Abstract
The disturbed cytokine–chemokine network could play an important role in the onset of diseases with inflammatory processes such as chronic idiopathic urticaria (CIU). Our main objectives were to evaluate the relation between proinflammatory chemokine serum levels from CIU patients and their response to autologous skin test (ASST) and basophil histamine release (BHR). We also aimed to assess the chemokine secretion by peripheral blood mononuclear cells (PBMC) upon polyclonal stimulus and to evaluate chemokine C–C ligand 2/C-X-C chemokine 8 (CCL2/CXCL8) and Toll-like receptor-4 (TLR-4) expression in monocytes. We observed significantly higher serum levels of the CXCL8, CXCL9, CXCL10 and CCL2 in CIU patients compared to the healthy group, regardless of the BHR or ASST response. The basal secretion of CCL2 by PBMC or induced by Staphylococcus aureus enterotoxin A (SEA) was higher in CIU patients than in the control group, as well as for CXCL8 and CCL5 secretions upon phytohaemagglutinin stimulation. Also, up-regulation of CCL2 and CXCL8 mRNA expression was found in monocytes of patients upon SEA stimulation. The findings showed a high responsiveness of monocytes through CCL2/CXCL8 expression, contributing to the creation of a proinflammatory environment in CIU.
Keywords: chemokines, chronic idiopathic urticaria, innate immunity, monocytes
Introduction
Chronic urticaria (CU) is a skin disorder characterized by recurrent and transitory itchy weals that last for 6 weeks or longer. CU can be accompanied by angioedema in 40% of the cases, has a higher incidence in women (2:1) and a prevalence of approximately 0·1%, with an average episode length of 3–5 years in adults [1]. Despite the extensive search for underlying causes or triggering factors, the aetiology of CU remains unclear in at least 80–90% of the patients; therefore, it is referred to as chronic idiopathic urticaria (CIU). In addition, the term chronic autoimmune urticaria has been proposed for those 35–40% of the CU patients who develop anti-immunoglobulin (Ig)E IgG to the receptor alpha subunit and for an additional 5–10% that produce anti-IgE IgG autoantibodies [2,3].
The functional properties of antibodies can be evidenced in vitro with the release of histamine from basophils and mast cells elicited in the presence of serum from CIU patients [2,4]. In vivo evidence can be achieved by intradermal injection of autologous serum (autologous serum skin test, ASST) that is able to induce a weal-and-flare response in patients with CIU [5]. Nonetheless, the ASST has been considered an autoreactivity test rather than a specific test for autoimmunity [6].
It has been suggested that inflammation in CU patients does not result solely from the consequences of mast cells (or basophil) degranulation [7]. In CIU patients, inflammatory aetiopathogenesis has been related to an increase in the interleukin (IL)-4, IL-13 and IL-18 serum levels or in factors such as neopterin [8–10]. Our previous findings support the concept of immunological dysregulation in CIU with disturbed cytokine production by T cells – related mainly to IL-17 and IL-10 production – regardless of skin reactivity to ASST [11]. The possibility of other factors, such as chemokines, acting in parallel to the cytokines is not excluded and should be investigated for CIU.
Chemokines are proinflammatory chemotactic cytokines that recruit leucocytes to inflammation sites, but also play important roles in tumour growth, angiogenesis, organ sclerosis and autoimmunity [12,13]. Chemokines are a superfamily of small proteins (8–14 kD) classified on the basis of their structural properties regarding the number and position of conserved cystein residues [14]. C-X-C chemokine 8 (CXCL8)/IL-8, which is the best-characterized C-X-C chemokine, is chemotactic for neutrophils, T lymphocytes and monocytes [15–17].
CXCL9/Mig, a monokine induced by interferon (IFN)-γ, acts as chemoattractant for T helper type 1 (Th1) cells and blocks eosinophil chemoattraction, the platelet-activating factor and leukotriene B4-induced responses [18]. Both CXCL9 and CXCL10/IFN-γ-induced protein 1 (IP-10) are considered type 1 chemokines and display strong chemoattraction for Th1 cells [19,20]. In contrast, the C-C chemokines, of which chemokine C–C ligand 2 (CCL2) (monocyte chemoattractant protein-1) is a prototypic example, activate a variety of cells, including monocytes, macrophages, lymphocytes, eosinophils and basophils, and have been implicated in chronic inflammatory diseases [13,21]. CCL2 is a crucial factor for the development of adaptive Th2 responses [22] and also causes degranulation of mast cells and, mainly, basophils [23]. Together with other factors, CCL2, CCL5 and CXCL8 have been suggested to play a fundamental role in histamine and serotonin generation in mast cells and are mediators of acute inflammatory responses [24]. Considering the important roles of chemokines and their receptors in the inflammatory process of several skin diseases [25–27], it is possible that the proinflammatory chemokines together with other factors, such as like cytokines, contribute to the immune dysregulation in CIU.
Due to the role of CXCL8, CXCL9, CXCL10, CCL2 and CCL5 in innate immune response and inflammation, we evaluated the level of these chemokines in sera from CIU patients and compared their ASST and BHR responses. Moreover, to evaluate the chemokines produced by monocytes and T cells, we assessed (i) the ability of peripheral blood mononuclear cells (PBMC) to secrete CXCL8 and CCL2 in response to superantigen, a stimulus to monocytes plus T cells and polyclonal T cells, and (ii) the relative levels of mRNA and protein in CD14+ cells and CD4+ T cells.
Materials and methods
Study subjects and ASST
CIU patients who had recurrent and transitory itchy weals for more than 6 weeks, at least four times a week, were selected from the Dermatological Outpatient Clinic of the Hospital das Clínicas de São Paulo. Patients with physical urticaria, urticarial vasculitis or food allergies were excluded as described previously [28]. Anti-histamine medications were withdrawn 48 h before the study. The CU patients and healthy control (HC) group who participated in our study did not take corticosteroids or immunosuppressive drugs. Samples were obtained from 38 patients with CIU (32 females and six males, aged 22–80 years, median age 48·50 years) and 32 healthy controls (24 females and eight males, aged 23–60 years, median age 38·81 years). The HC group consisted of laboratory staff who do not take corticosteroids or immunosuppressive drugs with no clinical signs of urticarial, asthma/rhinitis and food allergies. All subjects gave written informed consent, and the study protocol was approved by the institutional ethics committee of the HC-FMUSP.
The ASST (autologous serum skin test) was performed according to standard methods [5], using fresh autologous serum injected intradermally into the volar forearm skin. A positive test was defined as a serum-induced weal ≥1·5 mm diameter more than that elicited by the saline response after 30 min.
Basophil histamine release assay
BHR was performed using leucocyte suspension from healthy blood donors, as described by Luquin and collaborators [29]. Whole blood was collected in ethylenediamine tetraacetic acid (EDTA) (40 ml), sedimented in 6% dextran-3% glucose-0·15 m NaCl saline solution. Fifty µl of leucocytes (20 × 106 cells/ml) diluted in phosphate-buffered saline (PBS) containing 3% bovine serum albumin (BSA) with 2 mm CaCL2 and 1 mm MgCl2 were incubated with 50 µl of serum in 96-well microplates (Costar, Cambridge, MA, USA) for 40 min at 37°C. The positive control reaction was incubated with 50 µl of goat anti-human IgE (12·5 µg/ml) (Calbiochem, Darmstadt, Germany) in PBS. For determining total histamine, two replicates of cell aliquots were boiled for 5 min. Reaction was stopped by the addition of 25 µl of 20 mm/ml EDTA and ice-cold buffer, and centrifuged. Histamine concentration was measured with enzyme-linked immunosorbent assay (ELISA) based on the competition principle, according to the manufacturer's instructions (IBL, Hamburg, Germany). The optical density reading was performed at 450 nm in an ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA) with a SOFT max PRO program. The results are expressed as percentage release using the formula: (sample-basal)/(total-basal) × 100. The cut-off values for each assay were determined as the percentage of histamine release comparing to the media obtained from healthy serum (n = 27) plus 2 standard deviations (SD).
Serum chemokine measurements
Serum obtained from CIU patients and healthy subjects were measured using the human chemokine cytometric bead array kit (Becton Dickinson, San Diego, CA, USA) and analysed by flow cytometry [fluorescence activated cell sorter (FACS)Calibur; Becton Dickinson]. CCL5 measurement was performed by ELISA (R&D Systems, Minneapolis, MN, USA). The optical density reading was performed at 450 nm in an ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA) with a SOFT max PRO program.
In vitro induction of chemokine secretion by PBMC
PBMC were obtained from heparinized venous blood by Ficoll-Hypaque gradient centrifugation (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and diluted in RPMI-1640 medium supplemented with 10% AB human serum (Sigma, St Louis, MO, USA). Cultures of PBMC (3·0 × 106 cells/well) were incubated in 24-well plates (Costar, Cambridge, MA, USA) with medium or phytohaemagglutinin (PHA, 2·5 µg/ml) or Staphylococcus aureus enterotoxin A (SEA, 40 ng/ml) for 24 h at 37°C and 5% CO2. Cell-free supernatants were harvested and stored at −80°C until chemokine measurement by ELISA (R&D Systems). The limits for chemokine detection by ELISA are as follows: 7·81 pg/ml for CXCL8 and CCL5 and 23·44 pg/ml for CCL2.
Flow cytometry
For intracellular CXCL8 and CCL2 staining, PBMC (3·0 × 106) were cultivated in RPMI-1640 medium with 10% AB human serum (Sigma) in 24-well plates (Costar) for 18 h at 37°C and 5% CO2 with medium or SEA (1 µg/ml) and brefeldin A (Sigma, 10 µg/ml). After incubation with 10 µl of human IgG for 10 min at 4°C, cells were labelled with anti-CD14 PC5 (BD PharMingen). The cells were fixed with fixation medium (Fix & Perm medium A; Invitrogen, Calsbad, CA, USA), washed and then permeabilized with permeabilization medium (Fix & Perm medium B; Invitrogen). Samples were incubated with anti-CXCL8 phycoerythrin (PE) or anti-CCL2 PE (BD Pharmingen) with their respective isotype controls. Analyses were performed in a Coulter EPICS XL-ML flow cytometer (Coulter, Miami, FL, USA) with System II software.
Real-time polymerase chain reaction (PCR)
CD4+ T cells and CD14+ monocytes were isolated by magnetic cell sorting using negative and positive selection, respectively, with magnetic microbeads (Miltenyi Biotech, Paris, France). The purity of the two cell populations was 95% as assessed by flow cytometry. CD14+ and CD4+ cells were cultivated in RPMI-10% AB human serum (Sigma) in 96-well plates (Costar) with medium or SEA (40 ng/ml) or PHA (2·5 µg/ml), respectively, for 2 h at 37°C and 5% CO2. Total RNA from 1 × 106 cells was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and reverse transcription was performed with Sensiscript reverse transcriptase (Qiagen). The real-time PCR condition was performed as described previously [11], with the following primers: albumin sense: 5′-GCT-GTC-ATC-TCT-TGT-GGG-CTG-T-3′, albumin anti-sense: 5′-AAA-CTC-ATG-GGA-GCT-GCT-GGT-T-3′, melting temperature (Tm, 79°C); CCL2 sense: 5′-TCT-GTG-CCT-GCT-GCT-CAT-AG-3′, CCL2 anti-sense: 5′-CAG-ATC-TCC-TTG-GCC-ACA-AT-3′, Tm 83°C; CCL5 sense: 5′-ACC-ACA-CCC-TGC-TGC-TTT-GC-3′, CCL5 anti-sense: 5′-CCG-AAC-CCA-TTT-CTT-CTC-TGG-3′, Tm 84°C; CXCL8 sense: 5′-TGT-GTG-TAA-ACA-TGA-CTT-CCA-AGC-T-3′, CXCL8 anti-sense: 5′-GCA-AAA-CTG-CAC-CTT-CAC-ACA-G-3′, Tm 81°C; and Toll-like receptor-4 (TLR-4) sense 5′-CAG-AGT-TTC-CTG-CAA-TGG-ATC-A-3′, TLR-4 anti-sense: 5′-GCT-TAT-CTG-AAG-GTG-TTG-CAC-AT-3′, Tm 78°C. The copy number of chemokines messenger was normalized with the housekeeping gene, albumin, and presented as a cytokine to albumin copy-number ratio [30].
Statistical analysis
The Mann–Whitney U-test was used to compare variables between patients with CIU and healthy controls. Wilcoxon's test, a non-parametric paired test, was used to compare the difference between the expression levels under basal and stimulated conditions. P values less than 0·05 were considered significant.
Results
Circulating chemokines in CIU
Considering the active role chemokines play in inflammatory process, we evaluated the level of the CXCL8, CXCL9 and CXCL10 and the CCL2 and CCL5 in serum from patients with CIU. There was a significant increase in the level of CXCL8, 9, 10 and CCL2 in CIU sera in comparison with the HC group (Fig. 1). The CCL5 chemokine was detected at the highest concentration, but no difference was observed between the levels found in sera from CIU or HC groups. The relation among levels of chemokines in the CIU group was CCL5 > CXCL10 = CXCL9 > CCL2 > CCL8, and in the HC group was CCL5 > CXCL10 > CXCL9 > CCL2 > CCL8. In fact, in HC the level of CXCL10 was higher than that of CXCL9 (CXCL10 > CXCL9), whereas in CIU the level of CXCL10 was found to be similar to that of CXCL9 (CXCL10 = CXCL9).
Fig. 1.
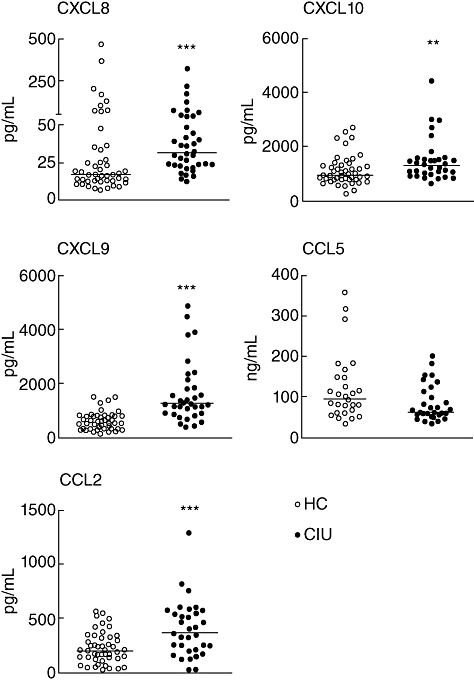
Circulating chemokine levels in patients with chronic idiopathic urticaria (CIU). Serum samples from healthy controls (HC) and CIU patients were used to determine the presence of CXCL8, CXCL10, CCL2 and CXCL9 by cytometric bead array. Determination of CCL5 levels was performed by enzyme-linked immunosorbent assay (ELISA). Horizontal line represents the median. ***P ≤ 0·001; **P ≤ 0·01 compared with the HC group.
The circulating levels of chemokines in CIU patients were tested through the ASST or the BHR assay. Table 1 shows that of 38 patients, 21 (55·3%) had a positive response to ASST and 44·7% were negative. For the BHR, 10 of the 38 patients (26·3%) were positive, of whom six (60%) were both BHR+ and ASST+, and the remaining four were BHR+ and ASST-. No significant difference was found regarding the levels of circulating chemokines between patients positive or negative for the ASST response; similarly, no difference was found between BHR-positive or BHR-negative. Furthermore, the levels of chemokines were compared with the clinical evidence for angioedema. No significant difference was found in the chemokine levels of patients with or without angioedema (Table 1).
Table 1.
Chemokine serum levels in patients with chronic idiopathic urticaria (CIU)
| Patients | CXCL10 | CXCL9 | CXCL8 | CCL2 | CCL5 |
|---|---|---|---|---|---|
| (n) | pg/ml | pg/ml | pg/ml | pg/ml | ng/ml |
| ASST+ (21) | 1747 ± 252·1 | 1933 ± 366·7 | 35·4 ± 5·0 | 433·6 ± 87·1 | 84·3 ± 15·1 |
| ASST- (17) | 1487 ± 194·7 | 1639 ± 217·4 | 72·5 ± 18·8 | 425·6 ± 66·8 | 84·3 ± 10·2 |
| BHR+ (10) | 1294 ± 134·8 | 1936 ± 469·0 | 54·1 ± 23·5 | 414·6 ± 144·3 | 85·3 ± 19·0 |
| BHR- (24) | 1618 ± 181·5 | 1700 ± 242·0 | 57·2 ± 12·6 | 507·1 ± 63·2 | 86·7 ± 8·6 |
| ANG+ (16) | 1403 ± 159 | 1424 ± 271·9 | 42·29 ± 5·26 | 474·0 ± 97·5 | 82·0 ± 10·5 |
| ANG- (11) | 1343 ± 155 | 1387 ± 197·9 | 64·83 ± 21·39 | 351·0 ± 58·0 | 85·4 ± 23·7 |
Autologous serum skin test (ASST) or basophil histamine release assay (BHR) in 38 patients with CIU expressed as mean ± standard error of the mean. Association with angioedema (ANG+).
In vitro chemokine secretion by PBMC
Considering the broad influence of CCL2, CXCL8 and CCL5 on the lymphocyte and monocyte populations, we evaluated the level of chemokines secreted by PBMC using PHA or SEA as stimulus. As shown in Fig. 2, the spontaneous secretion of CCL2 by PBMC from CIU patients was significantly higher than that from the HC group. In addition, in CIU patients, CCL2 secretion was greatly enhanced by SEA stimulation, and CCL5 and CXCL8 levels were increased using PHA as stimulus in comparison with HC individuals (Fig. 2). The CXCL8 secretion induced by SEA was not significantly different from the control values.
Fig. 2.
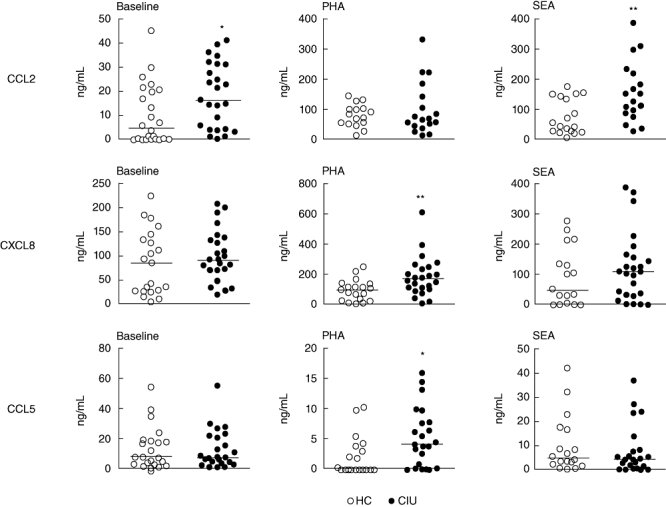
In vitro chemokine secretion by peripheral blood mononuclear cells (PBMC) from patients with chronic idiopathic urticaria (CIU). PBMC from healthy controls (HC) and CIU patients were incubated with medium (baseline) or with phytohaemagglutinin (PHA) (2·5 µg/ml) or Staphylococcus aureus enterotoxin A (SEA) (40 ng/ml) for 24 h. Supernatants were taken to determine the presence of CCL2, CXCL8 and CCL5 by enzyme-linked immunosorbent assay (ELISA). Horizontal line represents the median. **P ≤ 0·01; *P ≤ 0·05 compared with the HC group.
Up-regulation of CXCL8 and CCL2 and down-regulation of TLR-4 in monocytes of CIU patients
Monocytes from PBMC secreting CCL2 and CXCL8 were evaluated by flow cytometry after stimulation with SEA and in the presence of brefeldin. Also, levels of CCL2 and CXCL8 mRNA from CD14+ cells treated with SEA were analysed by real-time PCR. We found that in PBMC the CD14+ cells were the main source of CXCL8 in both groups (Fig. 3a), as the analysis of CD4+ and CD8+ T cells showed fewer than 2% of positive cells for CXCL8 secretion (data not shown).
Fig. 3.
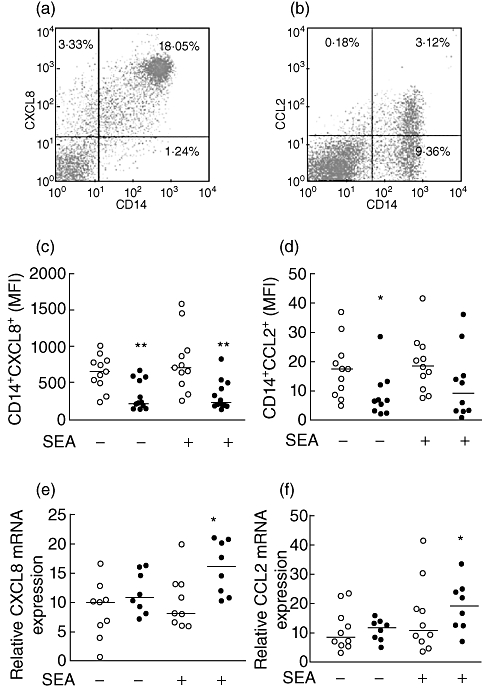
Up-regulation of CXCL8 and CCL2 mRNA levels in monocytes upon Staphylococcus aureus enterotoxin A (SEA) stimulation in patients with chronic idiopathic urticaria (CIU). Peripheral blood mononuclear cells (PBMC) from healthy controls (HC) (open symbol) and CIU patients (closed symbol) were incubated with medium (baseline) or with SEA and brefeldin A for 18 h; CD14+ cells secreting intracellular CXCL8 and CCL2 were evaluated by flow cytometry. Dot-plot graphs illustrate non-stimulated CD14+ cells from HC individuals expressing CXCL8 (a) or CCL2 (b). The mean fluorescence intensity (MFI) for CXCL8 (c) and CCL2 (d) expression on CD14+ cells is shown. CD14+ cells were purified from HC and CIU patients, stimulated with SEA for 2 h, and CXCL8 (e) and CCL2 (f) mRNA levels were determined by real-time polymerase chain reaction. The horizontal line represents the median. **P ≤ 0·01; *P ≤ 0·05 compared with the HC group.
The mean fluorescence intensity (MFI) of intracellular CXCL8+ in CD14+ cells with or without SEA stimulation was significantly lower in CIU than in HC subjects (Fig. 3c). Similar results were observed for the percentage of CD14+ cells secreting CXCL8 (data not shown).
In contrast, the mRNA analysis from CD14+ cells stimulated with SEA (Fig. 3e) revealed an increased CXCL8 expression in CIU patients compared to HC. The up-regulation of the CXCL8 mRNA and protein levels detected in the supernatant of cell cultures suggests that activated monocytes in CIU individuals secrete the chemokine in vivo quickly; this could interfere with the in vitro capture of the intracellular protein.
CCL2 was secreted mainly by CD14+ cells (Fig. 3b) and its basal expression decreased in CIU patients whereas, after SEA stimulation, similar levels were observed for both CIU and HC groups (Fig. 3d). Analysis of CCL2 expression in CD4+ T and CD8+ T cells, naive or memory B cells, as well as basophils, were negative (data not shown).
Similarly to CXCL8, the CCL2 mRNA level increased after SEA stimulation in CD14+ cells from the CIU group compared to HC (Fig. 3f). These findings show the activation status of monocytes in CIU and hyperresponsiveness to SEA stimulation. Moreover, considering that Gram-positive bacterial superantigenic exotoxins can up-regulate the transcription and membrane expression of TLR-4 in human monocytes [27], we evaluated the ex vivo TLR-4 expression in CD14+ T cells. Interestingly, TLR-4 expression on monocytes from patients was reduced significantly compared to HC CD14+ cells (Fig. 4a).
Fig. 4.
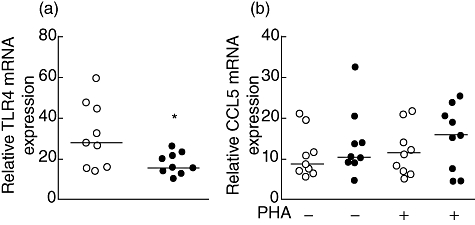
Toll-like receptor-4 (TLR-4) mRNA expression on monocytes and CCL5 mRNA expression on CD4+ T cells of patients with chronic idiopathic urticaria (CIU). Purified non-stimulated CD14+ cells from healthy controls (HC) (open symbol) and CIU patients (closed symbol) were evaluated for TLR-4 mRNA levels (a) or for CCL5 mRNA expression on purified CD4+ T cells stimulated with phytohaemagglutinin (PHA) for 2 h (b) by real-time polymerase chain reaction. The horizontal line represents the median. *P ≤ 0·05 compared with the HC group.
In view of the high levels detected for CCL5 secreted by PBMC, we evaluated the expression of the CCL5 mRNA levels in CD4+ T cells by real-time PCR (Fig. 4b). The CCL5 mRNA levels in CD4+ T cells with or without PHA stimulation were similar in both patients and control groups (Fig. 4b). Also, the expression of the CXCR5 receptor on CD4+ T cells, evaluated by flow cytometry, showed similar MFI between groups (data not shown).
Discussion
The combination of chemokines may exert a key influence in the innate and adaptive immune response and could explain why T cells or other cell subtypes become activated in CIU. We have shown previously in CIU a disturbance in the T cell response for IL-2, IL-4, IL-10 and IL-17a secretion [11]. Furthermore, we have also shown a disturbance in the innate immune response in CIU related to cytokine secretion by plasmacytoid dendritic cells though TLR-9 activation [31]. In this sense, we demonstrated that the altered chemokine synthesis is related to the dysfunction of the innate immune response in CIU. Therefore, we evaluated the chemokines that exhibit a broad effect on monocytes, a subset scarcely studied in CIU. Accordingly, CXCL8 was chosen, as it is chemotactic for neutrophils, T lymphocytes and monocytes [15,17], and also CXCL10, as it is chemotactic for IFN-γ-secreting Th1 lymphocytes [20] and CCL2, secreted mainly by monocytes, and a crucial factor for the development of adaptive Th2 responses [22].
Increases in CXCL8, 9 and 10 and CCL2 levels were detected in the majority of the CIU patients, regardless of ASST or BHR response. Moreover, chemokine up-regulation was not correlated with clinical parameters such as angi-oedema, which was detected in 59% in the cohort of CIU patients. Of these chemokines, we focused on CCL2, in view of its increased serum levels in CIU, the spontaneously active secretion by PBMC, and the high secretion levels induced by SEA stimulation. The crucial source of CCL2 was monocytes, as shown by the increased mRNA expression in CD14+ cells in CIU. CCL2 is one of the key factors involved in the initiation of inflammation, triggering chemotaxis and transendothelial migration of monocytes to inflammatory lesions by interacting with the membrane CC chemokine receptor 2 (CCR2) in monocytes [32]. CCL2 also has a potent basophil histamine release activity [22]; in CIU, a normal basophil response to CCL2 has been reported, whereas it cannot be ruled out that CCL2 might be involved in histamine release [33]. In fact, it seems that serum factor(s) could be involved in the activation of basophils, as basophils from patients with CIU are hyperresponsive to serum, but such a factor is yet to be identified [29]. Previously, we observed that basophils from patients with CIU displayed up-regulation of the activation/degranulation markers, CD203c and CD63, and high responsiveness to IL-3 stimulation [28]. This basophil-activated profile was due probably to an in vivo priming triggered by a potent basophil-activating factor such as CCL2; therefore, we observed increased CCL2 serum levels associated with active secretion by monocytes that, together, could contribute, at least in part, to the activated status of basophils in CIU patients.
In addition, several other chemokines may also be involved in the recruitment/activation of monocytes. In this regard, it has been demonstrated that the stimulation of CCL5 leads to recruitment of monocytes/macrophages which express CCR5 and CCR1, both of which bind CCL5 with high affinity [34]. In CIU the target cells are mast cells and basophils, in which mast cell activation requires an assembly of circulating chemokines, such as CCL5, CCL2 and CXCL8 that together play a fundamental role in histamine and serotonin generation [24]. In this sense, not only CCL5, but CCL2 and CXCL8 were also significantly more abundant in CIU; up-regulation of these chemokines in patients with CIU may possibly contribute to the maintenance of activated status of cell subsets, such as basophils, monocytes and T cells. It is probable that the disturbance of chemokine synthesis may be one of the triggering factor(s) of the inflammatory status in the CIU.
The activated status of monocytes in CIU was reinforced by hyperresponsiveness to SEA stimulation. Superantigens bind directly to specific regions of major histocompatibility complex (MHC) class II molecules on the antigen-presenting cells and to the variable region of the β chain of the T cell receptor [35]. Interestingly, a decreased TLR-4 expression on CD14+ cells from CIU patients was observed in ex vivo conditions. It has been described that histamine could inhibit the expression of CD14 on monocytes through the stimulation of H2-receptors [36]. TLR-4 requires CD14 to participate in the process of lipopolysaccharide (LPS)-induced signalling; whether there is a direct action from histamine released by skin mast cells, or by a secondary effect of H1-anti-histamine on regulation of TLR-4 expression on monocytes, needs to be explored further. Together, these findings suggest that there is an ongoing inflammatory process in CIU.
In conclusion, the results showed that patients with CIU present an altered chemokine secretion pattern that is potentially connected to their chronic inflammatory status. Up-regulation of CCL2 and CXCL8 genes, measured by mRNA levels and cell-secreted protein in serum, indicates high responsiveness from monocytes, contributing to the creation of a proinflammatory environment in CIU. These findings suggest that the innate immune system through chemokines and monocytes could lead to the immune activation contributing to the pathogenesis of CIU.
Acknowledgments
We thank Dr Gabriela Ribeiro-dos-Santos for reviewing the manuscript. This work was supported by FAPESP (2007/58485–9) and LIM-56/HCFMUSP.
Disclosure
The authors declare no conflict of interest.
References
- 1.Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664–72. doi: 10.1067/mai.2000.105706. [DOI] [PubMed] [Google Scholar]
- 2.Hide M, Francis DM, Grattan CE, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328:1599–604. doi: 10.1056/NEJM199306033282204. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan AP, Greaves M. Pathogenesis of chronic urticaria. Clin Exp Allergy. 2009;39:777–87. doi: 10.1111/j.1365-2222.2009.03256.x. [DOI] [PubMed] [Google Scholar]
- 4.Sabroe RA, Francis DM, Barr RM, et al. Anti-Fc(episilon)RI auto antibodies and basophil histamine releasability in chronic idiopathic urticaria. J Allergy Clin Immunol. 1998;102(Pt 1):651–8. doi: 10.1016/s0091-6749(98)70283-0. [DOI] [PubMed] [Google Scholar]
- 5.Sabroe RA, Grattan CE, Francis DM, Barr RM, Kobza Black A, Greaves MW. The autologous serum skin test: a screening test for autoantibodies in chronic idiopathic urticaria. Br J Dermatol. 1999;140:446–52. doi: 10.1046/j.1365-2133.1999.02707.x. [DOI] [PubMed] [Google Scholar]
- 6.Guttman-Yassky E, Bergman R, Maor C, Mamorsky M, Pollack S, Shahar E. The autologous serum skin test in a cohort of chronic idiopathic urticaria patients compared to respiratory allergy patients and healthy individuals. J Eur Acad Dermatol Venereol. 2007;21:35–9. doi: 10.1111/j.1468-3083.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan A. Inflammation in chronic urticaria is not limited to the consequences of mast cell (or basophil) degranulation. Clin Exp Allergy. 2010;40:834–5. doi: 10.1111/j.1365-2222.2010.03489.x. [DOI] [PubMed] [Google Scholar]
- 8.Caproni M, Cardinali C, Giomi B, et al. Serological detection of eotaxin, IL-4, IL-13, IFN-gamma, MIP-1alpha, TARC and IP-10 in chronic autoimmune urticaria and chronic idiopathic urticaria. J Dermatol Sci. 2004;36:57–9. doi: 10.1016/j.jdermsci.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Ciprandi G, De Amici M, Berardi L, et al. Serum neopterin levels in spontaneous urticaria and atopic dermatitis. Clin Exp Dermatol. 2011;36:85–7. doi: 10.1111/j.1365-2230.2010.03914.x. [DOI] [PubMed] [Google Scholar]
- 10.Tedeschi A, Lorini M, Suli C, Asero R. Serum interleukin-18 in patients with chronic ordinary urticaria: association with disease activity. Clin Exp Dermatol. 2007;32:568–70. doi: 10.1111/j.1365-2230.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 11.Dos Santos JC, Azor MH, Nojima VY, et al. Increased circulating pro-inflammatory cytokines and imbalanced regulatory T-cell cytokines production in chronic idiopathic urticaria. Int Immunopharmacol. 2008;8:1433–40. doi: 10.1016/j.intimp.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Mackay CR. Chemokines: immunology's high impact factors. Nat Immunol. 2001;2:95–101. doi: 10.1038/84298. [DOI] [PubMed] [Google Scholar]
- 13.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 14.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 15.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–9. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerszten RE, Garcia-Zepeda EA, Lim YC, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–23. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 17.Taub DD, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. Chemokines and T lymphocyte activation: I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156:2095–103. [PubMed] [Google Scholar]
- 18.Fulkerson PC, Zhu H, Williams DA, Zimmermann N, Rothenberg ME. CXCL9 inhibits eosinophil responses by a CCR3- and Rac2-dependent mechanism. Blood. 2005;106:436–43. doi: 10.1182/blood-2005-02-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotondi M, Rosati A, Buonamano A, et al. High pretransplant serum levels of CXCL10/IP-10 are related to increased risk of renal allograft failure. Am J Transplant. 2004;4:1466–74. doi: 10.1111/j.1600-6143.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- 21.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 22.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–36. [PubMed] [Google Scholar]
- 23.Rose CE, Jr, Sung SS, Fu SM. Significant involvement of CCL2 (MCP-1) in inflammatory disorders of the lung. Microcirculation. 2003;10:273–88. doi: 10.1038/sj.mn.7800193. [DOI] [PubMed] [Google Scholar]
- 24.Castellani ML, De Lutiis MA, Toniato E, et al. Impact of RANTES, MCP-1 and IL-8 in mast cells. J Biol Regul Homeost Agents. 2010;24:1–6. [PubMed] [Google Scholar]
- 25.Gros E, Bussmann C, Bieber T, Förster I, Novak N. Expression of chemokines and chemokine receptors in lesional and nonlesional upper skin of patients with atopic dermatitis. J Allergy Clin Immunol. 2009;124:753–60. doi: 10.1016/j.jaci.2009.07.004. e1. [DOI] [PubMed] [Google Scholar]
- 26.Homey B, Meller S. Chemokines and other mediators as therapeutic targets in psoriasis vulgaris. Clin Dermatol. 2008;26:539–45. doi: 10.1016/j.clindermatol.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Meller S, Gilliet M, Homey B. Chemokines in the pathogenesis of lichenoid tissue reactions. J Invest Dermatol. 2009;129:315–19. doi: 10.1038/jid.2008.251. [DOI] [PubMed] [Google Scholar]
- 28.Lourenço FD, Azor MH, Santos JC, et al. Activated status of basophils in chronic urticaria leads to interleukin-3 hyper-responsiveness and enhancement of histamine release induced by anti-IgE stimulus. Br J Dermatol. 2008;158:979–86. doi: 10.1111/j.1365-2133.2008.08499.x. [DOI] [PubMed] [Google Scholar]
- 29.Luquin E, Kaplan AP, Ferrer M. Increased responsiveness of basophils of patients with chronic urticaria to sera but hypo-responsiveness to other stimuli. Clin Exp Allergy. 2005;35:456–60. doi: 10.1111/j.1365-2222.2005.02212.x. [DOI] [PubMed] [Google Scholar]
- 30.Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- 31.Futata E, Azor M, Dos Santos J, et al. Impaired IFN-α secretion by plasmacytoid dendritic cells induced by TLR9 activation in chronic idiopathic urticaria. Br J Dermatol. 2011;164:1271–9. doi: 10.1111/j.1365-2133.2010.10198.x. [DOI] [PubMed] [Google Scholar]
- 32.O'Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J. 2008;409:635–49. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- 33.Puccetti A, Bason C, Simeoni S, et al. In chronic idiopathic urticaria autoantibodies against Fc epsilonRII/CD23 induce histamine release via eosinophil activation. Clin Exp Allergy. 2005;35:1599–607. doi: 10.1111/j.1365-2222.2005.02380.x. [DOI] [PubMed] [Google Scholar]
- 34.Weber C, Belge KU, von Hundelshausen P, et al. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000;67:699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- 35.Choi YW, Herman A, DiGiusto D, Wade T, Marrack P, Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990;346:471–3. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi HK, Morichika T, Iwagaki H, et al. Histamine downregulates CD14 expression via H2 receptors on human monocytes. Clin Immunol. 2003;108:274–81. doi: 10.1016/s1521-6616(03)00140-2. [DOI] [PubMed] [Google Scholar]


