Abstract
Purpose
To determine the maximum tolerated dose of combined therapy using an yttrium-90 labeled anti-CEA antibody with gemcitabine in patients with advanced CEA producing solid tumors.
Experimental Design
The chimeric human/murine cT84.66 is an anti-CEA intact IgG1, with high affinity and specificity to CEA. This was given at a fixed yttrium-90 labeled dose of 16.6 mCi/m2 to subjects who had and an elevated CEA in serum or in tumor by immunohistochemistry. Also required was a tumor that imaged with an 111In labeled cT84.66 antibody. Patients were treated with escalating doses of gemcitabine given intravenously over 30 minutes on day 1 and 3 after the infusion of the yttrium-90 labeled antibody. Patients were treated in cohorts of 3. The maximum tolerated dose was determined as the highest level at which no more than 1 of 6 patients experienced a dose limiting toxicity.
Results
A total of 36 patients were enrolled, and all but one had prior systemic therapy. The maximum tolerated dose of gemcitabine in this combination was 150mg/m2. Dose limiting toxicities at a gemcitabine dose of 165mg/m2 included a grade 3 rash and grade 4 neutropenia. One partial response was seen in a patient with colorectal cancer, and 4 patients had a > 50% decrease in baseline CEA levels associated with stable disease. Human antichimeric antibody responses were the primary reason for stopping treatment in 12 patients.
Conclusions
feasibility of combining gemcitabine with an yttrium-90 labeled anti-CEA antibody is demonstrated with preliminary evidence of clinical response.
Keywords: Radioimmunotherapy, gemcitabine, CEA
Introduction
Radiolabeled monoclonal antibodies have been studied as a possible treatment for human malignancies. Monoclonal antibodies have shown potential to act as therapeutic agents and have shown efficacy especially with hematologic malignancies as evidenced by the approval of rituximab and more recently of yttrium-90-labeled ibritumomab tiuxetan for low-grade non-Hodgkin’s lymphoma (1-3). Solid tumors have been treated with immune- guided radiotherapy, albeit with lower response rates due to complex factors related to tumor targeting, tumor vasculature, vascular permeability, and therapeutic index (4,5).
Radiosensitization has been a strategy to increase the efficacy of immune-guided radiotherapy. A recent study has demonstrated the feasibility of combining a 120-hour infusion of 5-fluorouracil with the anti-CEA yttrium-labeled IgG1 murine monoclonal antibody designated T84.66 (6). Stable disease and 2 mixed responses were seen. Other studies have also demonstrated the feasibility of this approach (7).
Gemcitabine is currently FDA approved for a variety of tumors including pancreas, breast, ovarian and lung cancer. Laboratory studies have demonstrated strong radiosensitization properties possibly due to inhibition of ribonucleotide reductase, effects on deoxyribonucleotide pool composition, and to incorporation into DNA with subsequent early chain termination. Preclinically radiosensitization was greatest when cells were exposed to gemcitabine between 2 to 24-48 hours before radiation. Radiosensitization was observed for approximately 2 days after exposure (8,9). The maximum radiosensitization correlated with a drop in adenosine diphospate and occurred at relatively low gemcitabine doses (10). Gemcitabine has also demonstrated significant radioenhancing properities with immune-guided radiotherapy in vivo (11-13).
Clinical studies combining gemcitabine with radiation have confirmed potent radiosensitizing properties. In head and neck patients, doses of gemcitabine needed de-escalation from a starting weekly dose of 300 mg/m2 (10). At doses of 30 mg/m2, gemcitabine triphosphate levels were in the same range as with the 150 mg/m2 dose. The levels of dFdCTP in biopsy specimens were similar to those seen in in vitro radiosensitizing experiments suggesting that significant interactions were occurring at these dose levels. Other studies report tolerance of radiation and gemcitabine in upper gastrointestinal tumors, also with less than full systemic doses (14,15). Studies with lung cancer have also be reported, albeit with increased esophagitis (16).
Based on this information we designed this study to determine the tolerance of a combination of gemcitabine and 90Y-T84.66 anti-CEA antibody. Gemcitabine was given in two equal doses 48 hours apart to maximize radiation sensitization with starting doses based on previous phase I data on twice weekly dosing schedules (15,17)
Material and Methods
Antibody Production and Conjugation
Human/murine cT84.66 is an anti-CEA intact IgG1, with high affinity (KA = 1.16 × 1011 M-1) and specificity to CEA. Details of its production, characterization, purification, conjugation, and radiolabeling have been reported previously (18). Briefly, for this study, cT84.66 was conjugated to isothiocyanatobenzyl DTPA. Preparation of the radiolabeled dose involved incubation of 111In at a ratio of 1 mCi to 1 mg and yttrium (90Y) at a ratio of 10 mCi to 1 mg followed by size exclusion HPLC purification. All administered doses demonstrated radiolabeling > 90%, endotoxin levels < 1 unit/ml, and immunoreactivity > 95%. The final vialed lot of purified conjugated antibody met standards set by the FDA. Investigational New Drug applications for 111In-DTPA-cT84.66 and 90Y-DTPA-cT84.66 are currently on file with the FDA.
Clinical Trial Design
The primary objective of this trial was determine the maximum tolerated dose (MTD) and associated toxicities of gemcitabine in combination with 90Y-DTPA-cT84.66. Patients were enrolled in cohorts of 3 with escalating doses of gemcitabine (Table 1). Gemcitabine was administered intravenously over 30 minutes beginning on day 1 and on day 3 after infusion of the therapeutic dose of 90Y-DTPA-cT84.66 (16.6 mCi/m2). This therapeutic dose was determined in a previous phase I study of 90Y-DTPA-cT84.66 as single agent (19). Gemcitabine dose escalation continued until a dose-limiting toxicity (DLT) was noted defined as any treatment-related grade III non-hematologic toxicity not reversible to grade II or less within 24 hours, or any grade IV toxicity. Up to 3 cycles of therapy were allowed with DLTs determined based on first cycle tolerance. Toxicity was graded using the NCI common toxicity criteria (CTC) version 2.0. Further patients were entered and further dose escalation continued if no further DLTs were noted in a completed cohort of 6 patients. The MTD was defined as the highest level at which ≤ 1 of 6 patients experienced a DLT. Biodistribution, tumor targeting, absorbed radiation dose estimates, and clearance of the antibody were also evaluated through serial blood samples, 24-h urine collection, and nuclear scans performed at time points out to 7 days after antibody infusion.
Table 1.
Gemcitabine Dose Escalation Schema
| Level | Dose (mg/m2) |
|---|---|
| 1 | 30 |
| 2 | 45 |
| 3 | 60 |
| 4 | 75 |
| 5 | 90 |
| 6 | 105 |
| 7 | 120 |
| 8 | 135 |
| 9 | 150 |
| 10 | 165 |
The following studies were performed before antibody administration: complete blood count and platelet count; complete metabolic panel; creatinine clearance; electrocardiogram; pulmonary function tests; urinalysis; serum HIV testing; serum pregnancy testing if indicated; plasma CEA levels; and serum human anti-chimeric antibody (HACA) response. Additionally, chest X-ray, and CT scans of relevant anatomical locations corresponding to areas of metastatic or suspected metastatic disease were obtained. If clinically indicated, bone scan, magnetic resonance imaging, or positron emission tomography images were also performed to assess disease location and extend. All blood studies were done within 2 weeks and all radiological studies within 6 weeks of antibody infusion.
For the initial cycle of therapy, each patient first received an imaging dose of 5 mCi/5 mg 111In-DTPA-cT84.66, which was used to track antibody activity and evaluate tumor targeting. The therapeutic dose of 90Y-DTPA-cT84.66 was subsequently given within 2 weeks and included 5 mCi of indium-111 labeled 90Y-DTPA-cT84.66. Initially, a test does of 100 μg of radiolabeled antibody was administered i.v. over 5 min. After 15 min., if there were no side effects, the remainder of the antibody was administered over 30 minutes. Subsequent cycles of therapy were not preceded by a separate imaging infusion. Serial blood samples were taken for pharmacokinetics at 30 min, 1 hr, 4 hr, and at each scan time after antibody infusion. Urine collections (24 h) were done daily for 5 consecutive days after antibody administration for pharmacokinetic analysis. Blood and urine samples were counted for 111In activity on a Packard gamma counter (Model 5530; Packard, Inc., Downers Grove, IL) with a window setting of 150-500 keV and were processed on a size exclusion HPLC Superose 6 column. Planar and whole body imaging studies were performed at 6 h, 24 h, 48 h, and 4-7 days after antibody administration using a Toshiba dual head 7200 camera with SPECT capability. In all cases, 20% energy windows were set over each of the two γ-ray energies of 111In. A medium energy high-resolution collimator was used throughout. Scan speed of 20 cm/min over a distance of 200 cm was used for the whole body imaging. SPECT scans were performed of relevant areas of 48 h and 4-7 days after antibody administration.
Gemcitabine in escalating doses was given intravenously on day 1 over 30 minutes, followed within 5 hours by the therapeutic dose of 90Y-DTPA-cT84.66, which was 16.6 mCi/m2 in all patients. A second dose of gemcitabine was given on day 3.
DTPA as a calcium salt was given at a dose of 250 mg/m2/24 hours in divided doses every 12 hours for 3 days after the dose of 90Y-DTPA-cT84.66 in an effort to reduce hematologic toxicity. This approach has been used in previous trials including the trial that established the MTD of 90Y-DTPA-cT84.66 as monotherapy (19). A previous trial using 90Y-DOTA-c84.66 documented an increase in hematologic toxicity with the omission of the Ca-DTPA infusion(20).
Radiological studies, including CT scans, were repeated at 5-6 weeks post-therapy to assess tumor response. Response criteria were defined as follows: complete response, disappearance of all measurable and evaluable disease and no new lesions; partial response, ≥ 50% decrease from baseline in the sum of the products of perpendicular diameters of all measurable lesions, with no progression of evaluable disease or development of new lesions; stable disease, does not qualify for complete response, partial response, or progression; progressive disease, 25% increase in the sum of products of measurable lesions over the smallest sum observed, or reappearance of any lesion that had disappeared, or appearance of any new lesion/site.
HACA Response
Serum HACA responses to cT84.66 and cT84.66-DTPA were assayed before infusion and at 2 weeks, 1, 3, and 6 months post-infusion using a double capture solid-phase quantitative, radioimmunoassay as described previously (18). Serum samples incubated with 111In-DTPA-cT84.66 were also examined by size exclusion HPLC using two tandem Superose 6 columns to detect possible immune responses not found by radioimmunoassay. Patients were felt to have anti-idiotype response if serum samples were positive by HPLC assay but were negative by radioimmunoassay.
Pharmacokinetic Analysis and Absorbed Dose Estimates
Blood and urine samples were counted for 111In activity on a gamma counter and were processed on a HPLC size-exclusion Superose 6 column. Samples containing both 111In and 90Y were counted sequentially in γ and β well counters. In the latter case, Cerenkov radiation was used with quench correction to determine the amount of 90Y present. Samples were homogenized in aqueous media and bleached before counting. Standards were used to calibrate the absolute accuracy of the counting systems.
For those organs seen in both projections, 111In activity in normal organs was estimated using parallel-opposed nuclear images to construct the geometric mean uptake as a function of time. Otherwise, single view images were acquired. All resultant curves demonstrating 111In activity versus time were corrected for background and patient attenuation. Attenuation was estimated using each patient’s CT scans and attenuation coefficients obtained from a separate series of experiments involving gamma camera efficiency in counting a planar 111In phantom source as a function of tissue-equivalent absorber thickness. Given the geometric mean or single view uptake values and measured blood and urine activity, a five-compartment modeling analysis was performed to estimate residence times for 111In and 90Y activity in blood, urine, liver, and whole body. Details of this compartmental model have been published previously (21). 90Y radiation doses to normal organs based on biodistribution of 111In-cT84.66 were estimated with the medical internal radiation dose method (22) using S values obtained from the MIRDDOSE3 program (23). Doses were calculated using male and female phantom organ sizes in these estimates. As previously reported, 90Y-DTPA-cT84.66 and 111In-DTPA-cT84.66 biodistributions were comparable in the mouse model (24). Red marrow radiation dose estimates were performed using the American Association of Physicists in Medicine algorithm (25) based on blood residence times determined from the five compartmental model.
Tumor absorbed radiation doses were estimated using 111In uptake versus time curves determined from serial nuclear imaging data. Regions of interest were drawn around each tumor lesion, and the conjugate view method (26) was used to estimate activity. Trapezoidal interpolation was used to integrate the time activity curve and estimate residence time. CT scans were used to define tumor volume as well as the effective attenuation factor for the conjugate view method. For lesions not clearly defined by CT scans, nuclear medicine region of interest (length and width) was used to estimate the tumor volume, assuming an ellipse with the third dimension defined by the geometric mean of the length and width. Absorbed fraction was a function of tumor size and determined via separate Monte Carlo simulation. Edge effects were thus taken into account (27). Uniform uptake was assumed within the tumor. This methodology still uses the medical internal radiation dose strategy but requires that we compute the effective β loss caused by the finite range of 90Y β radiation (28) using the formula:
where Eβ is the mean β energy of 90Y or 0.93 MeV, area under the curve (residence time) is in hours and tumor mass is in grams.
Results
A total of 36 patients were enrolled. The majority of patients were diagnosed with colorectal cancer and other gastrointestinal malignancies and all but one had previous systemic treatment (Table 2). Four patients did not image with 111In-DTPA-cT84.66 and no further treatment was given except in one case. This one patient was initially felt to image at the right adrenal gland, a site of known disease. However, it was subsequently determined that imaging represented a dilated gallbladder and a false positive on target and the patient received no further therapy beyond the first course.
Table 2.
Patient Characteristics
| Characteristic | No. of Patients |
|---|---|
| Gender | |
| Male | 20 |
| Female | 16 |
| Median Age | 62.7 |
| Race/Ethnicity | |
| Caucasian | 32 |
| Asian | 3 |
| African American | 1 |
| Diagnoses | |
| Colorectal | 18 |
| Appendix | 1 |
| Gastric/esophagus | 3 |
| Pancreas | 2 |
| Lung | 7 |
| Breast | 2 |
| Medullary thyroid | 2 |
| Testicular | 1 |
| Prior Treatment | |
| Chemotherapy | 35 |
| Radiation | 10 |
| Surgery | 35 |
The MTD level was established at a gemcitabine dose of 150 mg/m2. Toxicities at one dose level above this (165 mg/m2) included grade 3 rash and grade 4 hematologic. Grade 3 rash was noted in a 53 year old female with metastatic pancreatic cancer who had had prior treatment with ifosfamide and etoposide was well as gemcitabine and capecitabine. The patient developed a pruritic erythematous rash over the chest, back, abdomen and extremities beginning on day 3 after administration of the 90Y-DTPA-cT84.66 and resolved over the next 2 weeks. The patient had stable disease after the first course, but developed a HACA reaction and no further protocol therapy was given. The second patient developed grade 4 neutropenia but recovered uneventfully. This patient was a 76 year old female with stage IV non-small cell lung cancer with no previous radiation, but with previous systemic therapy including carboplatin/paclitaxel, carboplatin/docetaxel, pemetrexed, and gemcitabine.
Three of 36 patients completed the protocol maximum of 3 treatment cycles with four further patients completing 2 cycles. Twelve patients developed a HACA response precluding further therapy, all after the first treatment. Reasons for stopping therapy in 33 treated patients are listed in Table 3 with progressive disease or HACA being the most common.
Table 3.
Reason for Treatment Cessation
| HACA | 12 |
| Protocol completion* | 4 (one with 2 cycles) |
| Disease progression | 13 |
| Toxicity | 3 |
The protocol had included 2 cycles of treatment but was amended to allow up to 3 cycles.
The most frequent first cycle toxicities were hematologic. There was a trend for more leucopenia with increasing dose of gemcitabine with scattered instances of thrombocytopenia. Rash was also seen at various levels, but reached a grade 3 DLT level with a gemcitabine dose of 150 mg/m2. Rash occurred between day 1-5, and in all cases resolved when treatment was stopped. Other toxicities, including nausea, fluid retention, fever or pulmonary toxicity were not seen. See Table 4 for further details.
Table 4.
Cycle 1 Toxicities (grade 3/4)
| Treatment Level | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Leukopenia | 1 | 2 | 2 | 1 | 2 | 2 | ||||
| Lymphopenia | 2 | 3 | 1 | 2 | 2 | 1 | ||||
| Platelet | 1 | 1 | 1 | |||||||
| Rash | 1* | 1* | 1 | |||||||
| Anemia | 2 |
grade 2
A 59 year old female patient with colon cancer with pelvic and peri-pancreatic disease had a partial response after the first cycle of treatment and received 2 treatment cycles with a gemcitabine dose level of 45 mg/m2. Her CEA level was stable during treatment staying within 15% of baseline. She had been diagnosed with T3N2 colon cancer 4 years earlier and had received 1 year of adjuvant 5-fluorouracil. Subsequent treatment for recurrent disease included capecitabine, irinotecan, 5-fluorouracil and leucovorin (IFL), capecitabine/gemcitabine, as well as post-surgical radiation to a mass causing left hydronephrosis.
CEA levels were tabulated and correlated with clinical outcome. Progressive disease was noted in 6 of 7 patients with a 50% or greater rise in CEA, and stable disease was seen in all 4 cases with a CEA decrease of more than 50%.
Individual tumor radiation doses were estimated in cases where a clear delineation of a target lesion was possible, and responses for each of these lesions were recorded (Table 5, 6). Lesions where a partial response was noted had a higher average dose (8702±1411 rad), but a reverse trend was seen in comparing stable (2834±5763) and progressive lesions (5872±4216), with large standard deviations in each case. Estimated organ and total body doses are similar to previous reports (Table 7) (19). Tumor size with estimated Y-90 dose is presented in Table 5. When doses are tabulated by tumor volumes, the tumors with the highest dose included those below 10 cc with all tumors receiving a dose greater than 100 rad/mCi in this size range (Fig 1).
Table 5.
Summary of Y-90 Dose to Tumors
| Patient (dose1) | Diagnosis | Location | Volume2 (cc) | Y-90 AUC (mCi* hr/mCi) | Y-90 Dose (rad/mCi) | Delivered Dose (rad) | Response |
|---|---|---|---|---|---|---|---|
| 1 (45) | colorectal | peri-hilar | 10.3 | 0.162 | 31.2 | 989.7 | stable |
| pancreas | 203.7 | 0.648 | 6.3 | 200.1 | stable | ||
| periclavicular | 9.1* | 0.029 | 6.4 | 202.0 | - | ||
|
| |||||||
| 2 (60) | colorectal | left lingular | 0.4 | 0.076 | 392.9 | 11079.1 | progression |
| peri-hilar | 0.3 | 0.054 | 403.1 | 11368.7 | progression | ||
| right hepatic | 149.2 | whole liver dose | 37.7 | 1064.1 | stable | ||
|
| |||||||
| 3 (90) | NSCLC | mediastinum | 14.1 | 0.873 | 122.5 | 3392.2 | progression |
|
| |||||||
| 4 (120) | colorectal | subcarinal | 36.1 | 0.649 | 35.7 | 3502.4 | progression |
| right lung | 17.2 | 0.152 | 17.6 | 1728.1 | stable | ||
| left lung | 3.6 | 0.179 | 98.9 | 9700.3 | PR | ||
|
| |||||||
| 5 (120) | NSCLC | right lower lobe | 179.6 | 2.121 | 23.4 | 552.9 | stable |
|
| |||||||
| 6 (135) | colorectal | mesenteric node | 4.0 | 0.517 | 253.4 | 7704.2 | PR |
| subcarinal | 3.4 | 0.079 | 46.5 | 1414.8 | stable | ||
|
| |||||||
| 7 (135) | teratoma | left lung | 688.3 | 3.729 | 10.8 | 433.2 | stable |
|
| |||||||
| 8 (150) | colorectal | Peri-hilar | 12.7 | 0.321 | 50.0 | 1795.7 | progression |
|
| |||||||
| 9 (150) | colorectal | right hepatic | 470.5 | whole liver dose | 40.4 | 1005.1 | stable |
| paratracheal | 3.5 | 0.293 | 164.4 | 4093.9 | progression | ||
|
| |||||||
| 10 (150) | colorectal | lung nodule | 0.5 | 0.192 | 740.0 | 191667.0 | stable |
| right liver | 89.5 | 3.115 | 69.0 | 1788.5 | stable | ||
gemcitabine dose level – mg/m2
By CT scan except as noted
By nuclear medicine scan
Table 6.
Summary of Y-90 Dose to Tumors
| Volume (cc) | Y-90 Dose (rad/mCi) | Delivered Dose (rad) | |
|---|---|---|---|
| Average | 99.8 | 134.2 | 4272.8 |
| STD | 184.4 | 190.6 | 5172.5 |
| Maximum | 688.3 | 740.0 | 19167.0 |
| Median | 12.7 | 46.5 | 1788.5 |
Table 7.
Organ Y-90 Dose Estimates
| Organ (n = 23) | Average (rad/mCi) | Standard Deviation |
|---|---|---|
| Marrow | 3.00 | 1.28 |
| Liver | 21.78 | 8.86 |
| Kidney | 11.15 | 3.55 |
| Total body | 2.09 | 0.45 |
Fig. 1.
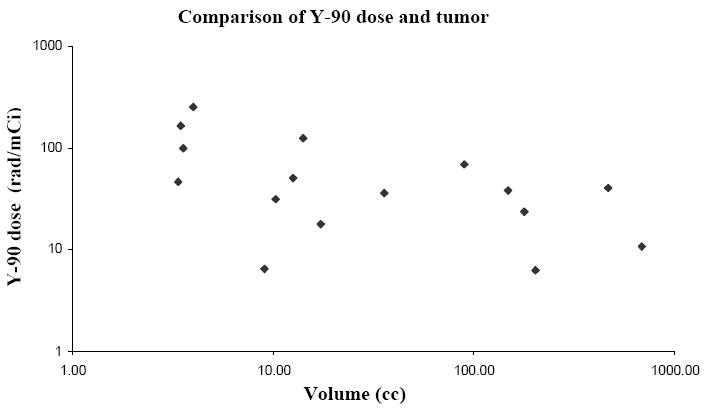
Y-90 dose (rad/mCi) to individual tumors compared with the volume of the individual tumors.
Discussion
This study demonstrates the feasibility of combining gemcitabine with the 90Y-DTPA-cT84.66 anti-CEA antibody. The MTD was defined at a gemcitabine dose of 150 mg/m2 given on day 1 and 3 of radioimmunotherapy. Overall the primary toxicity was hematologic, which was well tolerated with resolution before 6 weeks and with no instances of neutropenic fever.
Rash was noted in several patients at several dose levels. This rash occurred between 1 to 5 days after initiation of treatment and resolved within 1 to 2 weeks. Rash has been noted with single agent gemcitabine in the past and it is possible that its occurrence could be related to gemcitabine alone (29). However interaction with radiation and skin toxicity has been noted with gemcitabine in the context of radiation recall (30) and with increased skin toxicity during radiation (10). It is difficult to determine if the skin toxicity is clearly greater than what might be caused by gemcitabine alone due to the small number of cases, but an interaction is possible. Rash has also been reported with immune-guided radiotherapy alone, possibly due to an immune mediated process (31;32). This remains a possibility in this study as instances of rash appeared sporadic and not clearly associated with higher doses of gemcitabine. A HACA response was associated with the rash in 2 of 3 cases, but not seen in the DLT defining case.
The gemcitabine dose escalation scheme was conservative due to evidence for a strong interaction with radiation, and one of the concerns was that of increased hematologic effects. Results, including the lack of neutropenic fever, do not suggest a prohibitive interaction between RIT and gemcitabine. In addition there were no clinically significant instances of thrombocytopenia and no patient required a platelet transfusion. This is despite the use of doses that have been documented to be radiosensitizing in other studies (10).
It is of interest that the patient with a partial response was treated at a relatively low dose of gemcitabine (45 mg/m2) and had received and progressed through previous gemcitabine. As noted, in head and neck cancer patients treated with weekly gemcitabine and radiation, dFdCTP levels in tumor biopsies obtained during treatment were similar in patients receiving gemcitabine doses of 50 to 300 mg/m2. The fact that more objective responses were not seen at higher levels is likely due to the heavily pretreated nature of the study population. However, 4 patients did have at least a 50% decrease in CEA level that was associated with stable disease in all cases. These cases were spread through out various gemcitabine dose levels and are a further indication of possible clinical benefit. Although CEA levels are not measurable in terms of response, levels, especially if changed by more than 50% have been associated with clinical response (33;34). Of note, 2 of 4 patients with an initial increase in CEA maintained stable disease during further treatment. This may indicate that in some cases an increased CEA early in treatment may have been due to tumor necrosis and may predict response rather than progression (35;36).
There was a lack of clear correlation with radiation dose and response when individual tumors were evaluated. This may again be because to the heavily pretreated and heterogeneous nature of the patients enrolled on this study. It is of interest that the 2 tumors showing a partial response did receive relatively high radiation doses, and also higher levels of gemcitabine. However, tumors with stable disease when compared progressive lesions, received a lower dose on average across a broad range of gemcitabine doses. There was a tendency for smaller tumors to have a higher Y-90 dose suggesting better antibody penetration possibly based on better perfusion and lower interstitial pressure. However, among the 6 lesions with a dose greater than 100 rad/mCi, there were 1 partial response, 1 stable disease, and 4 instances of progressive disease again suggesting a lack of correlation between dose and response. Tumor doses ranged between 6.3 to 740.0 rad/mCi indicating an overall good therapeutic ratio when compared to red marrow and total body estimates.
In several cases, grade 3 lymphopenia was noted, scattered across treatment levels (Table 3). Radiation therapy has been associated with lymphocyte depletion in the past with differing effects on various lymphocyte subsets (37). There is also evidence that chemotherapy in combination with external beam radiation may increase lymphocytopenia (38). No specific clinical consequence related to increased rates of infection were noted during the course of this study. Among patients with grade 3 lymphopenia, 8 of 11 developed a HACA response.
HACA responses continue to be a problem, and were the primary reason for stopping treatment in 12 patients. It is possible that a number of these cases could have had a more prolonged period of stable disease or experienced a response with continued treatment. The primary strategies to overcome this would include using more humanized antibodies, use of antibody fragments and potentially use of immunosuppressive agents such as cyclosporine (5). These strategies would potentially allow the repetitive dosing that would allow for more effective treatment.
Although the formal definition of DLT was met, further dose escalation might have been possible given the nature of toxicities seen. However several factors contributed to our decision to forgo further dose escalation. First, as noted, the dose of gemcitabine was in the range noted to be radiosensitizing in other studies, and there was evidence for clinical activity, including a partial response, at levels below the ultimate MTD. Second, HACA responses, as noted, were limiting and we were transitioning to a humanized version of the 90Y-DTPA-cT84.66 antibody. A clinical trial has since been initiated evaluating the 90Y-labeled humanized - T84.66
Responses consisted mainly of stable disease, with one patient showing an objective response. Although this combination appears feasible, activity as a primary therapy in patients with bulky disease appears limited. This may be related to tumor factors including heterogeneity of vasculature and interstitial pressure that impede macromolecule penetration, although the degree of IgG penetration into solid tumors in not yet completely defined. Strategies for the future include studying combination therapy in minimal disease, use of antibody fragments with potentially improved therapeutic ratios, and using RIT in combination with full dose chemotherapy as a chemosensitizer(5;39;40).
Statement of Translational Relevance.
This manuscript describes the feasibility of immune-guided radiotherapy using a monoclonal anti-CEA antibody combined with gemcitabine at radiosensitizing doses. Importantly, evidence of clinical activity was seen in this cohort of heavily pretreated patients. This combination could be applied to less heavily pretreated patients in future trials, which could lead to higher response rates with better demonstration of clinical benefits. This regimen might have even greater utility in low volume tumor states, including in the adjuvant setting in CEA positive gastrointestinal cancer or other sites penetration of bulky tumors would not be an issue. In these settings immune guided-radiotherapy would represent a systemic agent with a unique mechanism of action that could potentially be integrated into multimodality treatment.
Acknowledgments
Grant Support: This work was supported in part by PO1 Grant: NIH PO1 CA 43904 and Cancer Center Grant: 5P30 CA 3354
Reference List
- 1.Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002 May 15;20(10):2453–63. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 2.Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol. 2002 Aug 1;20(15):3262–9. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Nademanee A, Forman S, Molina A, Fung H, Smith D, Dagis A, et al. A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood. 2005 Oct 15;106(8):2896–902. doi: 10.1182/blood-2005-03-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain M, Venkatraman G, Batra SK. Optimization of radioimmunotherapy of solid tumors: biological impediments and their modulation. Clin Cancer Res. 2007 Mar 1;13(5):1374–82. doi: 10.1158/1078-0432.CCR-06-2436. [DOI] [PubMed] [Google Scholar]
- 5.Wong JY. Basic immunology of antibody targeted radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(2 Suppl):S8–14. doi: 10.1016/j.ijrobp.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 6.Wong JY, Shibata S, Williams LE, Kwok CS, Liu A, Chu DZ, et al. A Phase I trial of 90Y-anti-carcinoembryonic antigen chimeric T84.66 radioimmunotherapy with 5-fluorouracil in patients with metastatic colorectal cancer. Clin Cancer Res. 2003 Dec 1;9(16 Pt 1):5842–52. [PubMed] [Google Scholar]
- 7.DeNardo SJ, Denardo GL. Targeted radionuclide therapy for solid tumors: an overview. Int J Radiat Oncol Biol Phys. 2006;66(2 Suppl):S89–S95. doi: 10.1016/j.ijrobp.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence TS, Eisbruch A, McGinn CJ, Fields MT, Shewach DS. Radiosensitization by gemcitabine. Oncology (Williston Park) 1999 Oct;13(10 Suppl 5):55–60. [PubMed] [Google Scholar]
- 9.Latz D, Fleckenstein K, Eble M, Blatter J, Wannenmacher M, Weber KJ. Radiosensitizing potential of gemcitabine (2’,2’-difluoro-2’-deoxycytidine) within the cell cycle in vitro. Int J Radiat Oncol Biol Phys. 1998 Jul 1;41(4):875–82. doi: 10.1016/s0360-3016(98)00105-9. [DOI] [PubMed] [Google Scholar]
- 10.Eisbruch A, Shewach DS, Bradford CR, Littles JF, Teknos TN, Chepeha DB, et al. Radiation concurrent with gemcitabine for locally advanced head and neck cancer: a phase I trial and intracellular drug incorporation study. J Clin Oncol. 2001 Feb 1;19(3):792–9. doi: 10.1200/JCO.2001.19.3.792. [DOI] [PubMed] [Google Scholar]
- 11.Okazaki S, T M, Colcher D. Combination radioimmunotherapy and chemotherapy using 131I-B72.3 and gemcitabine. Proceedings of the American Association for Cancer Research. 1998;39 [Google Scholar]
- 12.Gold P, Schutsky K, Cardillo TM. Successful treatment of pancreatic cancer by combined gemcitabine and low dose radioimmunotherapy with 90Y-labeled PAM4 antibody. Proceeding of the American Association for Cancer Research. 2002;43 [Google Scholar]
- 13.Graves SS, Dearstyne E, Lin Y, Zuo Y, Sanderson J, Schultz J, et al. Combination therapy with Pretarget CC49 radioimmunotherapy and gemcitabine prolongs tumor doubling time in a murine xenograft model of colon cancer more effectively than either monotherapy. Clin Cancer Res. 2003 Sep 1;9(10 Pt 1):3712–21. [PubMed] [Google Scholar]
- 14.Nanfro JJ. Phase I trial dosing of gemcitabine and concurrent involved field irradiation in patients with localized pancreatic carcinomas. J Clin Oncol. 1999 Nov;17(11):3692. [PubMed] [Google Scholar]
- 15.Blackstock AW, Bernard SA, Richards F, Eagle KS, Case LD, Poole ME, et al. Phase I trial of twice-weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. J Clin Oncol. 1999 Jul;17(7):2208–12. doi: 10.1200/JCO.1999.17.7.2208. [DOI] [PubMed] [Google Scholar]
- 16.Vokes EE, Gregor A, Turrisi AT. Gemcitabine and radiation therapy for non-small cell lung cancer. Semin Oncol. 1998 Aug;25(4 Suppl 9):66–9. [PubMed] [Google Scholar]
- 17.Poplin EA, Corbett T, Flaherty L, Tarasoff P, Redman BG, Valdivieso M, et al. Difluorodeoxycytidine (dFdC)--gemcitabine: a phase I study. Invest New Drugs. 1992 Aug;10(3):165–70. doi: 10.1007/BF00877241. [DOI] [PubMed] [Google Scholar]
- 18.Wong JY, Thomas GE, Yamauchi D, Williams LE, Odom-Maryon TL, Liu A, et al. Clinical evaluation of indium-111-labeled chimeric anti-CEA monoclonal antibody. J Nucl Med. 1997 Dec;38(12):1951–9. [PubMed] [Google Scholar]
- 19.Wong JYC, Chu DZ, Yamauchi DM, Williams LE, Liu A, Wilczynski S, et al. A phase I radioimmunotherapy trial evaluating 90yttrium-labeled anti-carcinoembryonic antigen (CEA) chimeric T84.66 in patients with metastatic CEA-producing malignancies. Clin Cancer Res. 2000 Oct;6(10):3855–63. [PubMed] [Google Scholar]
- 20.Wong JY, Chu DZ, Williams LE, Liu A, Zhan J, Yamauchi D, et al. A Phase I Trial of 90Y-DOTA-Anti-CEA Chimeric T84.66 (cT84.66) Radioimmunotherapy in Patients with Metastatic CEA-Producing Malignancies. Cancer Biother Radiopharm. 2006;21(2):88–100. doi: 10.1089/cbr.2006.21.88. [DOI] [PubMed] [Google Scholar]
- 21.Odom-Maryon TL, Williams LE, Chai A, Lopatin G, Liu A, Wong YC, et al. Pharmacokinetic modeling and absorbed dose estimation for chimeric anti-CEA antibody in humans. J Nucl Med. 1997 Dec;38(12):1959–66. [PubMed] [Google Scholar]
- 22.Loevinger R, B M. A schema for absorbed-dose calculations for biologically-distributed radionuclides. J Nucl Med. 1968;9(Suppl 1):7–14. J Nucl Med 1968;MIRD pamphlet no.1(9 (Suppl 1)):7-14. [PubMed] [Google Scholar]
- 23.Stabin MG. MIRDOSE: personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 1996 Mar;37(3):538–46. [PubMed] [Google Scholar]
- 24.Williams LE, Primus FJ, Wong JYC, Wu AM, Odom-Maryon TL, Johnson DK, Hefta LJF, Shively JE, Raubitschek AA. Biodistribution of an In-111 or Y-90 labeled chimeric anti-CEA monoclonal antibody (cT84.66) following its large scale production in a bioreactor. Tumor Targeting. 1996;2:116–24. [Google Scholar]
- 25.Siegel JA, Wessels BW, Watson EE, Stabin MG, Vriesendorp HM, Bradley EW, Badger CC, Brill AB, Kwok CS, et al. Bone marrow dosimetry and toxicity for radioimmunotherapy. Antibody Immunoconj Radiopharm. 1990;3:213–33. [Google Scholar]
- 26.Thomas SR, Maxon HR, Kereiakes JG. In vivo quantitation of lesion radioactivity using external counting methods. Med Phys. 1976 Jul;03(04):253–5. doi: 10.1118/1.594287. [DOI] [PubMed] [Google Scholar]
- 27.Loevinger R, Japha EM, Brownell GL. Discrete radioisotope sources. In: Hine GJ, Brownell GL, editors. Radiation Dosimetry, 732-739. New York: Academic Press; 1956. pp. 732–9. [Google Scholar]
- 28.Loevinger R, Japha EM, Brownell GL. Internally administered radioisotopes. In: Hine GJ, Brownell GL, editors. Radiation Dosimetry. Academic Press; New York: 1956. p. 824. [Google Scholar]
- 29.Green MR. Gemcitabine safety overview. Semin Oncol. 1996 Oct;23(5 Suppl 10):32–5. [PubMed] [Google Scholar]
- 30.Jeter MD, Janne PA, Brooks S, Burstein HJ, Wen P, Fuchs CS, et al. Gemcitabine-induced radiation recall. Int J Radiat Oncol Biol Phys. 2002 Jun 1;53(2):394–400. doi: 10.1016/s0360-3016(02)02773-6. [DOI] [PubMed] [Google Scholar]
- 31.Chong G, Lee FT, Hopkins W, Tebbutt N, Cebon JS, Mountain AJ, et al. Phase I trial of 131I-huA33 in patients with advanced colorectal carcinoma. Clin Cancer Res. 2005 Jul 1;11(13):4818–26. doi: 10.1158/1078-0432.CCR-04-2330. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs AJ, Fer M, Su FM, Breitz H, Thompson J, Goodgold H, et al. A phase I trial of a rhenium 186-labeled monoclonal antibody administered intraperitoneally in ovarian carcinoma: toxicity and clinical response. Obstet Gynecol. 1993 Oct;82(4 Pt 1):586–93. [PubMed] [Google Scholar]
- 33.Wang WS, Lin JK, Lin TC, Chiou TJ, Liu JH, Yen CC, et al. Tumor marker CEA in monitoring of response to tegafur-uracil and folinic acid in patients with metastatic colorectal cancer. Hepatogastroenterology. 2002 Mar;49(44):388–92. [PubMed] [Google Scholar]
- 34.Hanke B, Riedel C, Lampert S, Happich K, Martus P, Parsch H, et al. CEA and CA 19-9 measurement as a monitoring parameter in metastatic colorectal cancer (CRC) under palliative first-line chemotherapy with weekly 24-hour infusion of high-dose 5-fluorouracil (5-FU) and folinic acid (FA) Ann Oncol. 2001 Feb;12(2):221–6. doi: 10.1023/a:1008378412533. [DOI] [PubMed] [Google Scholar]
- 35.Ailawadhi S, Sunga A, Rajput A, Yang GY, Smith J, Fakih M. Chemotherapy-induced carcinoembryonic antigen surge in patients with metastatic colorectal cancer. Oncology. 2006;70(1):49–53. doi: 10.1159/000091184. [DOI] [PubMed] [Google Scholar]
- 36.Sorbye H, Dahl O. Transient CEA increase at start of oxaliplatin combination therapy for metastatic colorectal cancer. Acta Oncol. 2004;43(5):495–8. doi: 10.1080/02841860410032380. [DOI] [PubMed] [Google Scholar]
- 37.Louagie H, Van EM, Philippe J, Thierens H, de RL. Changes in peripheral blood lymphocyte subsets in patients undergoing radiotherapy. Int J Radiat Biol. 1999 Jun;75(6):767–71. doi: 10.1080/095530099140113. [DOI] [PubMed] [Google Scholar]
- 38.Bachtiary B, Herbacek I, Zideck T, Knocke TH, Dimopoulos J, Poetter R, et al. Impact of radiotherapy with and without concurrent cisplatin on lymphocyte subpopulations in cervical cancer patients. Anticancer Res. 2005 Nov;25(6C):4673–8. [PubMed] [Google Scholar]
- 39.Wong JY. Systemic targeted radionuclide therapy: potential new areas. Int J Radiat Oncol Biol Phys. 2006;66(2 Suppl):S74–S82. doi: 10.1016/j.ijrobp.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 40.Meredith RF, Wong JYC, Knox SJ. Radioimmunotherapy. In: Gunderson LG, Tepper JE, editors. Clinical Radiation Oncology. Philadelphia: 2006. [Google Scholar]


