Abstract
Dysregulations concerning the composition and function of regulatory T cells (Tregs) are assumed to be involved in the pathophysiology of complicated pregnancies. We used six-colour flow cytometric analysis to demonstrate that the total CD4+CD127low+/−CD25+forkhead box protein 3 (FoxP3)+ Treg cell pool contains four distinct Treg subsets: DRhigh+CD45RA-, DRlow+CD45RA-, DR-CD45RA- Tregs and naive DR-CD45RA+ Tregs. During the normal course of pregnancy, the most prominent changes in the composition of the total Treg cell pool were observed between the 10th and 20th weeks of gestation, with a clear decrease in the percentage of DRhigh+CD45RA- and DRlow+CD45RA- Tregs and a clear increase in the percentage of naive DR-CD45RA+ Tregs. After that time, the composition of the total Treg cell pool did not change significantly. Its suppressive activity remained stable during normally progressing pregnancy, but decreased significantly at term. Compared to healthy pregnancies the composition of the total Treg cell pool changed in the way that its percentage of naive DR-CD45RA+ Tregs was reduced significantly in the presence of pre-eclampsia and in the presence of preterm labour necessitating preterm delivery (PL). Interestingly, its percentage of DRhigh+CD45RA- and DRlow+CD45RA- Tregs was increased significantly in pregnancies affected by pre-eclampsia, while PL was accompanied by a significantly increased percentage of DR-CD45RA- and DRlow+CD45RA- Tregs. The suppressive activity of the total Treg cell pool was diminished in both patient collectives. Hence, our findings propose that pre-eclampsia and PL are characterized by homeostatic changes in the composition of the total Treg pool with distinct Treg subsets that were accompanied by a significant decrease of its suppressive activity.
Keywords: pre-eclampsia, pregnancy, preterm labour, regulatory T cells, subsets of regulatory T cells
Introduction
For decades, immunologists have been engaged in the investigation of the cellular mechanisms which prevent the rejection of the semi-allogeneic fetus during the normal course of pregnancy. It is known that special tolerance-inducing mechanisms, such as the expression of Fas-ligand (FasL), human leucocyte antigen-G (HLA-G) and indolamine-2,3-dioxygenase (IDO) in the syncytiotrophoblast act locally at the feto–maternal interface [1–3]. However, a bi-directional feto–maternal trafficking of cells during pregnancy is now well established [4], and it was shown that the maternal immune system is increasingly stimulated with ongoing pregnancy [5]. Therefore, further systemic changes are necessary to antagonize maternal immune reactions which could cause the rejection of the fetus, expressing paternal alloantigens. Currently, regulatory T cells (Tregs) are assumed primarily to be involved in the maintenance of tolerance during pregnancy. It has been reported that murine pregnancy is associated with elevated Treg numbers in the circulation [6], and that these Tregs prevent the immunological rejection of the fetus both in mice and humans [7,8]. However, in humans, changes of peripheral Treg cell counts during the normal course of pregnancy still remain a controversial issue. Earlier studies document increased Treg cell counts in pregnant women compared to non-pregnant women [9], while more recently it was postulated that Treg cell counts decreased during the normal course of pregnancy [10–12]. Obviously, the number of Tregs depends strongly on the gating strategy by which the Tregs were characterized after staining cells for flow cytometric analysis and on the moment at which the Treg cell numbers were determined during the course of pregnancy. Similarly, reports evaluating functionality of Tregs are also inconsistent. A decrease of their suppressive activity only at the end of normal pregnancy is reported by our group [13], while most others did not detect any differences in the suppressive activity between non-pregnant and pregnant women [11,14].
As Treg cells were shown to guarantee the maintenance of feto–maternal tolerance during the normal course of pregnancy, it is assumed that dysregulations concerning their number and function are involved in the pathogenesis of complications, such as gestation-associated hypertensive diseases and preterm intrauterine activation. Currently, decreased peripheral Treg cell numbers are reported for pregnant women with pre-eclampsia [14–16], deficiencies of their functional activity were ascertained by our group, especially for patients with pre-eclampsia [16], and for patients with preterm labour necessitating preterm delivery [13]. Thereby, we demonstrated that magnetically selected CD4+CD127low+/−CD25+ Treg cells contain a distinct Treg cell subset expressing very high levels of forkhead box transcription factor protein 3 (FoxP3) and human leucocyte antigen-DR (HLA-DR). HLA-DR expression of these FoxP3+DR+ Treg cells was reduced significantly, not only in preterm-labouring women, but also in kidney transplanted patients with acute rejection. The suppressive activity of the whole Treg pool declined in parallel to the number of FoxP3+DRhigh+ Treg cells, disclosing the important role of this Treg subpopulation for the maintenance of tolerance [13]. Recently, such non-proliferating DR+ Tregs were shown to express higher levels of FoxP3 and induced a more vigorous rapid T cell suppression than the Tregs that lack HLA-DR expression [17]. In particular, these highly suppressive DR+ Tregs were found to become inactivated by highly activated CD4+ responder T cells which produced high amounts of Granzyme B, and it was shown that these DR+ Tregs were much more prone to undergo Granzyme B-induced cell death than the DR- Tregs[18]. Although the majority of DR+ and DR- Treg cells in adults represent CD45RO+ memory Treg cells, a considerable percentage of naive CD45RA+ Tregs can also be detected in the circulation of adults. However, this percentage decreases with increasing age [19]. Recently, an impaired functional activity of such naive CD45RA+ Tregs was ascertained for patients with multiple sclerosis (MS) [20], and there is a growing body of evidence that the composition of the total Treg cell pool with different Treg cell subsets potentially influences its suppressive activity.
In this study, we used six-colour flow cytometric analysis to demonstrate that the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool consists of four distinct Treg cell subsets: DRhigh+CD45RA-, DRlow+CD45RA-, DR-CD45RA- Tregs and naive DR-CD45RA+ Tregs. Compared to non-pregnant women, the composition of the total Treg pool changed in the way that its percentage of naive DR-CD45RA+ Tregs increased, while that of DRhigh+CD45RA- and DRlow+CD45RA- Tregs decreased during the normal course of pregnancy. In the presence of complications, such as pre-eclampsia or preterm labour necessitating preterm delivery (PL), the composition of the total Treg pool was strongly modified and its suppressive activity was strongly diminished. Therefore, it could be assumed that the occurrence of certain gestational diseases is associated with characteristic homeostatic changes in the composition of the total Treg cell pool and that such changes may have a potential effect on its suppressive activity.
Methods
Patient collectives and healthy volunteers
Blood samples were obtained from 31 non-pregnant fertile female volunteers (group A), 135 healthy pregnant women during the normal course of pregnancy [group 1 (11–14 weeks' gestation, group 2 (15–23 weeks' gestation), group 3 (24–36 weeks' gestation), group 4 (37 42 weeks' gestation)], 27 pregnant women affected by pre-eclampsia (group 5, 27–39 weeks' gestation), 15 pregnant women affected by haemolysis-elevated liver enzyme levels-low platelet count syndrome (HELLP) syndrome (group 6, 24–39 weeks' gestation) and 54 pregnant women affected by preterm intrauterine activation (23–36 weeks' gestation) which was diagnosed in the presence of cervical insufficiency or preterm labour. All these women received tocolytic and corticoid treatment until fetal lung maturity (34 weeks' gestation) was achieved. These patients were grouped as cervical insufficiency (CI) in cases of cervical shortening of less than 20 mm before 36 completed weeks' gestation in the absence of labour leading to preterm delivery (group 7, n = 30, 24–35 weeks' gestation). The diagnosis of preterm labour necessitating preterm delivery (PL) was made in the case of the occurrence of spontaneous preterm labour (before 36 completed weeks' gestation) which was resistant to tocolytic treatment and which led to irresistible preterm delivery (group 8, n = 24, 23–36 weeks' gestation). Blood samples from healthy pregnancies (groups 1–4) were collected from women who had routine ultrasonography to exclude fetal malformations (groups 1–3) and from women delivering spontaneously or by elective caesarean section at term (group 4). Blood samples from affected pregnancies were collected during their hospitalization due to diagnosis of pre-eclampsia (group 5), HELLP syndrome (group 6), CI (group 7) or PL (group 8). Pre-eclampsia was diagnosed as blood pressure higher than 140/90 mm Hg on two separate occasions, 6 h apart, along with significant proteinuria (>300 mg/l in a 24-h collection or a dipstick reading of >2+ on a voided random urine sample in the absence of urinary tract infection) in previously normotensive women. The diagnosis of HELLP syndrome was made on the basis of haemolysis, elevated liver enzyme levels and thrombocytopenia. The laboratory parameters of these patients were thrombocyte count < 150·000 cell/µl and aspartate aminotransferase and alanine aminotransferase > 30 U/l. All patients in this group had significant proteinuria and showed characteristic clinical symptoms such as left-sided epigastric pain, flickering in front of the eyes and hyperreflexia. The study was approved by the Regional Ethics Committee. All women were fully informed of the aim of the study and informed consent was obtained from all participants.
Both the percentage of CD4+CD127low+/−CD25+FoxP3+ Treg cells of total CD4+ T cells and the percentage of DRhigh+CD45RA- Tregs, DRlow+CD45RA- Tregs, DR-CD45RA- Tregs and naive DR-CD45RA+ Tregs within the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool were determined by six-colour flow cytometric analysis for all participants. In order to control whether the results obtained on the percentages of the different Treg subsets within the total Treg cell pool correspond to their absolute numbers within the CD4+ T cell pool, their percentages were additionally calculated relative to the total CD4+ T cell pool. For a total number of six healthy volunteers, 33 healthy pregnant women, 15 pregnant women affected by gestation-associated hypertensive diseases (pre-eclampsia and HELLP syndrome) and 18 pregnant women affected by preterm intrauterine activation (CI and PL) suppression assays were performed in order to test the suppressive capacity of the magnetically selected total CD4+CD127low+/−CD25+ Treg cell pool.
Fluorescence-activated cell sorter (FACS) staining
Venous blood samples (10 ml) from all participants were collected into ethylenediamine tetraacetic acid (EDTA)-containing tubes. Whole peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Amersham Bioscience, Freiburg, Germany) gradient centrifugation and analysed by six-colour flow cytometric analysis. Briefly, PBMCs (4 × 106 cells) were surface-stained with peridinin chlorophyll (PerCP)-conjugated anti-CD4 (BD Bioscience, Heidelberg, Germany), phycoerythrin (PE)-conjugated anti-CD127 (eBioscience, Frankfurt, Germany), allophycocyananin-cyanin 7 (APC-Cy7)-conjugated anti-CD25, PE-Cy7-conjugated anti-HLA-DR (BD Bioscience) and APC-conjugated anti-CD45RA (BD Bioscience) mouse monoclonal antibodies. Subsequently, intracellular staining for the detection of FoxP3 was performed using a fluorescein isothiocyanate (FITC)-labelled anti-human FoxP3 staining set (clone PCH101, eBioscience) according to the manufacturer's instructions. Both the percentage of CD4+CD127low+/−CD25+FoxP3+ Treg cells and the percentages of the four Treg subsets (DRhigh+CD45RA- Tregs, DRlow+CD45RA- Tregs, DR-CD45RA- Tregs and naive DR-CD45RA+ Tregs) within the total Treg cell pool were determined for all participants. For all women, the percentages of these Treg subsets were additionally calculated relative to the total CD4+ T cell pool. Negative control samples were incubated with isotype-matched antibodies. Dead cells were excluded by forward- and side-scatter characteristics. Cells were analysed by a FACS Canto cytometer (BD Bioscience). Statistical analysis was based on at least 100 000 gated CD4+ T cells.
Positive selection and staining of CD4+CD127low+/−CD25+ Treg cells
Whole peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood (50 ml) drawn in EDTA tubes by Ficoll-Hypaque (Amersham Bioscience) gradient centrifugation. CD4+CD127low+/−CD25+ Treg cells were purified using the CD4+CD127low+/−CD25+ Regulatory T cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions. First, CD4+CD127low+/− T cells were isolated by magnetic depletion of non-CD4+CD127high+ T cells. In a second step, the CD4+CD127low+/−CD25+ Treg cells were isolated by positive selection over two consecutive columns. The CD4+CD127low+/−CD25- T cells were obtained in the flow-through fraction and used as responder T cells (Tresp). The CD4+CD127low+/−CD25+ Treg cells were subsequently retrieved from the columns. The purified CD4+CD127low+/−CD25+ Treg cell fraction was analysed using four-colour flow cytometry. Briefly, 1 × 105 cells were stained with 10 µl PerCP-conjugated-anti-CD4, PE-conjugated anti-CD25, FITC-conjugated FoxP3 and biotin-conjugated anti-CD127 mouse monoclonal antibodies. Positive staining for CD127 was detected using APC-conjugated streptavidin molecules. On average, 85% of the isolated CD4+CD127low+/−CD25+ Treg cells were shown to be within the CD4+CD127low+/−CD25+Foxp3+ Treg cell population.
Co-culture suppression assay
Whole peripheral mononuclear cells (PBMCs) were isolated from peripheral blood drawn in EDTA tubes by Ficoll-Hypaque (Amersham Bioscience) gradient centrifugation. CD4+CD127low+/−CD25+ Treg cells were purified using the CD4+CD127low+/−CD25+ Regulatory T cell Isolation Kit II (Miltenyi Biotec) described above. In all assays, 2 × 104 Tresp were co-cultured with the purified CD4+CD127low+/−CD25+ Treg cells at ratios 1:1–1:64 in 96-well v-bottomed plates. Suppression assays were performed in a final volume of 100 µl/well of X-VIVO15 medium (Lonza, Verviers, Belgium). For T cell stimulation, the medium was supplemented with 1 µg/ml anti-CD3 and 2 µg/ml anti-CD28 antibodies (eBioscience). As controls, CD4+CD127low+/−CD25+ Treg cells and Tresp cells alone were cultured both with and without any stimulus. Cells were incubated at 37°C and 5% of CO2. After 4 days 1 µCi [3H]-thymidine was added to the cultures and cells were incubated further for 16 h. Cells were then harvested and [3H] incorporation was measured by scintillation counting. For each donor, the co-culture suppression assay was repeated six times and exhibited <10% standard error of the mean (s.e.m.). For each patient group, the co-culture suppression assays were performed with blood samples obtained from at least six different donors. In order to compare the suppressive capacity of the isolated CD4+CD127low+/−CD25+ Tregs between the different patient groups, we calculated the maximum suppressive activity (ratio of Treg cells to Tresp 1:1). In addition, we determined the suppressive activity of the isolated Treg cells with gradient ratios of Treg cells to Tresp cells (ratio of Treg cells to Tresp cells 1:1–1:64) and identified the ratio with which a minimum suppression of at least 15% could be achieved (titre Treg/Tresp). A minimum suppression value of 15% was determined, because this value was the lowest which was able to guarantee a significant reduction of the suppressive activity.
Sorting and functional testing of the DRhigh+CD45RA- Treg cell subset
Venous blood samples (100 ml) from three different healthy, non-pregnant volunteers were collected into EDTA-containing tubes. Whole peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Amersham Bioscience) gradient centrifugation. For fluorescence activated cell sorting of the DRhigh+CD45RA- Treg cell subset, CD4+CD127low+/−CD25+ Treg cells were purified using the CD4+CD127low+/−CD25+ Regulatory T cell Isolation Kit II (Miltenyi Biotec), as described above. Respectively, 5 × 105 cells of the isolated CD4+CD127low+/−CD25+ Treg cells were stained with 20 µl FITC-conjugated anti-CD4 (BD Bioscience), 10 µl PE-conjugated anti-CD25 (BD Bioscience) and 20 µl PE-Cy7-conjugated anti-HLA-DR (BD Bioscience) mouse monoclonal antibodies. Contaminating non-CD4+ T cells were excluded, while the remaining cells were sorted using a FACS-VantageSE-Sorter (BD Bioscience). Thereby, the CD4+CD127low+/−CD25+ Treg cells were divided into a Treg population consisting exclusively of DRhigh+CD45RA- Treg cells and a Treg population consisting of the remaining DRlow+CD45RA- Tregs, DR-CD45RA- Tregs and naive DR-CD45RA+ Tregs. Subsequently, the suppressive activity of both Treg populations was analysed using the above-described suppression assay.
Statistical analysis
Statistical comparison of the percentages of CD4+CD127low+/−CD25+FoxP3+ Treg cells within CD4+ T cells and of the percentages of the different Treg subsets (DRlow+CD45RA- Tregs, DRhigh+CD45RA- Tregs, DR-CD45RA- Tregs and naive DR-CD45RA+ Tregs) within both the total CD4+ T cell pool and the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool, between the different patient populations, was performed using the non-parametric Kruskal–Wallis H-test, which is used for simultaneous comparison of more than two sample populations. Each H-test was followed by a Dunn test. Comparison of the suppressive activity of purified CD4+CD127low+/−CD25+ Treg cells was also performed using the Kruskal–Wallis test. P < 0·05 was considered significant.
Results
The suppressive activity, but not the percentage of CD4+CD127low+/−CD25+FoxP3+ Treg cells within the total CD4+ T cell pool is reduced significantly in both patients affected by pre-eclampsia and patients affected by preterm labour leading to preterm delivery
Both the percentage of CD4+CD127low+/−CD25+FoxP3+ Treg cells within total CD4+ T cells and their composition with four distinct Treg cell subsets were determined in the circulation of non-pregnant female volunteers (group A), healthy pregnant women during the normal course of pregnancy (groups 1–4) and in pregnant women affected by pre-eclampsia (group 5), HELLP syndrome (group 6), CI (group 7) or PL (group 8). Peripheral blood mononuclear cells (PBMCs) obtained from each participant were stained with anti-CD4, anti-CD127, anti-CD25, anti-FoxP3, anti-HLA-DR and anti-CD45RA monoclonal antibodies and analysed by six-colour flow cytometric analysis. Figure 1 shows the gating strategy for these measurements. First, CD4+ T cells (Fig. 1a, P1) were analysed for their simultaneous expression of CD25, CD127 and FoxP3 (Fig. 1b, P2; c, P3). At first, the percentage of CD4+CD127low+/−CD25+FoxP3+ Treg cells (Fig. 1c, P3) within the total CD4+ T cell pool and their composition with four distinct subsets [DRhigh+CD45RA- Tregs (P4), DRlow+CD45RA- Tregs (P5), DR-CD45RA- Tregs (P6), and naive DR-CD45RA+ Tregs (P7)] were determined for each participant (Fig. 1d). Figure 1a–d shows the gating strategy of one representative experiment for healthy pregnancies; Fig. 1e–h shows the gating strategy of one representative experiment performed with patients affected by HELLP syndrome, pre-eclampsia, CI and PL, respectively.
Fig. 1.
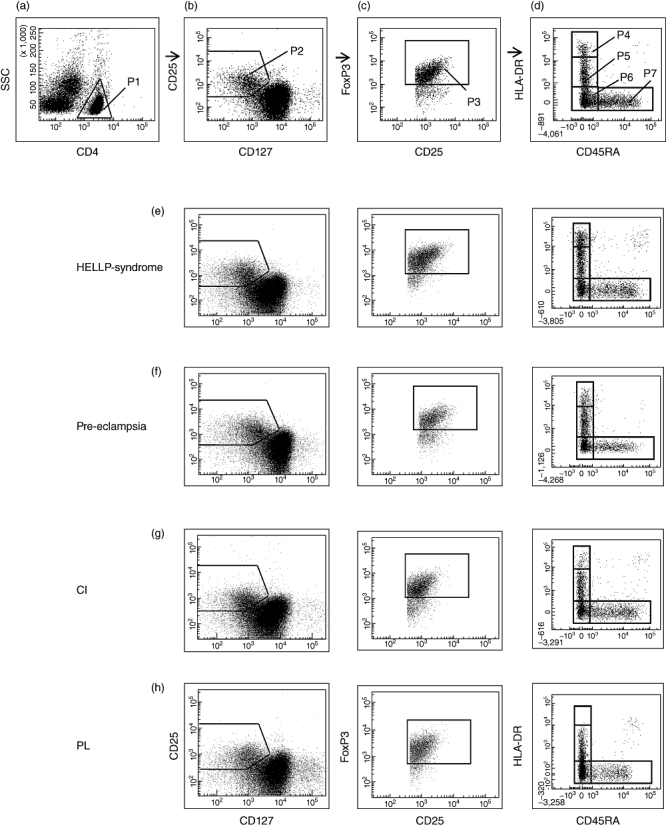
Gating strategy for six-colour flow cytometric detection of the total CD4+CD127low+/−CD25+forkhead box protein 3 (FoxP3+) regulatory T cell (Treg) cell pool and its percentage of naive DR-CD45RA+ Tregs, DR-CD45RA- Tregs, DRlow+CD45RA- Tregs and DRhigh+CD45RA- Tregs. (a) First, CD4+ T cells (P1) were gated by fluorescence intensity of CD4 versus side light-scatter (SSC). (b) CD4+CD127low+/−CD25+ Treg cells were gated by fluorescence intensity of CD25 versus CD127 (P2). (c) CD4+CD127low+/−CD25+FoxP3+ Treg cells were gated by excluding cells without FoxP3 expression (P3). (d) The percentage of the DRhigh+CD45RA- (P4), the DRlow+CD45RA- (P5), the DR-CD45RA- (P6) and the naive DR-CD45RA+ Treg subset (P7) was estimated by analysing CD4+CD127low+/−CD25+FoxP3+ Treg cells (P3) for their expression of human leucocyte antigen D-related (HLA-DR) and CD45RA. (a–d) Shows a representative experiment for healthy pregnancies, (e–h) show representative experiments for patients with HELLP syndrome (e), pre-eclampsia (f), CI (g) and PL (h). CI: cervical insufficiency; PL: preterm labour necessitating preterm delivery; HELLP syndrome: haemolysis-elevated liver enzyme level-low platelet count syndrome.
In analogy to our previous studies, when using three- or four-colour flow cytometric analysis for the detection of CD4+CD25+FoxP3+ Treg cells [16] or CD4+CD127low+/−CD25+ Treg cells [13], we observed a decreasing percentage of CD4+CD127low+/−CD25+FoxP3+ Tregs within the total CD4+ T cell pool during the normal course of pregnancy. Compared to healthy pregnant women in the first-trimester (group 1), the percentage of CD4+CD127low+/−CD25+FoxP3+ Treg cells was reduced significantly in term pregnant women (group 4) (Fig. 2a,b). However, in the present study, we did not detect a significantly decreased percentage of CD4+CD127low+/−CD25+FoxP3+ Treg cells of CD4+ T cells in patients with gestation-associated hypertensive diseases (pre-eclampsia, HELLP syndrome) or preterm intra-uterine activation (CI, PL) compared to healthy third-trimester pregnancies (groups 3 and 4) (Fig. 2a,b).
Fig. 2.
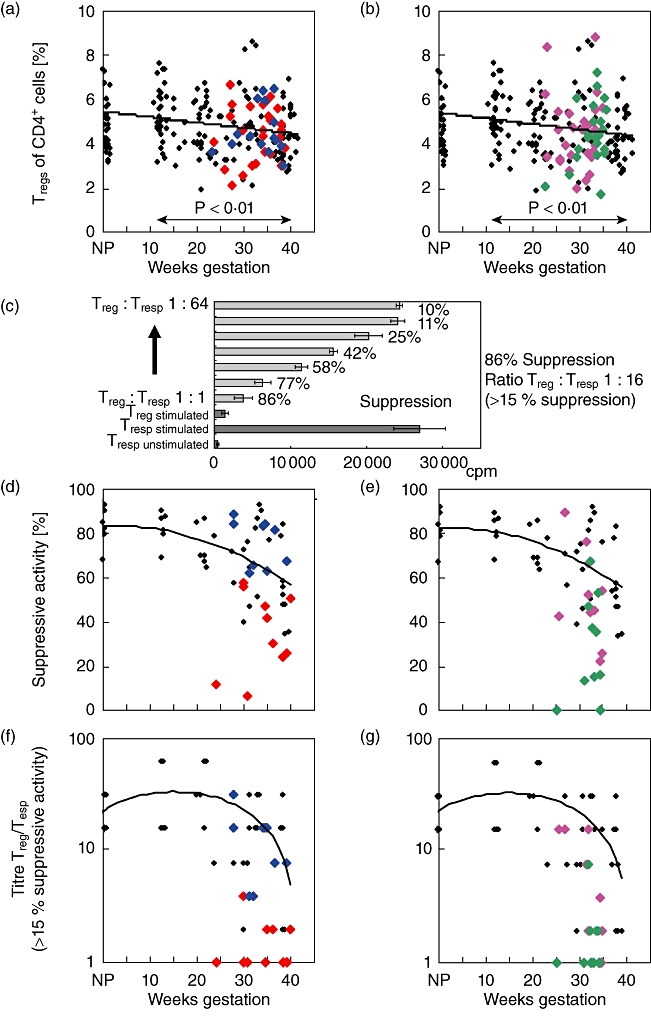
Detection of CD4+CD127low+/−CD25+forkhead box protein 3 (FoxP3+) Treg cells and their suppressive activity in healthy and complicated pregnancies. The percentage of CD4+CD127low+/−CD25+FoxP3+ Tregs of total CD4+ T cells was estimated in pregnancies affected by pre-eclampsia ( ) or HELLP syndrome (
) or HELLP syndrome ( ) (a), and in pregnancies affected by CI (
) (a), and in pregnancies affected by CI ( ) or PL (
) or PL ( ) (b), in comparison to healthy pregnancies (♦). CD4+CD127low+/−CD25+ Treg cells were isolated by the magnetic affinity cell sorting (MACS) technique and their suppressive activity was examined using suppression assays (c) (see Methods). The figure shows the maximum suppressive activity (Treg/Tresp = 1/1) (d,e) and the ratio of Treg/Tresp (titre) up to which the purified Treg cells could be diluted to achieve a minimum suppressive activity of at least 15% (f,g). The diagrams represent the individual data and the regression line obtained for healthy non-pregnant volunteers (group a; n = 6), healthy pregnancies during the normal course of pregnancy (groups 1–4; n = 36), pregnancies affected by pre-eclampsia (group 5, n = 10) or HELLP syndrome (group 6; n = 9) (d,f) and pregnancies affected by CI (group 7, n = 10) or PL (group 8; n = 10) (e,g). Compared to healthy third-trimester women (groups 3 and 4), the suppressive activity of the isolated CD4+CD127low+/−CD25+ Tregs from women with pre-eclampsia (group 5) and from women with PL (group 8) was reduced significantly, concerning both the maximum suppressive activity (P < 0·01) and the ratio of Treg/Tresp up to which a minimum suppressive activity of 15% could be achieved (P < 0·001). CI: cervical insufficiency; PL: preterm labour necessitating preterm delivery; HELLP syndrome: haemolysis-elevated liver enzyme level-low platelet count syndrome.
) (b), in comparison to healthy pregnancies (♦). CD4+CD127low+/−CD25+ Treg cells were isolated by the magnetic affinity cell sorting (MACS) technique and their suppressive activity was examined using suppression assays (c) (see Methods). The figure shows the maximum suppressive activity (Treg/Tresp = 1/1) (d,e) and the ratio of Treg/Tresp (titre) up to which the purified Treg cells could be diluted to achieve a minimum suppressive activity of at least 15% (f,g). The diagrams represent the individual data and the regression line obtained for healthy non-pregnant volunteers (group a; n = 6), healthy pregnancies during the normal course of pregnancy (groups 1–4; n = 36), pregnancies affected by pre-eclampsia (group 5, n = 10) or HELLP syndrome (group 6; n = 9) (d,f) and pregnancies affected by CI (group 7, n = 10) or PL (group 8; n = 10) (e,g). Compared to healthy third-trimester women (groups 3 and 4), the suppressive activity of the isolated CD4+CD127low+/−CD25+ Tregs from women with pre-eclampsia (group 5) and from women with PL (group 8) was reduced significantly, concerning both the maximum suppressive activity (P < 0·01) and the ratio of Treg/Tresp up to which a minimum suppressive activity of 15% could be achieved (P < 0·001). CI: cervical insufficiency; PL: preterm labour necessitating preterm delivery; HELLP syndrome: haemolysis-elevated liver enzyme level-low platelet count syndrome.
In order to examine whether the suppressive activity of the total CD4+CD127low+/−CD25+ Treg cell pool from affected pregnancies was impaired compared to healthy pregnancies, we used a co-culture suppression assay [13]. The suppression assays were performed using magnetic affinity cell sorting (MACS)-sorted CD4+CD127low+/−CD25+ Treg cells obtained from six non-pregnant volunteers (group A), 36 healthy pregnant women during the normal course of pregnancy (groups 1–4) and from 10 pregnant women affected by pre-eclampsia (group 5), HELLP syndrome (group 6), CI (group 7) and PL (group 8), respectively. Figure 2c shows the results of one representative experiment for healthy third-trimester women. Figure 2d–g and Table 2 show the results of these suppression assays for affected pregnancies in comparison to healthy pregnancies. The maximum suppressive capacity (Treg/Tresp = 1/1) of isolated CD4+CD127low+/−CD25+ Tregs obtained from healthy first- and second-trimester women was in the same range as that of non-pregnant women. It remained relatively stable during the normal course of pregnancy (groups 1, 2 and 3). However, after 30 weeks of gestation it decreased continuously and reached significantly reduced levels (P < 0·05) at the end of pregnancy near term (group 4, Fig. 2d,e). In addition, we determined the suppressive activity of the isolated Treg cells using gradient ratios of Treg cells to responder cells (Treg/Tresp 1:1–1:64) and identified the ratio with which a suppression of at least 15% could be achieved (titre Treg/Tresp). During the normal course of pregnancy, this ratio was in the same range as that of non-pregnant women, but it was also decreased significantly (P < 0·05) at the end of pregnancy near term (group 4) (Fig. 2f,g). Hence, the suppressive activity of the Treg cells ceased considerably near term.
Table 2.
Percentage of the CD4+CD127low+/−CD25+forkhead box protein 3 (FoxP3+) regulatory T cell (Treg) cell pool within CD4+ T cells, its suppressive activity and its composition with distinct Treg subsets in healthy and affected pregnancies
| Healthy pregnancy groups 3 and 4 | Pre-eclampsia group 5 | HELLP syndrome group 6 | CI group 7 | PL group 8 | |
|---|---|---|---|---|---|
| CD4+ T cells (%) | 29·7 (13·1–48·9) | 28·9 (7·1–42·6) | 29·7 (12·5–40·2) | 33·7 (9·7–52·1) | 22·0 ↓ (9·0–46·7) |
| Treg cells (%) | 4·4 (2·1–8·7) | 4·6 (2·1–6·7) | 4·3 (3·0–6·5) | 4·7 (2·1–8·9) | 4·5 (1·8–7·3) |
| Suppressive activity | |||||
| Max. suppr. activity (%) (Treg/Tresp 1/1) | 59·9 (35·8–93·8) | 37·3 ↓ (7·9–59·0) | 82·2 (62·9–89·9) | 50·9 (25·0–90·9) | 28·2 ↓ (0–68·9) |
| Titre of Treg (>15% suppr. activity) | 1:16 (1:2–1:32 | 1:1 ↓ (1:1–1:4) | 1:16 (1:4–1:32) | 1:4 (1:1–1:16) | 1:1 ↓ (1:1–1:8) |
| Subsets | |||||
| of the Treg cell pool (%) | |||||
| DR-CD45RA Tregs | 38·6 (19·1–68·9) | 27·9 ↓ (8·1–48·6) | 33·6 (15·0–57·6) | 35·4 (11·4–60·9) | 26·5 ↓ (7·1–39·8) |
| DR-CD45RA- Tregs | 30·4 (13·9–48·2) | 30·5 (18·8–46·8) | 25·4 (18·7–53·3) | 33·4 (16·1–46·5) | 36·1 ↑ (28·0–59·6) |
| DRlow+CD45RA- Tregs | 23·6 (12·4–39·6) | 27·1 ↑ (21·8–43·0) | 26·4 (15·5–40·0) | 24·8 (12·8–36·6) | 28·2 ↑ (20·1–55·5) |
| DRhigh+CD45RA- Tregs | 4·8 (1·4–11·1) | 6·1 ↑ (0·1–27·7) | 6·7 ↑ (3·7–18·0) | 4·1 (1·0–9·8) | 5·3 (0·9–10·3) |
| of the CD4+ T cell pool (%) | |||||
| DR-CD45RA+ Tregs | 2·4 (1·0–5·4) | 1·5 ↓ (0·5–4·0) | 1·7 (1·0–2·8) | 2·0 (1·1–4·4) | 1·6 ↓ (0·3–2·8) |
| DR-CD45RA- Tregs | 1·2 (0·5–2·5) | 1·2 (0·6–2·5) | 0·9 (0·5–1·9) | 1·4 (0·6–2·7) | 1·5 ↑ (0·6–2·5) |
| DRlow+CD45RA- Tregs | 1·1 (0·6–2·3) | 1·6 ↑ (0·8–2·5) | 1·2 (0·7–3·0) | 1·2 (0·5–3·9) | 1·7 ↑ (0·7–2·9) |
| DRhigh+CD45RA- Tregs | 0·2 (0–0·6) | 0·3 ↑ (0·1–1·2) | 0·3 ↑ (0·1–0·9) | 0·1 (0–0·5) | 0·2 (0–0·5) |
↑↓Significant differences were obtained compared to healthy pregnancies; CI: cervical insufficiency; HELLP: haemolysis-elevated liver enzyme levels-low platelet count syndrome; PL: preterm labour necessitating preterm delivery.
Tables 1 and 2 summarize the data regarding the maximum suppressive activity (Treg/Tresp 1:1) and the ratio of Treg/Tresp cells that led to a minimum suppressive activity of less than 15%. Compared to healthy third-trimester women (groups 3 and 4, 24–42 weeks' gestation), the suppressive activity of the isolated CD4+CD127low+/−CD25+ Tregs from women with pre-eclampsia (group 5) and from women with PL (group 8) was reduced strongly concerning the maximum suppressive activity (P < 0·01) and concerning the ratio (titre Treg/Tresp) with which a minimum suppressive activity of 15% could be achieved (P < 0·001) (Fig. 2d–g and Table 2). Surprisingly, the suppressive activity of Treg cells from women with HELLP syndrome (group 6) was in the normal range, similar to that of Treg cells from healthy third-trimester women (groups 3 and 4). The suppressive activity of Treg cells from women with CI (group 7) was slightly reduced. However, a significant difference compared to healthy third-trimester women (group 3) was not achieved (Fig. 2d–g and Table 2).
Table 1.
Percentage of the CD4+CD127low+/−CD25+FoxP3+ regulatory T cell (Treg) cell pool within CD4+ T cells, its suppressive activity and its composition with distinct Treg subsets in non-pregnant women and healthy pregnant women during the normal course of pregnancy
| Group A non-pregnant | Group 1 11–14 weeks | Group 2 15–23 weeks | Group 3 24–36 weeks | Group 4 37–42 weeks | |
|---|---|---|---|---|---|
| CD4+ T cells (%) | 37·4 (24·7–50·8) | 29·3 ↓ (21·2–46·8) | 31·9 ↓ (16·7–50·4) | 28·8 ↓ (16·7–48·9) | 32·8 ↓ (13·1–46·2) |
| Treg cells (%) | 4·7 (3·2–7·4) | 5·1 (3·2–7·7) | 4·5 (2·0–6·8) | 4·9 (2·1–8·7) | 4·3 (2·8–7·0) |
| Suppressive activity | |||||
| Max. suppr. activity (%) (Treg/Tresp 1/1) | 84·5 (69·7–94·1) | 84·8 (70·1–91·0) | 71·3 (66·1–87·6) | 74·1 (41·5–93·8) | 55·2 ↓ (35·8–85·0) |
| Titre of Treg (>15% suppr. activity) | 1:16 (1:16–1:32) | 1:32 (1:16–1:64) | 1:16 (1:8–1:64) | 1:16 (1:2–1:32) | 1:8 ↓ (1:2–1:32) |
| Subsets | |||||
| of the Treg cell pool (%) | |||||
| DR-CD45RA+ Tregs | 28·5 (9·8–51·5) | 31·9 (17·9–47·9) | 37·2 (21·0–55·9) | 36·2 (21·7–55·7) | 39·1 ↑ (19·1–68·9) |
| DR-CD45RA- Tregs | 30·7 (19·8–43·9) | 32·7 (21·4–42·3) | 31·6 (21·5–40·2) | 30·6 (18·7–48·2) | 30·2 (13·9–41·0) |
| DRlow+CD45RA- Tregs | 28·8 (20·6–44·4) | 29·3 (15·7–42·9) | 24·1 (11·6–36·9) | 23·8 (14·3–39·6) | 23·5 ↓ (12·4–31·4) |
| DRhigh+CD45RA- Tregs | 8·3 (4·2–20·0) | 8·0 (2·7–14·8) | 5·0 (1·9–8·8) | 5·4 (1·6–9·0) | 4·4 ↓ (1·4–11·1) |
| of the CD4+ T cell pool (%): | |||||
| DR-CD45RA+ Tregs | 1·9 (0·8–3·5) | 2·0 (1·0–3·8) | 2·3 (1·0–3·9) | 2·3 (1·0–5·4) | 2·4 ↑ (1·3–4·4) |
| DR-CD45RA- Tregs | 1·5 (0·7–2·4) | 1·6 (0·9–2·8) | 1·3 (0·9–2·1) | 1·2 (0·5–2·5) | 1·1 (0·1–2·1) |
| DRlow+CD45RA- Tregs | 1·5 (0·9–3·6) | 1·5 (0·7–3·3) | 1·3 (0·7–2·3) | 1·3 (0·6–2·3) | 1·1 ↓ (0·6–2·0) |
| DRhigh+CD45RA- Tregs | 0·3 (0·1–0·7) | 0·2 (0·1–0·6) | 0·2 (0·1–0·7) | 0·2 (0·1–0·5) | 0·2 ↓ (0–0·6) |
↑↓Sgnificant differences were obtained compared to non-pregnant women.
The normal course of pregnancy is associated with changes in the composition of the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool with distinct Treg cell subsets
In order to examine whether the reduced suppressive activity of the total CD4+CD127low+/−CD25+Foxp3+ Treg cell pool could be correlated with changes of its composition with distinct Treg cell subsets, we monitored their percentages during the normal course of pregnancy and in the presence of characteristic gestation-associated diseases. To this end, PBMCs were additionally stained with anti-HLA-DR- and anti-CD45RA-specific antibodies and analysed by six-colour flow cytometric analysis. This resulted in the detection of four different Treg subpopulations, as shown in Fig. 1d. The percentages of these Treg cell subsets within the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool were estimated in the circulation of healthy pregnant women from first trimester on until term. Figure 3 and Table 1 show the changes of these four Treg cell subsets during the normal course of pregnancy. The percentage of DRlow+CD45RA- and DRhigh+CD45RA- Treg cells (Fig. 3a,c) decreased significantly between the 10th and the 20th weeks of gestation and subsequently remained at the same level until term (Fig. 3b,d). Simultaneously, the percentage of naive DR-CD45RA+ Treg cells (Fig. 3e) increased significantly and also remained stable during the following course of pregnancy (Fig. 3f). The subset of DR-CD45RA- Treg cells (Fig. 3g) did not show any changes during the normal course of pregnancy (Fig. 3h). Obviously, these changes in the composition of the CD4+CD127low+/−CD25+FoxP3+ Treg cell pool between the 10th and 20th weeks of gestation were not accompanied by changes in its suppressive activity (Fig. 2d–g). However, the suppressive activity of the total CD4+CD127low+/−CD25+ Treg cell pool decreased significantly near term, at a gestational age when no further changes in the composition of the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool could be detected. Such findings may indicate that, besides the composition of the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool with distinct Treg cell subsets, further parameters may have an influence on its suppressive activity.
Fig. 3.
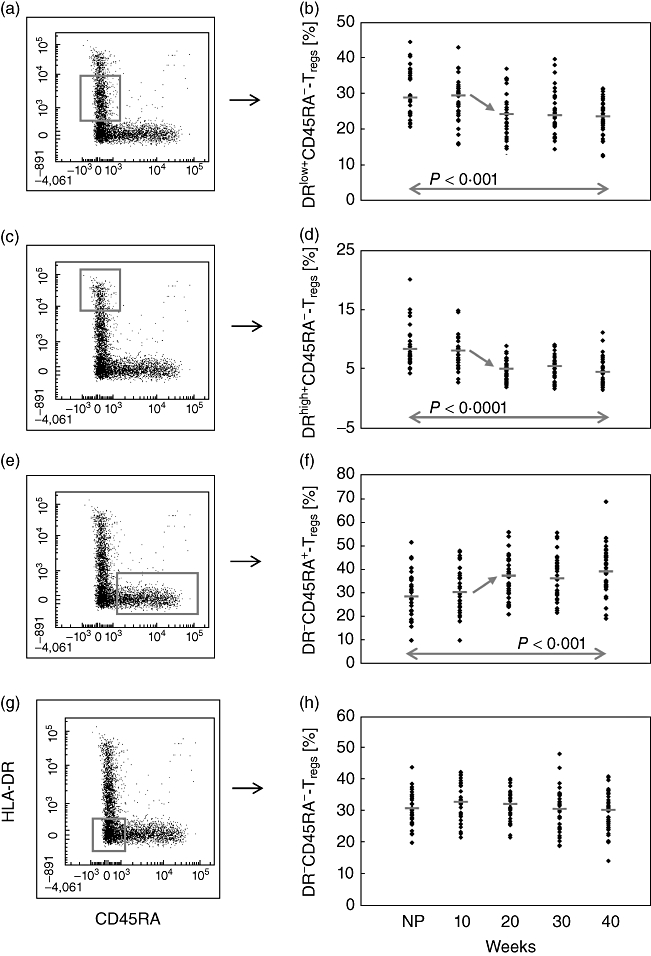
Detection of the percentages of the DRlow+CD45RA-, DRhigh+CD45RA-, DR-CD45RA- and DR-CD45RA+ Treg subsets within the total CD4+CD127low+/−CD25+forkhead box protein 3 (FoxP3+) Treg cell pool during the normal course of pregnancy. The figure shows the dot-blots of the gated DRlow+CD45RA-, DRhigh+CD45RA-, DR-CD45RA- and DR-CD45RA+ Treg subsets and their quantitative changes during the normal course of pregnancy. The diagram presents the individual and median data obtained for non-pregnant (NP) and healthy pregnant women. The percentage of the DRlow+CD45RA- Treg subset (a,b) and of the DRhigh+CD45RA- Treg subset (c,d) decreased significantly during the 10th and 20th weeks of gestation. In contrast, the percentage of the DR-CD45RA+ Treg subset increased during the same time (e,f). The percentage of the DR-CD45RA- Treg subset did not show any significant changes during the whole course of pregnancy (g,h).
Pre-eclampsia is associated with an increased percentage of DR+CD45RA- Treg cells, while PL is associated with an increased percentage of DR-CD45RA- Treg cells within both the total Treg cell pool and the total CD4+ T cell pool
In order to examine whether the discrepancies concerning the suppressive potency of the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool between healthy and affected pregnancies could be correlated with its composition with the above-described distinct Treg cell subsets (Fig. 1d), we assessed their percentages in pregnancies affected by pre-eclampsia (group 5), HELLP syndrome (group 6) CI (group 7) and PL (group 8), in comparison to healthy pregnancies in the third trimester (group 3 and group 4). Figure 4a–h show the quantitative alterations of these particular Treg subsets in the presence of gestation-associated hypertensive diseases (pre-eclampsia and HELLP syndrome; Fig. 4a–d) and in the presence of preterm intrauterine activation (CI and PL; Fig. 4e–h). Figure 4i–l and Table 2 summarize the data obtained for all patient groups and present significant differences between healthy and affected pregnancies.
Fig. 4.
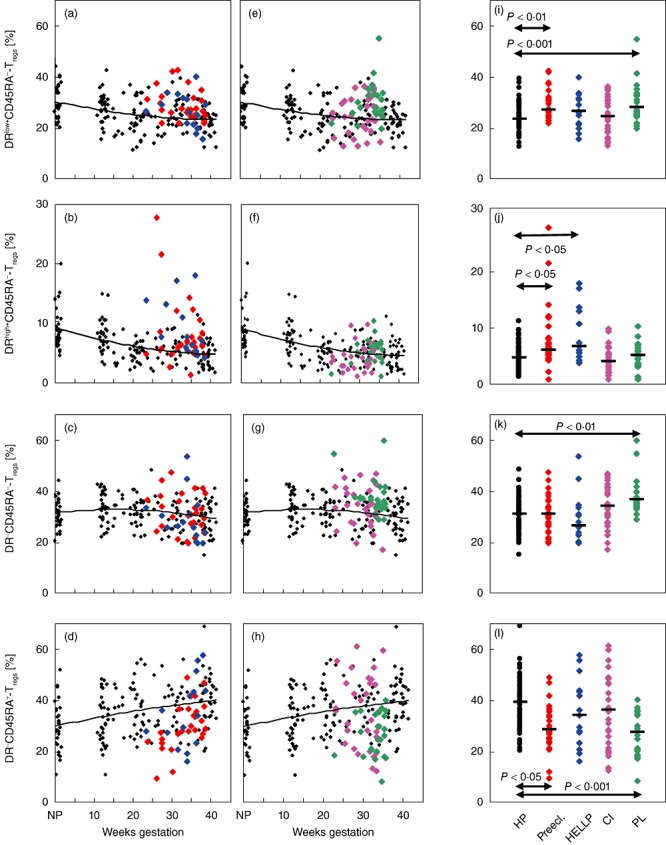
Detection of the percentages of the DRlow+CD45RA-, DRhigh+CD45RA-, DR-CD45RA- and DR-CD45RA+ Treg subsets within the total CD4+CD127low+/−CD25+forkhead box protein 3 (FoxP3+) Treg cell pool in healthy and complicated pregnancies. The figure presents the individual data (a–h) and median values (i–l) obtained for pregnancies affected by pre-eclampsia ( ) or HELLP syndrome (
) or HELLP syndrome ( ) (a–d), and for pregnancies affected by CI (
) (a–d), and for pregnancies affected by CI ( ) or PL (
) or PL ( ) (e–h), in comparison to healthy pregnancies (HP) (♦). Pregnancies affected by pre-eclampsia revealed significantly increased percentages of the DRlow+CD45RA- and DRhigh+CD45RA- Treg subsets (a,b,i,j), while in pregnancies affected by HELLP syndrome only the DRhigh+CD45RA- Treg subset was increased significantly (b,j). In contrast, the DR-CD45RA- and DRlow+CD45RA- Treg subsets were increased significantly in pregnancies affected by PL (e,g,i,k). The percentage of the naive DR-CD45RA+ Treg subset was decreased significantly in pregnancies affected by both pre-eclampsia and PL (d,h,l). CI: cervical insufficiency; PL: preterm labour necessitating preterm delivery; HELLP syndrome: haemolysis-elevated liver enzyme levels-low platelet count syndrome; MFI: mean fluorescence intensity.
) (e–h), in comparison to healthy pregnancies (HP) (♦). Pregnancies affected by pre-eclampsia revealed significantly increased percentages of the DRlow+CD45RA- and DRhigh+CD45RA- Treg subsets (a,b,i,j), while in pregnancies affected by HELLP syndrome only the DRhigh+CD45RA- Treg subset was increased significantly (b,j). In contrast, the DR-CD45RA- and DRlow+CD45RA- Treg subsets were increased significantly in pregnancies affected by PL (e,g,i,k). The percentage of the naive DR-CD45RA+ Treg subset was decreased significantly in pregnancies affected by both pre-eclampsia and PL (d,h,l). CI: cervical insufficiency; PL: preterm labour necessitating preterm delivery; HELLP syndrome: haemolysis-elevated liver enzyme levels-low platelet count syndrome; MFI: mean fluorescence intensity.
Compared to healthy pregnancies, we found a significantly increased percentage of DRlow+CD45RA- and DRhigh+CD45RA- Tregs in the Treg pool of pregnancies affected by pre-eclampsia. However, pregnancies affected by HELLP syndrome did not show an increased percentage of DRlow+CD45RA- Treg cells, but had a significantly increased percentage of DRhigh+CD45RA- Tregs in their Treg pool (Fig. 4a–b,i–j). In the presence of PL leading to preterm delivery, the percentages of DR-CD45RA- Tregs and of DRlow+CD45RA- Tregs were increased significantly (Fig. 4e,i,g,k), while the occurrence of CI was not associated with changes in the percentages of these Treg cell subsets (Fig. 4e–h,i–l). By contrast, we found that the presence of pre-eclampsia and PL, which were both associated with an increased percentage of DRlow+CD45RA- Tregs, were accompanied with a significantly decreased percentage of naive DR-CD45RA+ Treg cells within their Treg cell pool (Fig. 4d,h,l). As both patient collectives show a significantly reduced suppressive activity of their total CD4+CD127low+/−CD25+ Treg cell pool (Fig. 2d–g), it could be assumed that such changes could have an influence on its functional activity.
In order to examine whether the percentages of the different subsets within the total Treg cell pool correspond to their absolute numbers within the total CD4+ T cell pool, their percentages were additionally calculated relative to the total CD4+ T cell pool. Figure 5 depicts the results obtained for these measurements. Overall, there was a significant decrease of the DRlow+CD45RA- and DRhigh+CD45RA- Treg subsets and a significant increase of the naive DR-CD45RA+ Tregs subset during the normal course of pregnancy (Fig. 5). Compared to Fig. 4, exactly the same significant changes in the percentages of the different Treg subsets between healthy and affected pregnancies were documented both for the total Treg cell pool (Fig. 4) and the total CD4+ T cell pool (Fig. 5). Apparently, in the presence of complications such as pre-eclampsia and PL, DRlow+CD45RA-, DRhigh+CD45RA- Tregs and DR-CD45RA- Treg cells accumulated, while naive DR-CD45RA+ Treg cells declined within the CD4+ T cell pool. Such findings may propose that in the presence of pre-eclampsia or PL, the primarily existing naive DR-CD45RA+ Tregs cell compartment shrinks because of an increased conversion into either DR-CD45RA- or DR+CD45RA- Treg cells. As even the total CD4+ T cell pool may vary during the normal course of pregnancy and between the different patient groups, we estimated its percentage within PBMCs isolated after Ficoll-Hypaque gradient centrifugation. We found a significantly decreased percentage of CD4+ T cells between non-pregnant and pregnant women, irrespective of the gestational age (Table 1). Compared to healthy pregnancies, the percentage of CD4+ T cells was reduced significantly (P < 0·01) in patients affected by PL, but was not different in patients with cervical insufficiency, pre-eclampsia or HELLP syndrome (Table 2).
Fig. 5.
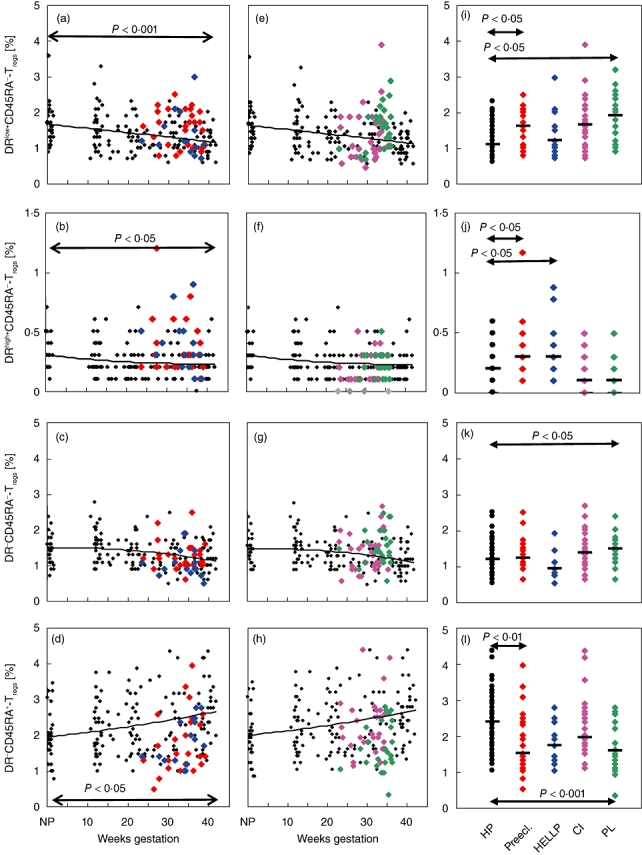
Detection of the percentages of the DRlow+CD45RA-, DRhigh+CD45RA-, DR-CD45RA- and DR-CD45RA+ Treg subsets within the total CD4+ T cell pool in healthy and complicated pregnancies. Concerning the changes in the percentages of the different Treg subsets in healthy and complicated pregnancies, similar results were obtained for the percentages of the different Treg subsets within both the total Treg cell pool and the CD4+ T cell pool (see Fig. 4).
The DRhigh+CD45RA- Treg cell subset is more suppressive than the pool of the residual Treg cell subsets
Our data demonstrate that the suppressive activity of the total Treg cell pool obtained from pregnancies affected by pre-eclampsia or PL is reduced significantly. Its composition in these patients is changed in the way that it contains a higher percentage of DRlow+CD45RA- and DRhigh+CD45RA- Tregs in the case of pre-eclampsia and a higher percentage of DR-CD45RA- and DRlow+CD45RA- Tregs in the case of PL. In contrast, its percentage of naive DR-CD45RA+ Tregs is diminished strongly in both patient collectives (Table 2). It is known that DR expression on Treg cells is associated with highly suppressive mature Treg cells [17]. Therefore, it could be assumed that the suppressive activity of the Treg pool depends mainly on the proportion of fully developed DRhigh+CD45RA- Tregs among the DR+CD45RA- Treg subset. Therefore, we examined whether these cells could have a potential effect of the suppressive activity of the total Treg cell pool. For that, magnetically isolated Treg cells obtained from three healthy, non-pregnant volunteers were stained with anti-CD4-, anti-CD25- and anti-HLA-DR-specific antibodies and sorted into a population of DRhigh+CD45RA- Tregs (Fig. 6a, R1) and into a population consisting of the remaining DRlow+CD45RA-, DR-CD45RA- and naive DR-CD45RA+ Tregs (Fig. 6a, R2). Subsequently, both Treg populations were analysed concerning their suppressive activity (Fig. 6b). Figure 6b,c demonstrate clearly that both the maximum suppressive activity and the titre (Treg/Tresp), with which a minimum suppressive activity of 15% was achieved, is increased strongly for the DRhigh+CD45RA- Tregs (R1) Tregs compared to the remaining Treg population consisting of DRlow+CD45RA-, DR-CD45RA- and naive DR-CD45RA+ Tregs (R2).
Fig. 6.
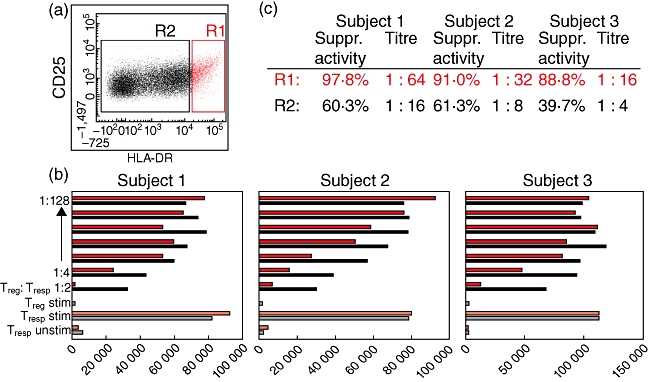
Positive selection and functional testing of the DRhigh+CD45RA- Treg subset. Magnetically isolated CD4+CD127low+/−CD25+ Treg were stained with anti-CD4, anti-CD25 and anti-human leucocyte antigen D-related (HLA-DR)-specific antibodies and sorted into a population of DRhigh+CD45RA- Tregs (a, R1) and into a population consisting of DRlow+CD45RA-, DR-CD45RA- and naive DR-CD45RA+ Tregs (a, R2). Subsequently, both Treg populations obtained from three different subjects were analysed concerning their suppressive activity (b). The maximum suppressive activity (Treg/Tresp = 1/1) and the minimum ratio of Treg/Tresp (titre) with at least 15% suppression were increased strongly for the DRhigh+CD45RA- Tregs compared to the remaining Tregs (R2) (c).
Discussion
Currently, great efforts are being made to investigate the role of the immunosuppressive CD4+CD127low+/−CD25+FoxP3+ Treg cell compartment for both the normal and the abnormal course of pregnancy. In this study, we thoroughly assessed Treg frequencies, their functional activity and their composition with distinct Treg cell subsets (DRlow+CD45RA-, DRhigh+CD45RA-, DR-CD45RA- and naive DR-CD45RA+ Tregs) in the circulation of healthy and complicated pregnancies. Similar to our previous studies, when Treg cells were simply detected on the basis of CD4, CD25 and FoxP3 [16] or on the basis of CD4, CD127 and CD25 [13], we observed that the percentage of CD4+CD127low+/−CD25+FoxP3+ Tregs among the total CD4+ T cell pool was decreasing during the normal course of pregnancy. Currently, such findings have been confirmed by other groups, although different gating strategies for the characterization of Treg cells were used. Mjösberg et al. detected CD4dim+CD25high+ Treg cells instead of CD4+CD127low+/−CD25+FoxP3+ Treg cells, but also found decreased percentages in second-trimester women compared to non-pregnant women [11]. Tilburgs et al. detected CD4+CD25high+ Treg cells and found that their percentage of FoxP3+ and HLA-DR+ cells was decreased significantly in second- and third-trimester women compared to non-pregnant women [10]. An even increased percentage of such cells was found in the decidua basalis and in the decidua parietalis, indicating that a selective Treg recruitment from the circulating pool into the placenta may occur, where these cells could act to prevent the acute allogeneic response towards the fetus [10].
With regard to functional studies of the circulating Treg pool, we did not find significant differences between non-pregnant and healthy pregnant women. Its suppressive activity was preserved approximately until the 30th week of gestation. Changes in the composition of the total Treg cell pool with the above-described Treg cell subsets were observed between the 10th and 20th weeks of gestation. However, these changes were not associated with alterations of its suppressive activity. A significant decline of the suppressive activity could be detected near term. However, that decline was not associated with characteristic changes of its composition with different Treg subsets. Therefore, further mechanisms may influence the functional activity of the Treg compartment. Recently, Mjösberg et al. demonstrated that FoxP3 expression of Treg cells could be influenced by the hormonal milieu and that it was already reduced in second-trimester women compared to non-pregnant women [11]. In particular, they were able to demonstrate that progesterone and 17β-oestradiol, which indeed reach maximum serum levels at the end of pregnancy, had the capacity to reduce FoxP3 expression of Treg cells in vitro. However, other in-vitro examinations demonstrated recently that oestradiol rather potentiates the suppressive activity of CD4+CD25+ Tregs[21]. In addition, in-vivo assays, monitoring CD4+CD25+FoxP3+ Treg cell numbers during the menstrual cycle, revealed tightly correlated increasing frequencies with increasing serum levels of oestradiol [22]. Moreover, most of these studies, supporting potential effects of hormones on the development, expansion and function of Treg cells, were performed on mice [23]. Currently, it still remains unclear whether such findings can be transferred to the human system.
Table 2 summarize our data concerning the frequency, the functional activity and the composition of the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool with distinct Treg cell subsets in the presence of characteristic gestation-associated diseases compared to healthy pregnancies. We were not able to detect a decreased percentage of CD4+CD127low+/−CD25+FoxP3+ Treg cells within the total CD4+ T cell pool in the presence of pre-eclampsia, HELLP syndrome, CI or PL. In contrast, most recent articles documented decreased Treg cell counts in patients affected by pre-eclampsia [14,16,24,25]. Such discrepancies may arise because human FoxP3 can be expressed transiently by in-vitro-activated T cells lacking regulatory function [26]. Recently, an inverse correlation between the expression of the IL-7 receptor α chain (CD127) and their suppressive function was shown for CD4+CD25+FoxP3+ Treg cells [27,28]. Therefore, simultaneous assessment of CD127, CD25 and FoxP3 expression by CD4+ T cells may help to exclude non-Treg cells and lead to accurate numbers of Tregs. Moreover, quantitative determinations of CD4+CD25high+ Treg cells in the absence of the FoxP3 marker confirm our results obtained for pregnancies affected by pre-eclampsia [29].
With regard to the functional activity of Treg cells from affected pregnancies, we observed a significantly reduced suppressive activity for patients with pre-eclampsia and for patients with PL, while the suppressive potency of patients with HELLP syndrome or CI was in the normal range. Astonishingly, patients with pre-eclampsia and PL had a significantly reduced percentage of naive DR-CD45RA+ Tregs within their total Treg cell compartment. Simultaneously, an increased percentage of DRhigh+CD45RA- Tregs and DRlow+CD45RA- Tregs was detected in patients with pre-eclampsia, while an increased percentage of DR-CD45RA- and DRlow+CD45RA- Tregs was found in patients with PL. Identical results were obtained concerning the percentages of these Treg cell subsets within the total CD4+ T cell pool, in fact, during the course of normal pregnancy and in the presence of pre-eclampsia or PL. Such findings suggest that naive DR-CD45RA+ Treg cells may be increasingly released by the thymus during the normal course of pregnancy, and that these cells may be converted into DR-CD45RA- DRlow+CD45RA- and DRhigh+CD45RA- Tregs in the presence of these diseases. Currently, we do not know whether the decrease of the naive DR-CD45RA+ Treg population contributes to the impaired suppressive activity of the total CD4+CD127+CD25+ Treg cell pool. So far, it is merely documented that naive RA+ Treg cells are more prevalent in early life [30]. Highest percentages were detected in the cord blood, while in adults the total Treg pool contained mainly RO+ memory Tregs[30]. Differences of the suppressive activity between naive RA+ Tregs and memory RO+ Tregs were not ascertained [31]. However, it was shown recently that the extent of the suppressive activity of RA+ Tregs depends decisively on its proportion of cells co-expressing CD31, a distinct Treg subset that enters the circulation as recent thymic emigrants [32]. Therefore, it may be possible that the suppressive capacity of naive DR-CD45RA+ Tregs may be different in pregnant and non-pregnant women.
Recently, we demonstrated that particularly the subset of DR+ Tregs contributes essentially to the suppressive activity of the total Treg cell pool [13]. Thereby, we showed that the reduced suppressive activity of Treg cells observed in patients with PL was not associated with a decreased percentage of DR+ Treg cells, but that the level of HLA-DR expression of their DR+ Treg cells (HLA-DR MFI) was strongly reduced [13]. Our current data clearly demonstrate that the percentage of DRlow+CD45RA- Tregs is increased significantly in these patients. As the percentage of DR-CD45RA- Tregs was also increased, one could assume that these patients could have deficiencies in the maturation of DR-CD45RA- Tregs into highly suppressive DRhigh+CD45RA- Tregs.
Astonishingly, we found that pre-eclamptic women, whose Treg pool and CD4+ T cell pool contained an increased percentage of DRhigh+CD45RA- Tregs and DRlow+CD45RA- Tregs concurrently exhibited a reduced suppressive activity. In contrast, the suppressive activity of the Treg pool in patients with HELLP syndrome was in the normal range, but contained an increased percentage of the DRhigh+CD45RA- Tregs. Obviously, the pre-eclamptic patients have deficiencies in the functional activity of their Treg pool, although it contains a high proportion of HLA-DR+ Tregs. Probably, in patients with HELLP syndrome the high proportion of fully developed, highly suppressive DRhigh+CD45RA- Tregs compensates this loss of suppressive activity observed in pre-eclamptic patients. It could also be possible that the responder T cells in pre-eclamptic patients could be resistant to Treg suppression. In either case, it seems that the DRhigh+CD45RA- Treg cells contribute potentially to the suppressive activity of the total Treg cell pool. Therefore, we examined the role of this Treg subset for the suppressive activity of the total Treg cell pool. Positive selection and analysis of its suppressive activity in comparison to the remaining Treg cells revealed that DRhigh+CD45RA- Treg cells are more suppressive than the pool of the residual Treg cells. Further studies may be necessary to evaluate the contribution of each of these Treg subsets to the suppressive activity of the total Treg cell pool.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft grant STE 885/3–2 (to A. Steinborn). The authors would like to thank C. Schneider and the nursing staff of the Delivery Room and the Ultrasound section of the Department of Obstetrics and Gynecology/University of Heidelberg, for arranging the collection of blood samples.
Disclosure
None of the authors has any conflict of interest related to this manuscript.
References
- 1.Guller S, LaChapelle L. The role of placental Fas ligand in maintaining immune privilege at maternal-fetal interfaces. Semin Reprod Endocrinol. 1999;17:39–44. doi: 10.1055/s-2007-1016210. [DOI] [PubMed] [Google Scholar]
- 2.Hunt JS, Petroff MG, Mc Intire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19:681–93. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 3.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1122–4. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi DW, Romero R. Biological implications of bi-directional fetomaternal cell traffic: a summary of a National Institute of Child Health and Human Development-sponsored conference. J Matern Fetal Neonatal Med. 2003;14:123–9. doi: 10.1080/jmf.14.2.123.129. [DOI] [PubMed] [Google Scholar]
- 5.Steinborn A, Rebmann V, Scharf A, Sohn C, Grosse-Wilde H. Soluble HLA-DR levels in the maternal circulation of normal and pathological pregnancy. Am J Obstet Gynecol. 2003;188:473–9. doi: 10.1067/mob.2003.55. [DOI] [PubMed] [Google Scholar]
- 6.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 7.Aluvihare VR, Kallikourdis M, Betz AG. Tolerance, suppression and the fetal allograft. J Mol Med. 2005;83:88–96. doi: 10.1007/s00109-004-0608-2. [DOI] [PubMed] [Google Scholar]
- 8.Aluvihare VR, Betz AG. The role of regulatory T cells in alloantigen tolerance. Immunol Rev. 2006;212:330–43. doi: 10.1111/j.0105-2896.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 9.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+CD4+ regulatory T cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilburgs T, Roelen DL, van der Mast BJ, et al. Evidence for a selective migration of fetus-specific CD25bright regulatory T cells from peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180:5737–45. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- 11.Mjösberg J, Svensson J, Johansson E, et al. Systemic reduction of functionally suppressive CD4dimCD25highFoxP3+ Tregs in human second trimester pregnancy is induced by progesterone and 17β-Estradiol. J Immunol. 2009;183:759–69. doi: 10.4049/jimmunol.0803654. [DOI] [PubMed] [Google Scholar]
- 12.Neuteboom RF, Verbraak E, Wierenga-Wolf AF, et al. Pregnancy-induced fluctuations in functional T-cell subsets in multiple sclerosis patients. Mult Scler. 2010;16:1073–8. doi: 10.1177/1352458510373939. [DOI] [PubMed] [Google Scholar]
- 13.Kisielewicz A, Schaier M, Schmitt E, et al. A distinct subset of HLA-DR+-regulatory T cells is involved in the induction of preterm labor during pregnancy and in the induction of organ rejection after transplantation. Clin Immunol. 2010;137:209–20. doi: 10.1016/j.clim.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Santer-Nanan B, Peek MJ, Khanam R, et al. Systemic increase in the ratio between FoxP3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–30. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki Y, Darmochwal-Kolarz D, Suzuki D, et al. Proportion of peripheral blood and decidual CD4+CD25bright regulatory T cells in preeclampsia. Clin Exp Immunol. 2007;149:139–45. doi: 10.1111/j.1365-2249.2007.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinborn A, Haensch GM, Mahnke K, et al. Distinct subsets of regulatory T cells during pregnancy: Is the imbalance of these subsets involved in the pathogenesis of preeclampsia? Clin Immunol. 2008;129:401–12. doi: 10.1016/j.clim.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 17.Baecher-Allan C, Wolf E, Hafler D. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–31. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 18.Ashley CW, Baecher-Allan C. Responder T cells regulate human DR+ effector regulatory T cell activity via Granzyme B. J Immunol. 2009;183:4843–7. doi: 10.4049/jimmunol.0900845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth NJ, McQuaid AJ, Sobande T, et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317–26. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 20.Venken K, Hellings N, Broekmans T, Hensen K, Rumens JL, Stinissen P. Natural naïve CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol. 2008;180:6411–20. doi: 10.4049/jimmunol.180.9.6411. [DOI] [PubMed] [Google Scholar]
- 21.Prieto GA, Rosenstein Y. Oestradiol potentiates the suppressive function of human CD4+CD25+ regulatory T cells by promoting their proliferation. Immunology. 2006;118:58–65. doi: 10.1111/j.1365-2567.2006.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+ and FoxP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572–8. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- 23.Tai P, Wang J, Jin H, et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214:456–64. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 24.Toldi G, Svec P, Vasarhelyi B, et al. Decreased number of FoxP3+ regulatory T cells in preeclampsia. Acta Obstet Gynecol Scand. 2008;87:1229–33. doi: 10.1080/00016340802389470. [DOI] [PubMed] [Google Scholar]
- 25.Prins JR, Boelens HM, Heimweg J, Van der Heide S, et al. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy. 2009;28:300–11. doi: 10.1080/10641950802601237. [DOI] [PubMed] [Google Scholar]
- 26.Banham AH, Powrie FM, Suri-Payer E. FOXP3+ regulatory T cells: current controversies and future perspectives. Eur J Immunol. 2006;36:2832–6. doi: 10.1002/eji.200636459. [DOI] [PubMed] [Google Scholar]
- 27.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, Putnam AL, XuYu Z, et al. CD127 expression inversely correlates with FOXP3 and suppressive function of human regulatory and activated T cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paeschke S, Chen F, Horn N, et al. Preeclampsia is not associated with changes in the levels of regulatory T cells in peripheral blood. Am J Reprod Immunol. 2005;54:384–9. doi: 10.1111/j.1600-0897.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 30.Seddiki N, Santner-Nanan B, Tangye SG, et al. Persistence of naïve regulatory T cells in adult life. Blood. 2006;107:2830–8. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- 31.Santer-Nanan B, Seddiki N, Zhu E, et al. Accelerated age-dependent transition of human regulatory T cells to effector memory phenotype. Int Immunol. 2008;20:375–83. doi: 10.1093/intimm/dxm151. [DOI] [PubMed] [Google Scholar]
- 32.Haas J, Fritzsching B, Trübswetter P, et al. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol. 2007;179:1322–30. doi: 10.4049/jimmunol.179.2.1322. [DOI] [PubMed] [Google Scholar]


