Abstract
Background
There remains little consensus on the link between vitamin D levels and muscle mass or strength. We therefore investigated the association of serum 25-hydroxyvitamin D (25(OH)D), 1,25-dihydroxyvitamin D (1,25(OH)2D), and parathyroid hormone (PTH) levels with skeletal muscle mass and strength.
Methods
We studied 311 men (mean age, 56 yrs; range, 23-91 yrs) and 356 women (mean age, 57 yrs; range, 21-97 yrs) representing an age-stratified, random sample of community adults. Multivariate linear regression models were used to examine the association of skeletal muscle mass (by total body dual-energy x-ray absorptiometry) and strength (handgrip force and isometric knee extension moment) with each of 25(OH)D, 1,25(OH)2D and PTH quartiles, adjusted for age, physical activity, fat mass and season.
Results
We found no consistent association between 25(OH)D or PTH and any of our measurements of muscle mass or strength, in either men or women. However, in subjects younger than 65 years, there was a statistically significant association between low 1,25(OH)2D levels and low skeletal mass in both men and women and low isometric knee extension moment in women, after adjustment for potential confounders.
Conclusion
Modestly low 25(OH)D or high PTH levels may not contribute significantly to sarcopenia or muscle weakness in community adults. The link between low 25(OH)D and increased fall risk reported by others may be due to factors that affect neuromuscular function rather than muscle strength. The association between low 1,25(OH)2D and low skeletal mass and low knee extension moment, particularly in younger people, needs further exploration.
Keywords: vitamin D; 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D; PTH; muscle; muscle strength; muscle mass
Introduction
A moderate, but consistent association has been reported between low vitamin D levels and diminished postural stability, poor functional performance and increased risk of falls (1-5). Speculation that this is due to a beneficial effect of vitamin D on muscle is plausible since human skeletal muscle is reported to have a receptor for 1,25 dihydroxyvitamin D (1,25(OH)2D) (6), and genotypic variations for this receptor have been associated with differences in muscle strength (7-10). Additionally, a reversible myopathy has been observed in individuals with osteomaIacia (11,12). Nevertheless, there remains a lack of consensus in the literature on the association between vitamin D metabolites and either muscle mass or strength. Randomized clinical trials performed to date have yielded differing conclusions on whether strength is improved with vitamin D supplementation (1,13-25). Similarly, observational studies (26-43) examining this association have demonstrated conflicting findings. These disparate results may reflect different baseline serum 25-hydroxyvitamin D (25(OH)D) levels in the populations examined, different vitamin D metabolites analyzed, or reliance on different strength assessments as outcome measures. Moreover, most studies focused on elderly subjects, and there is limited information regarding the effect of vitamin D on muscle mass or strength in younger adults.
Since vitamin D insufficiency is a major health problem in men and women of all ages (44), a better understanding of the influence of vitamin D on muscle mass and strength in all adults would be of clinical relevance. While the diagnosis of vitamin D deficiency is made on the basis of serum 25(OH)D levels, 1,25(OH)2D is the biologically active metabolite and the form of vitamin D reported to bind to receptors on human muscle tissue. Some have suggested that parathyroid hormone (PTH) may influence muscle mass and strength, independent of either 25(OH)D or 1,25(OH)2D levels (33). Therefore, in a age-stratified, random sample of community adult men and women, we investigated the association of both 25(OH)D and 1,25(OH)2D, as well as PTH, with skeletal muscle mass and strength.
Methods
Study Subjects
Following approval by the Mayo Institutional Review Board and written informed consent, we recruited subjects from an age-stratified, random sample of Rochester, Minnesota, residents (45). This population is highly characteristic of the U.S. White population, but Blacks and Asians are under-represented (46). The sample spanned ages from 21–97 yrs and included 325 men and 375 women, 98% of whom were White, reflecting the ethnic composition of the community. For this analysis, we excluded 33 subjects who had either severe renal insufficiency (GFR<30 ml/min/1.73m2 (47)), untreated primary hyperparathyroidism, or muscle weakness related to a known disorder (e.g., stroke, multiple sclerosis, myasthenia gravis, etc.), were current corticosteroids users or were missing values for both 25(OH)D and 1,25(OH)2D. Exclusions were based on self-reported information and review of medical records. Thus, the final study sample included 311 men and 356 women.
Subjects were interviewed in accordance with a standard protocol to collect demographic, clinical and lifestyle data. At that time, each subject also underwent anthropometric assessment, which included measurement of height to the nearest 0.1 cm and weight in light clothes without shoes to the nearest 0.1 kg. Total fat mass (kg) was measured by total body dual-energy x-ray absorptiometry (DXA) scan (Lunar Prodigy, Madison, WI; CV, 2.6%). Caloric expenditure estimates, for physical activity status, were based on body weight, duration of activity and published MET values (48). Fasting serum blood samples were obtained between 0800-0900h. All samples were stored at −70°C until analyzed.
Vitamin D and PTH Measurements
Serum 25(OH)D was measured by radioimmunoassay (RIA) (DiaSorin, Stillwater, MN; interassay CV, <14%), as was serum 1,25(OH)2D (DiaSorin, Stillwater, MN; CV, < 16%). Serum PTH was measured using a two-site immunoassay for intact PTH (Diagnostic Products Corporation, Los Angeles, CA; interassay CV, < 13%).
Muscle Mass Measurements
Total lean body mass (kg) was determined by DXA (CV, 1.6%). We estimated total skeletal muscle mass (SM, kg) as described by Wang et al. (49) by determining appendicular lean mass (kg) of the “arms” plus “legs” regions of the whole body scan and, assuming that this figures represents 75% of total SM, multiplying the result by 1.33.
Muscle Strength Measurements
Isometric handgrip force was assessed quantitatively with a dynamometer (NK DIGIT-Grip Device; NK Biotechnical Corp., Minneapolis, MN). Handgrip strength was defined as the highest single force recorded (±0.98N) during three trials with a 1-2 minute rest period between each. Isometric knee extension force was measured in the upright seated position using a custom-built dynamometer chair with an ankle strap connected to a force transducer. Subjects’ chest, waist and thigh were stabilized to isolate the quadriceps muscle group, and knee angle was standardized to 90 degrees of flexion using an inclinamometer placed along the tibia. Using their non-dominant leg, unless prior injury or pain necessitated testing the opposite leg, subjects were instructed to maximally extend their knee until a peak force was achieved (approximately 3-5 seconds) during three trials, with a 1-2 minute rest period in between (50). The maximum knee extension force was defined as the highest single force recorded (±0.98N). To calculate knee extension moment, force was then multiplied by tibia length, measured from the DXA scan (51). The reproducibility of the tibia length measurements was 0.98. We also examined normalized knee extension moment, dividing knee extension moment by the product of total lean body mass and height (52,53).
Statistical Analyses
Given differences in muscle strength between men and women, we stratified analyses by sex. However, quartiles of 25(OH)D, 1,25(OH)2D and PTH were created based on men and women combined so comparable vitamin D metabolite and PTH levels could be studied for each sex. Multivariable linear regression models were used to calculate the least-squares (LS) means of muscle mass and strength measures by 25(OH)D, 1,25(OH)2D and PTH quartiles after adjusting for age, height, physical activity, season of the baseline visit and fat mass, and tests for trend were used to evaluate the significance of these models. We performed identical analyses in men and women below and above the age of 65 years to explore whether findings were consistent between young and old.
In addition, we fit generalized additive models for 25(OH)D, 1,25(OH)2D and PTH separately, predicting muscle mass and strength variables, adjusted for covariates, and allowing 25(OH)D, 1,25(OH)2D and PTH, respectively, to vary using a loess function; results from these models were then plotted.
All analyses were performed using SAS version 9 (SAS Institute, Cary, NC) and Splus (TIBCO Corporation, Palo Alto, CA).
Results
We studied 311 men (mean ± SD age, 56 ±19 yrs; range, 22-93 yrs) and 356 women (mean age, 57 ±18 yrs; range, 21-97 yrs). The proportion of participants who had their serum 25(OH)D, 1,25(OH)2D and PTH measured between November and February was 23% in men and 44% in women.
25-HYDROXYVITAMIN D
The mean serum 25(OH)D level was 23.0 ± 8.2 ng/mL in men and 22.1 ± 10.0ng/mL in women. The proportion of subjects with vitamin D deficiency (levels < 20 ng/mL) (44, 54) was 42% (37% in men; 45% in women). Severe vitamin D deficiency (< 10 ng/mL) was present in only 4.5% (2.6% in men; 6.2% in women), while the proportion with 25(OH)D ≥ 30 ng/mL was 18% (17% in men; 19% in women).
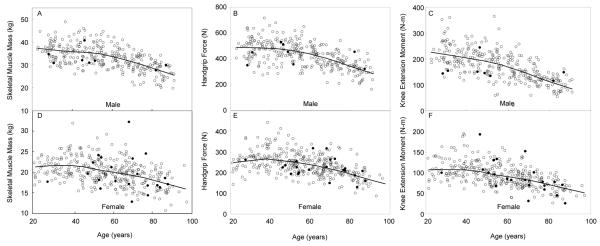
While we had too few men and women with 25(OH)D levels below 10 ng/ml (8 men and 22 women) to perform any robust analyses on whether very low 25(OH)D levels were associated with lower skeletal muscle mass or strength measures, we have provided a plot of skeletal muscle mass, handgrip force and knee extension moment by age and have indicated on these plots men and women with levels of 25(OH)D < 10 ng/ml (see Figure 1).
Figure 1.
Skeletal muscle mass, handgrip force and isometric knee extension moment for 311 men (panels A-C) and 356 women (panels D-F), representing an age-stratified random sample of the community, plotted by age. Men and women with 25-hydroxyvitamin D levels below 10 ng/ml are represented using solid circles.
Table 1 shows baseline characteristics by 25(OH)D quartiles. Both men and women in quartiles with lower 25(OH)D levels tended to have significantly lower 1,25(OH)2D levels, higher BMI, body fat mass and PTH levels and a higher proportion were assessed during the winter. Women, but not men, with lower levels of 25(OH)D tended to be significantly older. In neither sex was 25(OH)D associated with any measure of muscle mass or muscle strength, even after adjustment for age, physical activity, season, and total body fat mass (Table 1). The results for knee extension force were similar to those seen with knee extension moment (data not shown).
Table 1.
Baseline characteristics [mean(SD)] and adjusted muscle mass and strength measures [LS means] by 25(OH)D quartiles in an age-stratified sample of 311 men and 356 women from Rochester, Minnesota.
| Characteristics | Total (N=311) |
Men 25(OH)D (ng/mL) |
p value | Total (N=356) |
Women 25(OH)D (ng/mL) |
p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-16 (N=62) |
17-21 (N=94) |
22-27 (N=81) |
28-68 (N=74) |
5-16 (N=109) |
17-21 (N=81) |
22-27 (N=80) |
28-68 (N=86) |
|||||
| Age, yrs | 56.3 (18.5) | 55.9 (20.5) | 58.2 (18.8) | 58.0 (16.7) | 52.4 (18.2) | 0.224 | 57.2 (17.7) | 59.5 (15.2) | 57.0 (17.4) | 60.5 (17.2) | 51.4 (20.1) | 0.011 |
| Height, m | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 0.868 | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 0.399 |
| BMI, Kg/m2 | 28.5 (4.6) | 29.9 (4.9) | 28.8 (4.9) | 28.0 (3.8) | 27.7 (4.6) | 0.004 | 27.9 (6.3) | 30.1 (7.2) | 27.9 (5.2) | 27.4 (5.8) | 25.5 (5.3) | <0.001 |
| Fat mass, kg | 30.1(11.0) | 33.2(12.7) | 30.4(10.5) | 29.5(9.6) | 28.1(11.4) | 0.008 | 33.7(13.0) | 37.9(13.4) | 34.5(12.0) | 33.1(11.8) | 28.3(12.7) | <0.001 |
| Physical activity, total Kcal/week | 36462.3 (12516.8) |
37162.5 (13417.4) |
35638.0 (12771.0) |
36480.8 (12051.6) |
36897.9 (12086.5) |
0.926 | 27533.5 (9275.3) |
28243.5 (11556.1) |
29269.0 (9021.7) |
25996.9 (7503.8) |
26434.7 (7325.9) |
0.058 |
| Season (Nov-Feb) N[%] | 71 [22.8] | 22 [35.5] | 21 [22.3] | 17 [21.0] | 11 [14.9] | 0.007 | 155 [43.5] | 53 [48.6] | 39 [48.1] | 32 [40.0] | 31 [36.0] | 0.049 |
| PTH, pmol/L | 3.9 (1.8) | 4.6 (2.3) | 4.1 (1.8) | 3.7 (1.7) | 3.1 (1.2) | <0.001 | 3.6 (1.7) | 4.4 (1.8) | 3.5 (1.8) | 3.3 (1.1) | 2.8 (1.2) | <0.001 |
| 1,25(OH)D, pg/mL | 40.8 (13.3) | 34.8 (13.7) | 39.1 (11.7) | 41.5 (11.2) | 47.4 (14.2) | <0.001 | 38.4 (15.0) | 34.3 (13.0) | 36.4 (13.0) | 37.2 (12.3) | 46.8 (18.0) | <0.001 |
| Skeletal muscle mass, Kg | ||||||||||||
| Unadjusted model | 33.2 (5.3) | 33.16 | 33.21 | 32.89 | 33.36 | 0.927 | 20.0 (3.2) | 19.93 | 20.53 | 19.80 | 19.79 | 0.511 |
| Age and Height adjusted | 33.27 | 33.25 | 33.07 | 33.02 | 0.614 | 20.03 | 20.38 | 19.94 | 19.68 | 0.267 | ||
| Age, Height , PA, season and FM adjusted | 33.09 | 33.38 | 33.22 | 33.15 | 0.965 | 19.70 | 20.20 | 20.02 | 20.17 | 0.237 | ||
| Handgrip force, N | ||||||||||||
| Unadjusted model | 428.7 (96.2) | 429.36 | 425.09 | 431.50 | 429.74 | 0.852 | 238.9 (54.5) | 237.37 | 249.73 | 227.51 | 241.10 | 0.766 |
| Age adjusted | 426.53 | 431.65 | 436.88 | 417.96 | 0.594 | 240.71 | 249.39 | 232.54 | 232.58 | 0.088 | ||
| Age, PA, season and FM adjusted | 421.48 | 431.81 | 438.93 | 420.47 | 0.990 | 241.34 | 246.30 | 235.20 | 233.78 | 0.164 | ||
| Isometric knee extension moment, Nm | ||||||||||||
| Unadjusted model | 172.2 (58.7) | 166.03 | 170.65 | 173.75 | 177.15 | 0.263 | 93.7 (28.8) | 90.67 | 96.49 | 93.62 | 94.75 | 0.426 |
| Age adjusted | 162.84 | 174.80 | 177.51 | 170.34 | 0.390 | 93.12 | 95.98 | 96.50 | 89.54 | 0.449 | ||
| Age, PA, season and FM adjusted | 161.44 | 175.58 | 178.75 | 170.79 | 0.295 | 89.97 | 94.48 | 98.63 | 91.93 | 0.301 | ||
| Normalized knee extension moment | ||||||||||||
| Unadjusted model | 0.178 (0.050) | 0.170 | 0.176 | 0.181 | 0.184 | 0.085 | 0.162 (0.039) | 0.155 | 0.164 | 0.164 | 0.166 | 0.067 |
| Age adjusted | 0.167 | 0.179 | 0.184 | 0.179 | 0.109 | 0.158 | 0.164 | 0.167 | 0.159 | 0.494 | ||
| Age, PA, season and FM adjusted | 0.168 | 0.179 | 0.184 | 0.178 | 0.181 | 0.157 | 0.163 | 0.169 | 0.160 | 0.347 | ||
Note: BMI=body mass index; PTH=parathyroid hormone; PA=physical activity; FM=fat mass; p-values are from tests for trend
Among subjects under 65 years of age (202 men and 225 women), the mean serum 25(OH)D was 23.6 ± 8.8 ng/mL in men and 22.8 ± 10.6 ng/mL in women. In both unadjusted and adjusted models, no association was observed between 25(OH)D and most muscle mass or strength measurements in men or women, with the exception that low skeletal muscle mass in women was significantly associated with lower quartiles of 25(OH)D after adjustment for potential confounders (LS means from lowest to highest quartile: 20.30, 20.75, 20.90, 21.45 kg, p-value for trend =0.008).
In those aged 65 years or older (109 men and 131 women), the mean serum 25(OH)D level was 22.0 ± 7.1 ng/mL in men and 20.8 ± 8.8 ng/mL in women. No significant associations were observed in men. In women, following adjustment for potential confounders, low levels of 25(OH)D was associated with higher handgrip force only (LS means from lowest to highest quartile: 217.24, 214.89, 205.37, 190.01 N, p = 0.011).
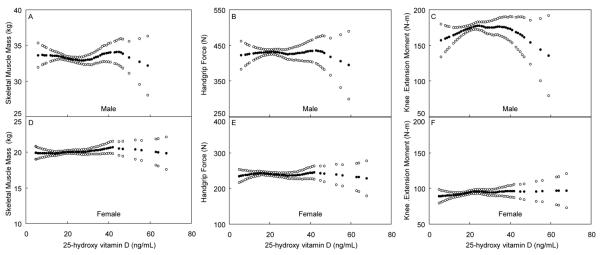
Using the loess regression plots (Figure 2), we did not visually identify any other cutoff levels for serum 25(OH)D that could be associated with lower muscle mass or strength, even when stratified by age (data not shown).
Figure 2.
Skeletal muscle mass, handgrip force and isometric knee extension moment, adjusted for potential confounders (solid circles), and 95% confidence interval (open circles), for 311 men (panels A-C) and 356 women (panels D-F), representing an age-stratified random sample of the community, by 25(OH) D levels. All were adjusted for age, physical activity, season and fat mass, and skeletal muscle mass was additionally adjusted for height.
1,25-DIHYDROXYVITAMIN D
The mean serum 1,25(OH)2D level was 40.8 ± 13.3 pg/mL in men and 38.4 ± 15.0 pg/mL in women. Baseline characteristics by 1,25(OH)2D quartiles are presented in Table 2. Both men and women in the lower quartiles of 1,25(OH)2D tended to be significantly older, have significantly higher body fat mass and significantly lower levels of 25(OH)D.
Table 2.
Baseline characteristics [mean(SD)] and adjusted muscle mass and strength measures [LS means] by 1,25(OH)2D quartiles in an age-stratified sample of 311 men and 356 women from Rochester, Minnesota.
| Characteristics | Total (N=311) |
Men 1,25(OH)2D (pg/mL) |
p value | Total (N=356) |
Women 1,25(OH)2D (pg/mL) |
p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10-30 (N=63) |
31-38 (N=68) |
39-48 (N=98) |
49-120 (N=82) |
10-30 (N=113) |
31-38 (N=99) |
39-48 (N=75) |
49-120 (N=69) |
|||||
| Age, yrs | 56.3 (18.5) | 67.5 (18.7) | 54.0 (18.2) | 55.3 (17.5) | 51.0 (16.5) | <0.001 | 57.2 (17.7) | 63.9 (15.7) | 56.5 (17.9) | 57.5 (16.6) | 46.9 (16.9) | <0.001 |
| Height, m | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 0.663 | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 0.116 |
| BMI, Kg/m2 | 28.5 (4.6) | 29.1 (4.3) | 29.0 (4.9) | 28.2 (4.3) | 28.1 (4.9) | 0.106 | 27.9 (6.3) | 29.4 (6.3) | 28.3 (6.4) | 26.6 (5.9) | 26.2 (5.8) | <0.001 |
| Fat mass, kg | 30.1 (11.0) | 33.5 (11.4) | 30.6 (12.5) | 29.0 (9.7) | 28.5 (10.5) | 0.005 | 33.7 (13.0) | 37.0 (13.0) | 34.9 (13.1) | 31.1 (11.6) | 29.2 (12.9) | <0.001 |
| Physical activity, total Kcal/week | 36462.3 (12516.8) |
33902.5 (12936.4) |
38644.4 (13481.3) |
36060.4 (11936.7) |
37085.5 (11878.3) |
0.349 | 27533.5 (9275.3) |
27824.3 (10036.4) |
27978.0 (9538.6) |
26076.5 (8075.0) |
27905.6 (8768.7) |
0.668 |
| Season (Nov-Feb), N[%] | 71 [22.8] | 12 [19.0] | 22 [32.4] | 23 [23.5] | 14 [17.1] | 0.420 | 155 [43.5] | 47 [41.6] | 46 [46.5] | 30 [40.0] | 32 [46.4] | 0.738 |
| PTH, pmol/L | 3.9 (1.8) | 4.3 (2.5) | 3.7 (1.7) | 3.8 (1.5) | 3.7 (1.4) | 0.090 | 3.6 (1.7) | 3.7 (1.6) | 3.5 (1.5) | 3.8 (1.9) | 3.3 (1.7) | 0.303 |
| 25(OH)D, ng/mL | 23.0 (8.3) | 19.6 (6.4) | 21.7 (7.0) | 23.0 (8.0) | 26.7 (9.3) | <0.001 | 22.1 (10.0) | 19.2 (8.8) | 21.1 (8.4) | 21.8 (7.9) | 28.3 (13.0) | <0.001 |
| Skeletal muscle mass, Kg | ||||||||||||
| Unadjusted model | 33.2 (5.3) | 31.72 | 33.75 | 33.26 | 33.62 | 0.077 | 20.0 (3.2) | 19.50 | 20.08 | 19.89 | 20.85 | 0.013 |
| Age and Height adjusted | 32.89 | 33.64 | 33.17 | 32.92 | 0.737 | 19.99 | 19.98 | 19.77 | 20.31 | 0.675 | ||
| Age, Height , PA, season and FM adjusted | 32.77 | 33.47 | 33.43 | 33.12 | 0.676 | 19.68 | 19.91 | 20.02 | 20.66 | 0.012 | ||
| Handgrip force, N | ||||||||||||
| Unadjusted model | 428.7 (96.2) | 406.13 | 433.50 | 433.13 | 436.33 | 0.093 | 238.9 (54.5) | 228.19 | 240.38 | 240.52 | 252.58 | 0.005 |
| Age adjusted | 441.21 | 426.43 | 430.04 | 419.78 | 0.184 | 237.95 | 239.34 | 241.13 | 237.43 | 0.936 | ||
| Age, PA, season and FM adjusted | 439.28 | 420.00 | 433.55 | 422.93 | 0.483 | 236.83 | 238.72 | 243.42 | 239.72 | 0.540 | ||
| Isometric knee extension moment, Nm | ||||||||||||
| Unadjusted model | 172.2 (58.7) | 148.10 | 170.02 | 183.62 | 178.51 | 0.001 | 93.7 (28.8) | 86.77 | 93.94 | 94.63 | 102.69 | <0.001 |
| Age adjusted | 170.78 | 166.89 | 180.25 | 168.00 | 0.810 | 92.54 | 93.20 | 95.32 | 94.20 | 0.535 | ||
| Age, PA, season and FM adjusted | 169.73 | 164.45 | 182.29 | 169.90 | 0.439 | 89.71 | 92.63 | 97.41 | 95.98 | 0.036 | ||
| Normalized knee extension moment | ||||||||||||
| Unadjusted model | 0.178 (0.050) | 0.155 | 0.174 | 0.191 | 0.183 | <0.001 | 0.162 (0.039) | 0.151 | 0.164 | 0.164 | 0.172 | <0.001 |
| Age adjusted | 0.171 | 0.172 | 0.188 | 0.176 | 0.189 | 0.158 | 0.163 | 0.164 | 0.163 | 0.314 | ||
| Age, PA, season and FM adjusted | 0.172 | 0.171 | 0.189 | 0.176 | 0.254 | 0.157 | 0.162 | 0.166 | 0.163 | 0.180 | ||
Note: BMI=body mass index; PTH=parathyroid hormone; PA=physical activity; FM=fat mass; p-values are from tests for trend
Although in unadjusted models, men with lower quartiles of 1,25(OH)2D had significantly lower isometric knee extension moment and normalized moment, this association was no longer observed following adjustment for potential confounding variables. In women, lower quartiles of 1,25(OH)2D tended to be significantly associated with lower skeletal muscle mass, handgrip force, and knee extension moment and normalized moment in unadjusted models. This association between 1,25(OH)2D and skeletal muscle mass, as well as knee extension moment, remained significant even after adjustment for potential confounders (Table 2). The findings for knee extension force were similar to those seen with knee extension moment (data not shown).
For subjects < 65 years, the mean serum 1,25(OH)2D was 43.4 ± 12.5 pg/mL for men and 41.2 ± 16.1 pg/mL for women. After adjustment for potential confounders, there was an association between lower quartiles of 1,25(OH)2D and lower skeletal muscle mass in both men (LS means from lowest to highest quartile: 33.38, 35.55, 35.48, 35.65 kg, p = 0.041) and women (LS means from lowest to highest quartile: 20.28, 20.67, 20.71, 21.71 kg, p = 0.001), but lower knee extension moment (LS means from lowest to highest quartile: 96.88, 101.98, 104.67, 108.30 Nm, p = 0.018), as well as force (data not shown; p = 0.026) in women only. No other significant associations were observed.
Considering subjects ≥ 65 years or older, the mean serum 1,25(OH)2D was 36.0 ± 13.3 pg/mL for men and 33.7 ± 11.4 pg/mL for women. In men, but not women, lower quartiles of 1,25(OH)2D were associated with significantly higher levels of skeletal muscle mass (LS means from lowest to highest quartile: 30.21, 29.49, 28.86, 27.39 kg, p < 0.001) and higher hand grip force (LS means from lowest to highest quartile: 368.66, 363.57, 344.47, 334.09 N, p = 0.037) after adjustment for potential confounders. No other significant associations were observed.
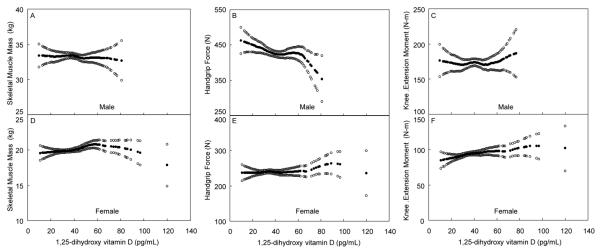
From our plots (Figure 3) and taking into account confidence limits, we did not visually identify any other cutoff levels for serum 1,25(OH)2D that could be associated with lower muscle mass or strength, even when we stratified by age (data not shown).
Figure 3.
Skeletal muscle mass, handgrip force and isometric knee extension moment, adjusted for potential confounders (solid circles), and 95% confidence interval (open circles), for 311 men (panels A-C) and 356 women (panels D-F), representing an age-stratified random sample of the community, by 1,25(OH)2 D levels. All were adjusted for age, physical activity, season and fat mass, and skeletal muscle mass was additionally adjusted for height.
PTH
The mean serum PTH level was 3.9 ± 1.8 pmol/L in men and 3.6 ±1.6 pmol/L in women. Baseline characteristics by PTH quartiles are presented in Table 3. Men with higher quartiles of PTH levels tended to be significantly older, have lower levels of 25(OH)D and a higher proportion were assessed in the winter. Women with higher PTH tended to be significantly older, have lower levels of 25(OH)D and have significantly higher BMI and fat mass.
Table 3.
Baseline characteristics [mean (SD)] and adjusted muscle mass and strength measures [LS means] by PTH quartiles in an age-stratified sample of 311 men and 356 women from Rochester, Minnesota
| Characteristics | Total (N=311) |
Men PTH (pmol/L) |
p value | Total (N=356) |
Women PTH (pmol/L) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5-2.5 (N=75) |
2.6-3.4 (N=75) |
3.5-4.5 (N=78) |
4.6-15.0 (N=83) |
0.5-2.5 (N=106) |
2.6-3.4 (N=80) |
3.5-4.5 (N=91) |
4.6-15.0 (N=79) |
|||||
| Age, yrs | 56.3 (18.5) | 51.2 (18.7) | 52.2 (17.4) | 56.0 (17.8) | 64.9 (17.4) | <0.001 | 57.2 (17.7) | 52.9 (18.9) | 57.3 (16.7) | 57.7 (18.1) | 62.4 (15.1) | <0.001 |
| Height, m | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 0.324 | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 0.148 |
| BMI, Kg/m2 | 28.5 (4.6) | 28.4 (4.7) | 28.3 (4.4) | 28.9 (4.6) | 28.5 (4.8) | 0.687 | 27.9 (6.3) | 26.1 (5.1) | 27.7 (5.7) | 28.6 (6.7) | 29.6 (7.1) | <0.001 |
| Fat mass, kg | 30.1 (11.0) | 29.4 (10.5) | 29.1 (12.1) | 31.5 (11.2) | 30.4 (10.4) | 0.324 | 33.6 (13.0) | 29.8 (12.1) | 34.5 (12.2) | 35.5 (14.1) | 36.1 (12.8) | <0.001 |
| Physical activity, total Kcal/week | 36462.3 (12516.8) |
37425.8 (14773.0) |
37242.0 (11590.5) |
37585.9 (13212.3) |
33792.2 (10007.6) |
0.090 | 27533.5 (9275.3) |
27513.2 (8577.0) |
26803.4 (6983.8) |
29008.5 (11755.8) |
26567.3 (8841.4) |
0.937 |
| Season (Nov-Feb), N [%] | 71 [22.8] | 26 [34.7] | 16 [21.3] | 15 [19.2] | 14 [16.9] | 0.010 | 155 [43.5] | 50 [47.2] | 39 [48.8] | 33 [36.3] | 33 [41.8] | 0.210 |
| 25(OH)D, ng/mL | 23.0 (8.3) | 24.9(9.4) | 25.6(8.2) | 22.3(7.5) | 19.6(6.5) | <0.001 | 22.1 (10.0) | 26.6 (10.6) | 21.6 (8.5) | 21.9 (9.8) | 16.7 (7.9) | <0.001 |
| 1,25(OH)D, pg/mL | 40.8 (13.3) | 38.2(13.0) | 45.3(13.0) | 41.8(14.0) | 38.2(12.0) | 0.520 | 38.4 (15.0) | 39.7 (15.8) | 38.7 (13.9) | 39.7 (16.1) | 34.9 (12.9) | 0.070 |
| Skeletal muscle mass, Kg | ||||||||||||
| Unadjusted model | 33.2 (5.3) | 33.56 | 34.25 | 32.96 | 31.95 | 0.021 | 20.0 (3.2) | 19.91 | 20.80 | 19.85 | 19.47 | 0.210 |
| Age and Height adjusted | 32.86 | 33.68 | 33.06 | 33.01 | 0.925 | 19.78 | 20.40 | 19.94 | 19.99 | 0.778 | ||
| Age, Height , PA, season and FM adjusted | 32.94 | 33.78 | 32.94 | 33.23 | 0.995 | 19.97 | 20.50 | 19.74 | 19.86 | 0.377 | ||
| Handgrip force, N | ||||||||||||
| Unadjusted model | 428.7 (96.2) | 442.09 | 451.04 | 421.98 | 402.69 | 0.002 | 238.9 (54.5) | 245.03 | 238.55 | 235.30 | 235.13 | 0.178 |
| Age adjusted | 427.39 | 439.05 | 421.36 | 427.52 | 0.679 | 238.63 | 238.67 | 236.10 | 242.68 | 0.725 | ||
| Age, PA, season and FM adjusted | 424.54 | 441.08 | 420.52 | 429.35 | 0.867 | 236.80 | 240.07 | 236.39 | 245.19 | 0.384 | ||
| Isometric knee extension moment, Nm | ||||||||||||
| Unadjusted model | 172.2 (58.7) | 183.62 | 183.53 | 170.92 | 152.71 | <0.001 | 93.7 (28.8) | 95.25 | 98.71 | 92.63 | 87.28 | 0.045 |
| Age adjusted | 171.01 | 175.89 | 171.04 | 170.79 | 0.809 | 91.37 | 98.85 | 92.87 | 92.23 | 0.923 | ||
| Age, PA, season and FM adjusted | 170.10 | 176.90 | 170.64 | 172.32 | 0.994 | 91.82 | 98.91 | 92.74 | 90.72 | 0.523 | ||
| Normalized knee extension moment | ||||||||||||
| Unadjusted model | 0.178 (0.050) | 0.189 | 0.185 | 0.179 | 0.160 | <0.001 | 0.162 (0.039) | 0.163 | 0.163 | 0.165 | 0.154 | 0.203 |
| Age adjusted | 0.180 | 0.180 | 0.179 | 0.174 | 0.373 | 0.158 | 0.163 | 0.166 | 0.159 | 0.624 | ||
| Age, PA, season and FM adjusted | 0.178 | 0.180 | 0.180 | 0.174 | 0.577 | 0.158 | 0.163 | 0.166 | 0.160 | 0.553 | ||
Note: BMI=body mass index; PTH=parathyroid hormone; PA=physical activity; FM=fat mass; p-values are from tests for trend
In unadjusted models, higher quartiles of PTH tended to be significantly associated with lower skeletal muscle mass, handgrip force and knee extension moment and normalized moment in men, but only with lower knee extension moment in women (Table 3). However, in both sexes, after adjustment for confounders, particularly age, there was no longer a significant association between PTH levels and any of the muscle mass or strength variables. The results for knee extension force were similar to those seen with knee extension moment (data not shown).
In subjects < 65 years, the mean serum PTH was 3.5 ± 1.4 pmol/L in men and 3.4 ± 1.6 pmol/L in women. In neither sex was there an association between PTH and any measurement of muscle mass or strength, either in unadjusted or adjusted models (data not shown).
In subjects ≥ 65 years, the mean serum PTH was 4.6 ± 2.2 pmol/L in men and 3.9 ± 1.7 pmol/L in women. In women only, and both before and after adjustment for potential confounding variables, lower quartiles of PTH tended to be significantly associated with lower levels of handgrip force (LS means from lowest to highest quartile: 195.58, 203.28, 204.87, 223.46 N, p = 0.006) and lower normalized knee extension moment (LS means from lowest to highest quartile: 0.132, 0.140, 0.147, 0.148, p = 0.028). There was a similar trend for lower knee extension moment and force, but results were not statistically significant (p = 0.237 and p = 0.120, respectively).
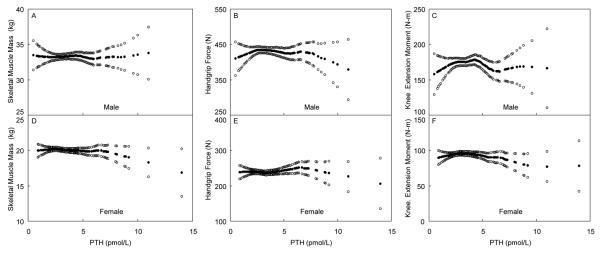
We again did not identify any other cutoffs for serum PTH that could be associated with lower muscle mass or strength (Figure 4), even when stratified by age (data not shown).
Figure 4.
Skeletal muscle mass, handgrip force and isometric knee extension moment, adjusted for potential confounders (solid circles), and 95% confidence interval (open circles), for 311 men (panels A-C) and 356 women (panels D-F), representing an age-stratified random sample of the community, by PTH levels. All were adjusted for age, physical activity, season and fat mass, and skeletal muscle mass was additionally adjusted for height.
Discussion
Although the 25(OH)D metabolite is considered the barometer for vitamin D status (54), we found no consistent association between serum 25(OH)D and any of our measurements of skeletal muscle mass or strength, even after adjustment for potential confounders, and despite the fact that 42% were vitamin D deficient. Moreover, when visually examining the loess regression plots to assess for alternative cutoffs, rather than the quartiles used in analyses, we did not see any other threshold levels that seemed to be associated with low muscle mass or strength. Only in women younger than age 65 years did we observe lower skeletal muscle mass to be associated with lower 25(OH)D levels, and differences were small. However, in men and women over the age of 65 years, we found no trend towards lower muscle mass or strength with lower 25(OH)D levels. Instead, there was a trend for higher grip strength among older women with lower 25(OH)D levels.
Our findings related to 25(OH)D levels and muscle are in line with some cross-sectional studies (26,33,34,39,42,43) but not with others (27,28,30-32,35-37), however methodologies varied widely among these studies. For example, most cross-sectional studies that found a significant association between low 25(OH)D levels and low muscle mass or strength were conducted outside the United States, so whether genetics, latitude or other environmental factors contributed to these differences is unclear. Furthermore, a higher proportion of these studies focused on institutionalized elderly subjects, and there was a tendency to see lower baseline serum 25(OH)D levels among studies reporting significant positive associations when compared with null studies. Similar differences were also noted between clinical trials where supplemental vitamin D was associated with higher muscle strength (15,16,19,22,23,25), compared with those trials that did not find such an association (14,17,18,20,21,24). Indeed, a recent systematic review of randomized controlled trials suggested that vitamin D supplementation did not have a significant effect on muscle strength in vitamin D replete adults, although it might have a role in adults with vitamin D deficiency (55). Most of the trials featured short follow-up periods (up to six months) or studied muscle strength as a secondary endpoint.
By contrast, in subjects under age 65 years, we found that lower levels of 1,25(OH)2D were associated with lower skeletal muscle mass in both men and women and lower knee extension force and moment in women, even after adjustment for potential confounders. In additional exploratory analyses, we examined whether moderate renal insufficiency (GFR<60 ml/min/1.73m2) could account for these results, but it did not explain our findings. These results are intriguing since 1,25(OH)2D is the active form of vitamin D, and receptors for 1,25(OH)2D have been reported in human skeletal muscle (6), although a recent study has now challenged this observation (56). Nevertheless, this reinforces the need to further explore our findings since the differences we identified in muscle mass and strength across quartiles were small and not consistent through all measurements of muscle strength. Furthermore, upon examining the loess regression plots, we did not find any clear threshold for 1,25(OH)2D below which muscle mass or strength was lower. To our knowledge, our study is the first to investigate the association between 1,25(OH)2D and muscle mass. Only one other cross-sectional study found a statistically significant, albeit modest, association between low 1,25(OH)2D and low muscle strength [30], whereas others have found no significant association with strength (29,32). In a randomized controlled trial in ambulatory elderly subjects, the administration of 0.5μg of 1,25(OH)2D for 6 months did not improve leg extension strength, although the dose used may not have been adequate since serum 1,25(OH)2D levels did not increase significantly in the treatment group (13).
A few studies have examined the association between PTH and muscle mass or strength, and these results are also inconsistent (26,27,31,33,40,41). Only one study found a statistically significant association between high PTH levels and low muscle strength independent of 25(OH)D, and they examined elderly subjects recruited from a falls clinic (33). Following adjustment for potential confounders, we did not find high PTH levels to be associated with lower muscle mass or strength measurements in either men or women. Instead, we observed lower levels of PTH to be significantly associated with low handgrip force and low normalized knee extension moment in women who were 65 years or older.
Our study has limitations insofar as results are based upon cross-sectional data, and we acknowledge that confirmation of our findings would require a longitudinal study design. However, we have studied a community-based sample of men and women, both young and old, and examined the role of two main vitamin D metabolites, 25(OH)D and 1,25(OH)2D, along with PTH, on muscle mass and strength. We do not know the duration subjects were at the 25(OH)D levels measured, and a relatively long period of vitamin D deficiency may be necessary to impair muscle strength (11, 57). Also, few subjects in our cohort had very low levels of 25(OH)D (4.5% < 10 ng/mL), limiting statistical power to detect an effect of severe vitamin D deficiency. A reversible myopathy has been observed in individuals with osteomalacia (11, 12), so very low levels of 25(OH)D, particularly of long duration, may still have an effect on muscle that could not be evaluated in our cohort. Tissue levels of vitamin D may be more important with respect to muscle mass or strength than the serum levels assessed here, although there is now controversy on whether skeletal muscle even possess receptors for vitamin D (56). Season may not only influence serum 25(OH)D levels relating to sun exposure, but could influence muscle mass and strength due to a potentially more sedentary lifestyle in winter or colder months. In additional exploratory analyses using different methods to adjust for season, our results remained similar. Finally, our observations in older subjects of an inverse relation between vitamin D metabolites with some muscle mass and strength measurements are challenging to interpret. We did note that subjects belonging to the highest vitamin D quartiles were more likely to be taking vitamin D supplements, compared to the lowest quartiles. Whether they were recommended vitamin D supplements because they were weak remains a possibility, but we cannot confirm this hypothesis since we do not know the indication for supplementation or the duration of use. Interestingly, a recent study in elderly women suggested there may be a U-shaped relation of 25(OH)D with frailty (which included low grip strength, among other factors, in the definition of frailty) (58). Thus, our observations may instead reflect that higher vitamin D levels are not necessarily beneficial to muscle mass or strength in the elderly.
Our findings are not necessarily inconsistent with results from a systematic review that showed that supplemental vitamin D might improve physical function (2) or a recent meta-analysis of randomized controlled trials showing that supplemental vitamin D modestly reduces the risk of falls among older individuals (3). It may be that vitamin D affects coordinative muscle function more than strength or mass, in which case the potential protective role of vitamin D on falls would be through improvement on balance and not muscle strength per se. An improvement in neuromuscular coordination through vitamin D supplementation is conceivable in light of the presence of 1,25(OH)2D receptors in both the animal and human nervous system, namely in the spinal cord and cerebellum (59-61), as well as the in vitro and in vivo induction of nerve growth factor synthesis by vitamin D derivatives (62). Furthermore, results from some epidemiological studies seem to show a relatively consistent association between vitamin D and functional performance and postural stability (4,5,17,18,33,34,57), supporting the hypothesis that vitamin D insufficiency may affect balance more than strength. We did not measure muscle function in our study, but others have suggested that the relationship between vitamin D and functional performance and postural stability may be independent from muscle strength (33,34,63).
In conclusion, in an age stratified community-based sample of both young and older adults, we found in younger subjects, especially women, a statistically significant association between low 1,25(OH)2D and low skeletal mass as well as low knee extension moment, which needs to be explored further. Importantly, we found no consistent association between modestly low 25(OH)D or high PTH levels with either low muscle mass or strength, particularly in older men and women. Modest levels of vitamin D deficiency may not significantly contribute to sarcopenia or muscle weakness in adult men and women from the general community and other reasons should be sought. Furthermore, the associations reported between vitamin D supplementation or 25(OH)D levels and fall risk may be due to factors that affect neuromuscular function, rather than muscle strength per se, and warrant further investigation.
Acknowledgments
This work was supported by Grants R01-AR27065 and M01-RR00585/ UL1-RR024150 from the National Institutes of Health, U.S. Public Health Service. Dr. Marantes was supported by a grant from the Fundação Calouste Gulbenkian.
The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Margaret Holets for the DXA measurements and for the muscle strength measurements; Lisa McDaniel, R.N., and Louise McCready, R.N., for assistance in recruitment and management of the study subjects and James M. Peterson for assistance with data management, file storage and figures.
This work was supported by research grants AR027065 (National Institute of Arthritis, Musculoskeletal and Skin Diseases) and M01-RR00585/UL1-RR024150 (Center for Translational Science Activities) from the National Institutes of Health, U.S. Public Health Service. Dr. Marantes was supported by a grant from the Fundação Calouste Gulbenkian.
Footnotes
Conflict of Interest Statement All authors state that they have no conflicts of interest with respect to this work.
REFERENCES
- 1.Latham NK, Anderson CS, Reid IR. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. J Am Geriatr Soc. 2003;51(9):1219–26. doi: 10.1046/j.1532-5415.2003.51405.x. [DOI] [PubMed] [Google Scholar]
- 2.Annweiler C, Schott AM, Berrut G, Fantino B, Beauchet O. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13(10):893–8. doi: 10.1007/s12603-009-0248-x. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, Dawson-Hughes B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80(3):752–8. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 5.Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15(6):1113–8. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin HB, Dick W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33(1):19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 7.Grundberg E, Brandstrom H, Ribom EL, Ljunggren O, Mallmin H, Kindmark A. Genetic variation in the human vitamin D receptor is associated with muscle strength, fat mass and body weight in Swedish women. Eur J Endocrinol. 2004;150(3):323–8. doi: 10.1530/eje.0.1500323. [DOI] [PubMed] [Google Scholar]
- 8.Roth SM, Zmuda JM, Cauley JA, Shea PR, Ferrell RE. Vitamin D receptor genotype is associated with fat-free mass and sarcopenia in elderly men. J Gerontol A Biol Sci Med Sci. 2004;59(1):10–5. doi: 10.1093/gerona/59.1.b10. [DOI] [PubMed] [Google Scholar]
- 9.Windelinckx A, De Mars G, Beunen G, Aerssens J, Delecluse C, Lefevre J, Thomis MA. Polymorphisms in the vitamin D receptor gene are associated with muscle strength in men and women. Osteoporos Int. 2007;18(9):1235–42. doi: 10.1007/s00198-007-0374-4. [DOI] [PubMed] [Google Scholar]
- 10.Geusens P, Vandevyver C, Vanhoof J, Cassiman JJ, Boonen S, Raus J. Quadriceps and grip strength are related to vitamin D receptor genotype in elderly nonobese women. J Bone Miner Res. 1997;12(12):2082–8. doi: 10.1359/jbmr.1997.12.12.2082. [DOI] [PubMed] [Google Scholar]
- 11.Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Andersen H, Charles P, Eriksen EF. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66(6):419–24. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- 12.Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet. 1976;1(7960):626–9. doi: 10.1016/s0140-6736(76)90428-1. [DOI] [PubMed] [Google Scholar]
- 13.Grady D, Halloran B, Cummings S, Leveille S, Wells L, Black D, Byl N. 1,25-Dihydroxyvitamin D3 and muscle strength in the elderly: a randomized controlled trial. J Clin Endocrinol Metab. 1991;73(5):1111–7. doi: 10.1210/jcem-73-5-1111. [DOI] [PubMed] [Google Scholar]
- 14.Kenny AM, Biskup B, Robbins B, Marcella G, Burleson JA. Effects of vitamin D supplementation on strength, physical function, and health perception in older, community-dwelling men. J Am Geriatr Soc. 2003;51(12):1762–7. doi: 10.1046/j.1532-5415.2003.51561.x. [DOI] [PubMed] [Google Scholar]
- 15.Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2008 doi: 10.1007/s00198-008-0662-7. [DOI] [PubMed] [Google Scholar]
- 16.Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, Nebiker M, Theiler R, Pfeifer M, Begerow B, Lew RA, Conzelmann M. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343–51. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 17.Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS) J Am Geriatr Soc. 2003;51(3):291–9. doi: 10.1046/j.1532-5415.2003.51101.x. [DOI] [PubMed] [Google Scholar]
- 18.Dhesi JK, Jackson SH, Bearne LM, Moniz C, Hurley MV, Swift CG, Allain TJ. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33(6):589–95. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 19.Verhaar HJ, Samson MM, Jansen PA, de Vreede PL, Manten JW, Duursma SA. Muscle strength, functional mobility and vitamin D in older women. Aging (Milano) 2000;12(6):455–60. doi: 10.1007/BF03339877. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KR, Jobber J, Stonawski BJ. Prophylactic vitamin D in the elderly. Age Ageing. 1980;9(2):121–7. doi: 10.1093/ageing/9.2.121. [DOI] [PubMed] [Google Scholar]
- 21.Honkanen R, Alhava E, Parviainen M, Talasniemi S, Monkkonen R. The necessity and safety of calcium and vitamin D in the elderly. J Am Geriatr Soc. 1990;38(8):862–6. doi: 10.1111/j.1532-5415.1990.tb05700.x. [DOI] [PubMed] [Google Scholar]
- 22.Songpatanasilp T, Chailurkit LO, Nichachotsalid A, Chantarasorn M. Combination of alfacalcidol with calcium can improve quadriceps muscle strength in elderly ambulatory Thai women who have hypovitaminosis D: a randomized controlled trial. J Med Assoc Thai. 2009;92(Suppl5):S30–41. [PubMed] [Google Scholar]
- 23.Moreira-Pfrimer LD, Pedrosa MA, Teixeira L, Lazaretti-Castro M. Treatment of vitamin D deficiency increases lower limb muscle strength in institutionalized older people independently of regular physical activity: a randomized double-blind controlled trial. Ann Nutr Metab. 2009;54(4):291–300. doi: 10.1159/000235874. [DOI] [PubMed] [Google Scholar]
- 24.Janssen HC, Samson MM, Verhaar HJ. Muscle strength and mobility in vitamin D-insufficient female geriatric patients: a randomized controlled trial on vitamin D and calcium supplementation. Aging Clin Exp Res. 22(1):78–84. doi: 10.1007/BF03324819. [DOI] [PubMed] [Google Scholar]
- 25.Zhu K, Austin N, Devine A, Bruce D, Prince RL. A randomized controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insufficiency. J Am Geriatr Soc. 58(11):2063–8. doi: 10.1111/j.1532-5415.2010.03142.x. [DOI] [PubMed] [Google Scholar]
- 26.Ceglia L, Chiu GR, Harris SS, Araujo AB. Serum 25-hydroxyvitamin D concentration and physical function in adult men. Clin Endocrinol (Oxf) doi: 10.1111/j.1365-2265.2010.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bischoff HA, Stahelin HB, Urscheler N, Ehrsam R, Vonthein R, Perrig-Chiello P, Tyndall A, Theiler R. Muscle strength in the elderly: its relation to vitamin D metabolites. Arch Phys Med Rehabil. 1999;80(1):54–8. doi: 10.1016/s0003-9993(99)90307-6. [DOI] [PubMed] [Google Scholar]
- 28.Zamboni M, Zoico E, Tosoni P, Zivelonghi A, Bortolani A, Maggi S, Di Francesco V, Bosello O. Relation between vitamin D, physical performance, and disability in elderly persons. J Gerontol A Biol Sci Med Sci. 2002;57(1):M7–11. doi: 10.1093/gerona/57.1.m7. [DOI] [PubMed] [Google Scholar]
- 29.Boonen S, Lysens R, Verbeke G, Joosten E, Dejaeger E, Pelemans W, Flamaing J, Bouillon R. Relationship between age-associated endocrine deficiencies and muscle function in elderly women: a cross-sectional study. Age Ageing. 1998;27(4):449–54. doi: 10.1093/ageing/27.4.449. [DOI] [PubMed] [Google Scholar]
- 30.Houston DK, Cesari M, Ferrucci L, Cherubini A, Maggio D, Bartali B, Johnson MA, Schwartz GG, Kritchevsky SB. Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62(4):440–6. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005;16(11):1425–31. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 32.Mowe M, Haug E, Bohmer T. Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc. 1999;47(2):220–6. doi: 10.1111/j.1532-5415.1999.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 33.Dhesi JK, Bearne LM, Moniz C, Hurley MV, Jackson SH, Swift CG, Allain TJ. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17(5):891–7. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 34.Pfeifer M, Begerow B, Minne HW, Schlotthauer T, Pospeschill M, Scholz M, Lazarescu AD, Pollahne W. Vitamin D status, trunk muscle strength, body sway, falls, and fractures among 237 postmenopausal women with osteoporosis. Exp Clin Endocrinol Diabetes. 2001;109(2):87–92. doi: 10.1055/s-2001-14831. [DOI] [PubMed] [Google Scholar]
- 35.Rinaldi I, Setiati S, Oemardi M, Aries W, Tamin TZ. Correlation between serum vitamin D (25(OH)D) concentration and quadriceps femoris muscle strength in Indonesian elderly women living in three nursing homes. Acta Med Indones. 2007;39(3):107–11. [PubMed] [Google Scholar]
- 36.Stewart JW, Alekel DL, Ritland LM, Van Loan M, Gertz E, Genschel U. Serum 25-hydroxyvitamin D is related to indicators of overall physical fitness in healthy postmenopausal women. Menopause. 2009;16(6):1093–101. doi: 10.1097/gme.0b013e3181a8f7ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inderjeeth CA, Glennon D, Petta A, Soderstrom J, Boyatzis I, Tapper J. Vitamin D and muscle strength in patients with previous fractures. N Z Med J. 2007;120(1262):U2730. [PubMed] [Google Scholar]
- 38.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57(12):M772–7. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 39.Shaunak S, Ang L, Colston K, Patel S, Bland M, Maxwell JD. Muscle strength in healthy white and Asian subjects: the relationship of quadriceps maximum voluntary contraction to age, sex, body build and vitamin D. Clin Sci (Lond) 1987;73(5):541–6. doi: 10.1042/cs0730541. [DOI] [PubMed] [Google Scholar]
- 40.Verreault R, Semba RD, Volpato S, Ferrucci L, Fried LP, Guralnik JM. Low serum vitamin d does not predict new disability or loss of muscle strength in older women. J Am Geriatr Soc. 2002;50(5):912–7. doi: 10.1046/j.1532-5415.2002.50219.x. [DOI] [PubMed] [Google Scholar]
- 41.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–72. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 42.Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 95(4):1595–601. doi: 10.1210/jc.2009-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Annweiler C, Beauchet O, Berrut G, Fantino B, Bonnefoy M, Herrmann FR, Schott AM. Is there an association between serum 25-hydroxyvitamin D concentration and muscle strength among older women? Results from baseline assessment of the EPIDOS study. J Nutr Health Aging. 2009;13(2):90–5. doi: 10.1007/s12603-009-0013-1. [DOI] [PubMed] [Google Scholar]
- 44.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 45.Riggs BL, Melton LJ, 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19(12):1945–54. doi: 10.1359/JBMR.040916. Iii. [DOI] [PubMed] [Google Scholar]
- 46.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 47.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 48.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr., Montoye HJ, Sallis JF, Paffenbarger RS., Jr. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Wang ZM, Visser M, Ma R, Baumgartner RN, Kotler D, Gallagher D, Heymsfield SB. Skeletal muscle mass: evaluation of neutron activation and dual-energy X-ray absorptiometry methods. J Appl Physiol. 1996;80(3):824–31. doi: 10.1152/jappl.1996.80.3.824. [DOI] [PubMed] [Google Scholar]
- 50.Sale DG. Testing of strength and power. In: MacDougall JD, Wenger HA, Green HJ, editors. Physiological testing of the high-performance athlete. 2nd edition Human Kinetics; Champaign, Ill., USA: 1991. pp. 21–106. [Google Scholar]
- 51.Chinappen-Horsley U, Blake GM, Fogelman I, Spector TD. A method for determining skeletal lengths from DXA images. BMC Musculoskelet Disord. 2007;8:113. doi: 10.1186/1471-2474-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Folland JP, Mc Cauley TM, Williams AG. Allometric scaling of strength measurements to body size. Eur J Appl Physiol. 2008;102(6):739–45. doi: 10.1007/s00421-007-0654-x. [DOI] [PubMed] [Google Scholar]
- 53.Jaric S. Muscle strength testing: use of normalisation for body size. Sports Med. 2002;32(10):615–31. doi: 10.2165/00007256-200232100-00002. [DOI] [PubMed] [Google Scholar]
- 54.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin d from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab. 96(1):53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. doi: 10.1007/s00198-010-1407-y. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Deluca HF. Is the Vitamin D Receptor Found in Muscle? Endocrinology [Google Scholar]
- 57.Gloth FM, 3rd, Smith CE, Hollis BW, Tobin JD. Functional improvement with vitamin D replenishment in a cohort of frail, vitamin D-deficient older people. J Am Geriatr Soc. 1995;43(11):1269–71. doi: 10.1111/j.1532-5415.1995.tb07404.x. [DOI] [PubMed] [Google Scholar]
- 58.Ensrud KE, Ewing SK, Fredman L, Hochberg MC, Cauley JA, Hillier TA, Cummings SR, Yaffe K, Cawthon PM. Circulating 25-hydroxyvitamin D levels and frailty status in older women. J Clin Endocrinol Metab. 95(12):5266–73. doi: 10.1210/jc.2010-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGrath JJ, Feron FP, Burne TH, Mackay-Sim A, Eyles DW. Vitamin D3-implications for brain development. J Steroid Biochem Mol Biol. 2004;89-90(1-5):557–60. doi: 10.1016/j.jsbmb.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 60.Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16(2):135–45. doi: 10.1016/s0891-0618(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 61.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Neveu I, Naveilhan P, Jehan F, Baudet C, Wion D, De Luca HF, Brachet P. 1,25-dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res Mol Brain Res. 1994;24(1-4):70–6. doi: 10.1016/0169-328x(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 63.Annweiler C, Schott AM, Montero-Odasso M, Berrut G, Fantino B, Herrmann FR, Beauchet O. Cross-sectional association between serum vitamin D concentration and walking speed measured at usual and fast pace among older women: the EPIDOS study. J Bone Miner Res. 25(8):1858–66. doi: 10.1002/jbmr.80. [DOI] [PMC free article] [PubMed] [Google Scholar]