Abstract
AIM
This study compared the responses between patients with unilateral sciatica and pain-free volunteers following administration of intradermal capsaicin.
METHODS
Fourteen patients with unilateral sciatica and 12 pain-free volunteers received one injection per hour over 4 h of 1 µg and 10 µg capsaicin, into each calf. For each dose, spontaneous pain (10 cm VAS), area of flare (cm2) and the sum of allodynia and hyperalgesia radii across eight axes (cm) were recorded pre-injection and at 5, 15, 30, 45 and 60 min post injection.
RESULTS
Sciatica subjects experienced higher spontaneous pain and hyperalgesia responses in both legs compared with pain-free volunteers. The largest mean difference in spontaneous pain was 2.8 cm (95% CI 1.6, 3.9) at 5 min in the unaffected leg following 10 µg. The largest mean difference in hyperalgesia was 19.7 cm (95% CI 12.4, 27.0) at 60 min in the unaffected leg following 10 µg. Allodynia was greater in patients than in controls with the largest mean difference of 2.9 cm (95% CI 1, 4.8) at 5 min following 10 µg in the affected leg. Allodynia was also higher in the affected leg compared with the unaffected leg in sciatica patients with the highest mean difference of 3.0 cm (95% CI 1.2, 4.7) at 5 min following 10 µg.
CONCLUSIONS
The responses to intradermal capsaicin are quantitatively and qualitatively different in unilateral sciatica patients compared with pain-free controls.
Keywords: intradermal capsaicin, neuropathic pain, pain model, sciatica
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The intradermal capsaicin model of neuropathic pain has been previously developed and used in healthy volunteers.
Few previous studies have used this model in pain patients.
WHAT THIS STUDY ADDS
We have compared the response to intradermal capsaicin in the painful and nonpainful legs of patients with unilateral sciatica and compared these with healthy, pain-free control subjects. Pain and hyperalgesia responses were enhanced in both legs of patients with unilateral sciatica compared with healthy controls. The time course of hyperalgesia was different in sciatica patients, demonstrating a slower and larger evolution.
Qualitative and quantitative differences between pain patients and healthy controls suggest that the use of a neuropathic pain model such as intradermal capsaicin to screen for novel antineuropathic agents might be superior in patients with pre-existing neuropathic pain syndromes.
Introduction
Experimental pain models have been proposed as tools for potential new therapies for neuropathic pain [1]. However, most work using experimental pain models has been in healthy volunteers [2, 3]. This makes their relevance questionable since the mechanisms by which chronic pain is maintained may not be active in pain-free people. Capsaicin, the hot constituent of the capsicum pepper, is a transient receptor potential vanilloid 1 (TRPV1) receptor agonist [4], expressed on C-fibre polymodal receptors [5]. Altered central nociceptive signal processing, called central sensitization [6], is experimentally reproduced with intradermal (i.d.) capsaicin, through widening of the receptive field of dorsal horn neurons [7–9] to Aβ-fibre input, enabling innocuous touch stimuli to be perceived as painful in allodynia. Increased responsiveness to Aδ inputs [8–12] allows hyperalgesia to occur in the uninjured skin surrounding tissue injury [13, 14].
Local application of capsaicin has some attractions as a model for neuropathic pain as it reliably reproduces some of the key symptoms of neuropathic pain, i.e. allodynia and hyperalgesia. Allodynia is due to an increased central response to a given input from rapidly conducting low-threshold Aβ-mechanoreceptors [15] and hyperalgesia is mediated through C-fibres and high threshold, short-diameter Aδ-fibres [15]. Additionally, surrounding the injection site there is an area of flare, or redness, lasting between 30–90 min, due to a local axon reflex [16].
Intradermal capsaicin has been found to have a dose-dependent relationship with flare, allodynia and hyperalgesia responses in pain-free volunteers [8], replicated in further studies [7, 12, 13, 17–22]. Intradermal rather than topical capsaicin is preferred because of superior spatial resolution of response. Intradermal capsaicin has been shown to detect the effects of a single dose of pregabalin in healthy volunteers [23]. Hence the i.d. capsaicin model is potentially a useful tool to study the mechanisms of neuropathic-type symptoms [9, 11, 15, 24], and the efficacy of analgesic drugs in relieving these symptoms [7, 23, 25].
Despite the extensive literature on the use of capsaicin models in pain-free volunteers, little has been published using i.d. capsaicin in pain patients. In a previous study, increased allodynia and hyperalgesia in vulvodynia-afflicted women was observed compared with pain-free controls following 10 µg of i.d. capsaicin [26]. A study in rheumatoid arthritis patients showed similar responses in patients and controls [27].
No study has compared the response of i.d. capsaicin in the affected and non-painful skin areas in patients with unilateral neuropathic limb pain. We have examined the response to i.d. capsaicin in patients with unilateral sciatica. Although the pathology of pain in sciatica is debated, and is certainly heterogeneous, it is a readily recruitable population and a poorly treated painful condition in which a component of neuropathic pain is present in many patients [28, 29]. We hypothesized that a) in patients with unilateral sciatica, the response to i.d. capsaicin would be amplified compared with pain-free controls and that b) responses in the affected and unaffected limbs may differ. We also wished to assess the acceptability of the technique in patients with chronic pain. Our hypothesis was that response would be greater in patients with pain.
Methods
Participants
Patients with unilateral sciatica and pain-free volunteers were recruited from direct advertising at the Royal Adelaide Hospital (RAH) and a database of subjects who previously enrolled into studies at the Pain and Anaesthesia Research Clinic (PARC).
Sciatica was diagnosed on clinical grounds by the presence of pain in the L5/S1 dermatomal distribution accompanied by dysaesthesia of a shocking or burning quality of pain. Patients were to have negligible pain symptoms in the contralateral leg.
Inclusion and exclusion criteria
Key inclusion criteria included having both lower limbs present, being greater than 18 years of age and having fair skin colour required to observe the flare response. Subjects on paracetamol or non-steroidal anti-inflammatory drugs (NSAIDs) were required to withhold medication for 24 h prior to the study to exclude any medication effect on the capsaicin response. Subjects were excluded if using controlled-release opioids within 2 weeks prior to the study, or if using gabapentin, pregabalin or tricyclic antidepressants within 6 weeks of the trial. Other exclusion criteria included previous topical capsaicin treatment, previous enrolment into a study involving i.d. or topical capsaicin, a known allergy to capsaicin, being pregnant or breastfeeding, significant scarring or burns to the back of the legs and a urine drug screen inconsistent with the patient's medication history or positive for non-medical drugs. For patients with sciatica, additional inclusion criteria included negligible pain symptoms in the unaffected leg, a minimum duration of symptoms of 3 months and sciatica to be their dominant pain problem. Pain-free volunteers were excluded if they suffered from a clinically significant painful condition. Ethics approval was obtained from the RAH Research Ethics Committee.
Capsaicin preparation
Capsaicin in 38% hydroxypropyl-β-cyclodextrin solution was prepared and dispensed as described previously [19]. The doses were selected as they were well tolerated and had shown reproducible response profiles in healthy volunteers in an earlier study [19]. For each injection, 10 µl of solution containing either 1 µg or 10 µg of capsaicin was drawn into a 0.3 ml sterile insulin syringe (BD Ultra-Fine II).
Familiarization
As part of the screening session, subjects were familiarized with the four outcome measures of spontaneous pain, area of flare, hyperalgesia and allodynia, described below, in response to a single-blinded saline injection containing no capsaicin. Subjects were told they would receive a ‘very low dose of capsaicin’ with all outcome measures tested prior to, and 5 min post injection. If a subject had an increased response following the dose, they were excluded from the study.
Assessment procedures
This randomized, double-blind study was conducted over a single 4 h session, either in the morning or afternoon. Subjects lay prone on a bed, so that they could not observe the test assessments on the back of the calf, minimizing potential bias. The skin temperature of the test site was fixed at 34–36°C, using a 250 W infrared heat lamp positioned 50 cm from the back of the calf, consistent with a previous study demonstrating this fixed temperature reduced variability in subject response [21]. The temperature was monitored using a thermode on the adjacent skin. Participants received four injections of i.d. capsaicin (1 and 10 µg into each leg) separated by 1 h intervals. Injections were into the upper or lower third of the back of the calf in either the affected leg or unaffected leg. This was to avoid repeated injection in the same site to avoid C-fibre desensitization [30]. The order was according to a random code for site (upper vs. lower) and dose but this was constrained so that left and right sides alternated so that injections into the same leg were separated by 2 h. In the patients with unilateral sciatica, the affected leg was defined as the painful leg and in pain-free volunteers, the affected leg was defined as the dominant leg. No site was injected twice for any subject. Both investigator and participant were blinded to all doses administered.
Assessments
All four assessments (described below) were measured before each capsaicin injection and at 5, 15, 30, 45 and 60 min post injection in the following order: spontaneous pain, area of flare, allodynia and finally hyperalgesia, the most provocative measurement. All measurements except flare were recorded by the same assessor to reduce observer bias.
Spontaneous pain
Spontaneous pain was assessed using a 10 cm visual analogue scale (VAS), where subjects were instructed to mark the severity of their pain from a score of 0, indicating ‘no pain’ to 10, signifying ‘the worst pain imaginable’. This length was recorded with a ruler (cm).
Flare
Area of flare (cm2) was assessed by tracing the visually identified area of reddened skin directly onto an overlying transparent acetate sheet, using a soft-tipped pen. The area was then calculated using digital planimetry.
Hyperalgesia
The sum of hyperalgesia radii (cm) was measured using a calibrated von Frey hair, number 5.46 [19, 22], a plastic rod that bends at a defined pressure of 26 g (TouchTest 800-821-9319, Semmes Weinstein, Stoelting, IL, USA). The von Frey hair was applied along eight compass directions, starting peripherally 10 cm away from the injection site, reapplied at a rate of 1 cm s–1 towards the area of injection site [20]. The participant was instructed to say ‘yes’ at the point where an increase in sensation was noted, and this point was marked on the skin with a soft-tipped pen. The sum of each radii between marked point and injection site was determined. This was more appropriate than measuring an area of hyperalgesia or allodynia, as not all assessments in the eight compass directions resulted in a positive response.
Allodynia
The sum of allodynia radii (cm) was assessed using a foam paintbrush (Foam brush 2*ROYMAC, Australia), gently stroked across the skin along eight compass directions, at a rate of 1 cm s–1 as described above for hyperalgesia. Subjects were instructed to say ‘yes’ if they noticed a ‘change in sensation’, and the sum of these points were then recorded as described for hyperalgesia.
Statistics
A linear mixed model was fitted to each outcome of spontaneous pain (cm), area of flare (cm2), hyperalgesia (sum of radii, cm) and allodynia (sum of radii, cm), predicted by group (sciatica or pain-free), dose (1 µg or 10 µg), location of injection (affected leg or unaffected leg) and time, and their interactions. Data from the upper and lower sites of the calf injected with capsaicin were combined for each leg. Statistical analysis was performed using the SAS 9.1 Program, SAS Institute Inc., Cary, NC, USA.
Results
Participants
Fourteen patients with unilateral sciatica (six males and eight females, mean (SD) age 55.1 ± 9.2 years) and 12 pain-free volunteers (six males and six females, mean (SD) age 52.3 ± 10.4 years) completed this study. In the sciatica group the duration of the disease ranged from 2–35 years, with a mean (SD) of 14.3 ± 12.2 years. The pain severity at rest ranged from 0–4 out of a maximum of 10 on the VAS pain score, and at its worst, the pain was in the range of 7–8.5 out of 10.
Tolerability and safety
Capsaicin doses were well tolerated by all sciatica patients, and no adverse reactions were reported or observed other than the expected local responses.
The mean effect–time curves are shown in Figure 1 for the 1 µg dose and Figure 2 for the 10 µg dose.
Figure 1.
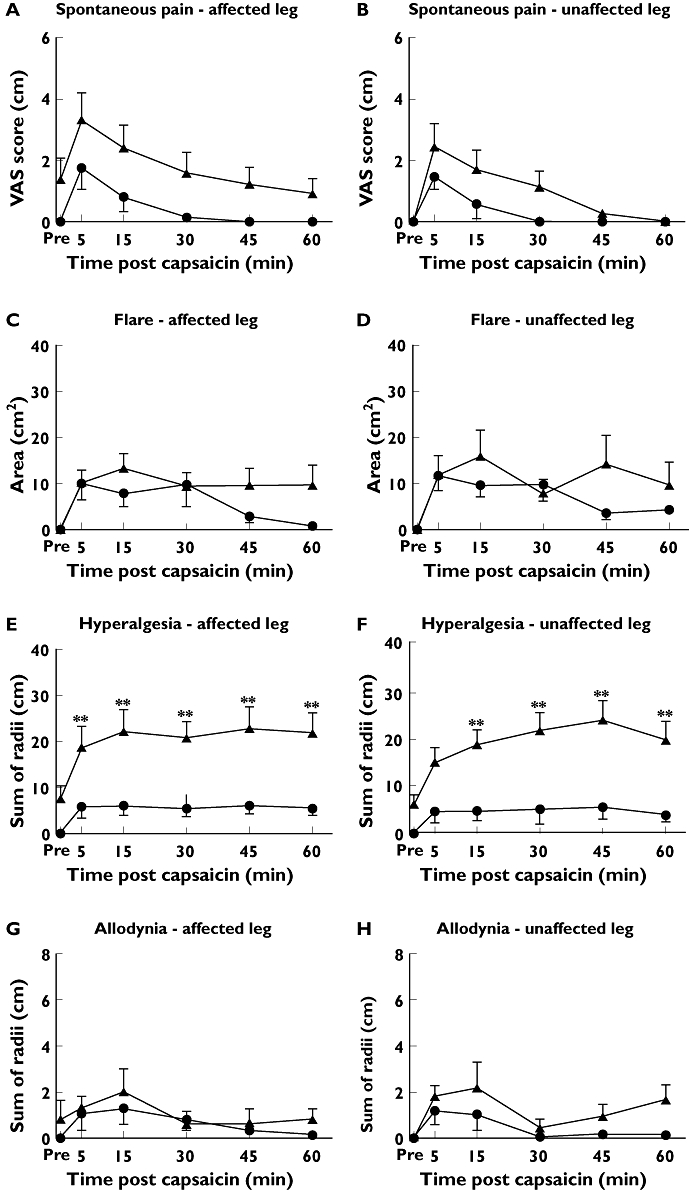
Time course of VAS, flare, hyperalgesia and allodynic response in affected (A, C, E, G) and unaffected legs (B, D, F, H) following 1 µg of intradermal capsaicin to sciatica patients (▴) and pain-free controls (•). *P < 0.01; **P < 0.001
Figure 2.
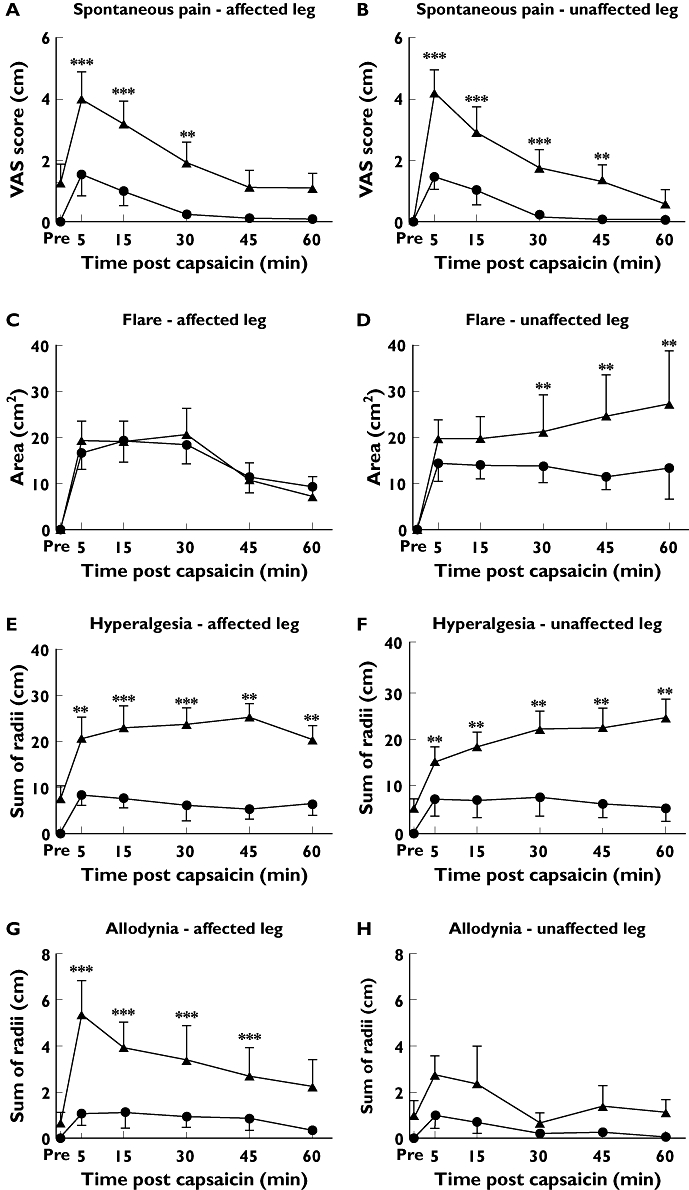
Time course of VAS, flare, hyperalgesia and allodynic response in affected (A, C, E, G) and unaffected legs (B, D, F, H) following 10 µg of intradermal capsaicin to sciatica patients (▴) and pain-free controls (•). *P < 0.01; **P < 0.001
There was generally a greater response to the 10 µg dose compared with 1 µg except that the values for hyperalgesia were comparable. The dose–response relationship was not formally tested as this was not a study objective.
VAS score of spontaneous pain
Effect–time profiles are shown in Figures 1 and 2 panels A and B.
In pain-free controls, baseline values of pain in both legs were zero. In patients, the VAS score values were zero in the unaffected leg, confirming the unilateral nature of symptoms. However, the mean baseline value was 1.2 (95% CI –0.2, 2.6) in the affected leg in patients, indicating a low baseline level of symptoms. The shape of the effect–time profiles for the VAS scores were similar in the sciatica patients and pain-free controls, with their highest mean occurring at t= 5 min, followed by a gradual decline over time. Following a 10 µg dose sciatica patients had a greater response than controls at all time points. The highest mean difference of 2.8 cm (95% CI 1.6, 3.9, P < 0.001) occurred at 5 min in the unaffected leg. In the affected leg, the greatest mean difference was 1.7 cm (95% CI 0.7, 2.8, P < 0.01), at 5 min. VAS scores of spontaneous pain did not differ in the affected vs. the unaffected leg. Following 1 µg, there were no differences between patients and controls at any time point in the affected leg but in the unaffected leg patients had a higher response at 5 and 15 min. The maximum difference of 1.5 cm (95% CI 0.4, 2.6, P < 0.01) was at 5 min.
Flare
Effect–time profiles are shown in Figures 1 and 2 panels C and D. All baseline flare values were zero. In the controls, flare was generally maximal at the first post dose assessment with a decline over time, at both doses and in both limbs. The exception was a flat profile in the unaffected leg following 10 µg. In patients, flare declined more slowly following 1 µg in both limbs so that significant differences from control were observed after 30 min in both legs. The highest mean difference of 12.6 cm2 (95% CI 0.47, 24.7, P < 0.05) occurred at 60 min in the unaffected leg. Following 10 µg administered to the affected leg, the time profile in both patients and controls followed a steady decline and were not different at any time point. In contrast, in the unaffected leg, controls showed a flat profile but flare steadily increased over time in patients with a maximum mean difference from controls of 17.4 cm2 (95% CI 5.2, 29.5, P= 0.005) which occurred at 60 min.
Hyperalgesia
Effect–time profiles are shown in Figures 1 and 2 panels E and F. All baseline hyperalgesia values were zero in the controls, but there were mean positive values in the patients, more in the affected leg. Sciatica patients had significantly higher hyperalgesia responses than pain-free volunteers at all times in both legs at both 1 µg and 10 µg doses and the time profile hyperalgesia differed between sciatica patients and controls. In controls, mean hyperalgesia response was highest at 5 min, and decreased slightly over time at both 1 µg and 10 µg doses. In contrast, sciatica patients had the lowest post-baseline response at 5 min, with responses increasing very gradually over time in the affected limb, and increasing along a higher gradient in the unaffected limb. This was greatest in the unaffected leg at the 10 µg dose, with sciatica patients having a mean of 19.7 cm higher hyperalgesia radius sum (95% CI 12.4, 27.0, P < 0.001) compared with the unaffected leg in controls at 60 min.
Allodynia
Effect–time profiles are shown in Figures 1 and 2, panels G and H. All baseline allodynia values were zero in the controls but mean positive values were recorded for the patients. Allodynia response was maximal at 5 min and decreased over time in both sciatica patients and controls. Sciatica patients had higher allodynia responses than controls across all time points and at both 1 µg and 10 µg doses, however this was only significant in the affected leg following 10 µg, with sciatica patients having a 2.9 cm greater (95% CI 1.0, 4.8) sum of radii than pain-free volunteers at 5 min (P < 0.01). In sciatica patients, comparing limbs, the affected leg had significantly higher allodynia responses at all time points up to t= 45 min (P < 0.001), with the peak mean difference of 3.0 cm (95% CI 1.2, 4.7) at 5 min.
Discussion
This study primarily aimed to determine if there was a difference in spontaneous pain, area of flare, hyperalgesia and allodynia responses between patients with unilateral sciatic pain and pain-free subjects in response to i.d. capsaicin and in the patients whether the response in the affected and unaffected limbs differed. In order to investigate this, it was necessary that sciatica patients could tolerate the capsaicin doses used and we were concerned that a higher response in patients would not be tolerated and hence dose selection was an important study design consideration.
The 1 µg and 10 µg doses chosen in this study were much lower than the maximum doses used in previous studies investigating pain-free volunteers [20, 21] and equal to the maximum dose used in one study investigating vulvodynia-afflicted women [19]. The dose used in a previous study in rheumatoid arthritis patients was much higher (33-fold higher concentration and 333-fold higher dose) [27]. Our previous work in healthy volunteers suggested that satisfactory responses would be observed in the controls at the doses used in the current study [19]. Placebo was not used in the main study session as it has been previously reported that subjects could identify the characteristic initial burning sensation of the capsaicin injection compared with placebo [5], potentially introducing bias due to unblinding.
There was increased pain in both legs in sciatica patients compared with pain-free controls. Mean pre dose VAS scores of approximately 1 cm are consistent with negligible pain (31). However the mean peak post capsaicin values of approximately 4 cm following a 10 µg dose came within a range generally accepted as being at the lower level of clinically significant pain, used as an entry criterion for treatment studies [31]. This is not due to high baseline levels and hence represents an exaggerated response compared with the controls confirming our hypothesis. This also confirms that the cautious approach in dose selection was appropriate and warranted.
Increased hyperalgesia responses in sciatica patients were significant at all time points, suggesting that chronic pain sufferers have central inputs that are more sensitive to Aδ-fibre input compared with pain-free volunteers. The reduction of hyperalgesia over time in pain-free volunteers is as expected from previous studies [20, 21]. However, in sciatica patients the hyperalgesia response increased steadily over time, particularly in the unaffected leg. This is a novel finding, and may suggest spinal cord wind-up mechanisms in subjects with chronic pain. Increased responses in sciatica patients compared with pain-free subjects have been previously demonstrated in the non-painful volar forearm skin areas of vulvodynia-afflicted women [26]. An explanation proposed is that the sensitized spinal cord in chronic pain patients may be highly sensitive to contralateral inputs from Aδ-fibres following i.d. capsaicin. Contralateral hyperalgesia input is supported by a recent study which identified contralateral hyperalgesia and allodynia responses in both rheumatoid arthritis subjects, an inflammatory type pain, and pain-free volunteers lasting for up to 1 h following 1 µg i.d. capsaicin [27]. The assessment of hyperalgesia response in the unaffected limb of neuropathic pain subjects may be a superior model of spinal cord wind-up using the i.d capsaicin model. Future studies should incorporate a longer duration of assessments following i.d. capsaicin, as the increasing trajectory of hyperalgesia in the unaffected leg of pain subjects may become significantly higher than the affected leg at greater than 1 post injection. Increasing the time between each injection may also reduce potential influences of the i.d. capsaicin in the contralateral leg.
In sciatica patients, allodynia response in the affected leg was significantly greater than in the unaffected leg at all time points, suggesting Aβ-fibres in chronic pain subjects may be received on the unilateral side of the sensitized dorsal horn neuron.
Flare responses showed the smallest differences between pain patients and controls. There were no differences in the affected limb at 10 µg and small differences in the other leg. As the flare is due to a local axon reflex, the small differences in this variable suggest that the differences observed with the other variables were not due to increase response at the level of peripheral nerve.
The strategic function of this study was to assess whether the i.d. capsaicin model in patients is worthy of further development as a screening model for the evaluation of novel analgesics in neuropathic pain. The key question is: What incremental response value does the injected capsaicin provide over standard quantitative sensory testing? This pain model is increasingly being evaluated as a biomarker for patient stratification and assessment of treatment response [32–37]. Firstly, although our patients reported significant functional interference with their daily living as a result of their sciatica, baseline pain scores on the treatment day were relatively low and would not generally qualify such patients for clinical trials. This enables recruitment of the patient population likely to be relatively representative of the true broad community of patients rather than selecting people at the severe end of the spectrum introducing potential bias. Secondly, daily baseline pain is likely to be affected by multiple environmental factors which will vary from day to day. The concept behind giving a standardized stimulus is to try to elicit more reproducibly a response in a controlled manner [38]. Similar logic applies in other provocative stimulation models and it should be noted that provocation technically occurs during standard quantitative sensory testing to thermal and punctate stimuli. However, these are short lived and do not necessarily reflect the sensitization that occurs in neuropathic pain. In this study we have demonstrated enhanced slowly developing and long-lasting hyperalgesia to i.d. capsaicin which may more closely reflect the pathological state of sensitization, potentially suitable for detecting an analgesic drug effect [39]. Future studies should compare the sensitivity of the i.d. capsaicin model to standard quantitative sensory testing to detect treatment responses.
In conclusion, this is the first study to investigate directly the i.d. capsaicin pain model in patients with unilateral sciatica. Capsaicin was well tolerated at 1 µg and 10 µg doses. Higher spontaneous pain and hyperalgesia responses were greatest in sciatica patients compared with pain-free controls, particularly in the unaffected leg. This suggests that patients with pre-existing neuropathic pain have fundamental differences in central nervous system processing compared with pain-free controls, and potentially limits the utility of neuropathic pain models in pain-free volunteers. Future studies using the i.d. capsaicin model should utilize neuropathic pain subjects to help identify neuropathic pain mechanisms and evaluate analgesic efficacy in clinical drug development.
Acknowledgments
The study was funded by the Pain and Anaesthesia Research Clinic, University of Adelaide.
We would like to thank the staff of the Pain and Anaesthesia Research Clinic for assistance with the conduct of the study. We would also like to acknowledge the support of the Florey Honours Bachelor Medical Sciences Scholarship for support of the study.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Arendt-Nielsen L, Curatolo M, Drewes A. Human experimental pain models in drug development: translational pain research. Curr Opin Investig Drugs. 2007;8:41–53. [PubMed] [Google Scholar]
- 2.Staahl C, Olesen AE, Andresen T, Arendt-Nielsen L, Drewes AM. Assessing efficacy of non-opioid analgesics in experimental pain models in healthy volunteers: an updated review. Br J Clin Pharmacol. 2009;68:322–41. doi: 10.1111/j.1365-2125.2009.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staahl C, Olesen AE, Andresen T, Arendt-Nielsen L, Drewes AM. Assessing efficacy of non-opioid analgesics in experimental pain models in healthy volunteers: an updated review. Br J Clin Pharmacol. 2009;68:149–68. doi: 10.1111/j.1365-2125.2009.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 5.Lynn B. Capsaicin: actions on the nociceptive C-fibres and therapeutic potential. Pain. 1990;41:61–9. doi: 10.1016/0304-3959(90)91110-5. [DOI] [PubMed] [Google Scholar]
- 6.Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66:105–8. [PubMed] [Google Scholar]
- 7.Park KM, Max MB, Robinovitz E, Gracely RH, Bennett GJ. Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain. 1995;63:163–72. doi: 10.1016/0304-3959(95)00029-R. [DOI] [PubMed] [Google Scholar]
- 8.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 9.Torebjörk HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992;448:765–80. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- 11.LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1999;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- 12.Sang CN, Gracely RH, Max MB, Bennett GJ. Capsaicin-evoked mechanical allodynia and hyperalgesia cross nerve territories. Evidence for a central mechanism. Anesthesiology. 1996;85:491–96. doi: 10.1097/00000542-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Ali Z, Meyer RA, Campbell JN. Secondary hyperalgesia to mechanical but not heat stimuli following a capsaicin injection in hairy skin. Pain. 1996;68:401–11. doi: 10.1016/s0304-3959(96)03199-5. [DOI] [PubMed] [Google Scholar]
- 14.Raja SN, Campbell JN, Meyer RA. Evidence for different mechanisms of primary and secondary hyperalgesia following heat injury to the glabrous skin. Brain. 1984;107:1179–88. doi: 10.1093/brain/107.4.1179. [DOI] [PubMed] [Google Scholar]
- 15.Koltzenburg M, Lundberg LE, Torebjörk HE. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain. 1992;51:207–19. doi: 10.1016/0304-3959(92)90262-A. [DOI] [PubMed] [Google Scholar]
- 16.Kalman S, Linderfalk C, Wårdell K, Eintrei C, Lisander B. Differential effect on vasodilation and pain after intradermal capsaicin in humans during decay of intravenous regional anesthesia with mepivacaine. Reg Anesth Pain Med. 1998;23:402–8. doi: 10.1016/s1098-7339(98)90015-3. [DOI] [PubMed] [Google Scholar]
- 17.Gazerani P, Anderson OK, Arendt-Nielsen L. Site-specific, dose-dependent, and sex-related responses to the experimental pain model induced by intradermal injection of capsaicin to the foreheads and forearms of healthy humans. J Orofac Pain. 2007;21:289–302. [PubMed] [Google Scholar]
- 18.Gazerani P, Kaeseler A, Arendt-Nielsen L. A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain. 2005;118:155–63. doi: 10.1016/j.pain.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Gustafsson H, Akesson J, Lau CL, Williams D, Miller L, Yap S, Rolan P. A comparison of two formulations of intradermal capsaicin as models of neuropathic pain in healthy volunteers. Br J Clin Pharmacol. 2009;68:511–7. doi: 10.1111/j.1365-2125.2009.03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes A, Macleod A, Growcott J, Thomas I. Assessment of the reproducibility of intradermal administration of capsaicin as a model for inducing human pain. Pain. 2002;99:323–31. doi: 10.1016/s0304-3959(02)00161-6. [DOI] [PubMed] [Google Scholar]
- 21.Liu M, Max MB, Robinovitz E, Gracely RH, Bennett GJ. The human capsaicin model of allodynia and hyperalgesia: sources of variability and methods for reduction. J Pain Symptom Manage. 1998;16:10–20. doi: 10.1016/s0885-3924(98)00026-8. [DOI] [PubMed] [Google Scholar]
- 22.Scanlon GC, Wallace MS, Ispirescu JS, Schulteis G. Intradermal capsaicin causes dose-dependent pain, allodynia, and hyperalgesia in humans. J Investig Med. 2006;54:238–44. doi: 10.2310/6650.2006.05046. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Bolognese J, Calder N, Baxendale J, Kehler A, Cummings C, Connell J, Herman G. Effects of morphine and pregabalin compared with diphenhydramine hydrochloride and placebo on hyperalgesia and allodynia induced by intradermal capsaicin in healthy male subjects. J Pain. 2008;9:1088–95. doi: 10.1016/j.jpain.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Sumikura H, Anderson OK, Drewes AM, Arendt-Nielsen L. Spatial and temporal profiles of flare and hyperalgesia after intradermal capsaicin. Pain. 2003;105:285–91. doi: 10.1016/s0304-3959(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 25.Gottrup H, Juhl G, Kristensen AD, Lai R, Chizh BA, Brown J, Bach FW, Jensen TS. Chronic oral gabapentin reduces elements of central sensitization in human experimental hyperalgesia. Anesthesiology. 2004;101:1400–08. doi: 10.1097/00000542-200412000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Foster DC, Dworkin RH, Wood RW. Effects of intradermal foot and forearm capsaicin injections in normal and vulvodynia-afflicted women. Pain. 2005;117:128–36. doi: 10.1016/j.pain.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Shenker NG, Haigh RC, Mapp PI, Harris N, Blake DR. Contralateral hyperalgesia and allodynia following intradermal capsaicin injection in man. Rheumatology. 2008;47:1417–21. doi: 10.1093/rheumatology/ken251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron R, Binder A. Wie neuropathisch ist die Lumboischialgie? Orthopade. 2004;33:568–75. doi: 10.1007/s00132-004-0645-0. [DOI] [PubMed] [Google Scholar]
- 29.Scholz J, Mannion RJ, Hord DE, Griffin RS, Rawal B, Zheng H, Scoffings D, Phillips A, Guo J, Laing RJC, Abdi S, Decosterd I, Woolf CJ. A novel tool for the assessment of pain: validation in low back pain. PLoS Med. 2009;6:e1000047. doi: 10.1371/journal.pmed.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci. 1998;18:8947–59. doi: 10.1523/JNEUROSCI.18-21-08947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407–14. doi: 10.1016/s1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 32.Andresen T, Staahl C, Oksche A, Mansikka H, Arendt-Nielsen L, Drewes A. Effect of transdermal opioids in experimentally induced superficial, deep and hyperalgesic pain. Br J Pharmacol. 2010;10:1476–5381. doi: 10.1111/j.1476-5381.2010.01180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraft B, Frickey NA, Kaufmann RM, Reif M, Frey R, Gustorff B, Kress HG. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology. 2007;109:101–10. doi: 10.1097/ALN.0b013e31817881e1. [DOI] [PubMed] [Google Scholar]
- 34.Wallace M, Schulteis G, Atkinson JH, Wolfson T, Lazaretto D, Bentley H, Gouaux B, Abramson I. ‘Gold standard’ Studies show that inhaled marijuana is medically safe and effective. Anesthesiology. 2007;107:785–96. doi: 10.1097/01.anes.0000286986.92475.b7. [DOI] [PubMed] [Google Scholar]
- 35.Pöyhiä R, Vainio A. Topically administered ketamine reduces capsaicin-evoked mechanical hyperalgesia. Clin J Pain. 2006;22:32–6. doi: 10.1097/01.ajp.0000149800.39240.95. [DOI] [PubMed] [Google Scholar]
- 36.Gottrup H, Juhl G, Kristensen AD, Lai R, Chizh BA, Brown J, Bach FW, Jensen TS. Chronic oral gabapentin reduces elements of central sensitization in human experimental hyperalgesia. Anesthesiology. 2004;101:1400–8. doi: 10.1097/00000542-200412000-00021. [DOI] [PubMed] [Google Scholar]
- 37.Wallace MS, Quessy S, Schulteis G. Lack of effect of two oral sodium channel antagonists, lamotrigine and 4030W92, on intradermal capsaicin-induced hyperalgesia model. Pharmacol Biochem Behav. 2004;78:349–55. doi: 10.1016/j.pbb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory tsting applied to skin, muscles and viscera. J Pain. 2009;10:556–72. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Chizh BA, Priestley T, Rowbotham M, Schaffler K. Predicting therapeutic efficacy – Experimental pain in human subjects. Brain Res Rev. 2009;60:243–54. doi: 10.1016/j.brainresrev.2008.12.016. [DOI] [PubMed] [Google Scholar]


