Abstract
Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) is a rare syndrome due to a mutation in the forkhead box protein 3 gene (FOXP3) leading to an impaired regulatory T cell (Treg) activity associated both with skewed T helper type 2 (Th2) response and autoreactive phenomena. The purpose of this study was to describe a combined proteomics and genomics approach to comprehensively evaluate clinical and immunological phenotypes of patients affected by IPEX. T cell receptor (TCR)-Vβ repertoire and peripheral blood lymphocytes phenotype from three brothers affected by IPEX were studied by flow cytometry. Specific immunoglobulin (Ig)E were evaluated by means of an allergenic molecules microarray [immuno solid-phase allergen chip (ISAC)]. Total RNA was extracted and hybridized to Affymetrix oligonucleotide arrays to obtain quantitative gene-expression levels. No FOXP3 protein was detectable within CD127-CD25highCD4+ T cells from peripheral blood. A T cell-naive phenotype (CD62L+CD45R0-) associated with a reduction of both CD26 and CD7 expression and a TCR-Vβ 8 and 22 family expansions were found. B lymphocytes were mainly CD5+ (B1) cells expressing a naive phenotype (tcl1+CD27-). The three IPEX patients had severe food allergy and specific IgE reactivity to cow's milk allergens, a hen's egg allergen and a wheat allergen. Gene expression profile analysis revealed a dysregulation associated mainly with Th1/Th2 pathways. The multiplexing evaluation reported in this study represents a comprehensive approach in the assessment of genetic conditions affecting the immune system such as the IPEX syndrome, paving the way for the development of diagnostic tools to improve the standard clinical and immunological profiling of the disease.
Keywords: immunodeficiency, IPEX syndrome, specific IgE
Introduction
Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX, OMIM 304790) is a rare, recessive syndrome that affects male infants, often characterized by a rapidly fatal course [1]. Hallmark features of IPEX syndrome are protein-losing enteropathy with refractory and sometimes life-threatening diarrhoea, chronic eczematous dermatitis with sometimes elevated levels of total immunoglobulin (Ig)E and polyendocrinopathies such as type 1 diabetes (insulin-dependent diabetes mellitus, IDDM) and hypothyroidism, haemolytic anaemia and thrombocytopenia, usually with the presence of autoantibodies [2]. No effective therapy for IPEX syndrome patients is currently available.
IPEX syndrome is due to mutation in the forkhead box protein 3 gene (FOXP3) located on chromosome Xp11.23. However, in approximately 50% of patients with clinical findings compatible with IPEX clinical phenotype, no mutation could be demonstrated [3]. The gene encodes a 431-amino acid, 48 kDa, protein, also named scurfin, belonging to the forkhead/winged-helix family of transcription factors, essential for the generation of CD4+CD25+ regulatory T lymphocytes (Treg) in the thymus [4–6]. A growing body of experimental and in-vivo evidence has shown that the absence or the dysfunction of a proper Tregactivity could lead to a dysregulated immune response characterized by both IgE-mediated reaction due to a skewed T helper type 2 (Th2) response [7] and autoreactive phenomena due to the presence of self-reactive T cell activation and proliferation [8,9]. The autoimmune imbalance has been widely described in this disease in terms of specific autoantibody response, while only limited data are currently available on the specific IgE response to environmental allergens [10,11].
We report herein a combined proteomics and genomics approaches to comprehensively evaluate the clinical and immunological phenotypes. This combined approach was first applied to three brothers affected by IPEX syndrome, all presenting multiple severe allergic reactions to foods and chronic eczema.
Materials and methods
Clinical cases
Three brothers, born in 1980 (Y1), 1990 (Y2) and 1994 (Y3), after uneventful pregnancies to otherwise healthy non-consanguineous parents, and diagnosed previously as affected by IPEX in 2003, asked for a consultation at the Center for Molecular Allergology (IDI-IRCCS, Rome, Italy) in 2007. An older brother, born in 1978, died at 10 months of age for causes which could not be detailed by the parents. He was affected by severe diffuse eczema and complicated enterocolitis with intractable diarrhoea. No information is available regarding whether or not the baby presented additional symptoms, such as endocrinopathy. No autopsy was performed.
Patient Y1, the eldest living brother, had no problems during the neonatal period while he was exclusively breastfed, but in the course of the first year of life he developed abdominal pain, watery bloody diarrhoea, accompanied by severe eczema and urticaria/angioedema after ingestion, or even inhalation, of cow's milk. Similar symptoms were observed after the ingestion of small amounts of hen's egg at 3 years of age. At the age of 14, high titres of anti-thyroperoxidase and anti-thyroglobulin antibodies were detected. The autoimmune thyroiditis was followed by clinical hypothyroidism and 3 years later an autoimmune sclerosing cholangitis was diagnosed. In 2006 he presented a painless, slowly growing mass in the right palatine tonsil. A histological diagnosis of non-Hodgkin's B cell lymphoma, diffuse large cell type, was made after a biopsy of the lesion. The patient was treated with two courses of chemotherapy, including methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine and dexamethasone (m-BACOD). After chemotherapy, an impressive improvement of chronic eczema was recorded, as reported by the patient and his parents.
Because of the older brother's medical history, the two younger brothers (Y2 and Y3) were exclusively breastfed and the mother's diet was restricted to exclude cow's milk proteins. Despite these preventive measures, during their first year of age they both developed eczema, severe watery bloody diarrhoea, urticaria and angioedema, even though exclusion was extended from cow's milk proteins to eggs, peanuts and fish. Immediate, severe generalized allergic reactions occurred in both children after the accidental ingestion of negligible traces of cow's milk or egg proteins. These reactions were characterized by immediate nausea and vomiting, accompanied by severe abdominal pain and watery diarrhoea. In patient Y3, several episodes of angioedema and lip swelling were observed upon ingestion of wheat-containing food. Autoimmune thyroiditis was diagnosed in both brothers at the age of 12 and 10 years, respectively. None of the three patients developed glucose intolerance or insulin-dependent diabetes mellitus at the time of our observation.
The avoidance of milk and egg ingestion was followed by the disappearance of urticaria, angioedema and a mild improvement of diarrhoea, but no direct effect on eczema was obtained.
In 2007 their major complaint was worsening of eczema and the development of rhinitis and asthma. Multiplex approaches including IgE determination using an allergen-based microarray, a microarray genomics testing, a comprehensive flow cytometry analysis, including T cell receptor (TCR)-Vβ and a broad panel of CD lymphocyte markers, were applied in order to define their immunological and allergy profiles. All subjects were enrolled into clinical protocols approved by the Ethical Committee of IDI-IRCCS and informed written consent was obtained in accordance with the Declaration of Helsinki.
FOXP3 gene analysis
DNA was isolated from peripheral blood by using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Eleven exons, including all intron–exon boundaries, were amplified from genomic DNA by means of polymerase chain reaction (PCR) with specific flanking intron primer pairs [2]. The amplified gene fragments were sequenced by using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) on an automated ABI PRISM 310 Genetic Analyzer (Applied Biosystems).
Fluorescence activated cell sorter (FACS) analysis
The TCR-Vβ repertoire and circulating lymphocytes phenotype were evaluated extensively using a panel of specific monoclonal antibodies, as reported in Table 1. Quantitative analysis for five-colour FC was carried out using FACS-Aria cytofluorimeter (Becton Dickinson Immunocytometry Systems, San Diego, CA, USA). The data were evaluated by means of the BD FACSDiva software (Becton Dickinson).
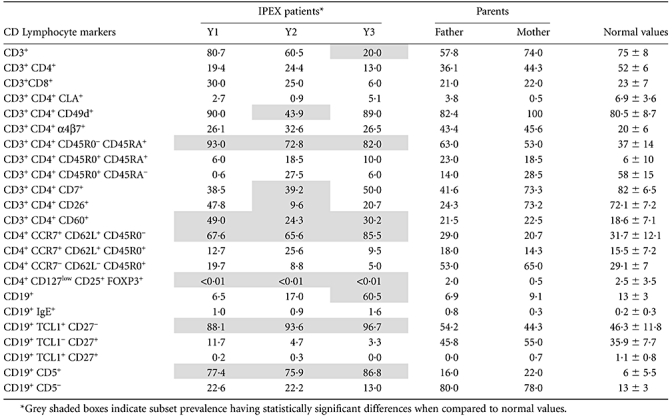
Table 1.
Flow cytometry-based lymphocyte CD phenotyping of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) patients and their parents. No detectable FOXP3 protein was found within CD127-CD25highCD4+ T cells subset [regulatory T cells (Treg)] in all patients. Raised levels of naive cells were also found both in T (CCR7+CD62L+CD45R0-CD45RA+) and B cell subsets (TCL1+CD27-). Raised levels of CD19+CD5+ B lymphocytes (B1 subset), were observed, in one case (Y3) associated with a clear increase of B cells
 |
Allergenic molecules proteomic microarray for specific IgE detection
A protein microarray having 89 native or recombinant allergenic molecules [immuno solid-phase allergen chip (ISAC); VBC-Genomics, Vienna, Austria] was used for specific IgE detection [12]. Microarray slides bearing allergens spotted in triplicate were placed into a humid chamber and 20 µl of undiluted serum from allergic patients were applied to the individual reaction sites. After 120 min incubation, allergen chips were washed and rinsed, and then probed with 20 µl of anti-human IgE antibody (Pharmingen, San Diego, CA, USA) for 60 min. Images were acquired by scanning the allergen chips using a ScanArray Gx Microarray scanner (Perkin Elmer Life Sciences, Boston, MA, USA), with two laser power settings, and evaluated using the software Microarray Image Analyzer 1·5 (VBC-Genomics).
Genomics microarray expression profile
Total RNA was extracted from the peripheral blood mononuclear cells (PBMC) of each IPEX syndrome patient, with both parents acting as controls. RNA-Trizol reagent (Invitrogen, Carlsbad, CA, USA) was used for the extraction procedure, according to the manufacturer's protocol. Total RNA was assessed by spectroscopy at 260/280 nm (NanoDrop; Thermo Scientific, Wilmington, DE, USA) and its quality analysed by gel electrophoresis.
Biotinylated cRNAs probes were prepared and hybridized to HG-U133A2·0 GeneChip oligonucleotide arrays following the Affymetrix protocol (Affymetrix, Santa Clara, CA, USA). Analysis was performed using the BRB-Array Tools software (Biometric Research Branch, National Cancer Institute), a comprehensive package for the visualization and statistical analysis of DNA microarray gene expression data. The random variance model was applied to the filtered data sets. This model assumes that the variance of the residuals in analysis of variance (anova) for each gene is a random variable from a common inverse gamma distribution with unknown parameters. To identify genes that were differentially expressed between the IPEX syndrome and parents groups, supervised analysis, using ‘class comparison tools’ of BRB array tools, was performed. The test, using random variance, compares the differences in mean log-intensities between classes relative to the expected variation in mean differences computed from the independent samples. Genes with P-values < 0·01 were considered statistically significant. The false discovery rate was also estimated for each gene using the method of Benjamini and Hochberg [13] to control for false positives. After significant genes were identified, annotation and functional clustering was performed using Database for Annotation, Visualization and Integrated Discovery (DAVID) [14,15]. The raw list of AffyIDs was submitted as a ‘gene list’ to DAVID and the data were then analysed using the ‘Functional Annotation Clustering’ tool using the ‘High’ classification stringency setting.
Results
As IPEX syndrome affected individuals carry the FOXP3 gene mutation, we analysed the relative nucleotide sequence. A single-base substitution (543 C > T) at the intron 4/exon 5 boundary that resulted in a silent mutation (S181S) was found in all three brothers. The 543 C > T transition is reported as a rare polymorphism (rs2232367) in the single nucleotide polymorphism (SNP) National Center for Biotechnology Information (NCBI) database and its consequences on FOXP3 mRNA processing, stability or expression are still not clarified and, therefore, cannot be totally excluded [16–20].
FACS analysis
An extensive FACS analysis revealed a profound imbalance of peripheral lymphocytes subsets in all subjects. No detectable levels of FOXP3 protein were found within the CD127-CD25highCD4+ T cells subset (Treg cells) in any of the three patients (Table 1). These data confirmed previous studies demonstrating that the lack of FOXP3 in Treg cells affects the peripheral blood lymphocytes subsets [19,21]. Most T cells had a naive phenotype (CCR7+CD62L+ CD45RA+CD45R0-) and a reduction of both CD26 and CD7 expression in T cells was recorded. TCR-Vβ repertoire evaluation showed the expansion of TCR-Vβ 8 and 22 in the two older brothers (Fig. 1). Raised levels of CD19+CD5+ B lymphocytes (B1 subset), with a naive phenotype (tcl1+CD27-) [22], were observed in all patients, but the youngest patient showed significantly higher levels of CD19+CD5+ cells (60·5%) in the peripheral blood.
Fig. 1.
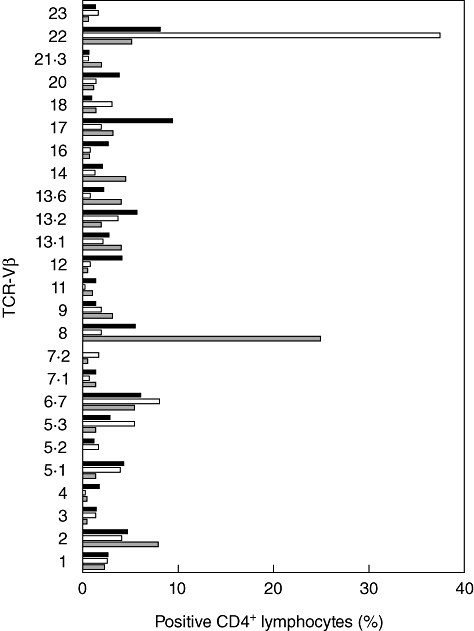
T cell receptor (TCR)-Vβ repertoire analysis of CD4+ T lymphocytes. Expansions of TCR-Vβ8 and TCR-Vβ22 were detected in the two older brothers affected by immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, Y1 and Y2, respectively, reflecting the clinical picture of the two patients. All remaining TCR-Vβ were in the normal range. Grey bar: IPEX patient Y1; white bar: IPEX patient Y2; black bar: IPEX patient Y3.
Allergenic molecule microarray IgE analysis
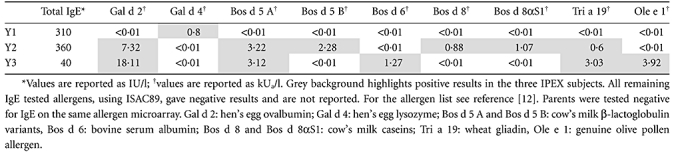
Because dysregulated total IgE production was already observed in patients affected by IPEX syndrome [1,5], we also evaluated the specific IgE profile in our patients. Low to slightly increased total IgE levels were found (Table 2). When measuring the specific IgE levels by means of the microarray system, IgE reactivity to hen's egg lysozyme (Gal d 4) was recorded in the older brother, whereas the presence of specific IgE to cow's milk allergens, namely the β-lactoglobulin, Bos d 5, the casein fraction, Bos d 8, and the αS1 casein, Bos d 8αS1, hen's egg ovalbumin and lysozyme (Gal d 2 and Gal d 4, respectively) and wheat gliadin (Tri a 19) were found in the second brother. A similar IgE profile was found in the youngest patient, recorded positive to β-lactoglobulin (Bos d 5), bovine serum albumin (Bos d 6), hen's egg ovalbumin and lysozyme (Gal d 2 and Gal d 4, respectively), wheat gliadin (Tri a 19) and the major olive pollen allergen, Ole e 1 (Table 2). None of the three patients showed an IgE reactivity to the most common allergenic molecules (e.g. Cup a 1, Der p 2, Der p 1, Phl p 1, Par j 2) if compared with the IgE reactivity of the allergic population of the same gender and in the same age range living in the same geographic area [12]. Parents were tested totally negative for IgE on the same allergen microarray.
Table 2.
Specific immunoglobulin (Ig)E results obtained by means of a proteomics microarray allergen-based system [immuno solid-phase allergen chip (ISAC89)]. As well as being tested on 89 different allergenic molecules, immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) patients were detected positive for IgE towards milk and hen's egg allergens
 |
Gene expression profile analysis
We then compared the gene expression profile in the peripheral blood mononuclear cells of the two younger IPEX patients with their parents. Unsupervised hierarchical cluster analysis revealed two distinct clustering of IPEX syndrome and parent samples, indicating that gene expression patterns clearly differentiate IPEX syndrome patients from their unaffected parents despite the common genetic background (data not shown).
In order to identify statistically significant genes whose differential expression was able to discriminate between the two groups, a supervised class comparison analysis was performed. The result, shown graphically in Fig. 2 as heatmap clusters, identified 67 significant genes expressed differentially in the IPEX group (P-value < 0·01) (Table 3). This list of genes was investigated further for pathway analysis with the aim of identifying molecular mechanisms that might be dysregulated in the IPEX syndrome. The 67 differentially expressed genes were grouped into categories according to subcellular localization, biological process or molecular function, as revealed by the gene ontology (GO) biological process description [23]. Using the functional annotation clustering tool of DAVID bioinformatics resources (http://david.abcc.ncifcrf.gov/) [14], 38 distinct clusters of differentially expressed gene categories having the best enrichment score (P-value < 0·01) were identified, including those related mainly to immune function, such as positive regulation of leucocyte activation and proliferation, regulation of T cell activation, positive regulation of apoptosis and regulation of phosphorylation. Interestingly, the first and most statistically significant (P-value < 1e−14) cluster of dysregulated cell transcripts contained 14 genes involved in positive regulation of immune system process.
Fig. 2.
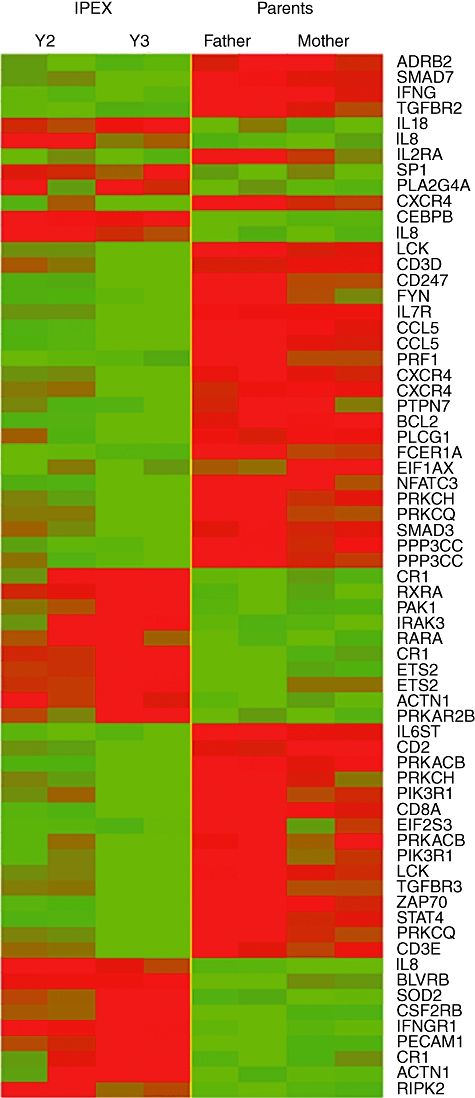
Clustered heatmap graphic representation of the 67 significant genes expressed differentially in the two youngest patients (on the left) and their parents (on the right). Details on gene-related proteins are given in Table 3. The green colour indicates down-regulated genes, red colour over-expressed genes.
Table 3.
List of the 67 significant genes differentially expressed comparing immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) patients and their parents. Up-regulated genes in IPEX patients included relevant immune response-related genes; interleukin (IL)-8, IL-18, CD31 and interferon (IFN)-γ-receptor 1, while FcεR-I, signal transducer and activator of transcription-4 (STAT4), CD3δ, CD3ε and CD3ζ, CXCR4 and CCL5 were among the most down-regulated genes
| Gene activation* | |||||||
|---|---|---|---|---|---|---|---|
| Probe set | Gene symbol | Name | IPEX | Parents | Fold change | P-value | |
| 1 | 210354_at | IFN-G | IFN-γ | 40·08 | 106·06 | 0·38 | 0·0000008 |
| 2 | 205758_at | CD8A | CD8a molecule | 184·79 | 752·34 | 0·25 | 0·0000028 |
| 3 | 204655_at | CCL5 | Chemokine (C-C motif) L5 | 1187·55 | 3769·35 | 0·32 | 0·0000045 |
| 4 | 1405_i_at | CCL5 | Chemokine (C-C motif) L5 | 1092·18 | 3233·23 | 0·34 | 0·0000088 |
| 5 | 212501_at | CEBPB | CCAAT/enhancer binding protein (C/EBP), β | 5270·75 | 2930·74 | 1·8 | 0·0000092 |
| 6 | 203685_at | BCL2 | B cell CLL/lymphoma 2 | 109·71 | 302·31 | 0·36 | 0·0000102 |
| 7 | 214032_at | ZAP70 | ζ-chain (TCR)-associated protein kinase 70 kDa | 271·16 | 686·86 | 0·39 | 0·0000111 |
| 8 | 206118_at | STAT4 | Signal transducer and activator of transcription 4 | 280·86 | 720·5 | 0·39 | 0·0000267 |
| 9 | 211676_s_at | IFN-GR1 | IFN-γ receptor 1 | 1446·24 | 925·76 | 1·56 | 0·000039 |
| 10 | 212195_at | IL-6ST | IL-6 signal transducer (gp130, oncostatin M receptor) | 511·1 | 829·61 | 0·62 | 0·0000432 |
| 11 | 211734_s_at | FCER1A | Fc fragment of IgE, high affinity I, receptor for; α polypeptide | 122·17 | 381·85 | 0·32 | 0·0000574 |
| 12 | 211506_s_at | IL-8 | IL-8 | 1229·78 | 700·46 | 1·76 | 0·0000608 |
| 13 | 202741_at | PRKACB | Protein kinase, cAMP-dependent, catalytic, β | 590·06 | 1080·34 | 0·55 | 0·0000901 |
| 14 | 208488_s_at | CR1 | Complement component (3b/4b) receptor 1 (Knops blood group) | 471·89 | 178·43 | 2·64 | 0·000195 |
| 15 | 202426_s_at | RXRA | Retinoid X receptor, α | 243·78 | 148·31 | 1·64 | 0·0002419 |
| 16 | 32541_at | PPP3CC | Protein phosphatase 3 (formerly 2B), catalytic subunit, γ isoform | 169·9 | 279·14 | 0·61 | 0·0002587 |
| 17 | 210556_at | NFATC3 | Nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 3 | 235·76 | 406·52 | 0·58 | 0·0003062 |
| 18 | 207334_s_at | TGF-BR2 | Transforming growth factor, β receptor II (70/80 kDa) | 43·52 | 85·6 | 0·51 | 0·0003105 |
| 19 | 208637_x_at | ACTN1 | Actinin, α 1 | 335·43 | 201·62 | 1·66 | 0·0003203 |
| 20 | 214617_at | PRF1 | Perforin 1 (pore forming protein) | 1949·45 | 3816·41 | 0·51 | 0·0003764 |
| 21 | 205831_at | CD2 | CD2 molecule | ′435·7 | 1111·33 | 0·39 | 0·0003955 |
| 22 | 205798_at | IL7R | IL-7 receptor | 1083·05 | 3854·88 | 0·28 | 0·0004234 |
| 23 | 206170_at | ADRB2 | Adrenergic, β-2, receptor, surface | 71·89 | 135·33 | 0·53 | 0·0004319 |
| 24 | 201329_s_at | ETS2 | v-ets erythroblastosis virus E26 oncogene homologue 2 (avian) | 423·64 | 238·09 | 1·78 | 0·0005064 |
| 25 | 202859_x_at | IL8 | IL-8 | 3278·21 | 2057·88 | 1·59 | 0·000712 |
| 26 | 210031_at | CD247 | CD247 molecule | 939·33 | 1980·45 | 0·47 | 0·0007122 |
| 27 | 208981_at | PECAM1 | Platelet/endothelial cell adhesion molecule (CD31 antigen) | 1539·05 | 973·44 | 1·58 | 0·0007861 |
| 28 | 204790_at | SMAD7 | SMAD family member 7 | 43·78 | 119·01 | 0·37 | 0·0008775 |
| 29 | 204891_s_at | LCK | Lymphocyte-specific protein tyrosine kinase | 683·74 | 1624·99 | 0·42 | 0·0009013 |
| 30 | 215223_s_at | SOD2 | Superoxide dismutase 2, mitochondrial | 1776·67 | 883·36 | 2·01 | 0·0010719 |
| 31 | 202201_at | BLVRB | Biliverdin reductase B [flavin reductase (NADPH)] | 1247 | 718·53 | 1·74 | 0·0010769 |
| 32 | 206099_at | PRKCH | Protein kinase C, η | 210·75 | 419·15 | 0·5 | 0·0011349 |
| 33 | 205159_at | CSF2RB | CSF2 R, β (granulocyte-macrophage) | 1594·02 | 837·8 | 1·9 | 0·0012975 |
| 34 | 220034_at | IRAK3 | IL-1 receptor-associated kinase 3 | 233·17 | 134·12 | 1·74 | 0·0013425 |
| 35 | 208636_at | ACTN1 | Actinin, α 1 | 627·36 | 341·48 | 1·84 | 0·0014019 |
| 36 | 202742_s_at | PRKACB | Protein kinase, cAMP-dependent, catalytic, β | 294·34 | 510·06 | 0·58 | 0·0016042 |
| 37 | 212249_at | PIK3R1 | Phosphoinositide-3-kinase, regulatory subunit 1 (p85 α) | 299·47 | 539·65 | 0·55 | 0·0016352 |
| 38 | 204890_s_at | LCK | Lymphocyte-specific protein tyrosine kinase | 371·51 | 864·54 | 0·43 | 0·0016548 |
| 39 | 206244_at | CR1 | Complement component (3b/4b) receptor 1 (Knops blood group) | 243·02 | 105·49 | 2·3 | 0·0018088 |
| 40 | 202789_at | PLCG1 | Phospholipase C, γ 1 | 102·32 | 224·48 | 0·46 | 0·001989 |
| 41 | 210038_at | PRKCQ | Protein kinase C, θ | 266·17 | 666·04 | 0·4 | 0·0020766 |
| 42 | 207000_s_at | PPP3CC | Protein phosphatase 3 (formerly 2B), catalytic subunit, γ isoform | 159·99 | 280·98 | 0·57 | 0·0021735 |
| 43 | 211919_s_at | CXCR4 | Chemokine (CXC motif) receptor 4 | 2143·05 | 3372 | 0·64 | 0·0022819 |
| 44 | 203749_s_at | RARA | Retinoic acid receptor, α | 201·54 | 137·24 | 1·47 | 0·00261 |
| 45 | 206341_at | IL-2RA | IL-2 receptor, α | 26·57 | 47·78 | 0·56 | 0·0029684 |
| 46 | 218764_at | PRKCH | Protein kinase C, eta | 539·32 | 1044·76 | 0·52 | 0·0035346 |
| 47 | 206295_at | IL-18 | IL-18 (IFN-γ-inducing factor) | 101·68 | 67·22 | 1·51 | 0·0038569 |
| 48 | 203680_at | PRKAR2B | Protein kinase, cAMP-dependent, regulatory, type II, β | 279·27 | 182·61 | 1·53 | 0·0039945 |
| 49 | 209545_s_at | RIPK2 | Receptor-interacting serine-threonine kinase 2 | 725·53 | 520·11 | 1·39 | 0·0040329 |
| 50 | 205321_at | EIF2S3 | Eukaryotic translation initiation factor 2, subunit 3 γ, 52 kDa | 381·03 | 593·83 | 0·64 | 0·0042516 |
| 51 | 217028_at | CXCR4 | Chemokine (CXC motif) receptor 4 | 3897·23 | 5792·91 | 0·67 | 0·0047524 |
| 52 | 212239_at | PIK3R1 | Phosphoinositide-3-kinase, regulatory subunit 1 (p85 α) | 514·72 | 969·17 | 0·53 | 0·0053206 |
| 53 | 217281_x_at | IL-8 | IL-8 | 91·46 | 47·08 | 1·94 | 0·0053833 |
| 54 | 214732_at | SP1 | Sp1 transcription factor | 53·16 | 32·09 | 1·66 | 0·0055414 |
| 55 | 217552_x_at | CR1 | Complement component (3b/4b) receptor 1 (Knops blood group) | 755·77 | 221·14 | 3·42 | 0·0057036 |
| 56 | 210039_s_at | PRKCQ | Protein kinase C, theta | 163·02 | 344·84 | 0·47 | 0·0057336 |
| 57 | 216033_s_at | FYN | FYN oncogene related to SRC, FGR, YES | 869·01 | 1260·7 | 0·69 | 0·0059456 |
| 58 | 209201_x_at | CXCR4 | Chemokine (CXC motif) receptor 4 | 1645·65 | 2668·73 | 0·62 | 0·0059718 |
| 59 | 204731_at | TGF-BR3 | Transforming growth factor, β receptor III | 394·58 | 829·35 | 0·48 | 0·0060871 |
| 60 | 205456_at | CD3E | CD3e molecule, epsilon (CD3–TCR complex) | 293·32 | 722·44 | 0·41 | 0·0062648 |
| 61 | 210145_at | PLA2G4A | Phospholipase A2, group IVA (cytosolic, calcium-dependent) | 52·4 | 35·04 | 1·5 | 0·0067767 |
| 62 | 209615_s_at | PAK1 | p21/Cdc42/Rac1-activated kinase 1 (STE20 homologue, yeast) | 212·87 | 152·72 | 1·39 | 0·0067792 |
| 63 | 201328_at | ETS2 | v-ets erythroblastosis virus E26 oncogene homologue 2 (avian) | 414·19 | 220·44 | 1·88 | 0·0068566 |
| 64 | 204852_s_at | PTPN7 | Protein tyrosine phosphatase, non-receptor type 7 | 108·76 | 170·37 | 0·64 | 0·0069018 |
| 65 | 201017_at | EIF1AX | Eukaryotic translation initiation factor 1A, X-linked | 177·87 | 324·3 | 0·55 | 0·0069616 |
| 66 | 213539_at | CD3D | CD3d molecule, δ (CD3–TCR complex) | 700·22 | 1956·46 | 0·36 | 0·0080763 |
| 67 | 218284_at | SMAD3 | SMAD family member 3 | 148·87 | 215·06 | 0·69 | 0·0089997 |
Geometric mean of detected fluorescence. NAPDH: nicotinamide adenine dinucleotide phosphate; cAMP: cyclic adenosine-5′-monophosphate; TCR: T cell receptor.
Up-regulated genes in IPEX patients included interleukin (IL)-8, IL-18 CD31 and interferon (IFN)-γ-receptor 1, while FcεR-I, signal transducer and activator of transcription-4 (STAT4), CD3δ, CD3ε and CD3ζ, CXCR4 and CCL5 were among the most down-regulated genes.
We then tested our list of differentially expressed genes for the presence of cancer related genes through a cancer genes database (http://cbio.mskcc.org/CancerGenes). Notably, 36 of 67 (53·7%) turned out to be cancer-related genes, including BCL2, LCK and FYN (data not shown).
Discussion
In this study we report three brothers affected by IPEX and having severe allergic reactions to food. Both genomics and proteomics approaches were used. IPEX is characterized by a complex combination of allergic, autoimmune and inflammatory disorders related to a lack of proper regulation of the immune response [19,24]. The main genetic feature of IPEX is represented by inability to synthesize the FOXP3 gene product, followed by a partial or total defect of the Treg function [11,24]. Accordingly, we observed the absence of FOXP3-positive cells (Treg) in the peripheral blood of our patients. The Treg deficiency may be caused by mutations localized within or near the forkhead domain, thus interfering with DNA binding [4,24], or located throughout the gene, therefore affecting FOXP3 function or mRNA splicing with markedly reduced FOXP3 messenger RNA (mRNA) levels [11,18]. All our IPEX patients display the 543 C > T nucleotide substitution at the junction between intron 4 and exon 5, possibly affecting FOXP3 mRNA production. This is a rare polymorphism (rs2232367) reported in the general population, and it cannot be excluded that its presence might increase susceptibility to disease development. In the case of mRNA splicing defect, the presence of multiple severe food allergies associated with autoimmune enteropathy has already been reported [11]. Similarly, our patients had severe allergic reactions to food, as also confirmed by specific IgE evaluation using a proteomic microarray system. As severe eczema hinders skin prick testing, and the IgE single-plexing test approach is not feasible as a screening test [25], the ISAC system allowed us to test our patients using a large panel of allergenic molecules, using small amounts of sera [26]. To our knowledge, this type of testing has never been used before, as the most recent report on IPEX describes not the immunological/allergy phenotype, but the clinical phenotype, of the selected patients [2,27]. Several other primary immunodeficiencies (PID) have been reported to be associated with IgE dysregulation (e.g. Omenn syndrome, Wiskott–Aldrich syndrome, Comel–Netherton syndrome, Job's syndrome and atypical complete DiGeorge syndrome) [28]. Total IgE levels are reported currently as proof of a skewed IgE dysregulation, while scant data are currently available on the allergen-specific IgE sensitization profile in patients affected by PID if we consider using allergenic molecules for such profiling. Unless IgE dysregulation could lead to a broad IgE recognition of environmental allergens, none of the most common inhalant allergens were found positive in the three brothers. Our IPEX patients showed IgE reactivity, directed mainly to cow's milk and hen's eggs allergens, rarely observed in a larger population in the same age range [12]. Such IgE sensitization could be the cause of skin [29] and bowel involvement [11], observed frequently in IPEX and other PIDs. As our patients were in the ‘normal’ allergic range for total IgE, in our opinion it would be more useful to evaluate not only total IgE levels, but also specific IgE reactivity in PID, in order to show the presence of an IgE dysregulation. Whether or not evaluation of specific IgE recognition might add further insight into each PID dysregulation requires larger co-operative studies. In fact, to translate our experimental findings into a reliable approach to IPEX and other PIDs, the same experimental procedure should be applied to a larger group of patients, evaluated longitudinally, leading to the design of useful diagnostic algorithms. Nevertheless, the overall clinical picture of patients affected by IPEX syndrome, including the development of autoimmune, allergic and, as described herein, lymphoproliferative diseases, undoubtedly requires an early disease-modifying intervention such as transplantation.
The gene expression profile revealed the involvement of genes related to the positive regulation of the immune system process, thereby confirming the presence of immune dysregulation, which is one of the main features of the IPEX syndrome.
Finally, an association with the development of lymphoproliferative diseases was observed in our patients. Accordingly, we observed a dysregulation of several cancer-related genes (Table 3). The development of lymphoma after immunosuppressive drugs such as rapamycin has already been observed [30], but the possible evolution of IPEX syndrome to lymphoma has not been reported to date. This is due probably to the early death of patients affected by the most aggressive form of IPEX; however, this evolution might possibly be observed in patients with a mild clinical picture. FACS analysis, through the evaluation of (i) the TCR-Vβ repertoire and/or (ii) the presence of CD19+ B cells bearing CD5 (B1 cells), represents a useful approach in order to verify the possibility of a lymphoproliferative evolution of the disease.
In conclusion, the proteomics (IgE and FACS analysis) and genomics evaluation described in this study could improve the procedures used currently in the diagnosis of IPEX concurrent diseases, thus helping the staging of IPEX itself. The same could also be extended to other PIDs, before and after any therapeutic intervention.
Acknowledgments
The authors would like to thank Chiara Rafaiani, Marina Liso and Paola Palazzo, who routinely performed the ISAC test during the time the study was carried out. This study was funded by the Italian Ministry of Health, Current Research Program 2008–2010.
Disclosure
The authors declare no conflict of interest.
References
- 1.Baud O, Goulet O, Canioni D, et al. Treatment of the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) by allogeneic bone marrow transplantation. N Engl J Med. 2001;344:1758–62. doi: 10.1056/NEJM200106073442304. [DOI] [PubMed] [Google Scholar]
- 2.Gambineri E, Perroni L, Passerini L, et al. Clinical and molecular profile of a new series of patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: Inconsistent correlation between forkhead box protein 3 expression and disease severity. J Allergy Clin Immunol. 2008;122:1105–12. doi: 10.1016/j.jaci.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira JB, Fleisher TA. 26. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125:297–305. doi: 10.1016/j.jaci.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett CL, Brunkow ME, Ramsdell F, et al. A rare polyadenylation signal mutation of the FOXP3 gene (AAUAAA–>AAUGAA) leads to the IPEX syndrome. Immunogenetics. 2001;53:435–9. doi: 10.1007/s002510100358. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 6.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 7.Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of T regulatory cells in immune regulation of allergic diseases. Eur J Immunol. 2010;40:1232–40. doi: 10.1002/eji.200940045. [DOI] [PubMed] [Google Scholar]
- 8.Ochs HD, Gambineri E, Torgerson TR. IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol Res. 2007;38:112–21. doi: 10.1007/s12026-007-0022-2. [DOI] [PubMed] [Google Scholar]
- 9.d'Hennezel E, Ben-Shoshan M, Ochs HD, et al. FOXP3 forkhead domain mutation and regulatory T cells in the IPEX syndrome. N Engl J Med. 2009;361:1710–13. doi: 10.1056/NEJMc0907093. [DOI] [PubMed] [Google Scholar]
- 10.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–46. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Torgerson TR, Linane A, Moes N, et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a non-coding region of the FOXP3 gene. Gastroenterology. 2007;132:1705–17. doi: 10.1053/j.gastro.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 12.Scala E, Alessandri C, Bernardi ML, et al. Cross-sectional survey on immunoglobulin E reactivity in 23 077 subjects using an allergenic molecule-based microarray detection system. Clin Exp Allergy. 2010;40:911–21. doi: 10.1111/j.1365-2222.2010.03470.x. [DOI] [PubMed] [Google Scholar]
- 13.Benjamini Y, Hochberg Y. Controlling the false discovery rate – a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 14.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 15.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr. 2001;13:533–8. doi: 10.1097/00008480-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–45. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–5. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Ochs HD, Ziegler SF, Torgerson TR. FOXP3 acts as a rheostat of the immune response. Immunol Rev. 2005;203:156–64. doi: 10.1111/j.0105-2896.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 20.Myers AK, Perroni L, Costigan C, Reardon W. Clinical and molecular findings in IPEX syndrome. Arch Dis Child. 2006;91:63–4. doi: 10.1136/adc.2005.078287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakaguchi S. The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J Clin Invest. 2003;112:1310–12. doi: 10.1172/JCI20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narducci MG, Pescarmona E, Lazzeri C, et al. Regulation of TCL1 expression in B- and T-cell lymphomas and reactive lymphoid tissues. Cancer Res. 2000;60:2095–100. [PubMed] [Google Scholar]
- 23.Gaudet P, Chrisholm R. The Gene Ontology's Reference Genome Project: a unified framework for functional annotation across species. PLoS Comput Biol. 2009;5:e1000431. doi: 10.1371/journal.pcbi.1000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen CJ, Jennings CE, Imrie H, et al. Mutational analysis of the FOXP3 gene and evidence for genetic heterogeneity in the immunodysregulation, polyendocrinopathy, enteropathy syndrome. J Clin Endocrinol Metab. 2003;88:6034–9. doi: 10.1210/jc.2003-031080. [DOI] [PubMed] [Google Scholar]
- 25.Mari A, Alessandri C, Bernardi ML, Ferrara R, Scala E, Zennaro D. Microarrayed allergen molecules for the diagnosis of allergic diseases. Curr Allergy Asthma Rep. 2010;10:357–64. doi: 10.1007/s11882-010-0132-0. [DOI] [PubMed] [Google Scholar]
- 26.Hiller R, Laffer S, Harwanegg C, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–16. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- 27.Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: forkhead box protein 3 mutations and lack of regulatory T cells. J Allergy Clin Immunol. 2007;120:744–50. doi: 10.1016/j.jaci.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 28.Ozcan E, Notarangelo LD, Geha RS. Primary immune deficiencies with aberrant IgE production. J Allergy Clin Immunol. 2008;122:1054–62. doi: 10.1016/j.jaci.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Halabi-Tawil M, Ruemmele FM, Fraitag S, et al. Cutaneous manifestations of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Br J Dermatol. 2009;160:645–51. doi: 10.1111/j.1365-2133.2008.08835.x. [DOI] [PubMed] [Google Scholar]
- 30.Lucas KG, Ungar D, Comito M, Groh B. Epstein Barr virus induced lymphoma in a child with IPEX syndrome. Pediatr Blood Cancer. 2008;50:1056–7. doi: 10.1002/pbc.21341. [DOI] [PubMed] [Google Scholar]


