google Scholar]
- 8.Gober LM, Eckman JA, Sterba PM, et al. Expression of activation markers on basophils in a controlled model of anaphylaxis. J Allergy Clin Immunol. 2007;119:1181–8. doi: 10.1016/j.jaci.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Kleine-Tebbe J, Erdmann S, Knol EF, MacGlashan DW, Jr, Poulsen LK, Gibbs BF. Diagnostic tests based on human basophils: potentials, pitfalls and perspectives. Int Arch Allergy Immunol. 2006;141:79–90. doi: 10.1159/000094495. [DOI] [PubMed] [Google Scholar]
- 10.Shreffler WG. Evaluation of basophil activation in food allergy: present and future applications. Curr Opin Allergy Clin Immunol. 2006;6:226–33. doi: 10.1097/01.all.0000225165.83144.2f. [DOI] [PubMed] [Google Scholar]
- 11.Mousallem T, Burks A. Immunotherapy for Food Allergy. Clin Exp Immunol. 2012;167:26–31. doi: 10.1111/j.1365-2249.2011.04499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishna MT, Huissoon AP. Clinical immunology review series: an approach to desensitization. Clin Exp Immunol. 2011;163:131–46. doi: 10.1111/j.1365-2249.2010.04296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nopp A, Cardell LO, Johansson SG, Oman H. CD-sens: a biological measure of immunological changes stimulated by ASIT. Allergy. 2009;64:811–14. doi: 10.1111/j.1398-9995.2008.01900.x. [DOI] [PubMed] [Google Scholar]
- 14.Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936. doi: 10.1002/14651858.CD001936.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebo DG, Hagendorens MM, Schuerwegh AJ, et al. Flow-assisted quantification of in vitro activated basophils in the diagnosis of wasp venom allergy and follow-up of wasp venom immunotherapy. Clin Cytom. 2007;72:196–203. doi: 10.1002/cyto.b.20142. [DOI] [PubMed] [Google Scholar]
- 16.Kim EH, Bird JA, Kulis M, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127:640–6 e1. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SM, Pons L, Roberts JL, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. doi: 10.1016/j.jaci.2009.05.022. e1-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikkelsen S, Bibby BM, Dolberg MK, Dahl R, Hoffmann HJ. Basophil sensitivity through CD63 or CD203c is a functional measure for specific immunotherapy. Clin Mol Allergy. 2010;8:2–9. doi: 10.1186/1476-7961-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schubert R, Eickmeier O, Garn H, et al. Safety and immunogenicity of a cluster specific immunotherapy in children with bronchial asthma and mite allergy. Int Arch Allergy Immunol. 2009;148:251–60. doi: 10.1159/000161585. [DOI] [PubMed] [Google Scholar]
- 20.Fujisawa T, Nagao M, Hiraguchi Y, et al. Biomarkers for allergen immunotherapy in cedar pollinosis. Allergol Int. 2009;58:163–70. doi: 10.2332/allergolint.09-RAI-0097. [DOI] [PubMed] [Google Scholar]
- 21.Eberlein-Konig B, Ullmann S, Thomas P, Przybilla B. Tryptase and histamine release due to a sting challenge in bee venom allergic patients treated successfully or unsuccessfully with hyposensitization. Clin Exp Allergy. 1995;25:704–12. doi: 10.1111/j.1365-2222.1995.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 22.Kucera P, Cvackova M, Hulikova K, Juzova O, Pachl J. Basophil activation can predict clinical sensitivity in patients after venom immunotherapy. J Inves Allergol Clin Immunol. 2010;20:110–16. [PubMed] [Google Scholar]
- 23.Peternelj A, Silar M, Erzen R, Kosnik M, Korosec P. Basophil sensitivity in patients not responding to venom immunotherapy. Int Arch Allergy Immunol. 2008;146:248–54. doi: 10.1159/000116361. [DOI] [PubMed] [Google Scholar]
- 24.Kosnik M, Silar M, Bajrovic N, Music E, Korosec P. High sensitivity of basophils predicts side-effects in venom immunotherapy. Allergy. 2005;60:1401–6. doi: 10.1111/j.1398-9995.2005.00894.x. [DOI] [PubMed] [Google Scholar]
- 25.Korosec P, Kosnik M. Predicting side-effects in venom immunotherapy by basophil activation: basophil sensitivity vs maximal response. Allergy. 2007;62:81. doi: 10.1111/j.1398-9995.2006.01244.x. [DOI] [PubMed] [Google Scholar]
- 26.Eberlein-Konig B, Schmidt-Leidescher C, Behrendt H, Ring J. Predicting side-effects in venom immunotherapy by basophil activation? Allergy. 2006;61:897. doi: 10.1111/j.1398-9995.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- 27.Eberlein-Konig B, Schmidt-Leidescher C, Rakoski J, Behrendt H, Ring J. In vitro basophil activation using CD63 expression in patients with bee and wasp venom allergy. J Invest Allergol Clin Immunol. 2006;16:5–10. [PubMed] [Google Scholar]
- 28.Plewako H, Wosinska K, Arvidsson M, et al. Basophil interleukin 4 and interleukin 13 production is suppressed during the early phase of rush immunotherapy. Int Arch Allergy Immunol. 2006;141:346–53. doi: 10.1159/000095461. [DOI] [PubMed] [Google Scholar]
- 29.Nagao M, Hiraguchi Y, Hosoki K, et al. Allergen-induced basophil CD203c expression as a biomarker for rush immunotherapy in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2008;146(Suppl 1):47–53. doi: 10.1159/000126061. [DOI] [PubMed] [Google Scholar]
- 30.Horak F, Zieglmayer P, Zieglmayer R, et al. Early onset of action of a 5-grass-pollen 300-IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol. 2009;124:471–7. doi: 10.1016/j.jaci.2009.06.006. 7 e1. [DOI] [PubMed] [Google Scholar]
- 31.Erdmann SM, Sachs B, Kwiecien R, Moll-Slodowy S, Sauer I, Merk HF. The basophil activation test in wasp venom allergy: sensitivity, specificity and monitoring specific immunotherapy. Allergy. 2004;59:1102–9. doi: 10.1111/j.1398-9995.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- 32.Brown SG, Haas MA, Black JA, Parameswaran A, Woods GM, Heddle RJ. In vitro testing to diagnose venom allergy and monitor immunotherapy: a placebo-controlled, crossover trial. Clin Exp Allergy. 2004;34:792–800. doi: 10.1111/j.1365-2222.2004.01949.x. [DOI] [PubMed] [Google Scholar]
- 33.Jutel M, Muller UR, Fricker M, Rihs S, Pichler WJ, Dahinden C. Influence of bee venom immunotherapy on degranulation and leukotriene generation in human blood basophils. Clin Exp Allergy. 1996;26:1112–18. [PubMed] [Google Scholar]
- 34.Siegmund R, Vogelsang H, Machnik A, Herrmann D. Surface membrane antigen alteration on blood basophils in patients with Hymenoptera venom allergy under immunotherapy. J Allergy Clin Immunol. 2000;106:1190–5. doi: 10.1067/mai.2000.110928. [DOI] [PubMed] [Google Scholar]
- 35.Ceuppens JL, Bullens D, Kleinjans H, van der Werf J. Immunotherapy with a modified birch pollen extract in allergic rhinoconjunctivitis: clinical and immunological effects. Clin Exp Allergy. 2009;39:1903–9. doi: 10.1111/j.1365-2222.2009.03379.x. [DOI] [PubMed] [Google Scholar]
- 36.Malveaux FJ, Conroy MC, Adkinson NF, Jr, Lichtenstein LM. IgE receptors on human basophils. Relationship to serum IgE concentration. J Clin Invest. 1978;62:176–81. doi: 10.1172/JCI109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen LH, Holm J, Lund G, Riise E, Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol. 2008;122:298–304. doi: 10.1016/j.jaci.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Lalek N, Kosnik M, Silar M, Korosec P. Immunoglobulin G-dependent changes in basophil allergen threshold sensitivity during birch pollen immunotherapy. Clin Exp Allergy. 2010;40:1186–93. doi: 10.1111/j.1365-2222.2010.03524.x. [DOI] [PubMed] [Google Scholar]
- 39.Gadermaier E, Staikuniene J, Scheiblhofer S, et al. Recombinant allergen-based monitoring of antibody responses during injection grass pollen immunotherapy and after 5 years of discontinuation. Allergy. 2011;66:1174–82. doi: 10.1111/j.1398-9995.2011.02592.x. [DOI] [PubMed] [Google Scholar]
- 40.Francis JN, James LK, Paraskevopoulos G, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. 2008;121:1120–5 e2. doi: 10.1016/j.jaci.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 41.Golden DB, Meyers DA, Kagey-Sobotka A, Valentine MD, Lichtenstein LM. Clinical relevance of the venom-specific immunoglobulin G antibody level during immunotherapy. J Allergy Clin Immunol. 1982;69:489–93. doi: 10.1016/0091-6749(82)90172-5. [DOI] [PubMed] [Google Scholar]
- 42.Muller U, Helbling A, Bischof M. Predictive value of venom-specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy. 1989;44:412–18. doi: 10.1111/j.1398-9995.1989.tb04172.x. [DOI] [PubMed] [Google Scholar]
- 43.Ejrnaes AM, Svenson M, Lund G, Larsen JN, Jacobi H. Inhibition of rBet v 1-induced basophil histamine release with specific immunotherapy -induced serum immunoglobulin G: no evidence that FcgammaRIIB signalling is important. Clin Exp Allergy. 2006;36:273–82. doi: 10.1111/j.1365-2222.2006.02442.x. [DOI] [PubMed] [Google Scholar]
- 44.Wurtzen PA, Lund G, Lund K, Arvidsson M, Rak S, Ipsen H. A double-blind placebo-controlled birch allergy vaccination study II: correlation between inhibition of IgE binding, histamine release and facilitated allergen presentation. Clin Exp Allergy. 2008;38:1290–301. doi: 10.1111/j.1365-2222.2008.03020.x. [DOI] [PubMed] [Google Scholar]
- 45.Flicker S, Steinberger P, Norderhaug L, et al. Conversion of grass pollen allergen-specific human IgE into a protective IgG(1) antibody. Eur J Immunol. 2002;32:2156–62. doi: 10.1002/1521-4141(200208)32:8<2156::AID-IMMU2156>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 46.James LK, Shamji MH, Walker SM, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509–16 e1-5. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 47.Scadding GW, Shamji MH, Jacobson MR, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40:598–606. doi: 10.1111/j.1365-2222.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- 48.Uermosi C, Beerli RR, Bauer M, et al. Mechanisms of allergen-specific desensitization. J Allergy Clin Immunol. 2010;126:375–83. doi: 10.1016/j.jaci.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 49.Michils A, Ledent C, Mairesse M, Gossart B, Duchateau J. Wasp venom immunotherapy changes IgG antibody specificity. Clin Exp Allergy. 1997;27:1036–42. doi: 10.1111/j.1365-2222.1997.tb01255.x. [DOI] [PubMed] [Google Scholar]
- 50.Cady CT, Powell MS, Harbeck RJ, et al. IgG antibodies produced during subcutaneous allergen immunotherapy mediate inhibition of basophil activation via a mechanism involving both FcgammaRIIA and FcgammaRIIB. Immunol Lett. 2010;130:57–65. doi: 10.1016/j.imlet.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kepley CL, Cambier JC, Morel PA, et al. Negative regulation of FcepsilonRI signaling by FcgammaRII costimulation in human blood basophils. J Allergy Clin Immunol. 2000;106:337–48. doi: 10.1067/mai.2000.107931. [DOI] [PubMed] [Google Scholar]
- 52.Wigginton SJ, Furtado PB, Armour KL, et al. An immunoglobulin E-reactive chimeric human immunoglobulin G1 anti-idiotype inhibits basophil degranulation through cross-linking of FcepsilonRI with FcgammaRIIb. Clin Exp Allergy. 2008;38:313–19. doi: 10.1111/j.1365-2222.2007.02896.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat Med. 2002;8:518–21. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu D, Kepley CL, Zhang K, Terada T, Yamada T, Saxon A. A chimeric human–cat fusion protein blocks cat-induced allergy. Nat Med. 2005;11:446–9. doi: 10.1038/nm1219. [DOI] [PubMed] [Google Scholar]
- 55.Kepley CL, Youssef L, Andrews RP, Wilson BS, Oliver JM. Syk deficiency in nonreleaser basophils. J Allergy Clin Immunol. 1999;104:279–84. doi: 10.1016/s0091-6749(99)70367-2. [DOI] [PubMed] [Google Scholar]
- 56.MacGlashan DW., Jr Relationship between spleen tyrosine kinase and phosphatidylinositol 5′ phosphatase expression and secretion from human basophils in the general population. J Allergy Clin Immunol. 2007;119:626–33. doi: 10.1016/j.jaci.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 57.Vilarino N, MacGlashan DW., Jr Actin cytoskeleton-dependent down-regulation of early IgE-mediated signaling in human basophils. J Leukoc Biol. 2004;75:928–37. doi: 10.1189/jlb.0903431. [DOI] [PubMed] [Google Scholar]
- 58.Kepley CL. Antigen-induced reduction in mast cell and basophil functional responses due to reduced Syk protein levels. Int Arch Allergy Immunol. 2005;138:29–39. doi: 10.1159/000087355. [DOI] [PubMed] [Google Scholar]
- 59.MacGlashan D, Jr, Vilarino N. Polymerization of actin does not regulate desensitization in human basophils. J Leukoc Biol. 2009;85:627–37. doi: 10.1189/jlb.1008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lund G, Jacobi H, Skov PS, Holm J, Lund K. Effect on cell surface markers following allergen-induced desensitization of human whole-blood basophils. Int Arch Allergy Immunol. 2010;153:323–34. doi: 10.1159/000316343. [DOI] [PubMed] [Google Scholar]
- 61.MacGlashan D, Jr, Undem BJ. Inducing an anergic state in mast cells and basophils without secretion. J Allergy Clin Immunol. 2008;121:1500–6. doi: 10.1016/j.jaci.2008.04.019. 6 e1–4. [DOI] [PubMed] [Google Scholar]
- 62.Sancho-Serra Mdel C, Simarro M, Castells M. Rapid IgE desensitization is antigen specific and impairs early and late mast cell responses targeting FcepsilonRI internalization. Eur J Immunol. 2011;41:1004–13. doi: 10.1002/eji.201040810. [DOI] [PubMed] [Google Scholar]
- 63.MacGlashan DW., Jr Endocytosis, recycling, and degradation of unoccupied FcepsilonRI in human basophils. J Leukoc Biol. 2007;82:1003–10. doi: 10.1189/jlb.0207103. [DOI] [PubMed] [Google Scholar]
- 64.Paterniti M, Kelly DC, Eckman JA, et al. Cat allergen-induced blood basophil reactivity in vitro predicts acute human nasal allergen challenge responses in vivo. Clin Exp Allergy. 2011;41:963–9. doi: 10.1111/j.1365-2222.2011.03719.x. [DOI] [PubMed] [Google Scholar]


