In this series we draw attention to medicines that have entered the European market with an entirely new mechanism of action. Publication is not to be confused with endorsement of use in clinical practice. Copyright to the images belongs to Leiden University, but use of the images (also available at http://coo.lumc.nl/trc) is free.
Indication
Tolvaptan [1, 2] (Samsca®) is indicated in Europe for the treatment of patients with hyponatremia secondary to syndrome of inappropriate antidiuretic hormone secretion (SIADH). In the US, tolvaptan is also indicated for hypervolemic and euvolemic hyponatremia (sodium concentration <125 mmol l–1) in patients with heart failure and cirrhosis.
Mechanism
Symptomatic hyponatremia can accompany heart failure, SIADH and liver cirrhosis. Increased concentrations of vasopressin (also known as antidiuretic hormone, ADH) contribute via overstimulation of vasopressin-2 (V2) receptors in renal collecting ducts (Figure 1A). Consequently, the synthesis and transport of aquaporin channels is enhanced, water is retained and plasma osmolality decreases as drinking continues (due to dietary habit rather than to thirst), urine volume is reduced but urinary Na+ excretion continues.
Figure 1.
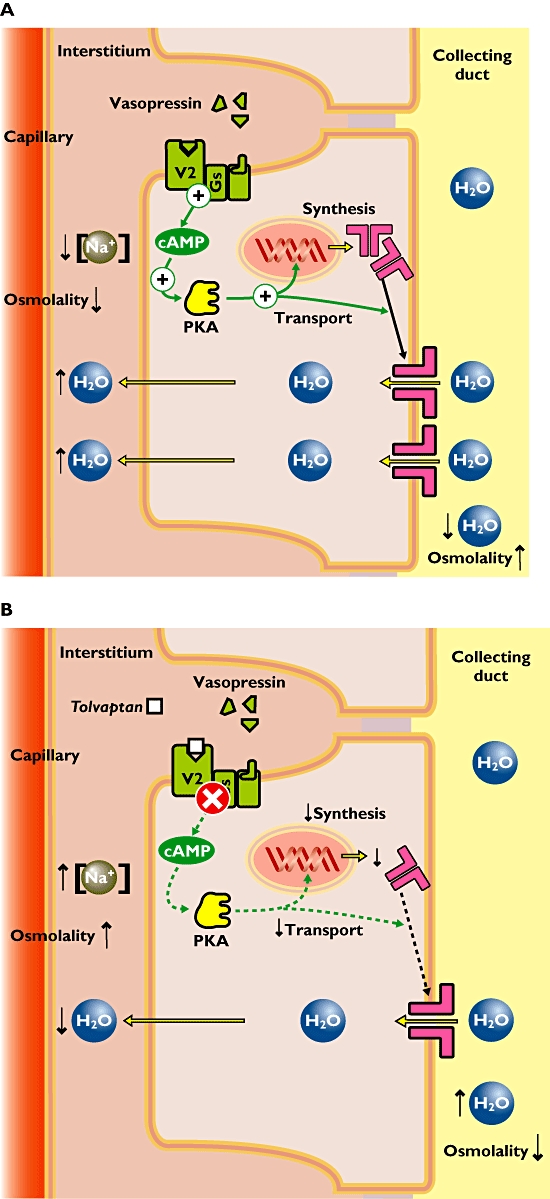
(A) Pathophysiology of hyponatremia. Increased concentrations of vasopressin (or antidiuretic hormone) evoke high activity of the vasopressin-2 (V2) receptors in the kidney. The signal transduction involves activation of the adenylate cyclase (not shown), increased cAMP and activation of protein kinase A (PKA). This results in enhanced expression of aquaporin channels and their transport towards the membrane. These channels transport water molecules from the collecting duct back into the circulation achieving both decreased plasma osmolality and decreased urine production. (B) Mechanism of action of tolvaptan in hyponatremia. The V2 receptor antagonist tolvaptan blocks the vasopressin effect in the kidney. This results in decreased expression of aquaporin channels and lower amounts of aquaporin channels in the apical membrane. Fewer water molecules are retained and more water is excreted in the urine. Eventually the plasma osmolality increases and normal plasma sodium concentrations are achieved
Tolvaptan is the first orally active, specific V2 receptor antagonist to be licensed for use in man. It counteracts the actions of vasopressin by blocking the V2 receptor, thereby decreasing the expression of the aquaporin channels (Figure 1B). This causes (i) an increase in free water clearance, (ii) a decrease in urine osmolality and (iii) an increase in serum sodium concentration.
The starting dose for tolvaptan is 15 mg. This may be increased up to 60 mg daily depending on serum sodium concentration and volume status.
Adverse effects
The water diuresis resulted in thirst, polyuria and dry mouth in up to 5% of the treated patients [3]. In some cases (mainly in heart failure patients) dehydration occurred. Serious effects, such as cardiogenic shock and pulmonary embolism, were slightly more frequent in tolvaptan-treated patients compared with placebo. Tolvaptan has teratogenic effects in animal models and is therefore contraindicated during pregnancy and breastfeeding.
REFERENCES
- 1. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000980/human_med_001046.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124.
- 2.Ghali JK, Hamad B, Yasothan U, Kirkpatrick P. Fresh from the pipeline: tolvaptan. Nat Rev Drug Discov. 2009;8:611–2. doi: 10.1038/nrd2946. [DOI] [PubMed] [Google Scholar]
- 3.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C, for the SALT Investigators Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]


