Abstract
Background
Worsening renal function (WRF) in the setting of heart failure has been associated with increased mortality. However, it is unclear if this decreased survival is a direct result of the reduction in glomerular filtration rate (GFR) or if the mechanism underlying the deterioration in GFR is driving prognosis. Given that WRF in the setting of angiotensin converting enzyme inhibitor (ACE-I) initiation is likely mechanistically distinct from spontaneously occurring WRF, we sought to investigate the relative early WRF associated mortality rates in subjects randomized to ACE-I or placebo.
Methods and Results
Subjects in the Studies Of Left Ventricular Dysfunction limited data set were studied (6,377 patients). The interaction between early WRF (decrease in estimated GFR ≥20% at 14 days), randomization to enalapril, and mortality was the primary endpoint. In the overall population, early WRF was associated with increased mortality (adjusted HR=1.2, 95% CI 1.0–1.4, p=0.037). When analysis was restricted to the placebo group, this association strengthened (adjusted HR=1.4, 95% CI 1.1–1.8, p=0.004). However, in the enalapril group, early WRF had no adverse prognostic significance (adjusted HR=1.0, 95% CI 0.8–1.3, p=1.0, p interaction=0.09). In patients that continued study drug despite early WRF, a survival advantage remained with enalapril therapy (adjusted HR=0.66, 95% CI 0.5–0.9, p=0.018).
Conclusions
These data support the notion that the mechanism underlying WRF is important in determining its prognostic significance. Specifically, early WRF in the setting of ACE-I initiation appears to represent a benign event which is not associated with a loss of benefit from continued ACE-I therapy.
Keywords: cardio-renal syndrome, worsening renal function, kidney, ACE inhibitor
Worsening renal function (WRF) has been associated with increased mortality in both inpatients and outpatients with cardiac failure.1–8 In the majority of prior research in this area, WRF has been studied as a single entity, regardless of the inciting mechanism. This may not be unreasonable given that the reduction in glomerular filtration rate (GFR) itself has been proposed as a direct pathophysiologic contributor to heart failure disease progression.9, 10 However, recent data suggest that WRF may actually represent a heterogeneous group of disorders with different underlying mechanisms and potentially prognostic implications.11–13 Notably, some patients appear to be intrinsically susceptible to the development of WRF, a predisposition which occurs independently of treatment such as aggressive diuresis.12 These observations raise the possibility that much of risk associated with WRF is simply a reflection of underlying disease severity and the reduction in GFR may be of secondary importance.
Initiation of an angiotensin converting enzyme inhibitor (ACE-I) therapy causes alterations in intra-glomerular hemodynamics, potentially leading to a decrease in filtration fraction and a resultant reduction in GFR.14 As such, WRF has been reported at an increased frequency following initiation of these agents. Despite this early deterioration in renal function, the long term renal outcomes may actually be superior in patients with early ACE-I induced WRF, with WRF potentially acting as a marker of baseline renal physiology poised to derive the most benefit from renin-angiotensin-aldosterone system antagonism.15–17 However, the effect of ACE-I induced WRF on mortality in the setting heart failure has yet to be determined. The Studies Of Left Ventricular Dysfunction (SOLVD) trials were randomized placebo controlled trials of enalapril in subjects with cardiac dysfunction.18, 19 Given that assignment to enalapril or placebo was random, we hypothesized that if the reduction in GFR is directly causative of the reduced survival associated with early WRF, a similar early WRF associated mortality would be expected in the placebo and enalapril groups. However, if the predominant driver for early WRF associated mortality is advanced cardio-renal disease found in these patients, then early WRF in the enalapril group (which is potentially treatment induced) should prognostically be of limited significance. The primary objective of this study was to compare the relative prognostic importance of early WRF in patients randomized to enalapril compared to placebo in the SOLVD population.
Methods
The of Studies Of Left Ventricular Dysfunction (SOLVD) prevention and treatment trials were National Heart, Lung and Blood Institute (NHLBI) sponsored, randomized, double-blind, placebo controlled trials of the effect of enalapril on in patients with asymptomatic and symptomatic left ventricular dysfunction and comprise the overall SOLVD population. Methods and results have been previously published.18, 19 Briefly, 4,228 patients were enrolled in the prevention trial and 2,569 patients in the treatment trial at 23 international centers (total n= 6797). Inclusion in either trial required an ejection fraction ≤ 35% and age between 21 and 80 years. Patients not receiving medication for the treatment of heart failure who demonstrated no evidence of heart failure at the end of a three week run-in period were eligible for the prevention trial. Eligibility for the treatment trial required a diagnosis of heart failure and the use of medications for this condition. Exclusion criteria included a baseline creatinine level >2.5 mg/dL, severe or unstable coronary or valvular disease, suspected renal artery stenosis, or any other disease that may shorten survival or impede participation in the long term trial. Prior use of angiotensin inhibitor therapy was not a contraindication to enrollment.
Estimated glomerular filtration rate (eGFR) was calculated using the Modified Diet and Renal Disease equation (MDRD).20 Early worsening renal function (early WRF) was defined as a 20% decrease in eGFR from baseline to 14 days after randomization to study drug, the first post-randomization serum creatinine.21 The entire SOLVD population was analyzed as a whole to maximize power given the lack of interaction between trials (prevention vs. treatment) and early WRF in the overall trial (p=0.38), the placebo group (p interaction=0.42), or the enalapril group (p=0.62). The SOLVD trials were conducted and supported by the NHLBI in collaboration with the SOLVD study investigators. This analysis was conducted using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the SOLVD investigators or the NHLBI.
Statistical Methods
The primary analyses in this study focused on the risk for mortality associated with early WRF in patients randomized to placebo or enalapril. The primary endpoint was the interaction between early WRF, mortality, and study drug assignment. Values reported are mean ± standard deviation, median (quartile 1 - quartile 4), and percentile. Independent Student’s t-test or the Mann-Whitney U test was used to compare continuous parameters. Pearson’s Chi Square was used to evaluate categorical variables. Proportional hazards modeling was used to evaluate time to event associations with all cause mortality. Candidate covariates for multivariable modeling were obtained by screening all baseline variables with a univariate association with mortality (p≤0.2). Covariates were removed using backwards elimination (likelihood ratio) and variables with a p<0.2 were retained.22 For the primary analyses, modeling was repeated using forced entry of all covariates producing similar results (data not shown). Goodness of fit was tested using the added variables version of the Groennesby and Borgan test and reported as the likelihood-ratio p value. Survival curves for death from any cause were plotted for the four combinations of groups between patients that did or did not experience early WRF and patients receiving placebo or enalapril. The same covariates used in the primary multivariable models were used in the adjusted survival curves. The x-axis was terminated when the remaining number at risk was <10%. Significance was defined as 2-tailed p<0.05 for all analyses excluding tests of interaction where p values < 0.1 were considered significant. Statistical analysis was performed with PASW Statistics version 18.0 (SPSS Inc, Chicago, Illinois) and STATA 11.0 (College Station, TX, U.S.A).
Results
Baseline characteristics and the effect of randomization to enalapril on mortality in the SOLVD trials have been previously reported.18, 19 Additionally, the strong association between baseline renal dysfunction and reduced survival has been previously described in both asymptomatic and symptomatic patients in the SOLVD dataset.23 Characteristics of the 6,377 patients with data on renal function both at baseline and 14 days are presented in Table 1. In total, 606 patients (9.5%) experienced early WRF between baseline and 14 days post randomization with a mean decrease in eGFR of 29.2 ± 9.8% in the enalapril group and 28.9 ± 9.3% in the placebo group. Patients experiencing early WRF at 14 days had a significant recovery of renal function by one year (p<0.0001) and the degree of recovery was similar between those assigned to enalapril or placebo (16.0 ± 34.1% vs. 18.2 ± 38.0%, p=0.52). Characteristics of patients with and without early WRF are presented in Table 1. Early WRF was not significantly associated with all cause mortality in a univariate model (HR=1.2, 95% CI 0.98–1.4, p=0.10). However, baseline eGFR was significantly higher in patients that ultimately developed early WRF (Table 1) potentially confounding this association. After adjustment for baseline eGFR, early WRF demonstrated a highly significant association with mortality (HR=1.4, 95% CI 1.2–1.7, p<0.0001). This association remained significant after extensive adjustment for baseline characteristics associated with mortality (age, race, ejection fraction, heart rate, diastolic blood pressure, New York Heart Association class, serum sodium, eGFR, history of diabetes, hypertension, stroke or myocardial infarction, loop diuretic, potassium sparing diuretic, digoxin, beta blocker use, and randomization to enalapril) (HR=1.2, 95% CI 1.0–1.4, p=0.037) (goodness of model fit test p=0.48).
Table 1.
Baseline patient characteristics of the overall cohort and patients with and without worsening renal function
| Characteristics | Overall Cohort (n=6,377 ) | Early WRF | P | |
|---|---|---|---|---|
| Yes (n=606, 9.5%) | No (n=5,771, 90.5%) | |||
| Demographics | ||||
| Age | 59.3 ± 10.2 | 59.8 ± 10.1 | 59.3 ± 10.2 | 0.237 |
| White race | 89.1% | 88.3% | 89.2% | 0.514 |
| Male | 85.7% | 82.0% | 86.1% | 0.006 |
| Past Medical History | ||||
| Hypertension | 38.4% | 40.6% | 38.2% | 0.251 |
| Diabetes | 19.0% | 21.6% | 18.7% | 0.087 |
| Stroke | 6.5% | 6.4% | 6.5% | 0.976 |
| Prior myocardial infarction | 75.2% | 73.6% | 75.4% | 0.332 |
| Physical Examination | ||||
| Heart rate | 76.1 ± 12.3 | 77.0 ± 12.6 | 76.0 ± 12.2 | 0.079 |
| Systolic blood pressure (mmHg) | 119.3 ± 16.7 | 119.1 ± 16.5 | 119.3 ± 16.8 | 0.685 |
| Diastolic blood pressure (mmHg) | 74.2 ± 9.9 | 74.1 ± 9.9 | 74.2 ± 9.9 | 0.790 |
| Medications (Baseline) | ||||
| Digoxin | 32.9% | 37.1% | 32.5% | 0.020* |
| Loop diuretic | 31.9% | 38.4% | 31.2% | <0.001* |
| Beta blocker | 18.0% | 14.5% | 18.3% | 0.020* |
| Potassium sparing diuretic | 6.1% | 6.8% | 6.0% | 0.439 |
| Enalapril | 49.8% | 53.5% | 49.5% | 0.060 |
| Laboratory Value | ||||
| Serum sodium (mmol/L) | 139.5 ± 3.0 | 139.4 ± 3.0 | 139.5 ± 3.0 | 0.447 |
| Estimated glomerular filtration rate (mL/min/1.73m2) | 65.6 ± 19.1 | 79.5 ± 28.0 | 64.1 ± 17.3 | <0.001* |
| Estimated glomerular filtration rate < 60 mL/min/1.73m2 | 39.8% | 22.9% | 41.6% | <0.001* |
| Blood urea nitrogen (mg/dL) | 18.8 ± 6.9 | 18.7 ± 7.1 | 18.8 ± 6.9 | 0.680 |
| Creatinine (mg/dL) | 1.2 ± 0.3 | 1.0 ± 0.3 | 1.2 ± 0.3 | <0.001* |
| Functional Status/Ejection Fraction | ||||
| Left ventricular ejection fraction (%) | 27.0 ± 6.3 | 26.4 ± 6.4 | 27.1 ± 6.2 | 0.011* |
| New York Heart Association class | 1.7 ± 0.7 | 1.8 ± 0.7 | 1.7 ± 0.7 | 0.001* |
Early WRF: Worsening renal function. Early WRF defined as a 20% reduction in glomerular filtration rate from baseline to 14 days post randomization.
Significant p value.
Of the 6377 patients in the current analysis, 49.8% were randomized to enalapril and 50.2% were randomized to placebo. The net deterioration in eGFR from baseline to 14 days after randomization was slightly greater in the enalapril group compared to placebo (−0.7 ± 14.2 mL/min/1.73m2 vs. 0.4 ± 15.4 mL/min/1.73m2, p=0.002). Early WRF tended to occur more frequently in the enalapril group but this difference did not meet statistical significance (OR=1.2, 95% CI 0.99–1.4, p=0.06). In patients assigned to placebo, early WRF was significantly associated with increased mortality (HR=1.3, 95% CI 1.1–1.7, p=0.012). This relationship was strengthened by adjusting for baseline eGFR (HR=1.7, 95% CI 1.4–2.2, p<0.0001) and persisted after extensively adjusting for baseline characteristics associated with mortality (age, race, ejection fraction, heart rate, diastolic blood pressure, New York Heart Association class, serum sodium, eGFR, history of diabetes, hypertension, stroke or myocardial infarction, loop diuretic, potassium sparing diuretic, digoxin, and beta blocker use) (HR=1.4, 95% CI 1.1–1.8, p=0.004) (goodness of model fit test p=0.34). However, in patients randomized to enalapril, early WRF was not associated with increased mortality (HR=1.0, 95% CI 0.78–1.3, p=1.0, p interaction=0.09) (Figure 1). This lack of association persisted after adjustment for baseline eGFR (HR=1.2, 95% CI 0.94–1.5, p=0.15, p interaction=0.04) and baseline characteristics associated with mortality (HR=1.0, 95% CI 0.78–1.3, p=1.0, p interaction=0.09). Goodness of fit test was p=0.26 for the model in the strata of patients randomized to enalapril and p=0.28 for the full interaction model. Analysis of alternative definitions of early WRF including larger reductions in renal function produced similar results (Table 2). Although not randomized interventions, interactions were not detectable between early WRF and the use of b-blockers (p=0.92) or loop diuretics (p=0.40) with respect to mortality. Similarly, no significant interactions between randomization to enalapril and baseline predictors of mortality were detected (data not shown) with the exception of baseline ejection fraction (interaction p=0.023). This interaction appeared to be predominantly driven by a greater efficacy of enalapril in patients with an ejection fraction below the median value of 28% (HR=0.78, 95% CI 0.68–0.88) compared to patients with an ejection fraction above the median (HR=1.0, 95% CI 0.88–1.2, p=0.61).
Figure 1. Kaplan Meier curves grouped by presence or absence of early worsening renal function and randomization to enalapril or placebo.
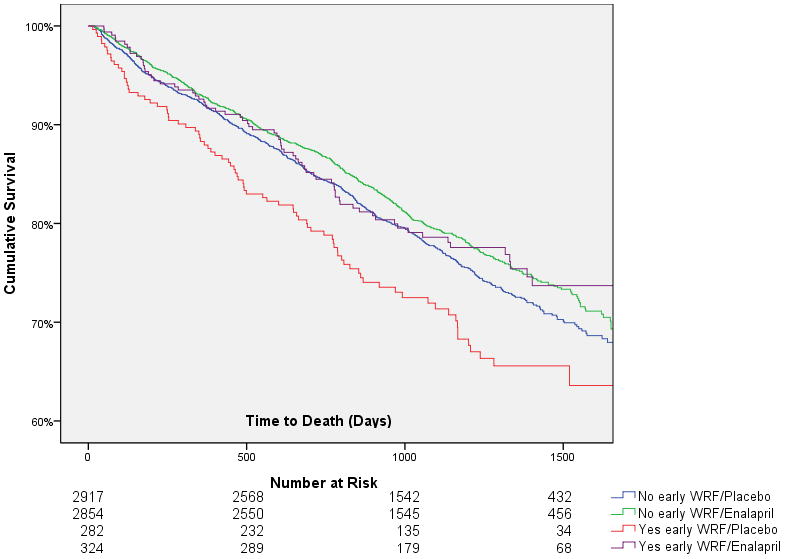
WRF: Worsening renal function. Early WRF defined as a 20% reduction in glomerular filtration rate from baseline to 14 days post randomization.
Table 2.
Incidence and risk of death associated with various definitions of early worsening renal function in patients randomized to placebo or enalapril.
| WRF Definition | Overall Incidence n (%) | Mean increase in creatinine mg/dl ± SD | OR (95% CI) for WRF with enalapril | p | Placebo group | Enalapril group | Adjusted Interaction p* | ||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted HR* (95% CI) | p | Adjusted HR* (95% CI) | p | ||||||
| ≥ 20% decrease in GFR | 606 (9.5) | 0.37 ± 0.23 | 1.2 (0.99–1.4) | 0.06 | 1.4 (1.1–1.8) | 0.004 | 1.0 (0.78–1.3) | 1.00 | 0.09 |
| ≥ 50% decrease in GFR | 30 (0.5) | 0.90 ± 0.46 | 1.5 (0.73–3.1) | 0.26 | 2.6 (1.2–5.7) | 0.015 | 0.75 (0.24–2.4) | 0.62 | 0.098 |
| ≥ 0.3 mg/dl increase in creatinine | 397 (6.2) | 0.46 ± 0.24 | 1.3 (1.1–1.6) | 0.01 | 1.5 (1.1–1.9) | 0.006 | 1.1 (0.83–1.4) | 0.55 | 0.16 |
| ≥ 25% increase in creatinine | 418 (6.6) | 0.43 ± 0.24 | 1.1 (0.9–1.4) | 0.28 | 1.6 (1.2–2.0) | 0.001 | 1.1 (0.8–1.4) | 0.66 | 0.07 |
| Both a ≥ 0.3 mg/dl and a 25% increase in creatinine | 316 (5.0) | 0.49 ± 0.26 | 1.2 (0.99–1.6) | 0.06 | 1.7 (1.3–2.4) | <0.001 | 1.1 (0.82–1.5) | 0.46 | 0.05 |
| ≥ 0.5 mg/dl increase in creatinine | 71 (1.1) | 0.86 ± 0.33 | 1.5 (0.9–2.4) | 0.11 | 2.1 (1.3–3.6) | 0.004 | 1.1 (0.68–1.9) | 0.61 | 0.13 |
WRF: Worsening renal function, SD: Standard deviation, OR: Odds ratio, HR: Hazard ratio, CI: Confidence interval. eGFR: Estimated glomerular filtration rate. All WRF definitions are calculated from change in creatinine from baseline to 14 days post randomization. Covariates adjusted for: age, race, ejection fraction, heart rate, diastolic blood pressure, NYHA class, serum sodium, eGFR, history of diabetes, hypertension, stroke or myocardial infarction, loop diuretic, potassium sparing diuretic, digoxin, and beta blocker use.
Overall 12.7% of the population had a reduction in dose or discontinuation of study drug within 1 month of the 14 day assessment of renal function; however, only 0.8% were coded as secondary to azotemia. Randomization to enalapril was associated with a significantly increased incidence of reduction/discontinuation of study drug for any reason (OR=1.3, p<0.001) or due to azotemia (OR=2.6, p=0.002). However, analysis of only patients whose study drug was not dose reduced/discontinued did not change the strong association between early WRF in the placebo group and death (adjusted HR=1.4, 95% CI 1.1–1.9, p=0.004) (goodness of fit test p=0.26) or the lack of association in the enalapril group (adjusted HR=0.95, 95% CI 0.7–1.3, p=0.70, p interaction=0.04). The goodness of fit test p=0.34 for the model in patients randomized to enalapril and p=0.28 for the full interaction model. Interestingly, amongst patients that continued study drug, the greatest survival advantage with randomization to enalapril vs. placebo seemed to be in patients that developed early WRF (adjusted HR=0.66, 95% CI 0.5–0.9, p=0.018) (goodness of fit test p=0.17) as opposed to the survival advantage of enalapril in patients without early WRF (adjusted HR=0.9, 95% CI 0.8–1.0, p=0.10) (goodness of fit p=0.46) (Figure 2). The above models were adjusted for age, race, ejection fraction, heart rate, diastolic blood pressure, New York Heart Association class, serum sodium, eGFR, history of diabetes, hypertension, stroke or myocardial infarction, loop diuretic, potassium sparing diuretic, digoxin, and beta blocker use. Notably, there were no significant differences in any baseline characteristic amongst patients with early WRF assigned to enalapril vs. placebo (data not shown).
Figure 2. Adjusted curves grouped by randomization to enalapril or placebo and subsequent early worsening renal function status in patients who did not discontinue or dose reduce the study drug in proximity to worsening renal function.
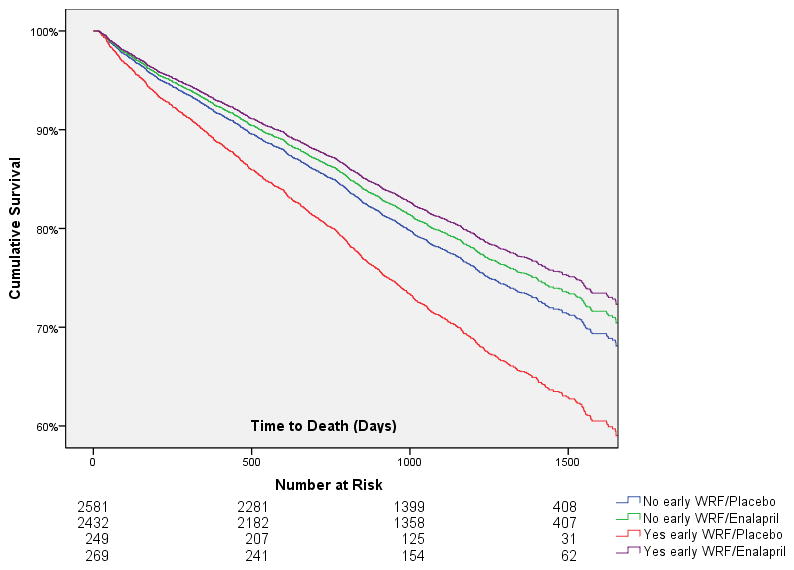
WRF: Worsening renal function. Early WRF defined as a 20% reduction in glomerular filtration rate from baseline to 14 days post randomization. Covariates adjusted for: age, race, ejection fraction, heart rate, diastolic blood pressure, NYHA class, serum sodium, eGFR, history of diabetes, hypertension, stroke or myocardial infarction, loop diuretic, potassium sparing diuretic, digoxin, and beta blocker use.
Discussion
The primary finding of this analysis is the strong interaction between early WRF associated mortality and randomization to enalapril or placebo in a large population with cardiac dysfunction. Early WRF was associated with significantly increased mortality in subjects randomized to placebo. However, in the group randomized to enalapril, early WRF was free from adverse prognostic significance. Notably, the development of early WRF did not appear to reduce the survival benefit imparted by enalapril. These results provide strong support for the concept that WRF is not a prognostically uniform syndrome and the mechanism underlying WRF may be critical in determining the subsequent prognosis.
The renin-angiotensin-aldosterone axis plays a critical role in the regulation of intra-renal hemodynamics.14 Particularly in the setting of heart failure, angiotensin II can have an important role in the preservation of GFR. This results from preferential vasoconstriction of the efferent arteriole leading to an increased filtration fraction and maintenance of GFR, despite an overall decrease in renal blood flow.24–26 However, the degree to which a compensatory increase in filtration fraction occurs is variable and as a result changes in GFR secondary to ACE inhibition is highly unpredictable with some patients experiencing a significant reduction in GFR and some with a substantial improvement.27–29 This inter-patient variability is the result of the linear relationship between GFR, renal plasma flow, and filtration fraction (GFR = renal plasma flow * filtration fraction) since ACE-I reliably cause an increase in renal plasma flow but a variable decrease in filtration fraction.28, 30
The finding of a strong differential influence of early WRF on mortality between patients randomized to enalapril and placebo in the setting of a small nonsignificant increase in the incidence of early WRF can likely be explained by the above referenced physiology. One possible explanation would be that initiation of enalapril did not directly cause any new cases of early WRF but somehow completely eliminated the negative prognostic effects of early WRF. This scenario seems unlikely given that ACE inhibitor use has been prevalent in the largely contemporary populations where WRF associated mortality has been described. A more likely explanation is that enalapril initiation was the direct cause of some cases of early WRF, but this was offset by a lower rate of spontaneously occurring early WRF facilitated by the well known positive effects of ACE inhibition on systemic and renal hemodynamics. As a result, despite the similar incidence of early WRF in both groups, the underlying mechanism causing early WRF may have been different, potentially explaining the differential mortality between groups.
Regardless of whether enalapril caused a shift in the underlying mechanism or mitigated the resultant mortality, the fact remains that patients randomized to enalapril did not experience increased mortality associated with early WRF whereas those randomized to placebo had substantially worsened survival with early WRF. Exclusion of patients that had their study drug dose reduced/stopped in proximity to the early WRF did not eliminate this finding. Furthermore, at one year, recovery of renal function in patients with early WRF was significant and similar between patients assigned to placebo or enalapril. Notably, the survival benefit associated with enalapril remained present in patients that developed early WRF. As a result, these data provide some reassurance that even relatively large (i.e. ~30%) early deteriorations in renal function following initiation of an ACE-I may not indicate an adverse clinical event or that the patient will not derive benefit from continuation of the medication. Notably, even patients with a 50% reduction in GFR (average increase in serum creatinine of 0.9 mg/dl) after enalapril initiation did not have increased mortality associated with early WRF. However, the American Heart Association scientific statement regarding ACE-I initiation in heart failure patients recommends that an increase in serum creatinine of ≥ 0.5 mg/dl may be an indication to discontinue ACE-I therapy.31 Although the current data indicate that further study of this phenomenon is needed, the small number of large increases in creatinine in the SOLVD population limits the conclusions that can be drawn from these analyses. As such, best clinical judgment on a case by case basis should be used in instances of large increases in creatinine after ACE-I initiation. Nonetheless, these data provide evidence to suggest that ACE-I therapy should not be withdrawn after an early reduction in renal function of moderate severity.
Limitations
This study was a post hoc retrospective analysis and as a result residual confounding cannot be excluded. The SOLVD trial was not designed to investigate early WRF and given that treating physicians were not blinded to renal data, treatment strategies were likely modified in response to these variables. It is impossible to discern what percentage of the enalapril/early WRF group had early WRF as a direct result of randomization to enalapril. As a result, there are an unknown percentage of subjects in the enalapril group that likely had spontaneous early WRF unrelated to enalapril, possibly reducing the effect size. Patients with severe renal insufficiency (creatinine >2.5 mg/dL) were excluded from SOLVD limiting generalization to this group of patients. Although randomization to enalapril was associated with a greater survival advantage in patients that developed early WRF, this finding was the result of a post-randomization subgroup analysis and thus causality cannot be determined. As a result, this result should be interpreted with caution. Although the average decrease in eGFR was not small in the early WRF group, larger potentially clinically significant deteriorations in renal function likely triggered modifications in therapy and thus the outcome of continuation of ACE inhibitor in patients with large reductions in eGFR cannot be determined from this analysis. The limited number of patients with severe heart failure also limits generalization. Although the SOLVD trials took place prior to the routine use of medications such as beta blockers and aldosterone antagonists, and replication prospectively in contemporary populations would be valuable; the lack of clinical equipoise in denying ACE-I therapy to patients with cardiac dysfunction will likely make this impossible.
Conclusion
In patients with left ventricular dysfunction, early WRF in the setting of ACE-I initiation is free of adverse prognostic significance, as opposed to early WRF in patients not treated with ACE-I which is associated with significantly reduced survival. Notably, patients with early WRF in the setting of ACE-I initiation do not appear to lose the survival benefit imparted by use of the ACE-I therapy. These findings add further evidence to the notion that WRF is a heterogeneous disorder and provide reassurance that ACE-I associated early WRF may be free of prognostic importance.
Acknowledgments
Sources of Funding
NIH Grant 5T32HL007843-15: The funding source had no role in study design, data collection, analysis or interpretation.
Footnotes
Disclosures
None.
References
- 1.Akhter MW, Aronson D, Bitar F, Khan S, Singh H, Singh RP, Burger AJ, Elkayam U. Effect of elevated admission serum creatinine and its worsening on outcome in hospitalized patients with decompensated heart failure. Am J Cardiol. 2004;94:957–960. doi: 10.1016/j.amjcard.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 2.Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B, Investigators P. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: Results of the prospective outcomes study in heart failure (posh) Eur Heart J. 2006;27:1216–1222. doi: 10.1093/eurheartj/ehi859. [DOI] [PubMed] [Google Scholar]
- 3.Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ, investigators C. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: Results from the coordinating study evaluating outcome of advising and counseling in heart failure (coach) European journal of heart failure: journal of the Working Group on Heart Failure of the European Society of Cardiology. 2009;11:847–854. doi: 10.1093/eurjhf/hfp108. [DOI] [PubMed] [Google Scholar]
- 4.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: Systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Logeart D, Tabet J, Hittinger L, Thabut G, Jourdain P, Maison P, Tartiere J, Solal A. Transient worsening of renal function during hospitalization for acute heart failure alters outcome. Int J Cardiol. 2008;127:228–232. doi: 10.1016/j.ijcard.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei Cas L. Worsening renal function in patients hospitalised for acute heart failure: Clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–195. doi: 10.1016/j.ejheart.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 8.van Kimmenade RR, Januzzi JL, Jr, Baggish AL, Lainchbury JG, Bayes-Genis A, Richards AM, Pinto YM. Amino-terminal pro-brain natriuretic peptide, renal function, and outcomes in acute heart failure: Redefining the cardiorenal interaction? J Am Coll Cardiol. 2006;48:1621–1627. doi: 10.1016/j.jacc.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 9.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Ronco C, McCullough PA, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House A, Katz NM, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Cardiorenal syndromes: An executive summary from the consensus conference of the acute dialysis quality initiative (adqi) Contrib Nephrol. 2010;165:54–67. doi: 10.1159/000313745. [DOI] [PubMed] [Google Scholar]
- 11.Aronson D, Burger AJ. The relationship between transient and persistent worsening renal function and mortality in patients with acute decompensated heart failure. J Card Fail. 2010;16:541–547. doi: 10.1016/j.cardfail.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Testani JM, Cappola TP, McCauley BD, Chen J, Shen J, Shannon RP, Kimmel SE. Impact of worsening renal function during the treatment of decompensated heart failure on changes in renal function during subsequent hospitalization. Am Heart J. 2011;161:944–949. doi: 10.1016/j.ahj.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner BM, Rector FC. Brenner & rector’s the kidney. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 15.Hillege HL, van Gilst WH, van Veldhuisen DJ, Navis G, Grobbee DE, de Graeff PA, de Zeeuw D. Accelerated decline and prognostic impact of renal function after myocardial infarction and the benefits of ace inhibition: The cats randomized trial. Eur Heart J. 2003;24:412–420. doi: 10.1016/s0195-668x(02)00526-2. [DOI] [PubMed] [Google Scholar]
- 16.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: Is this a cause for concern? Arch Intern Med. 2000;160:685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 17.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, Parving HH, Brenner BM, Shahinfar S, Lambers Heerspink HJ. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80:282–287. doi: 10.1038/ki.2011.79. [DOI] [PubMed] [Google Scholar]
- 18.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The solvd investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 19.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The solvd investigattors. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F Chronic Kidney Disease Epidemiology C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Testani JM, McCauley BD, Chen J, Shumski M, Shannon RP. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio-renal interactions. Cardiology. 2010;116:206–212. doi: 10.1159/000316038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 23.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 24.Packer M. Adaptive and maladaptive actions of angiotensin ii in patients with severe congestive heart failure. Am J Kidney Dis. 1987;10:66–73. [PubMed] [Google Scholar]
- 25.Suki WN. Renal hemodynamic consequences of angiotensin-converting enzyme inhibition in congestive heart failure. Arch Intern Med. 1989;149:669–673. [PubMed] [Google Scholar]
- 26.Badr KF, Ichikawa I. Prerenal failure: A deleterious shift from renal compensation to decompensation. N Engl J Med. 1988;319:623–629. doi: 10.1056/NEJM198809083191007. [DOI] [PubMed] [Google Scholar]
- 27.Cody RJ, Ljungman S, Covit AB, Kubo SH, Sealey JE, Pondolfino K, Clark M, James G, Laragh JH. Regulation of glomerular filtration rate in chronic congestive heart failure patients. Kidney Int. 1988;34:361–367. doi: 10.1038/ki.1988.189. [DOI] [PubMed] [Google Scholar]
- 28.Creager MA, Halperin JL, Bernard DB, Faxon DP, Melidossian CD, Gavras H, Ryan TJ. Acute regional circulatory and renal hemodynamic effects of converting-enzyme inhibition in patients with congestive heart failure. Circulation. 1981;64:483–489. doi: 10.1161/01.cir.64.3.483. [DOI] [PubMed] [Google Scholar]
- 29.Packer M, Lee WH, Medina N, Yushak M, Kessler PD. Functional renal insufficiency during long-term therapy with captopril and enalapril in severe chronic heart failure. Ann Intern Med. 1987;106:346–354. doi: 10.7326/0003-4819-106-3-346. [DOI] [PubMed] [Google Scholar]
- 30.Munger MA. Renal functional alterations induced by angiotensin-converting enzyme inhibitors in heart failure. Ann Pharmacother. 1993;27:205–210. doi: 10.1177/106002809302700216. [DOI] [PubMed] [Google Scholar]
- 31.Schoolwerth AC, Sica DA, Ballermann BJ, Wilcox CS. Renal considerations in angiotensin converting enzyme inhibitor therapy: A statement for healthcare professionals from the council on the kidney in cardiovascular disease and the council for high blood pressure research of the american heart association. Circulation. 2001;104:1985–1991. doi: 10.1161/hc4101.096153. [DOI] [PubMed] [Google Scholar]


