Abstract
Background
In the setting of acute decompensated heart failure, worsening renal function (WRF) and improved renal function (IRF) have been associated with similar hemodynamic derangements and poor prognosis. Our aim was to further characterize IRF and its associated mortality risk.
Methods and Results
Consecutive patients with a discharge diagnosis of congestive heart failure at the Hospital of the University of Pennsylvania were reviewed. IRF was defined as a ≥20% improvement and WRF as a ≥20% deterioration in glomerular filtration rate. Overall, 903 patients met eligibility criteria, 31.4% experiencing IRF. Baseline venous congestion/right sided cardiac dysfunction was more common (p≤0.04) and volume of diuresis (p=0.003) was greater in patients with IRF. IRF was associated with a greater incidence of pre-admission (OR=4.2, 95% CI 2.6–6.7, p<0.0001) and post-discharge (OR=1.8, 95% CI 1.2–2.7 p=0.006) WRF. IRF was associated with increased mortality (adjusted HR=1.3, 95% CI 1.1–1.7, p=0.011), a finding largely restricted to patients with post-discharge recurrence of renal dysfunction (p interaction=0.038).
Conclusion
IRF is associated with significantly worsened survival and may represent the resolution of venous congestion induced pre-admission WRF. Unlike WRF, the renal dysfunction in IRF patients occurs independent of the confounding effects of acute decongestion and may provide incremental information for the study of cardio-renal interactions.
Keywords: Cardio-renal syndrome, Worsening renal function, Venous congestion
Introduction
Worsening renal function (WRF) during the treatment of acute decompensated heart failure has been associated with adverse outcomes such as death in multiple recent studies.[1–8] However, despite significant study of this phenomenon, little progress has been made toward a mechanistic or therapeutic understanding of cardio-renal interactions through the study of WRF. This limited success may relate to the fact that, in the setting of aggressive decongestion, some decreases in glomerular filtration may represent a normal physiologic response to intravascular contraction and be free of adverse prognostic significance.[9] Since decongestion is the primary goal of most decompensated heart failure admissions, the confounding effects of treatment makes WRF a complex entity to study. Further progress towards an understanding of cardio-renal syndromes (CRS) could likely be accomplished by identifying CRS at a time prior to, or in the absence of, the physiologic derangements induced by acute treatment.
We have recently reported that patients experiencing improvement in renal function (IRF) during the treatment of decompensated heart failure have a similarly increased rate of mortality to patients that develop WRF.[10] A possible explanation for the increased mortality in patients with IRF could either be that WRF occurred prior to admission and/or they have a recurrence of renal dysfunction after discharge. The primary aim of this study was to validate our previous observation that IRF is associated with significantly increased mortality and to further investigate the clinical profile of these patients. Additionally, we sought to test the hypothesis that patients with IRF likely experienced WRF as an outpatient prior to admission. We further hypothesized that the improvement in renal function is likely transient, possibly driving the adverse prognosis observed in these patients.
Methods
Consecutive admissions from 2004 to 2009 to the cardiology and internal medicine services at the Hospital of the University of Pennsylvania with a primary discharge diagnosis of congestive heart failure were reviewed. Inclusion required an admission B-type natriuretic peptide level > 100 pg/mL within 24 hours of admission, a length of stay 3 to 14 days, in addition to admission and discharge serum creatinine levels. Exclusion criteria included renal replacement therapy or admission to interventional cardiology services (to avoid confounding from contrast nephropathy). In the event of multiple hospitalizations in a single patient, the first admission that the patient underwent right heart catheterization was given priority to maximize available right heart catheterization data. If right heart catheterization did not occur, the first admission was retained. The primary analyses investigating the direct association between IRF and mortality were conducted in first admissions only (without preference for right heart catheterization) to ensure that the RHC enrollment criteria did not introduce bias into the survival analyses. Results of the same analyses in the RHC enriched population can be found in Supplementary Table 1. Values for echocardiographic and right heart catheterization derived variables were obtained from their respective clinical reports.
Estimated glomerular filtration rate (GFR) was calculated using the Modified Diet and Renal Disease equation.[11] IRF was defined as a ≥ 20% increase in GFR consistent with prior published literature investigating IRF.[9, 10, 12] Given the non-linear relationship between serum creatinine and renal function, and to maintain consistency with the IRF definition, WRF was defined as a ≥20% decrease in GFR.[13] Changes occurring at any time during the hospitalization were evaluated, unless specifically stated otherwise. Transient-IRF was defined as the occurrence of IRF at any time during hospitalization but deterioration in GFR prior to discharge leaving the admission to discharge improvement in GFR < 20%. Persistent-IRF was defined the as a continued ≥ 20% improvement in GFR at discharge. All cause mortality was determined via the Social Security Death Index.[14] Pre and post-discharge creatinine values were obtained by searching electronic medical records which provide access to data for the University of Pennsylvania health system which includes 3 hospitals in the Philadelphia area and the majority of the associated outpatient facilities. In an attempt to capture the patients’ pre and post discharge compensated renal function, creatinine values were collected if they were within 1 year of admission, greater than 7 days prior to or after the hospitalization, and obtained when the patient was an outpatient. Loop diuretic doses were converted to furosemide equivalents with 1 mg bumetanide = 20 mg torsemide = 80 mg furosemide for oral diuretics, and 1 mg bumetanide = 20 mg torsemide = 40 mg furosemide for intravenous diuretics. Data on net fluid output (total fluid out-total fluid in) was obtained by summing the daily fluid in/out flow sheets on all days of hospitalization. This study was approved by the institutional review board of the Hospital of the University of Pennsylvania.
Statistical Methods
The primary analyses in this study focused on; 1) description of the clinical characteristics associated with IRF, 2) evaluation of the relative change in pre-admission to admission GFR, 3) evaluation of the relative change in GFR occurring post discharge, and 4) investigation of the risk for mortality associated with IRF and its interaction with post IRF changes in renal function. Values reported are mean ± standard deviation, median (quartile 1 - quartile 4) and percentile. Independent Student’s t-test or the Mann-Whitney U test was used to compare continuous parameters. The Wilcoxon signed ranks test was used to evaluate paired data associations. Pearson’s Chi Square was used to evaluate associations between categorical variables. Proportional hazards modeling was used to evaluate time to event associations with all cause mortality. Candidate covariates for multivariable models adjusting for baseline characteristics were obtained by screening all baseline variables with missing data <5% and a univariate association with mortality (p≤ 0.2). Covariates were removed using backwards elimination (likelihood ratio) and variables with a p<0.2 were retained.[15] Covariates for other multivariable models were entered using forced entry of theoretically relevant variables. Given that the primary hypothesis was that IRF represents baseline cardio-renal dysfunction, discharge rather than baseline indices of renal function (i.e. GFR and blood urea nitrogen) were used to control for the potential influence of chronic renal insufficiency. Survival curves for death from any cause were plotted for patients that did not experience IRF, patients with transient-IRF and patient with persistent-IRF. Additional survival curves were plotted for the four combinations of groups between yes/no IRF and yes/no deterioration in renal function post discharge. Given that the focus of these plots was on change in GFR rather than absolute GFR, all survival curve plots were adjusted for discharge GFR. The x axis was terminated when the remaining number at risk was <10%. Proportional hazard models for the primary analysis were subjected to 1000 bootstrap replications (with replacement) to derive p values and 95% confidence intervals. Significance was defined as 2-tailed p<0.05 for all analyses excluding tests of interaction where p values <0.1 was considered significant. Statistical analysis was performed with PASW Statistics version 18.0 (SPSS Inc, Chicago, Illinois).
Results
Overall, 903 patients met eligibility criteria. Baseline characteristics are presented in Table 1. In total, 31.4% of the population experienced IRF during the hospitalization with 18.1% still meeting criteria for IRF at the time of discharge. Patients experiencing IRF had a mean improvement in GFR of 43.7 ± 27.1% compared to the remainder of the cohort that experienced only a 5.3 ± 6.7 % improvement in GFR from admission to the highest GFR during hospitalization. At the time of discharge, IRF patients had a 25.4 ± 29.6% mean improvement in GFR compared to a 9.6 ± 14.8% deterioration in GFR in the remainder of the cohort.
Table 1.
Patient characteristics and their association with improved renal function
| Characteristics | Overall Cohort (n=903) | IRF | p | |
|---|---|---|---|---|
| No (n=624) | Yes (n=279) | |||
| Demographics | ||||
| Age (years) | 62.9 ± 15.8 | 63.3 ± 15.5 | 61.8 ± 16.4 | 0.183 |
| White race | 34.1% | 30.6% | 41.3% | 0.002* |
| Males | 54.3% | 51.6% | 60.4% | 0.014* |
| Medical History | ||||
| Hypertension | 74.0% | 76.9% | 68.2% | 0.006* |
| Diabetes | 39.4% | 41.3% | 35.7% | 0.115 |
| Coronary artery disease | 43.0% | 42.6% | 44.3% | 0.637 |
| Ischemic etiology | 24.7% | 23.5% | 27.6% | 0.186 |
| Ejection fraction ≥40% | 35.0% | 38.3% | 28.1% | 0.003* |
| Admission Physical Exam | ||||
| Heart rate (bpm) | 89.6 ± 20.0 | 90.4 ± 19.4 | 87.8 ± 21.1 | 0.060 |
| Systolic blood pressure (mm Hg) | 138.8 ± 34.5 | 143.5 ± 34.6 | 128.6 ± 32.1 | <0.001* |
| Jugular venous distention | 35.8% | 33.5% | 40.5% | 0.052 |
| Moderate to severe edema | 15.3% | 14.2% | 17.3% | 0.235 |
| Hepatojugular reflux | 18.7% | 13.6% | 31.3% | <0.001* |
| Medications | ||||
| β-Blocker | 67.1% | 64.4% | 73.1% | 0.009* |
| ACE inhibitor or ARB | 61.6% | 61.0% | 62.9% | 0.587 |
| Digoxin | 22.4% | 20.4% | 27.1% | 0.024* |
| Spironolactone | 15.5% | 12.1% | 22.9% | <0.001* |
| Loop diuretic dose (mg) | 40 (0–80) | 40 (0–80) | 40 (0–80) | 0.147 |
| Laboratory Findings | ||||
| Serum sodium (mEq/L) | 138.6 ± 4.4 | 138.9 ± 4.1 | 137.9 ± 4.9 | 0.002* |
| Hemoglobin (g/dL) | 12.2 ± 2.1 | 12.1 ± 2.0 | 12.4 ± 2.1 | 0.057 |
| B-type natriuretic peptide (pg/mL) | 1636.1 ±1207.4 | 1538.3 ±1153.0 | 1854.3 ±1296.6 | 0.002* |
| Blood urea nitrogen (admission) (mg/dL) | 28.4 ± 20.8 | 25.8 ± 17.9 | 34.1 ± 25.1 | <0.001* |
| Blood urea nitrogen (discharge) (mg/dL) | 31.1 ± 20.7 | 31.8 ± 21.4 | 29.5 ± 19.0 | 0.290 |
| Glomerular filtration rate (admission) (mL/min) | 60.3 ± 28.6 | 63.2 ± 30.1 | 54.0 ± 23.8 | <0.001* |
| Glomerular filtration rate (discharge) (mL/min) | 59.3 ± 27.6 | 56.6 ± 27.1 | 65.2 ± 28.0 | <0.001* |
| Right Heart Catheterization Parameters† | ||||
| Right atrial pressure (mmHg) | 10.4 ± 6.7 | 9.9 ± 5.9 | 11.0 ± 7.6 | 0.274 |
| Pulmonary capillary wedge pressure (mmHg) | 21.8 ± 9.2 | 21.5 ± 9.6 | 22.3 ± 8.6 | 0.530 |
| Cardiac index (L/min/m²) | 2.1 ± 0.6 | 2.1 ± 0.5 | 2.1 ± 0.7 | 0.996 |
| Systemic vascular resistance (dyn·s/cm5) | 1605 ± 586 | 1612 ± 538 | 1595 ± 645 | 0.834 |
| Mean pulmonary artery pressure (mmHg) | 33.3 ± 10.4 | 33.2 ± 10.4 | 33.4 ± 10.5 | 0.918 |
| Pulmonary vascular resistance (Wood units) | 3.2 ± 3.0 | 3.2 ± 3.4 | 3.2 ± 2.3 | 0.981 |
| Echocardiographic Parameters‡ | ||||
| Ejection fraction (%) | 27.5 (15.0–45.0) | 30.0 (17.5–50.0) | 25.0 (15.0–40.0) | 0.001* |
| Left atrial size (cm) | 4.8 ± 0.8 | 4.8 ± 0.8 | 4.8 ± 0.8 | 0.398 |
| Left ventricular internal diastolic dimension (cm) | 5.9 ± 1.2 | 5.9 ± 1.1 | 6.0 ± 1.2 | 0.100 |
| Moderate to severe right ventricular dysfunction | 38.5% | 33.6% | 49.1% | <0.001* |
| Moderate to severe right ventricular dilation | 25.3% | 22.6% | 31.3% | 0.006* |
| Moderate to severe right atrial dilation | 9.3% | 7.1% | 13.5% | 0.003* |
| Moderate to severe tricuspid regurgitation | 35.7% | 33.3% | 40.8% | 0.041* |
| Lack of inspiratory inferior vena cava collapse | 14.1% | 11.9% | 18.7% | 0.015* |
Improved renal function defined as a 20% improvement in glomerular filtration rate at any time during hospitalization. All right heart catheterization and echocardiographic parameters were obtained from their respective clinical reports and non-continuous variables represent qualitative assessment by the interpreting physician. IRF: Improved renal function, ACE: Angiotensin converting enzyme, ARB: Angiotensin receptor blocker.
Significant p value.
Data available in n=211, (IRF=91 no IRF=120).
Data available n=771, (IRF=240 no IRF=531).
Characteristics of patients experiencing IRF and comparisons to the remainder of the cohort are presented in Table 1. Notably, the IRF group had multiple baseline indices consistent with a higher heart failure disease severity including lower baseline GFR, serum sodium, ejection fraction, and systolic blood pressure in addition to a higher B-type natriuretic peptide level (Table 1). Patients experiencing IRF also had a higher rate of heart failure medication utilization such as beta blockade, spironolactone, and digoxin (Table 1). All baseline echocardiographic parameters indicative of right sided volume overload and dysfunction (data available n=771, median time from admission to echocardiography = 1 day) were significantly associated with incident IRF (Table 1). Physical examination findings such as presence of edema (data available n=890), jugular venous distention (data available n=840), and hepatojugular reflux (data available n=684) tended to occur more frequently in IRF patients, however this association only met statistical significance for hepatojugular reflux (Table 1). Left atrial size and left ventricular internal diastolic dimension had no relationship with IRF despite the strong association noted with their right sided analogs (Table 1). Right heart catheterization parameters (data available n=211) were not significantly different between groups (Table 1). Notably, right atrial pressure was also not significantly higher in WRF patients (11.2 ± 7.1 mmHg vs. 9.9 ± 6.4 mmHg, p=0.17) compared to patients without WRF. Patients experiencing IRF had a greater volume of fluid lost during hospitalization (5.7 ± 5.5 L vs. 4.2 ± 6.2 L, p=0.003). Peak [80 (40–160) mg vs. 80 (40–160) mg, p=0.54] and total [240 (80–540) mg vs. 200 (80–480) mg, p=0.31] loop diuretic doses were similar. Length of stay was significantly longer in the group with IRF [7 (4–10) days vs. 5 (4–7) days, p<0.001] and utilization of inotropes was greater (OR=3.3, 95% CI 2.3–5.0, p<0.001).
Associations with pre-hospitalization renal function
In total, 41.3% (n=373) of patients had outpatient creatinine levels available within 1 year prior to but ≥7 days from the admission. The median time between hospital admission and the patient’s closest available creatinine was 60 (26–150) days and the lowest creatinine level (i.e. best pre admission renal function) was 136 (45–248) days. Patients with and without prior creatinine levels available were equally likely to experience IRF (OR=0.92, 95% CI 0.7–1.2, p=0.55). Amongst patients who experienced in hospital IRF, pre-admission GFR was significantly higher than admission GFR (Table 2). The change in GFR from pre-admission to admission was significantly greater in patients experiencing IRF compared to those that did not (Figure 1). The incidence of pre-admission WRF was significantly greater in patients who experienced inpatient IRF (closest GFR WRF OR=4.2, 95% CI 2.5–7.0, p<0.0001; lowest GFR WRF OR=4.2, 95% CI 2.6–6.7, p<0.0001).
Table 2.
Renal function prior to and after hospitalization in patients experiencing improved renal function
| Pre-Admission Renal Function (n=373) | Post-Discharge Renal Function (n=454) | |||||
|---|---|---|---|---|---|---|
| Admission GFR | Closest GFR | Best GFR | Discharge GFR | Closest GFR | Worst GFR | |
| IRF (any time) | 52.5 ± 25.0 | 59.6 ± 25.5* | 65.4 ±27.0* | 64.8 ± 28.9 | 61.3 ± 25.2† | 52.2 ± 25.1* |
| IRF (discharge) | 47.8 ± 22.3 | 58.8 ± 24.7* | 63.9 ± 25.0* | 67.9 ± 31.7 | 59.9 ± 27.6* | 51.2 ± 23.7* |
IRF: Improved renal function, GFR: Estimated glomerular filtration rate. IRF (any time) refers to a ≥20% improvement in GFR over the admission value at any time during the hospitalization; IRF (discharge) indicates that the ≥20% improvement in GFR remained at the time of discharge. GFR was calculated using creatinine values that were obtained as an outpatient within 1 year but greater than 7 days from admission or discharge. Closest GFR represents the closest eligible laboratory values prior to admission or after discharge for pre and post discharge renal function respectively. Best GFR represents the lowest and worst the highest eligible laboratory value prior to or after hospitalization respectively.
Comparison to admission GFR for pre-admission and comparison to discharge GFR for post-discharge, p<0.0001 for all.
Comparison to admission GFR p<0.011.
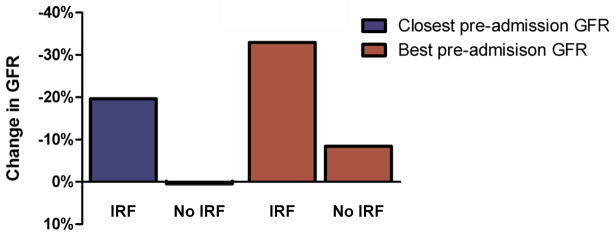
Figure 1.
Pre-admission to admission change in estimated glomerular filtration rate in patients with and without in-hospital IRF. IRF: Improved renal function. IRF defined as a 20% improvement in glomerular filtration rate at any time during hospitalization. Comparison of change in GFR between patients with and without IRF p<0.0001 for both closest and best pre-admission GFR.
Associations with post-hospitalization renal function
In total, 50.3% (n=454) of the population had an outpatient serum creatinine level available within 1 year after but ≥7 days from hospital discharge. The median time between hospital discharge and the patient’s closest available creatinine was 45 (16–109) days and the highest creatinine level (i.e. worst post discharge renal function) was 132 (48–248) days. Patients with and without a creatinine available after discharge were equally likely to experience IRF (OR=0.97, 95% CI 0.7–1.3 p=0.82). Amongst patients who experienced IRF during hospitalization, their closest and worst post-admission GFR was significantly lower than the discharge GFR (Table 2). This was particularly true for patients that still met criteria for IRF at the time of discharge (Table 2). Deterioration in GFR after discharge was significantly greater in patients experiencing IRF compared to those that did not, a finding which was more pronounced for patients meeting IRF criteria at the time of discharge (Figures 2A and 2B). The incidence of post discharge WRF was significantly greater in patients who experienced inpatient IRF at any time (OR=1.8, 95% CI 1.2–2.7 p=0.006) and who still met the criteria at discharge (OR=2.7, 95% CI 1.5–4.0 p<0.001) using the worst GFR as the comparator. These associations did not meet significance for the closest GFR (IRF at any time p=0.15, IRF at discharge p=0.057). Amongst patients experiencing IRF, the GFR at hospital admission and the lowest post discharge GFR were not different (54.8 ± 24.8 mL/min vs. 52.2 ± 25.1 mL/min, p=0.12).
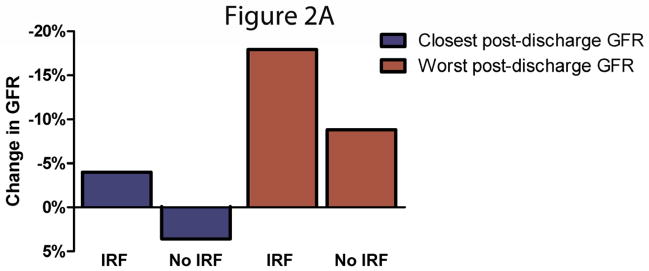
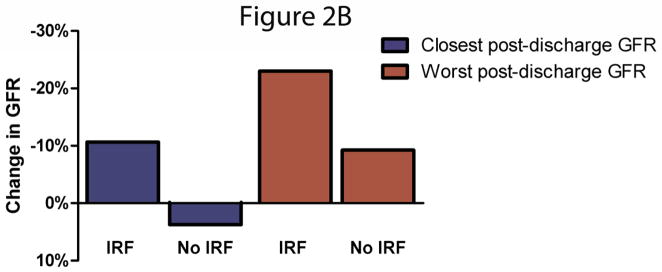
Figure 2.
A/B Discharge to post-discharge change in estimated glomerular filtration rate in patients with and without in-hospital improved renal function. Panel A represents patients with improved renal function at any time during hospitalization and panel B represents patients that continued to meet criteria at the time of discharge. IRF: Improved renal function. IRF defined as a 20% improvement in glomerular filtration rate. Comparison of change in GFR between patients with and without IRF p<0.0001 for all comparisons aside for the closest GFR in patients with and without IRF at discharge where p=0.001.
Associations with mortality
Overall, 44.0% of the population died over a median of 2.6 years (1.2–4.2 years) after hospital discharge. Patients experiencing IRF had a significantly greater incidence of all cause mortality (Table 3 and Supplementary Table 1). Controlling for baseline characteristics, in-hospital treatment characteristics, right ventricular dysfunction, physical exam findings consistent with volume overload, or discharge medications did not eliminate the independent association between IRF and increased mortality (Table 3 and Supplementary Table 1). Of note, IRF was associated with a significantly greater risk for long term mortality than WRF (HR=1.5, 95% CI 1.2–2.0, p=0.001), however WRF itself was not associated with a long term risk for mortality even after excluding patients with IRF from the referent population (HR=0.92, 95% CI 0.72–1.2, p=0.52). However, the short term (30 day) risk for mortality was significantly increased for patients with WRF (HR=3.4, 95% CI 1.1–10.8, p=0.028) but was not significantly different between patients with IRF and WRF (HR=1.1, 95% CI 0.48–2.7, p=0.77).
Table 3.
Association between improved renal function and mortality in consecutively admitted patients
| Association | IRF (Any Time) | IRF (Discharge) | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | P | |
| Unadjusted | 1.4 (1.1–1.7) | 0.002 | 1.5 (1.2–2.0) | <0.001 |
| Adjusted for Baseline Characteristics* | 1.3 (1.1–1.7) | 0.011 | 1.4 (1.1–1.7) | 0.017 |
| Adjusted for In-hospital Treatment Characteristics†,‡ | 1.3 (1.0–1.7) | 0.037 | 1.4 (1.0–1.9) | 0.046 |
| Adjusted for Right-Ventricular Function†,§ | 1.3 (1.0–1.6) | 0.029 | 1.3 (1.0–1.7) | 0.047 |
| Adjusted for Physical Examination c/w Volume Overload†,|| | 1.3 (1.0–1.7) | 0.024 | 1.4 (1.1–2.0) | 0.018 |
| Adjusted for Discharge Medications†,# | 1.3 (1.0–1.6) | 0.027 | 1.3 (1.0–1.7) | 0.047 |
IRF: Improved renal function, HR: Hazard ratio, CI: Confidence interval.
Adjusted for age, race, hypertension, diabetes, coronary artery disease, B-type natriuretic peptide level, serum sodium, hemoglobin, blood urea nitrogen (discharge), GFR (discharge), systolic blood pressure, heart rate, loop diuretic dose, angiotensin converting enzyme inhibitor or receptor blockers, beta blockers, digoxin, and spironolactone use.
Age, sex, race, systolic blood pressure, heart rate, discharge glomerular filtration rate history of diabetes, hypertension, and coronary artery disease forced into the model.
Adjusted for inotropes, peak intravenous loop diuretic dose, total intravenous loop diuretic dose, thiazide diuretics, beta blockers, digoxin, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, spironolactone, and net fluid output.
Adjusted for qualitative right ventricular function (complete data available n=837).
Adjusted for edema, hepatojugular reflux, and jugular venous distention (complete data available n=683).
Adjusted for loop diuretic dose, thiazide diuretic use, beta blockers, digoxin, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and spironolactone.
Inpatient deterioration of renal function following IRF
Given that 42.4% of the patients experiencing IRF during hospitalization had post IRF deterioration of renal function prior to discharge (transient-IRF) in conjunction with the fact that discharge IRF tended to be associated with the greatest risk for mortality, we sought to investigate if patients with transient-IRF may have a different mortality risk than those in which IRF was persistent at discharge. Patients with persistent and transient-IRF had a similar improvement in renal function from admission to best in-hospital GFR (37.1 ± 25.3% vs. 42.7 ± 26.7%, p=0.55) but experienced an average 23.5 ± 14.0% decrease in GFR from their best inpatient GFR to discharge. Notably, those with transient-IRF had a greater net volume loss (6.9 ± 6.1 L vs. 4.8 ± 5.0 L, p=0.004) and length of stay [8 (5–11) days vs. 6 (4–9) days, p=0.005] than patients with persistent-IRF. Discharge GFR (59.4 ± 24.1 mL/min) was not significantly different from admission GFR (58.6 ± 24.1 mL/min) in patients with transient-IRF (p=0.42). Notably, the risk for mortality was significantly lower in patients with transient-IRF compared to persistent-IRF (HR=0.69, 95% CI 0.49–0.97, p=0.032). This association remained after adjusting for discharge GFR (HR=0.63, 95% CI 0.44–0.89, p=0.007) (Figure 3). The risk for mortality amongst patients that had transient-IRF was not different from patients that did not experience IRF at any time point (HR=1.1, 95% CI 0.8–1.5, p=0.41). Given that incomplete decongestion may be a potential contributor to this differential association with mortality, the effect of discharge diuretic dose was examined. After adjusting for baseline characteristics, IRF patients with a deterioration in renal function prior to discharge (transient-IRF) had a significantly greater risk for mortality with higher doses of loop diuretics (HR=1.6 per 100 mg increase, 95% CI 1.1–2.3, p=0.013) whereas patients that met criteria for IRF at discharge (persistent-IRF) had no increased risk associated with higher loop diuretic doses (HR=1.0 per 100 mg, 95% CI 0.8–2.3, p=0.82, p interaction=0.003).
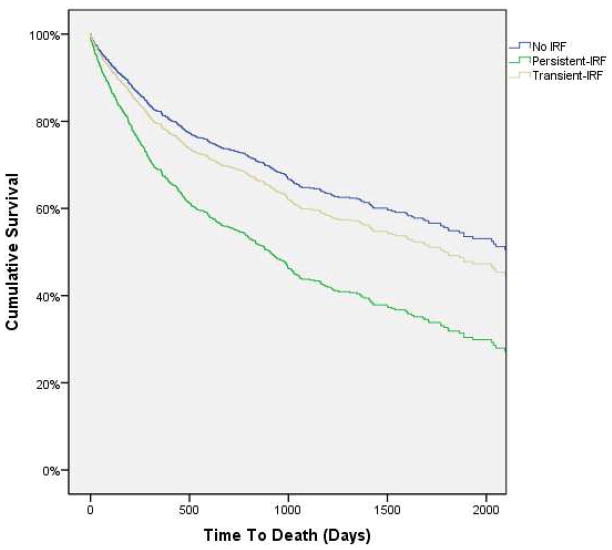
Figure 3.
Survival curves for patients with improvement in renal function persistent at discharge (persistent-IRF), transient improvement in renal function no longer present at discharge (transient-IRF), and no improvement in renal function (no IRF). IRF: Improved renal function. IRF defined as a ≥20% improvement in glomerular filtration rate. Survival curves are adjusted for discharge glomerular filtration rate. P=0.032 for the difference in mortality between patients with persistent-IRF and transient-IRF.
Outpatient deterioration of renal function following IRF
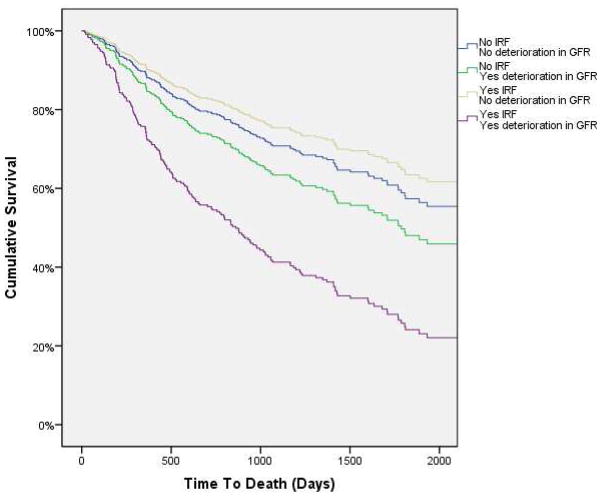
Given the poor prognosis associated with IRF and the high rate of deterioration in renal function after discharge, we sought to investigate if a differential mortality association was present between those whose renal function remained stably improved compared to those in which it deteriorated again. In patients who experienced a deterioration in GFR post discharge (highest post-discharge GFR < discharge GFR) there was a significantly greater incidence of death (HR=1.5, 95% CI 1.1–2.1, p=0.009). This association tended to strengthen after adjusting for discharge GFR (HR=1.7, 95% CI 1.2–2.2, p=0.002). Amongst patients meeting criteria for IRF at discharge, those with a deterioration in GFR had a significantly increased risk for mortality (adjusted for discharge GFR HR=2.1, 95% CI 1.2–3.5, p=0.006). However, IRF patients with stable renal function after discharge were not at increased risk for mortality (adjusted for discharge GFR HR=0.8, 95% CI 0.4–1.6, p=0.49, p interaction=0.038) (Figure 4).
Figure 4.
Survival curves for the combination of groups with or without improvement in renal function and with or without deterioration in renal function after discharge. IRF: Improved renal function. IRF defined as a ≥20% improvement in glomerular filtration rate at discharge. Deterioration in renal function defined as the highest available post discharge glomerular filtration rate less than the discharge glomerular filtration rate. Survival curves are adjusted for discharge glomerular filtration rate.
Discussion
The principal findings of this study are: 1) Validation of the concept that IRF is a common event during the treatment of acute decompensated heart failure and is associated with a significant, independent, increased risk of mortality. 2) Patients who experience IRF have multiple parameters consistent with venous congestion and right sided cardiac dysfunction at baseline and have a greater degree of diuresis as inpatients. 3) IRF patients frequently experience significant deterioration in renal function prior to admission in addition to a high incidence of post-IRF recurrence of renal dysfunction. 4) The association between IRF and mortality appears to interact with factors such as the degree of inpatient/post-discharge deterioration of renal dysfunction and the dose of discharge loop diuretics. Overall, these findings support the notion that, in the setting of acute decompensated heart failure, IRF identifies a subtype of cardio-renal syndrome with significant therapeutic, prognostic, and research potential.
The observation that IRF is highly correlated with measures of right sided cardiac dysfunction and venous congestion may be central to all of the observations noted in this study. Animal models demonstrate a rapid and pronounced decrease in renal blood flow, glomerular filtration rate, ultrafiltration coefficient, and increase in sodium avidity with experimentally induced venous congestion; aberrations in renal function that improve promptly with relief of the congestion.[16–21] In line with this physiology, we have previously demonstrated that patients with signs of significant venous congestion and right ventricular dysfunction at baseline have frequent improvement in renal function with aggressive diuresis, mirroring the physiology observed in animal models.[12] The finding that directly measured right atrial pressure did not show any detectable association with IRF, when volumetric echocardiographic indices were strongly associated, is not necessarily surprising in light of the well described disconnect between pressure and volume in the venous system.[22] These findings provide further evidence that right atrial pressure is likely a suboptimal tool for the study of venous congestion induced renal dysfunction in heart failure. Notably, the changes in renal function described in this study parallel the natural history of venous congestion/fluid overload frequently seen in heart failure; significant increase in venous congestion prior to presentation with acute decompensated heart failure (WRF as an outpatient), followed by rapid decongestion during hospitalization (IRF as an inpatient), then a reaccumulation of fluid after discharge (recurrent WRF as an outpatient).[23] These observations provide further support for the concept that venous congestion may be a central pathophysiologic factor/target in the cardio-renal syndrome.[9, 12, 24–26]
The mortality associations noted in this study have several potentially important implications. The most direct clinical application of this data is the incremental information for prognostication provided by IRF. Perhaps more importantly, the form of cardio-renal syndrome identified by IRF is, by definition, reversible. Although not testable from these data, it is possible that targeted therapy in these patients could further improve renal function or help to maintain the improvement achieved during standard therapy. The latter may be particularly relevant given that IRF patients who have a recurrence of renal dysfunction after discharge had a substantially increased risk for mortality whereas those who maintained the improved renal function did not have an increased risk for death. As a result, targeted therapy that can prevent recurrent deterioration of renal function (or perhaps more importantly correcting the underlying pathophysiology driving the recurrent renal dysfunction) could possibly reduce the substantial risk for death in these patients.
The observation that patients with transient-IRF did not have a significantly increased risk for mortality may also relate to venous congestion. There is preliminary data to support the notion that aggressive decongestion during the treatment of acute decompensated heart failure, sufficient to cause a reduction in intravascular volume and deterioration in renal function, is associated with significantly improved survival.[9] Notably, patients with transient-IRF had a 33% longer length of stay and a 44% greater net fluid loss but a similar initial improvement in GFR compared to patients with persistent-IRF. A possible explanation for these observations may be that a portion of the IRF associated mortality is being driven by inadequate decongestion. Additional support for the possibility that inadequate decongestion may be an important factor is the finding that higher doses of discharge loop diuretics were strongly associated with increased mortality in patients with transient-IRF (i.e. adequately decongested) but had no significant association with mortality in patients with persistent-IRF (i.e. inadequately decongested). Given the near ubiquitous finding of an association between loop diuretic dose and mortality in prior studies, and the recent description that some subgroups of patients may actually derive a survival benefit from high dose loop diuretics, this highly significant interaction provides additional support for the possibility that inadequate decongestion may be important in determining the outcomes of these patients.[27–31]
Limitations
The single center retrospective design of this study leads to several potential limitations, uncontrolled confounding cannot be excluded, and causality is impossible to demonstrate. Given the observational nature of these data, physicians were not blinded to renal function data points or their respective changes and may have modified treatment in response. Similarly, the extensive inclusion/exclusion criteria resulted in the exclusion of many heart failure admissions, potentially limiting generalizability of the findings. The results of the mortality subanalyses stratified by post-IRF changes in renal function all have significant methodologic limitations, are limited by sample size, and cannot exclude the possibility of selection bias as the primary cause. As a result these analyses should be regarded as hypothesis generating only. Specifically, despite the significantly longer length of stay and greater degree of diuresis in patients with transient vs. persistent-IRF, a cause and effect relationship cannot be proven. There is a significant quantity of missing data with respect to pre and post discharge creatinine levels. Although availability of these creatinine levels was not associated with incident IRF, availability or non-availability of these values was likely not a random event, potentially introducing bias. The analysis demonstrating a differential rate of mortality amongst patients that did or did not suffer deterioration of renal function post discharge may be particularly vulnerable to the above possibility. In addition, availability or non-availability of data points across all the variables studied was not insignificant given the retrospective nature of the study, limiting the ability to built comprehensive models. As a result, residual confounding cannot be excluded. The exclusion of patients admitted to interventional cardiology services does not capture all contrast exposures (i.e. computed tomography) and thus confounding by contrast nephropathy is still possible. The unavailability of cardiac transplantation and ventricular assist device placement events is a limitation. Additionally, the lack of data on rehospitalization is a significant limitation which should be addressed in future studies.
Conclusion
Improvement of renal function during the treatment of acute decompensated heart failure is a common event and serves as a marker of an increased risk for subsequent mortality. Patients that experience IRF during the treatment of decompensated heart failure have a high incidence of pre-admission WRF and evidence of baseline venous congestion. Thus, resolution of venous congestion induced pre-admission WRF may represent the mechanism for these improvements in GFR with treatment. However, the majority of these patients ultimately have recurrence of renal dysfunction and the poor prognosis seen in these patients appears to interact with these subsequent changes in renal function. Although this work is preliminary in nature and should be regarded primarily as hypothesis generating, further study in this area is warranted given the potential research and treatment implications of these findings.
Supplementary Material
Acknowledgments
Funding: NIH Grant 5T32HL007843-15
Footnotes
Disclosures: All authors have no potential conflicts of interest relevant to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akhter MW, Aronson D, Bitar F, Khan S, Singh H, Singh RP, et al. Effect of elevated admission serum creatinine and its worsening on outcome in hospitalized patients with decompensated heart failure. Am J Cardiol. 2004;94:957–60. doi: 10.1016/j.amjcard.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 2.Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B, Investigators P. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH) Eur Heart J. 2006;27:1216–22. doi: 10.1093/eurheartj/ehi859. [DOI] [PubMed] [Google Scholar]
- 3.Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ, et al. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH) European journal of heart failure : journal of the Working Group on Heart Failure of the European Society of Cardiology. 2009;11:847–54. doi: 10.1093/eurjhf/hfp108. [DOI] [PubMed] [Google Scholar]
- 4.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–7. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Logeart D, Tabet J, Hittinger L, Thabut G, Jourdain P, Maison P, et al. Transient worsening of renal function during hospitalization for acute heart failure alters outcome. Int J Cardiol. 2008;127:228–32. doi: 10.1016/j.ijcard.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, et al. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–95. doi: 10.1016/j.ejheart.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 8.van Kimmenade RR, Januzzi JL, Jr, Baggish AL, Lainchbury JG, Bayes-Genis A, Richards AM, et al. Amino-terminal pro-brain natriuretic Peptide, renal function, and outcomes in acute heart failure: redefining the cardiorenal interaction? J Am Coll Cardiol. 2006;48:1621–7. doi: 10.1016/j.jacc.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 9.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–72. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol. 2010;106:1763–9. doi: 10.1016/j.amjcard.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Testani JM, Khera AV, St John Sutton MG, Keane MG, Wiegers SE, Shannon RP, et al. Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am J Cardiol. 2010;105:511–6. doi: 10.1016/j.amjcard.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Testani JM, McCauley BD, Chen J, Shumski M, Shannon RP. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio-renal interactions. Cardiology. 2010;116:206–12. doi: 10.1159/000316038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn J, Kramer N, McDermott D. Validation of the Social Security Death Index (SSDI): An Important Readily-Available Outcomes Database for Researchers. West J Emerg Med. 2008;9:6–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 16.Doty JM, Saggi BH, Sugerman HJ, Blocher CR, Pin R, Fakhry I, et al. Effect of increased renal venous pressure on renal function. The Journal of trauma. 1999;47:1000–3. doi: 10.1097/00005373-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Abildgaard U, Amtorp O, Holstein-Rathlou NH, Agerskov K, Sjontoft E, Christensen NJ, et al. Effect of renal venous pressure elevation on tubular sodium and water reabsorption in the dog kidney. Acta Physiol Scand. 1988;132:135–42. doi: 10.1111/j.1748-1716.1988.tb08310.x. [DOI] [PubMed] [Google Scholar]
- 18.Dilley JR, Corradi A, Arendshorst WJ. Glomerular ultrafiltration dynamics during increased renal venous pressure. The American Journal of Physiology. 1983;244:F650–8. doi: 10.1152/ajprenal.1983.244.6.F650. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto T, Maekawa M, Miyazaki M, Yamamoto K, Ueda J. Effects of renal venous pressure elevation on renal hemodynamics, urine formation and renin release. Jpn Circ J. 1972;36:439–48. doi: 10.1253/jcj.36.439. [DOI] [PubMed] [Google Scholar]
- 20.Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet. 1988;1:1033–5. doi: 10.1016/s0140-6736(88)91851-x. [DOI] [PubMed] [Google Scholar]
- 21.Hamza SM, Kaufman S. Splenorenal reflex modulates renal blood flow in the rat. The Journal of physiology. 2004;558:277–82. doi: 10.1113/jphysiol.2004.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology. 2008;108:735–48. doi: 10.1097/ALN.0b013e3181672607. [DOI] [PubMed] [Google Scholar]
- 23.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–73. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 24.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–74. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 25.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–96. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein L, Massie BM, Leimberger JD, O’Connor CM, Pina IL, Adams KF, Jr, et al. Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Circ Heart Fail. 2008;1:25–33. doi: 10.1161/CIRCHEARTFAILURE.107.746933. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–9. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper HA, Dries DL, Davis CE, Shen YL, Domanski MJ. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation. 1999;100:1311–5. doi: 10.1161/01.cir.100.12.1311. [DOI] [PubMed] [Google Scholar]
- 29.Domanski M, Tian X, Haigney M, Pitt B. Diuretic use, progressive heart failure, and death in patients in the DIG study. J Card Fail. 2006;12:327–32. doi: 10.1016/j.cardfail.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E, et al. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 2003;42:705–8. doi: 10.1016/s0735-1097(03)00765-4. [DOI] [PubMed] [Google Scholar]
- 31.Testani JM, Cappola TP, Brensinger CM, Shannon RP, Kimmel SE. Interaction between Loop Diuretic Associated Mortality and Blood Urea Nitrogen Concentration in Chronic Heart Failure. J Am Coll Cardiol. 2010 doi: 10.1016/j.jacc.2011.01.052. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.