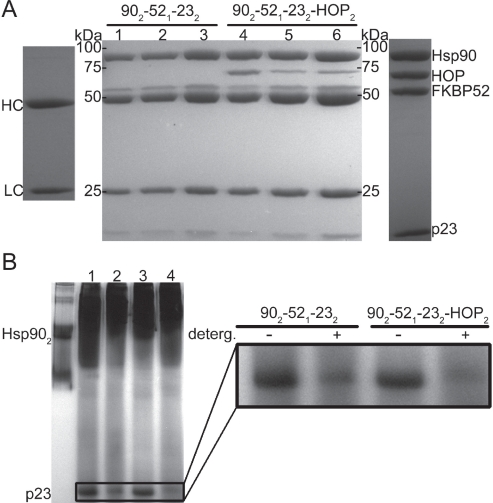
Figure 5. Co-immunoprecipitation and Native-PAGE experiments analyzing the relative degrees Hsp902-FKBP521-p232 and Hsp902-FKBP521-p232-HOP2 complex formation in the presence of varying buffering conditions.
(A) Lanes 1-3 represent the relative degree of Hsp902-FKBP521-p232 formation in the absence of ATP, in the presence of ATP and with ATP plus 0.01% of a non-ionic detergent, respectively. Lanes 4-6 equate to the same buffering conditions withheld in lanes 1-3 but with the additional incorporation of HOP into the Hsp902-FKBP521-p232-HOP2 complex. The SDS-PAGE image is surrounded by two additional lanes indicating the relative position of the Hsp90 antibody heavy (HC) and light (LC) chains (far left) as well as the anticipated distribution of the target proteins (far right). Results illustrated by 10% SDS-PAGE (B) Native-PAGE results revealing a greater degree of p23 incorporation into the respective Hsp902-FKBP521-p232 (Lanes 1-2) and Hsp902-FKBP521-p232-HOP2 (Lanes 3-4) complexes in the presence of 0.01% non-ionic dodecyl-maltopyranoside (Lanes 2 and 4).